Abstract
Fungal infections of the central nervous system (CNS) are associated with high mortality rates in immunocompromised patients. Surgical intervention is a mainstay of therapy, but not always possible. We describe the use of medical therapy for the treatment of CNS fungal infections in four pediatric cancer patients. Definitive resection was not performed in any patient. All patients initially received combination antifungal therapy with good clinical response; long-term survival was documented in two patients able to transition to long-term azole therapy. Prolonged antifungal therapy is an important option for treating invasive CNS fungal infections when surgery is not feasible.
Keywords: cancer, central nervous system, pediatric fungal infection, aspergillus, rhizopus
Introduction
Increasing utilization of new and prolonged immunosuppressive therapy has been associated with an increase in Aspergillus and other mold infections in children with underlying malignancies. [1, 2] Dissemination of mold infections to the central nervous system (CNS) is the most serious and life-threatening complication of these infections, with mortality rates of >90%. [3] Aggressive antifungal therapy combined with surgical debridement and long-term antifungal therapy is generally recommended. [3] but limited data are available. [2] The potential importance of surgical resection of CNS aspergillosis has been described [4–6] but no controlled studies performed. We report here the outcomes of children with CNS fungal infections treated with medical therapy.
Methods
All pediatric cancer patients with invasive CNS fungal infections diagnosed at Seattle Children’s Hospital between December, 2002 and December, 2012, were identified. During this period, an average of 240 new cancer patients were diagnosed annually. Patients with confirmed fungal diagnoses were identified through microbiology laboratory records; clinical records were reviewed. This study was approved by Seattle Children’s Institutional Review Board.
Case Descriptions
Patient 1
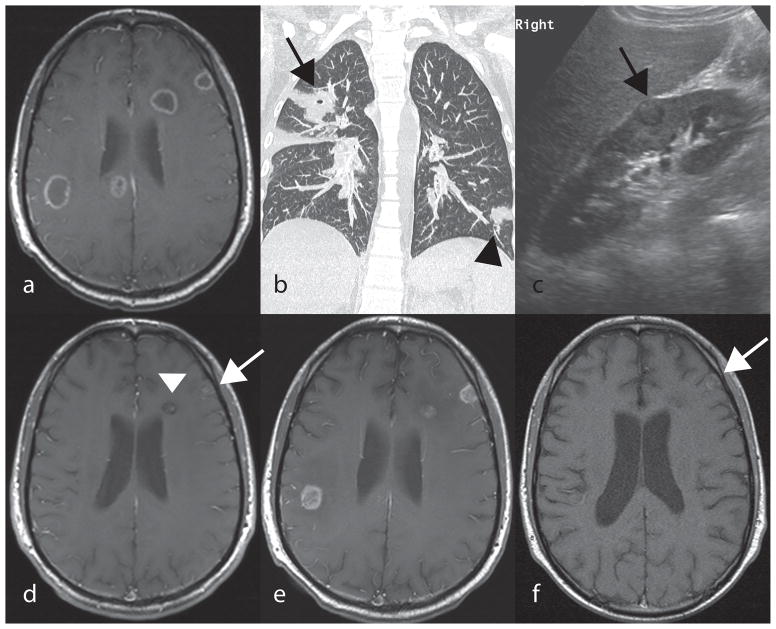
A 19 year old male with acute lymphoblastic leukemia (ALL) in CNS relapse, was undergoing reinduction chemotherapy when a chest CT for prolonged fever showed a pulmonary mass (Figure 1B). Voriconazole and liposomal amphotericin B (AmB) therapy was initiated. AmB was dosed at 10 mg/kg due to its tolerability and higher plasma concentration compared to standard dosing. [7] Lung biopsy specimens grew Aspergillus fumigatus. AmB was stopped and voriconazole continued. A brain MRI showed 9 ring-enhancing lesions with diffusion restriction in the bilateral supratentorial hemispheres. (Figure 1A) Voriconazole dosing was reduced due to visual disturbances and AmB resumed; micafungin was added temporarily several days later when renal fungal lesions were identified. (Figure 1C). A month later, an increase in size of multiple ring-enhancing supratentorial masses was noted. The patient was discharged to hospice on oral voriconazole. While in hospice care, he experienced substantial improvement and subsequently resumed chemotherapy, achieving a second complete remission.
