Abstract
Objectives
Cancer survivors often report cognitive problems. Furthermore, decreases in physical activity typically occur over the course of cancer treatment. Although physical activity benefits cognitive function in non-cancer populations, evidence linking physical activity to cognitive function in cancer survivors is limited. In our recent randomized controlled trial, breast cancer survivors who received a yoga intervention had lower fatigue and inflammation following the trial compared to a wait-list control group. This secondary analysis of the parent trial addressed yoga’s impact on cognitive complaints.
Methods
Post-treatment stage 0 – IIIA breast cancer survivors (N = 200) were randomized to a 12-week twice-weekly Hatha yoga intervention or a wait-list control group. Participants reported cognitive complaints using the Breast Cancer Prevention Trial (BCPT) Cognitive Problems scale at baseline, immediately post-intervention, and 3-month follow-up.
Results
Cognitive complaints did not differ significantly between groups immediately post-intervention (p = .250). However, at the 3-month follow-up, yoga participants’ BCPT Cognitive Problems scores were an average of 23% lower than wait-list participants’ scores (p = .003). These group differences in cognitive complaints remained after controlling for psychological distress, fatigue, and sleep quality. Consistent with the primary results, those who practiced yoga more frequently reported significantly fewer cognitive problems at the 3-month follow-up than those who practiced less frequently (p < 0.001).
Conclusions
These findings suggest that yoga can effectively reduce breast cancer survivors’ cognitive complaints, and prompt further research on mind-body and physical activity interventions for improving cancer-related cognitive problems.
Keywords: cancer, oncology, cognition, yoga, physical activity
Background
Breast cancer survivors commonly experience cognitive impairment during survivorship [1, 2]. Accumulating evidence suggests that cancer and its treatment can negatively impact neuropsychological test performance [3-5], although these findings are not without controversy [1, 6]. Cancer-related neuropsychological problems appear to dissipate over time; however, for a subset of cancer survivors, mild impairment may persist over the long-term in several focused cognitive domains, such as verbal ability [6].
In addition to poorer neuropsychological test performance, survivors often report cognitive problems [7]. Although subjective cognitive dysfunction is consistently associated with psychological distress [7, 8], the relationships between subjective and objective cognitive function is less clear. Cross-sectional studies indicate that cognitive complaints may parallel neuropsychological test performance in some domains. For example, breast cancer survivors who reported more memory problems had lower scores on a standardized verbal memory task than those who reported fewer memory problems [9]. On the other hand, breast cancer survivors who just stopped adjuvant endocrine therapy (e.g., tamoxifen or aromatase inhibitors) continued to report cognitive problems over the following year, despite improvement in objective neuropsychological test scores [10]. Whether or not perceptions are mirrored by objective neuropsychological measures, perceived cognitive dysfunction can be disruptive to cancer survivors. For example, one year after cancer treatment, women with more cognitive complaints had lower quality of life scores than those with fewer cognitive complaints [11]. Accordingly, subjective cognitive problems are bothersome for some cancer survivors.
Physical activity benefits cognitive function in non-cancer populations [12, 13], but evidence linking physical activity to cognitive complaints in cancer survivors is limited. Significant de-conditioning and decreases in physical activity typically occur over the course of cancer treatment [14, 15]. Consequently, cognitive complaints among cancer survivors may be at least partially linked to decreased physical activity. A recent meta-analysis concluded that physical activity interventions improved cancer survivors’ overall quality of life, but did not consistently affect their perceived cognitive problems [16]. However, several limitations of the meta-analysis precluded strong conclusions, including the small sample sizes of many studies, as well as the relatively limited number of trials that reported cognitive outcomes. Taken together, these findings suggest that further research is necessary to determine whether physical activity impacts cognitive function for cancer survivors.
Yoga is a particularly appealing exercise intervention for improving cognitive function in breast cancer survivors. With gentle physical activity, breathing practices, and meditation, yoga can be easily adapted for breast cancer survivors who may be experiencing common physical symptoms like pain or fatigue [17]. Indeed, recent randomized controlled trials (RCTs) demonstrated that cancer survivors derived both physical and psychological benefits from yoga [17-19]. In addition, healthy college-aged females performed better on a working memory and inhibitory control task immediately following a yoga practice session compared to a baseline or aerobic exercise session [20]. Yoga can also reduce inflammation [19], one proposed mechanism that may contribute to breast cancer survivors’ cognitive symptoms [21-23].
