Abstract
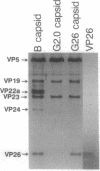
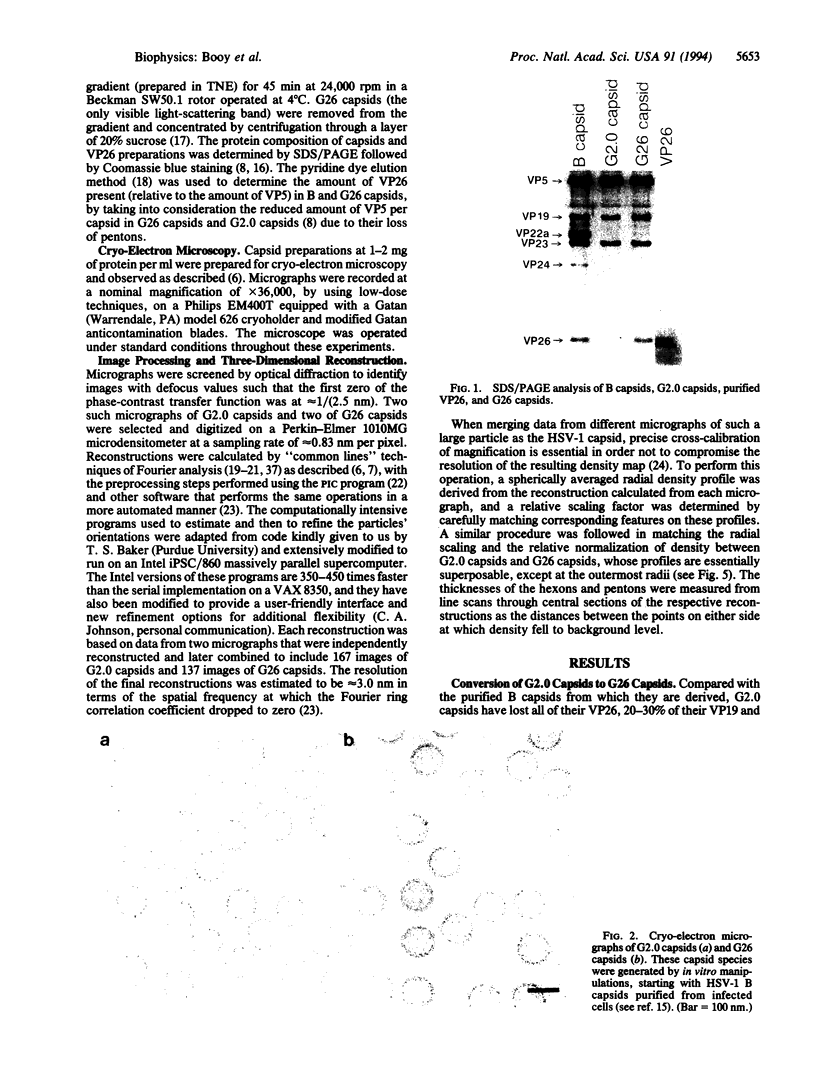
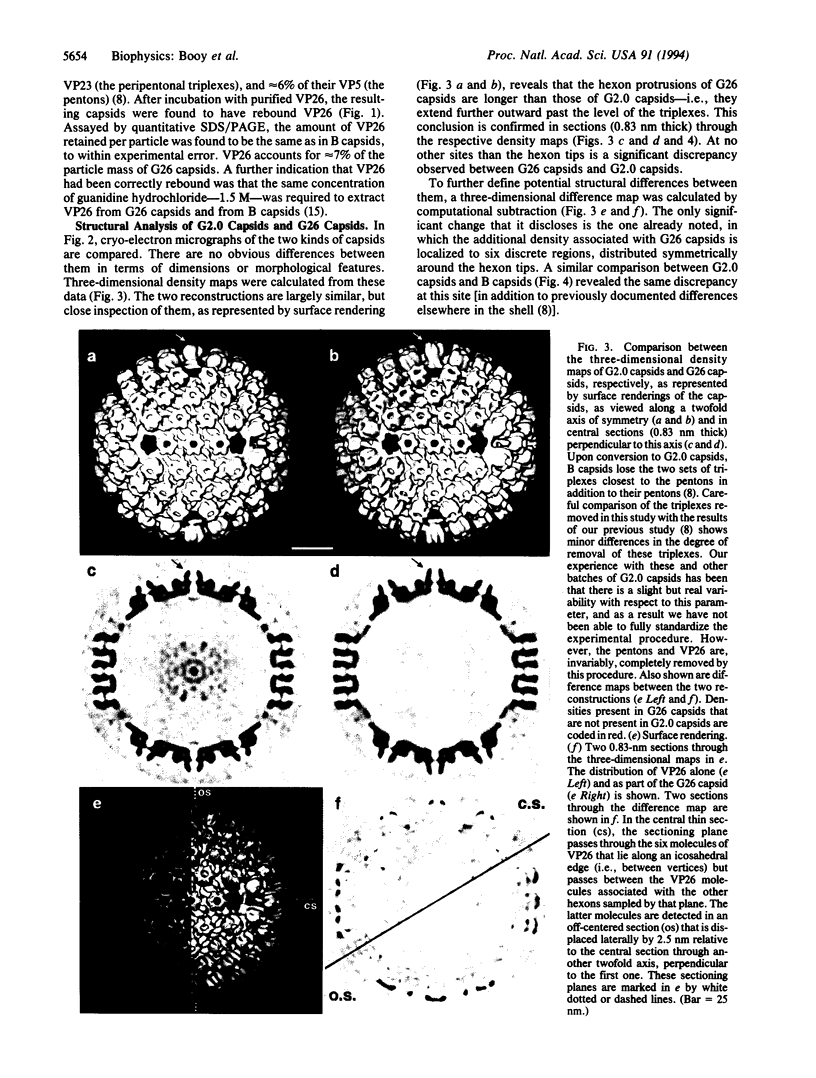
Macromolecular complexes that consist of homopolymeric protein frameworks with additional proteins attached at strategic sites for a variety of structural and functional purposes are widespread in subcellular biology. One such complex is the capsid of herpes simplex virus type 1 whose basic framework consists of 960 copies of the viral protein, VP5 (149 kDa), arranged in an icosahedrally symmetric shell. This shell also contains major amounts of three other proteins, including VP26 (12 kDa), a small protein that is approximately equimolar with VP5 and accounts for approximately 6% of the capsid mass. With a view to inferring the role of VP26 in capsid assembly, we have localized it by quantitative difference imaging based on three-dimensional reconstructions calculated from cryo-electron micrographs. Purified capsids from which VP26 had been removed in vitro by treatment with guanidine hydrochloride were compared with preparations of the same depleted capsids to which purified VP26 had been rebound and with native (undepleted) capsids. The resulting three-dimensional density maps indicate that six VP26 subunits are distributed symmetrically around the outer tip of each hexon protrusion on VP26-containing capsids. Because VP26 may be readily dissociated from and reattached to the capsid, it does not appear to contribute significantly to structural stabilization. Rather, its exposed location suggests that VP26 may be involved in linking the capsid to the surrounding tegument and envelope at a later stage of viral assembly.