Figure 1.
A,D,E,F: Post contrast axial T1 spin echo of the brain. B: Coronal CT of the chest. C: Ultrasound of the right kidney (long axis). A: Multiple ring enhancing lesions within the periventricular white matter and peripheral cortex of the brain seen at presentation. B: Nodular lung opacity within the left lower lobe (black arrowhead) and a cavitating lung opacity in the right upper lobe (black arrow). C: Round peripheral renal lesion (black arrow) with a hypoechoic rim. D: Significant decrease in number and size of brain lesions (white arrow and arrowhead) after 22 months of antifungal treatment E: Relapse with increase in size of brain lesions showing now rim enhancement following one month off voriconazole therapy due to chemotherapy. F: Significant decrease in number and size of brain lesions showing intrinsic T1 hyperintensity in one of the lesions (white arrow) with absence of enhancement prior to successful HCT transplant, following four months of therapy from relapse in Figure 3E, above.
One year after starting voriconazole, a MRI showed a marked decrease in all brain lesions (Figure 1D), but a second CNS ALL relapse occurred. Voriconazole was replaced by micafungin due to vincristine incompatibility; his fungal CNS lesions increased markedly one month later (Figure 1E). After re-initiation of voriconazole, MRI findings improved (Figure 1F). Six months later, the patient underwent a successful matched unrelated peripheral blood HCT while receiving voriconazole. Two years post-HCT, he remains clinically well on voriconazole with resolving CNS lesions.
Patient 2
A 17-year old male with ALL developed sinusitis while undergoing re-induction chemotherapy for a second marrow relapse 18 months following a mismatched unrelated hematopoietic stem cell transplant.. Endoscopic sinus debridement was performed; cultures grew Rhizopus, Acremonium, and Candida. Initial therapy consisted of high dose AmB (10 mg/kg, increased to 12.5 mg/kg), voriconazole, and continuation of caspofungin. Renal dysfunction ensued, with lower dose AmB given. After one month of antifungal therapy, CT scans revealed persistent sinus opacification but near resolution of pulmonary lesions. Surgical consensus concluded that further intervention would require radical complex resection, considered inappropriate in view of the underlying malignancy. Posaconazole was discontinued after elevation of liver function tests; AmB was continued. Progressive decrease in CNS fungal disease was documented radiographically, but 3 months after fungal diagnosis, the patient relapsed and expired two weeks later.
Patient 3
A 17 year old male with relapsed ALL undergoing salvage chemotherapy presented with fever and painful left-sided facial swelling. Radiographic studies demonstrated pan-sinusitis, osteomyelitis of multiple facial bones, and left temporal lobe abscess. Antibiotics, AmB (10 mg/kg) and micafungin were started. Subsequent MRI demonstrated an increase in temporal lobe abscess size, which was drained and grew Rhizopus species. Widespread sinus disease remained and erosive osteomyelitis of the skull base worsened. Six weeks after initiation of antifungal therapy, the patient developed an acute intracranial hemorrhage and expired.
Patient 4
A 12-year old female presented with post-operative acute hydrocephalus following surgical resection of a posterior fossa ependymoma. MRI studies showed enhancement within the lumbosacral spine and dura; surgical exploration was undertaken. Biopsy specimens demonstrated Aspergillus fumigatus by culture, histopathology, and PCR. Her antifungal regimen initially consisted of voriconazole and AmB 10 mg/kg. Due to increasing spinal cord dysfunction, intrathecal amphotericin B was started via a subdural catheter and escalated to a maximum of 1 mg/dose, but therapy was complicated by Staphylococcus aureus meningitis and the subdural catheter removed.