Several meta-analyses suggest that yoga improves cancer survivors’ fatigue, distress, and quality of life [24-26], but yoga’s impact on cognitive function following cancer treatment is unclear. Indeed, a recent meta-analysis of randomized controlled yoga trials for breast cancer survivors concluded that there was insufficient evidence to evaluate yoga’s cognitive effects, because too few trials reported cognitive outcomes [27]. Studies with larger samples of post-treatment breast cancer survivors, appropriate covariates, and supporting adherence data are necessary to evaluate whether yoga decreases subjective cognitive problems. In our recent RCT, a brief yoga intervention reduced fatigue and inflammation compared to a wait-list control group [19]. In the current paper, we report secondary analyses that examined whether yoga also affected self-reported cognitive complaints.
Methods
Participants
Female stage 0 - IIIA breast cancer survivors (N = 200) were recruited from breast cancer physicians and clinics, community announcements, and breast cancer groups and events for an RCT investigating yoga’s effects on inflammation, fatigue, and depression from 2007 to 2012 (ClinicalTrials.gov identifier: NCT00486525). The sample size was calculated to ensure adequate (80%) power to detect differences in these primary endpoints, requiring 85 participants per group and assuming a 15% attrition rate [19]. Participants’ cancer stage at diagnosis was determined using medical records. Women were eligible for the study if they had completed breast cancer treatment (except for tamoxifen/aromatase inhibitors) between 2 months and 3 years previously. Women were ineligible if they engaged in over 5 hours of vigorous physical activity per week, if they had a prior history of any other cancer (except basal or squamous cell skin cancer), or if they suffered from major medical conditions such as anemia, diabetes, multiple sclerosis, chronic obstructive pulmonary disease, symptomatic ischemic heart disease, uncontrolled hypertension, or liver or kidney failure. Women were also excluded if they had severe cognitive impairment (e.g., dementia, Alzheimer’s disease), or abused alcohol or drugs. Those who reported current yoga practice or prior yoga practice exceeding three months were also excluded. The recruitment and randomization procedures have been described in detail in the primary RCT paper [19]. The institutional review board approved this study, and each participant provided informed consent.
Procedures
Participants completed a variety of self-report measures (described below) during study visits at the Clinical Research Center. Following a baseline study visit, a data manager (who had no participant contact) used an online randomization program to assign participants to a 12-week Hatha yoga intervention (n = 100) or a wait-list control condition (n = 100). Immediately post-intervention and at the 3-month follow-up, participants completed additional questionnaires and provided fasting morning blood samples. Participants were asked not to share their group assignment with the study personnel during study visits.
Trained yoga instructors delivered the yoga intervention, which outlined poses for the 24, twice-weekly, 90-minute sessions (see [19] for detailed information on the yoga protocol). Each of the 25 yoga groups (i.e., cohorts) included between 4 and 20 participants. Sessions were audiotaped, and raters assessed 50% of the tapes for protocol drift. To maximize adherence, yoga instructors called women who missed a class to discuss missed material and to assess barriers for participation. Participants received pamphlets that detailed the poses and breathing exercises from class, and were encouraged to practice at home. Women were also given a commercial yoga video for cancer survivors as a home practice aide. Although instructors did not give specific instructions or requirements for the length of home practice, they gave suggestions for ways to complete the poses at home. Women in the yoga condition used weekly logs to record their combined yoga class and home practice time during the 12-week intervention period; the combined total was used to calculate their average daily minutes spent practicing yoga during the intervention period. Instructors also encouraged yoga participants to continue to practice yoga after the 12-week intervention period ended. However, participants did not log their yoga practice during the follow-up period. Wait-list control participants were told to continue normal activities and refrain from beginning any yoga practice; all participants reported adhering to this guideline. After their 3-month follow-up, women in the wait-list group were offered the option to participate in the yoga classes.