Full text
PDF

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aebi U., van Driel R., Bijlenga R. K., ten Heggeler B., van den Broek R., Steven A. C., Smith P. R. Capsid fine structure of T-even bacteriophages. Binding and localization of two dispensable capsid proteins into the P23* surface lattice. J Mol Biol. 1977 Mar 15;110(4):687–698. doi: 10.1016/s0022-2836(77)80084-3. [DOI] [PubMed] [Google Scholar]
- Aldroubi A., Trus B. L., Unser M., Booy F. P., Steven A. C. Magnification mismatches between micrographs: corrective procedures and implications for structural analysis. Ultramicroscopy. 1992 Oct;46(1-4):175–188. doi: 10.1016/0304-3991(92)90013-a. [DOI] [PubMed] [Google Scholar]
- Baker T. S., Drak J., Bina M. Reconstruction of the three-dimensional structure of simian virus 40 and visualization of the chromatin core. Proc Natl Acad Sci U S A. 1988 Jan;85(2):422–426. doi: 10.1073/pnas.85.2.422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T. S., Drak J., Bina M. The capsid of small papova viruses contains 72 pentameric capsomeres: direct evidence from cryo-electron-microscopy of simian virus 40. Biophys J. 1989 Feb;55(2):243–253. doi: 10.1016/S0006-3495(89)82799-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baker T. S., Newcomb W. W., Booy F. P., Brown J. C., Steven A. C. Three-dimensional structures of maturable and abortive capsids of equine herpesvirus 1 from cryoelectron microscopy. J Virol. 1990 Feb;64(2):563–573. doi: 10.1128/jvi.64.2.563-573.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Booy F. P., Newcomb W. W., Trus B. L., Brown J. C., Baker T. S., Steven A. C. Liquid-crystalline, phage-like packing of encapsidated DNA in herpes simplex virus. Cell. 1991 Mar 8;64(5):1007–1015. doi: 10.1016/0092-8674(91)90324-r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway J. F., Trus B. L., Booy F. P., Newcomb W. W., Brown J. C., Steven A. C. The effects of radiation damage on the structure of frozen hydrated HSV-1 capsids. J Struct Biol. 1993 Nov-Dec;111(3):222–233. doi: 10.1006/jsbi.1993.1052. [DOI] [PubMed] [Google Scholar]
- Crowther R. A. Procedures for three-dimensional reconstruction of spherical viruses by Fourier synthesis from electron micrographs. Philos Trans R Soc Lond B Biol Sci. 1971 May 27;261(837):221–230. doi: 10.1098/rstb.1971.0054. [DOI] [PubMed] [Google Scholar]
- Davison M. D., Rixon F. J., Davison A. J. Identification of genes encoding two capsid proteins (VP24 and VP26) of herpes simplex virus type 1. J Gen Virol. 1992 Oct;73(Pt 10):2709–2713. doi: 10.1099/0022-1317-73-10-2709. [DOI] [PubMed] [Google Scholar]
- Dryden K. A., Wang G., Yeager M., Nibert M. L., Coombs K. M., Furlong D. B., Fields B. N., Baker T. S. Early steps in reovirus infection are associated with dramatic changes in supramolecular structure and protein conformation: analysis of virions and subviral particles by cryoelectron microscopy and image reconstruction. J Cell Biol. 1993 Sep;122(5):1023–1041. doi: 10.1083/jcb.122.5.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenner C., Traut R. R., Mason D. T., Wikman-Coffelt J. Quantification of Coomassie Blue stained proteins in polyacrylamide gels based on analyses of eluted dye. Anal Biochem. 1975 Feb;63(2):595–602. doi: 10.1016/0003-2697(75)90386-3. [DOI] [PubMed] [Google Scholar]
- Fuller S. D. The T=4 envelope of Sindbis virus is organized by interactions with a complementary T=3 capsid. Cell. 1987 Mar 27;48(6):923–934. doi: 10.1016/0092-8674(87)90701-x. [DOI] [PubMed] [Google Scholar]
- Ishii T., Yanagida M. Molecular organization of the shell of the Teven bacteriophage head. J Mol Biol. 1975 Oct 5;97(4):655–660. doi: 10.1016/s0022-2836(75)80065-9. [DOI] [PubMed] [Google Scholar]