Two subsequent biopsies of the cerebellum showed evidence of Aspergillus by histology but negative cultures. Increased enhancement in the 3rd ventricle and spine was seen on MRI. Voriconazole was changed to micafungin and AmB continued, with temporary improvement. MRI studies one year later again showed an increase in the third ventricular lesion, and voriconazole replaced micafungin and AmB. MRI scans subsequently stabilized. Four years following her diagnosis, she was changed to single-agent therapy with oral posaconazole and remained on this over the next 6 years, with therapeutic levels maintained with routine monitoring. Following 10 years of antifungal therapy, and three years following stabilization of MRI studies, antifungal therapy was discontinued.
Discussion
We present the results of long-term medical management in four pediatric cancer patients. Radical resection of infected tissue within the brain was not attempted in any patient, and two patients had no CNS surgical intervention. Good response to antifungal therapy was documented, with potential mortality related to fungal infection in only one patient. Surgery is recommended for aspergillosis of the lung, sinuses, and bone, [3] and although limited data is available for CNS infections, twofold reduction in mortality was reported in children with CNS aspergillosis who received neurosurgical intervention. [4] However, some patients may be too ill or have too extensive disease to undergo surgery. While mortality of CNS aspergillosis approached 100% in earlier case series [8] superior safety profiles and CNS penetration of azoles have improved outcomes in CNS aspergillosis [2], and cerebral mucormycosis. [9] The ability to deliver well-tolerated oral antifungal therapy has enabled long-term oral treatment to take place, such that treatment can be potentially curative and not merely palliative. The feasibility of long-term single agent therapy is documented in several of our patients.
Despite the clear improvement in survival with the new azole therapies, potential drawbacks remain. Azole drugs interact with common chemotherapeutic drugs [10, 11] with vincristine toxicity significantly worsened by concomitant azole administration. [12] The replacement of voriconazole with an echinocandin in Patient 1 after one year of therapy during re-induction chemotherapy resulted in disease progression, although subsequent improvement was noted after voriconazole was restarted.
CNS mold infections are a potentially devastating complication in cancer patients, and no randomized controlled studies exist to guide management. In our series, children with substantial invasive CNS fungal disease were successfully treated with long-term antifungal therapy. Our small series does not address the utility of combination antifungal therapy, but after initial radiologic stabilization, our patients continued to improve on long-term oral azole therapy. Therapeutic drug monitoring and routine radiographic monitoring are essential components to this treatment strategy. While surgical intervention remains an important element of management, long-term survival with CNS fungal disease is possible with medical management alone.
Table I.
Patient characteristics and treatment outcome.
| Pt # | Age (YR), Sex |
Underlying disease |
Immunosuppressive medications within 1 month of fungal diagnosis |
Fungal Organism |
Organ System |
Diagnosis Method |
Treatment | Initial Radiologic Response |
Outcome |
|---|---|---|---|---|---|---|---|---|---|
| 01 | 19, M | 19, ALL, second CNS relapse | Prednisone taper, high dose methotrexate | Aspergillus fumigatus | Brain, Lungs, Kidney | Culture | AmB voriconazole micafungin; continues on voriconazole 18 M post-transplant | Improvement in lung and CNS disease after 1 month | Alive with resolving lesions 2 yrs s/p BMT |