Measures
Self-reported cognitive problems
Participants rated how much they were bothered by cognitive symptoms (i.e., forgetfulness, difficulty concentrating, and being easily distracted) in the past 4 weeks (0 “not at all” to 4 “extremely”) as part of the Breast Cancer Prevention Trial (BCPT) Symptom Checklist [28]. The BCPT Symptom Checklist contains several subscales, and factor analytic studies from 4 samples demonstrated that the 3-item BCPT Cognitive Problems Scale is psychometrically and conceptually appropriate for evaluating cognitive symptoms [29]. The individual item scores were averaged to index cognitive problems, with higher scores indicating more cognitive complaints. The scale demonstrates good internal consistency and discriminant validity [28]; Cronbach’s alpha in our sample was .91 at baseline, .91 at the post-intervention visit, and .93 at the 3-month follow-up.
Covariates
In our primary trial, yoga improved sleep quality and fatigue [19]. In addition, prior research has demonstrated that cognitive complaints are linked to depressive symptoms, anxiety symptoms, and fatigue [7, 29]. Accordingly, we assessed depressive symptoms, anxiety symptoms, fatigue, and sleep quality in order to account for the possibility that they could be responsible for yoga-related differences in self-reported cognitive function.
Women reported current levels of depressive symptoms using the Center for Epidemiological Studies Depression Scale (CES-D), a valid, reliable, and widely-used measure of depressive symptoms [30]. Anxiety symptoms were measured with the Beck Anxiety Inventory (BAI), which has well-established internal consistency and test-retest reliability [31]. Participants rated sleep quality and disturbances using the Pittsburgh Sleep Quality Index (PSQI), which has been used extensively in sleep assessment [32]; higher scores reflect poorer sleep quality. Participants reported vitality in the last month using the Medical Outcomes Study Short Form Health Survey (SF-36) Energy Scale [33], which provided a measure of general energy without assessing the overlapping construct of cognitive fatigue. Higher scores indicate greater vitality and thus lower fatigue.
Inflammation
As part of the parent RCT, fasting blood samples were assayed for lipopolysaccharide (LPS)-stimulated production of interleukin-6 (IL-6), interleukin-1β (IL-1β), and tumor necrosis factor alpha (TNF-α). LPS-stimulated cytokines were measured from isolated peripheral blood mononuclear cells according to Meso Scale Discovery kit instructions (see [19] for detailed methods).
General activity level
At each study visit, the Community Health Activities Model Program for Seniors (CHAMPS) questionnaire was used to assess the average frequency and duration of participants’ engagement in various physical activities in the last month [34, 35]. For each participant, weekly hours spent engaging in activities of moderate-to-high intensity were calculated.
Statistical analyses
In preliminary analyses, we tested for baseline between-group differences in cognitive complaints using an independent samples t-test. In primary analyses, linear mixed models tested whether self-reported cognitive function differed between groups following the intervention. Intervention group, visit, the group by visit interaction, and baseline cognitive complaints were entered as predictors of post-intervention cognitive complaints. To account for repeated post-intervention assessments of each participant and the yoga class cohorts (resulting in partially nested data), subject and intervention cohort were included as random effects. Significant group by visit interactions were decomposed using planned contrasts that tested whether cognitive complaints differed for the yoga and wait-list groups both immediately post-intervention and at the 3-month follow-up. A second set of planned contrasts tested the effect of visit within each group, addressing whether cognitive complaints changed significantly from the immediate post-intervention to 3-month follow-up visits for each group.
We conducted two sets of ancillary analyses. First, we examined whether potential confounds could account for yoga’s effect on cognitive function. To accomplish this goal, we simultaneously included levels of depression, anxiety, fatigue, and sleep quality in the primary model [7, 19, 29]. Because these variables were measured at each study visit, they were included as time-varying covariates.