- McNabb D. S., Courtney R. J. Posttranslational modification and subcellular localization of the p12 capsid protein of herpes simplex virus type 1. J Virol. 1992 Aug;66(8):4839–4847. doi: 10.1128/jvi.66.8.4839-4847.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNabb D. S., Courtney R. J. Posttranslational modification and subcellular localization of the p12 capsid protein of herpes simplex virus type 1. J Virol. 1992 Aug;66(8):4839–4847. doi: 10.1128/jvi.66.8.4839-4847.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molla A., Paul A. V., Wimmer E. Cell-free, de novo synthesis of poliovirus. Science. 1991 Dec 13;254(5038):1647–1651. doi: 10.1126/science.1661029. [DOI] [PubMed] [Google Scholar]
- Newcomb W. W., Brown J. C., Booy F. P., Steven A. C. Nucleocapsid mass and capsomer protein stoichiometry in equine herpesvirus 1: scanning transmission electron microscopic study. J Virol. 1989 Sep;63(9):3777–3783. doi: 10.1128/jvi.63.9.3777-3783.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb W. W., Brown J. C. Induced extrusion of DNA from the capsid of herpes simplex virus type 1. J Virol. 1994 Jan;68(1):433–440. doi: 10.1128/jvi.68.1.433-440.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb W. W., Brown J. C. Structure of the herpes simplex virus capsid: effects of extraction with guanidine hydrochloride and partial reconstitution of extracted capsids. J Virol. 1991 Feb;65(2):613–620. doi: 10.1128/jvi.65.2.613-620.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb W. W., Brown J. C. Use of Ar+ plasma etching to localize structural proteins in the capsid of herpes simplex virus type 1. J Virol. 1989 Nov;63(11):4697–4702. doi: 10.1128/jvi.63.11.4697-4702.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newcomb W. W., Trus B. L., Booy F. P., Steven A. C., Wall J. S., Brown J. C. Structure of the herpes simplex virus capsid. Molecular composition of the pentons and the triplexes. J Mol Biol. 1993 Jul 20;232(2):499–511. doi: 10.1006/jmbi.1993.1406. [DOI] [PubMed] [Google Scholar]
- Radermacher M. Three-dimensional reconstruction of single particles from random and nonrandom tilt series. J Electron Microsc Tech. 1988 Aug;9(4):359–394. doi: 10.1002/jemt.1060090405. [DOI] [PubMed] [Google Scholar]
- Ross P. D., Black L. W., Bisher M. E., Steven A. C. Assembly-dependent conformational changes in a viral capsid protein. Calorimetric comparison of successive conformational states of the gp23 surface lattice of bacteriophage T4. J Mol Biol. 1985 Jun 5;183(3):353–364. doi: 10.1016/0022-2836(85)90006-3. [DOI] [PubMed] [Google Scholar]
- Schrag J. D., Prasad B. V., Rixon F. J., Chiu W. Three-dimensional structure of the HSV1 nucleocapsid. Cell. 1989 Feb 24;56(4):651–660. doi: 10.1016/0092-8674(89)90587-4. [DOI] [PubMed] [Google Scholar]
- Steven A. C., Couture E., Aebi U., Showe M. K. Structure of T4 polyheads. II. A pathway of polyhead transformation as a model for T4 capsid maturation. J Mol Biol. 1976 Sep 5;106(1):187–221. doi: 10.1016/0022-2836(76)90307-7. [DOI] [PubMed] [Google Scholar]
- Thomsen D. R., Roof L. L., Homa F. L. Assembly of herpes simplex virus (HSV) intermediate capsids in insect cells infected with recombinant baculoviruses expressing HSV capsid proteins. J Virol. 1994 Apr;68(4):2442–2457. doi: 10.1128/jvi.68.4.2442-2457.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trus B. L., Newcomb W. W., Booy F. P., Brown J. C., Steven A. C. Distinct monoclonal antibodies separately label the hexons or the pentons of herpes simplex virus capsid. Proc Natl Acad Sci U S A. 1992 Dec 1;89(23):11508–11512. doi: 10.1073/pnas.89.23.11508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WILDY P., RUSSELL W. C., HORNE R. W. The morphology of herpes virus. Virology. 1960 Oct;12:204–222. doi: 10.1016/0042-6822(60)90195-1. [DOI] [PubMed] [Google Scholar]
- Way M., Weeds A. Actin-binding proteins. Cytoskeletal ups and downs. Nature. 1990 Mar 22;344(6264):292–294. doi: 10.1038/344292a0. [DOI] [PubMed] [Google Scholar]
- Yeager M., Berriman J. A., Baker T. S., Bellamy A. R. Three-dimensional structure of the rotavirus haemagglutinin VP4 by cryo-electron microscopy and difference map analysis. EMBO J. 1994 Mar 1;13(5):1011–1018. doi: 10.1002/j.1460-2075.1994.tb06349.x. [DOI] [PMC free article] [PubMed] [Google Scholar]