| 02 | 15, M | ALL, second marrow relapse | tacrolimus (taper), prednisone 1mg/kg | Rhizopus,, Candida, Acremonium | Brain, Sinus, Lung | Culture | voriconazole, caspofungin posaconazole | Continued decrease in frontal lobe and sinus lesions | Clinical response to therapy but died of ALL relapse |
| 03 | 11, M | ALL, relapsed, not in remission | Clofarabine, Etoposide, Cyclophosphamide | Rhizopus | Brain, Sinus | Pathology, Culture | voriconazole AmB micafungin caspofungin | Progressive disease over first month, with improvement seen after 2nd month | Died of intracranial hemorrhage, possibly related to fungus |
| 04 | 12, F | Posterior fossa ependymoma, post-surgical resection | none | Aspergillus fumigatus | Cerebellum, 3rd ventricle, spinal cord | Pathology, Culture, PCR | AmB caspofungin, voriconazole, posaconazole; total therapy for 10 years | Clinical response; disease free and off therapy for 2 years |
Acknowledgments
This investigation was supported by the National Institutes of Health under Ruth L. Kirschstein National Research Service Award T32CA009351
References
- 1.Dasbach EJ, Davies GM, Teutsch SM. Burden of aspergillosis-related hospitalizations in the United States. Clin Infect Dis. 2000;31:1524–1528. doi: 10.1086/317487. [DOI] [PubMed] [Google Scholar]
- 2.Zaoutis TE, Heydon K, Chu JH, et al. Epidemiology, outcomes, and costs of invasive aspergillosis in immunocompromised children in the United States, 2000. Pediatrics. 2006;117:e711–6. doi: 10.1542/peds.2005-1161. [DOI] [PubMed] [Google Scholar]
- 3.Walsh TJ, Anaissie EJ, Denning DW, et al. Treatment of aspergillosis: clinical practice guidelines of the Infectious Diseases Society of America. Clin Infect Dis. 2008;46:327–360. doi: 10.1086/525258. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz S, Ruhnke M, Ribaud P, et al. Improved outcome in central nervous system aspergillosis, using voriconazole treatment. Blood. 2005;106:2641–2645. doi: 10.1182/blood-2005-02-0733. [DOI] [PubMed] [Google Scholar]
- 5.Imai T, Yamamoto T, Tanaka S, et al. Successful treatment of cerebral aspergillosis with a high oral dose of itraconazole after excisional surgery. Intern Med. 1999;38:829–832. doi: 10.2169/internalmedicine.38.829. [DOI] [PubMed] [Google Scholar]
- 6.Denning DW, Stevens DA. Antifungal and surgical treatment of invasive aspergillosis: review of 2,121 published cases. Rev Infect Dis. 1990;12:1147–1201. doi: 10.1093/clinids/12.6.1147. [DOI] [PubMed] [Google Scholar]
- 7.Walsh TJ, Goodman JL, Pappas P, et al. Safety, tolerance, and pharmacokinetics of high-dose liposomal amphotericin B (AmBisome) in patients infected with Aspergillus species and other filamentous fungi: maximum tolerated dose study. Antimicrob Agents Chemother. 2001;45:3487–3496. doi: 10.1128/AAC.45.12.3487-3496.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lin SJ, Schranz J, Teutsch SM. Aspergillosis case-fatality rate: systematic review of the literature. Clin Infect Dis. 2001;32:358–366. doi: 10.1086/318483. [DOI] [PubMed] [Google Scholar]
- 9.Vehreschild JJ, Birtel A, Vehreschild MJGT, et al. Mucormycosis treated with posaconazole: review of 96 case reports. Crit Rev Microbiol. 2013;39:310–324. doi: 10.3109/1040841X.2012.711741. [DOI] [PubMed] [Google Scholar]
- 10.Nagappan V, Deresinski S. Reviews of anti-infective agents: posaconazole: a broad-spectrum triazole antifungal agent. Clin Infect Dis. 2007;45:1610–1617. doi: 10.1086/523576. [DOI] [PubMed] [Google Scholar]
- 11.Pemán J, Salavert M, Cantón E, et al. Voriconazole in the management of nosocomial invasive fungal infections. Ther Clin Risk Manag. 2006;2:129–158. doi: 10.2147/tcrm.2006.2.2.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Schie RM, Brüggemann RJM, Hoogerbrugge PM, et al. Effect of azole antifungal therapy on vincristine toxicity in childhood acute lymphoblastic leukaemia. J Antimicrob Chemother. 2011;66:1853–1856. doi: 10.1093/jac/dkr223. [DOI] [PubMed] [Google Scholar]