The second set of ancillary analyses examined whether women who practiced yoga more frequently derived more benefit from the intervention. To test this hypothesis, we repeated the primary analyses and replaced intervention group with the participants’ average minutes of yoga practice per day, which included time spent practicing in class and at home during the intervention. Significant practice by visit interactions were decomposed using planned contrasts that tested the effect of yoga practice at each post-intervention visit. To examine whether yoga practice was associated with change in cognitive complaints over time, a second set of planned contrasts tested the effect of visit at no yoga practice (wait-list participants, 0 minutes per day), lower frequency yoga practice (25th percentile, 18 minutes per day), and higher frequency yoga practice (75th percentile, 29 minutes per day).
Given prior research linking inflammation, physical activity, and cognitive function [13, 20], we also explored the possibility that inflammation mediated yoga’s effect on cognitive complaints. The parent RCT demonstrated that women in the yoga group had lower levels of LPS-stimulated IL-6, IL-1β, and TNF-α than women in the wait-list control group at the 3-month follow-up [19]. Levels of LPS-stimulated IL-6, IL-1β, and TNF-α did not differ between yoga and waitlist groups immediately post-intervention. In exploratory analyses, we tested whether levels of inflammation were associated with cognitive complaints. We tested this possibility in two ways. First, we added each inflammatory marker separately to the primary model individually as a time-varying covariate. These analyses allowed us to examine whether inflammation significantly predicted cognitive complaints. Next, we calculated changes in LPS-stimulated cytokines by subtracting 3-month follow-up values from baseline values; we investigated whether changes in inflammation predicted cognitive problems scores at the 3-month follow-up (controlling for baseline cognitive complaints). Levels of LPS-stimulated IL-6, IL-1β, and TNF-α were natural log-transformed to reduce skew.
Finally, to gain information about participants’ activity level during the follow-up period, we conducted a post-hoc exploratory analysis to test the effect of group on moderate-to-high physical activity levels following the intervention. To do so, we repeated the primary analyses and replaced the cognitive complaints variable with the activity level outcome variable while controlling for baseline activity levels.
Results
Sample description
Demographic and baseline characteristics of the sample are presented in Table 1. Participants were primarily employed (68.5%), Caucasian (88.5%), post-menopausal (81%) women. On average, participants were 10.9 (± 7.9 SD) months post-treatment, with the exception of hormonal therapy. Demographic and disease-related characteristics did not differ significantly between groups. Four women (two in the yoga group and two in the waitlist group) experienced a recurrence of their breast cancer during study enrollment. Importantly, BCPT Cognitive Problems Scale scores did not differ significantly between the two groups at baseline (t(198) = −.45, p = 0.654). On average, participants reported slight-to-moderate bother from cognitive symptoms, which is consistent with previous reports using the BCPT Cognitive Problems Scale [28].
Table 1.
Demographic and baseline characteristics of total sample.
| Total sample (n = 200) | Yoga (n = 100) | Wait-list (n = 100) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Variable | n | % | M | (SD) | n | % | M | (SD) | n | % | M | (SD) |
| Age (years) | 51.6 | (9.2) | 51.8 | (9.8) | 51.3 | (8.7) | ||||||
| BMI (kg/m2) | 27.8 | (5.7) | 27.9 | (5.3) | 27.6 | (6.0) | ||||||
| BCPT cognitive problems | 1.5 | (1.0) | 1.5 | (1.0) | 1.4 | (1.0) | ||||||
| CES-D depressive symptoms | 10.7 | (8.2) | 10.2 | (8.2) | 11.2 | (8.2) | ||||||
| BAI anxiety symptoms | 10.1 | (7.1) | 9.8 | (7.2) | 10.33 | (7.1) | ||||||
| SF-36 vitality | 46.5 | (20.6) | 48.6 | (20.2) | 44.4 | (20.9) | ||||||
| PSQ sleep quality | 7.5 | (3.5) | 7.9 | (3.9) | 7.2 | (3.1) | ||||||
| CHAMPS moderate-to-high intensity activity, hrs/week | 6.3 | (5.8) | 6.8 | (6.3) | 5.8 | (5.1) | ||||||
| Race/Ethnicity | ||||||||||||
| White | 176 | 88.5 | 88 | 88 | 88 | 88 | ||||||
| Black | 18 | 9 | 8 | 8 | 10 | 10 | ||||||
| Asian | 5 | 2.5 | 3 | 3 | 2 | 2 | ||||||
| Marital Status | ||||||||||||
| Single | 26 | 13 | 18 | 18 | 8 | 8 | ||||||
| Married | 140 | 70 | 68 | 68 | 72 | 72 | ||||||
| Separated/divorced | 29 | 14.5 | 14 | 14 | 15 | 15 | ||||||
| Widowed | 5 | 2.5 | 0 | 0 | 5 | 5 | ||||||
| Education level | ||||||||||||
| High school or less | 12 | 6 | 5 | 5 | 7 | 7 | ||||||
| Some college | 49 | 24.5 | 27 | 27 | 22 | 22 | ||||||
| College graduate | 62 | 31 | 29 | 29 | 33 | 33 | ||||||
| Postgraduate | 77 | 38.5 | 39 | 39 | 38 | 38 | ||||||
| Employment status | ||||||||||||
| Employed | 137 | 68.5 | 71 | 71 | 66 | 66 | ||||||
| Unemployed | 35 | 17.5 | 15 | 15 | 20 | 20 | ||||||
| Retired | 28 | 14 | 14 | 14 | 14 | 14 | ||||||
| Income level ($) | ||||||||||||
| 0 – 25,000 | 10 | 5.5 | 3 | 3 | 7 | 7 | ||||||
| 25,000 – 50,000 | 33 | 18 | 18 | 18 | 15 | 15 | ||||||
| 50,000 – 75,000 | 35 | 19.1 | 17 | 17 | 18 | 18 | ||||||
| 75,000-100,000 | 46 | 25.1 | 23 | 23 | 23 | 23 | ||||||
| >100,000 | 59 | 32.2 | 30 | 30 | 29 | 29 | ||||||
| No report | 17 | 8 | 9 | 9 | 8 | 8 | ||||||
| Type of treatment | ||||||||||||
| Surgery only | 26 | 13 | 13 | 13 | 13 | 13 | ||||||
| Surgery plus radiation | 52 | 26 | 28 | 28 | 24 | 24 | ||||||
| Surgery plus chemotherapy | 46 | 23 | 23 | 23 | 23 | 23 | ||||||
| Surgery plus radiation plus chemotherapy | 76 | 38 | 36 | 36 | 40 | 40 | ||||||
| Cancer stage at diagnosis | ||||||||||||
| 0 | 18 | 9 | 9 | 9 | 9 | 9 | ||||||
| I | 89 | 44.5 | 46 | 46 | 43 | 43 | ||||||
| IIA | 52 | 26 | 27 | 27 | 25 | 25 | ||||||
| IIB | 23 | 11.5 | 10 | 10 | 13 | 13 | ||||||
| IIIA | 18 | 9 | 8 | 8 | 10 | 10 | ||||||
| Tamoxifen/aromatase inhibitor use | 143 | 71.5 | 72 | 72 | 71 | 71 | ||||||
| Postmenopausal | 153 | 81 | 76 | 76 | 77 | 77 | ||||||
| Time since diagnosis (months) | 17.3 | (8.1) | 16.3 | (7.5) | 18.3 | (8.5) | ||||||
| Time since treatment (months) | 10.9 | (7.9) | 9.9 | (7.1) | 11.8 | (8.5) | ||||||
Protocol adherence
Of the 200 women in the initial sample, 186 provided post-intervention data across the wait-list (n = 90) and yoga (n =96) groups. Women who did not provide post-intervention data were more likely to be separated or divorced compared to women who provided data (X2 = 8.28, p = .041). Other demographic characteristics did not differ significantly between women who provided post-intervention data and those who did not (ps > .100). However, women who dropped out of the study had higher anxiety symptoms (t(198) = 2.57, p = .011) and worse sleep quality (t(197) = 1.94, p = .053), as well as slightly more cognitive complaints (t(198) = 1.68, p = .094) at baseline than those who completed the intervention.
On average, women who received the yoga intervention attended 18.13 (± 4.52 SD) of 24 classes (75.4%), and reported 24.69 (± 10.62 SD) minutes per day of yoga practice during the 12-week intervention. None of the wait-list control participants reported practicing yoga over the course of the intervention.
Primary analyses
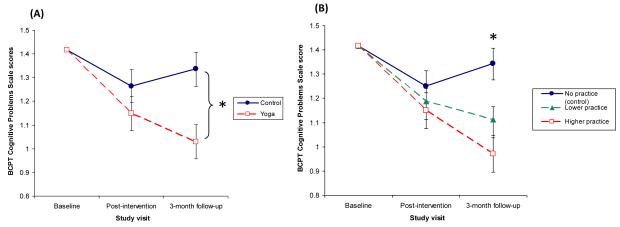
Table 2 summarizes the results from the primary linear mixed model, which tested group differences in cognitive complaints over time. The group by visit interaction was a significant predictor of self-reported cognitive problems, suggesting that change in cognitive complaints differed for yoga versus wait-list groups (F(1, 176) = 4.11, p = .044; see Table 2 and Figure 1A). The first set of planned contrasts tested group differences at each time point (see Table 3). Cognitive complaints did not differ significantly between yoga (M = 1.15) and wait-list (M = 1.26) groups immediately following the intervention (t(86) = 1.16, p = .250). However, at the 3-month follow-up visit, yoga participants (M = 1.03) reported 23% fewer cognitive problems than wait-list participants (M = 1.34; t(88) = −3.02, p = .003). A second set of contrasts tested the effect of visit within each group. For the control group, cognitive complaints did not differ significantly from immediately post-intervention to the 3-month follow-up visits (t(175) = 1.06, p = .291). However, cognitive complaints improved over time in the yoga group, although this effect approached significance (t(177) = −1.82, p = .071).
Table 2.
F-tests for all predictors of BCPT Cognitive Problems Scale scores at the post-intervention visits in primary and ancillary analyses.
| Effect | F | DF | P |
|---|---|---|---|
| Primary model | |||
| Baseline cognitive complaints | 243.73 | 1, 183 | <.001 |
| Visit* | .27 | 1, 176 | .608 |
| Group | 5.60 | 1, 55 | .022 |
| Visit × Group | 4.11 | 1, 176 | .044 |
| Ancillary model | |||
| Baseline cognitive complaints | 159.77 | 1, 198 | <.001 |
| Visit | .66 | 1, 172 | .417 |
| Group | 1.42 | 1, 59 | .238 |
| Visit × Group | 3.08 | 1, 173 | .081 |
| Depressive symptoms | 5.78 | 1, 347 | .017 |
| Anxiety symptoms | 8.32 | 1, 351 | .004 |
| Fatigue | 11.43 | 1, 323 | .001 |
| Sleep quality | .507 | 1, 343 | .477 |
Immediate post-intervention visit versus 3-month follow-up visit.
Figure 1.
(A) BCPT Cognitive Problems Scale scores at baseline, immediately post-intervention, and 3 months post-intervention in yoga and control groups. The plot shows estimated marginal means (± SE) from a linear mixed model adjusting for baseline BCPT Cognitive Problems Scale scores. Yoga participants reported significantly fewer cognitive problems at the 3-month follow-up visit compared to wait-list control participants (* indicates significant group contrast, p < .05). (B) BCPT Cognitive Problems Scale scores at baseline, immediately post-intervention, and 3-month follow-up based on yoga practice frequency. The plot shows estimated marginal means (± SE) from a linear mixed model adjusting for baseline BCPT Cognitive Problems Scale scores at no yoga practice (wait-list participants, 0 minutes per day), lower frequency yoga practice (25th percentile, 18 minutes per day), and higher frequency yoga practice (75th percentile, 29 minutes per day). At 3-month follow-up, those who practiced yoga more frequently reported fewer cognitive problems than those who practiced less frequently (* indicates significant slope of yoga practice, p < .05).
Table 3.
Contrasts comparing cognitive complaints across groups and over time from the primary linear mixed effects model.
| Mean Differences in BCPT Cognitive Scale scores |
||||
|---|---|---|---|---|
| Contrast | Mean Difference |
SE | 95% CI | p |
| Comparing groups | ||||
| Yoga vs. wait-list immediately post- intervention |
.12 | .10 | −.08 to .32 | .250 |
| Yoga vs. wait-list at 3-month follow-up | .31 | .10 | .11 to .51 | .003 |
| Comparing visits | ||||
| Immediately post-intervention to 3-month follow-up in yoga group |
.07 | .07 | −.06 to .20 | .291 |
| Immediately post-intervention to 3-month follow-up in wait-list control group |
.12 | .07 | −.01 to .25 | .071 |
Ancillary analyses
In secondary analyses, we adjusted for the concurrent effects of depression, anxiety, fatigue, and sleep quality (see Table 2). The results of the primary analysis remained the same, albeit slightly weaker; the group by visit interaction approached significance with all of the covariates included (F(1, 173) = 3.08, p = .081). Planned contrasts reflected the primary results. Specifically, cognitive complaints did not differ significantly between groups immediately following the intervention (t(97) = −.18, p = .858). However, yoga participants tended to report fewer cognitive problems than wait-list participants at the 3-month follow-up (t(101) = −1.89, p = .062). For the control group, cognitive complaints did not differ significantly from immediately post-intervention to 3-month follow-up (t(171) = .66, p = .511). However, cognitive complaints decreased from immediately post-intervention to 3-month follow-up for women in the yoga group, although again this effect was trending towards significance (t(173) = −1.84, p = .068).
Analyses that examined the effect of yoga practice on self-reported cognitive function bolstered the primary analyses examining the assigned intervention group (see Figure 1B). The yoga practice by visit interaction was significant (F(1, 174) = 8.81, p = .003). Follow-up tests revealed that the effect of yoga practice frequency on cognitive function was not significant immediately following the intervention (b = −.003 ± .003 SE, t(126) = −1.02, p = .308). However, women who spent more time practicing yoga during the course of the trial reported significantly fewer cognitive problems at the 3-month follow-up visit than those who practiced yoga less frequently (b = −.01 ± .003 SE; t(127) = −3.79, p < 0.001). A second set of contrasts examined the effect of visit on cognitive complaints for those with different levels of yoga practice. Among women who spent no time practicing yoga (i.e., wait-list controls, 0 minutes per day), cognitive complaints did not change significantly from immediately post-intervention (M = 1.25) to the 3-month follow-up (M = 1.34; t(175) = 1.53, p = .128). Similarly, those with lower yoga practice frequency (i.e., 25th percentile, 18 minutes per day) did not report significant changes from immediately post-intervention (M = 1.19) to the 3-month follow-up (M = 1.11; t(176) = −1.54, p = .125). However, among women with higher yoga practice frequency (i.e., 75th percentile, 29 minutes per day), cognitive complaints decreased significantly from immediately post-intervention (M = 1.15) to the 3-month follow-up (M = 0.97; t(175) = −2.58, p = .011). Adjusting for depression, anxiety, sleep quality, and fatigue did not change the results.
Exploratory analyses
Yoga decreased inflammation in the parent RCT; accordingly, we examined whether changes in inflammation contributed to group differences in cognitive complaints. We added each inflammatory marker to the primary model as a time-varying covariate; IL-6, IL-1β, and TNF-α levels were not significant predictors of cognitive complaints (ps > .369). Changes in IL-6, IL-1β, and TNF-α levels from baseline to 3-month follow-up did not significantly predict cognitive complaints at the 3-month follow-up (ps > .474).
We also examined whether general physical activity levels as measured by the CHAMPS differed between groups following the intervention. The main effect of group predicting moderate-to-high intensity activity hours was significant (F(1, 78) = 5.69, p = .019), and the group by visit interaction was not significant (p = .751). Immediately post-intervention, yoga participants (M = 6.60) tended to report greater moderate-intensity activity hours compared to wait-list participants (M = 5.23), and this effect approached significance (t(145) = 1.83, p =.068). At the 3-month follow-up, yoga participants (M = 6.80) reported significantly greater moderate-to-high intensity activity hours than wait-list participants (M = 5.17, t(148) = 2.17, p =.032).
Conclusions
On average, breast cancer survivors who received a brief yoga intervention had 23% lower self-reported cognitive problems scores than wait-list participants at the 3-month follow-up visit. Among women in the intervention group, those who practiced yoga more frequently during the intervention had larger decreases in cognitive complaints than those who practiced less frequently, suggesting that components of yoga were beneficial. The current findings suggest that yoga may be useful for reducing cognitive complaints in breast cancer survivors.
These results extend the current literature on cognitive function, yoga, and breast cancer survivorship in an important new direction. RCTs and meta-analyses have demonstrated that yoga reduces common behavioral symptoms for breast cancer survivors, such as psychological distress, fatigue, and sleep disturbances [17-19, 25, 26]. However, limited research has addressed yoga’s effect on perceived cognitive problems, another important aspect of cancer survivors’ well-being [27]. With good adherence (above 90%), inclusion of relevant covariates (i.e., psychological distress), and supporting yoga practice frequency data, this study addresses limitations of the few yoga intervention trials reporting cognitive outcomes. Importantly, group differences in cognitive complaints remained even after controlling for psychological distress, fatigue, and sleep quality, which are often related to perceived cognitive problems [7, 8]. Indeed, our results indicated that lower distress and fatigue may have contributed to yoga’s beneficial effect on cognitive function, but could not entirely explain it.
In this study, group differences in cognitive complaints were significant at the 3-month follow-up, but not immediately following the intervention. This pattern is consistent with the primary outcomes of this trial; yoga participants had significantly lower LPS-stimulated IL-6, IL-1β, and TNF-α, and fatigue than waitlist participants at the 3-month follow-up, but group differences were not significant immediately post-intervention. One possibility is that women may have continued to practice yoga beyond the intervention period, accruing its positive effects on physical, emotional, and cognitive well-being over time. Although women reported their at-home and in-class yoga practice during the intervention, we did not ask participants to track their yoga activities following the 12-week intervention period, a limitation of this study. However, participants reported their participation in other activities at each study visit, including the 3-month follow-up visit. Compared to waitlist participants, yoga participants reported more hours of moderate-to-high intensity activity both immediately post-intervention and at the 3-month follow-up. These data suggest that women who received the yoga intervention sustained greater overall physical activity levels over time, which could have produced the cognitive benefits that were evident at the 3-month follow-up. Future RCTs may be strengthened by including follow-up periods, and continuing to measure participants’ yoga practice after the intervention ends.
There are several plausible mechanisms through which yoga may reduce breast cancer survivors’ cognitive complaints. Prior research suggests that inflammation contributes to breast cancer survivors’ cognitive symptoms [21-23]. However, reductions in the inflammatory markers studied here did not explain yoga-related changes in cognitive complaints in the current study, which suggests that yoga likely affected cognitive complaints through other pathways. Physical activity can benefit cognitive function by increasing cerebral blood flow, neurogenesis, and neurotrophic factors that support neuronal health [37]. In addition, yoga may decrease cognitive complaints by reducing negative performance expectations. For example, women who received chemotherapy and were reminded about its negative cognitive effects performed more poorly on a subsequent memory task and reported more cognitive problems than those who did not receive such reminders [38]. Breathing exercises and meditation during yoga may help to focus attention to the present moment; emerging research suggests that mindfulness can impact cognitive function [39]. Accordingly, yoga may reduce perceived cognitive deficits by increasing physical fitness and/or mindfulness. Finally, it is possible that yoga participants’ expectations of the intervention’s benefits may have influenced their likelihood to engage in practice or perceive cognitive improvement. Comparing yoga to other physical activity interventions in future trials would help to further assess yoga’s utility in improving post-treatment cognitive problems, as well as the mechanisms through which yoga affects cognitive function.
Women in our study reported relatively low levels of cognitive problems; on average, they were “slightly” or “moderately” bothered by forgetfulness, difficulty concentrating, and distractibility. These data are consistent with breast cancer survivors’ reports in other studies [28, 40]. Those who dropped out of the intervention reported slightly more cognitive problems and fatigue [19] than those who completed the trial. Consequently, our results may actually underestimate the true effect of yoga on cognitive function, one limitation. Alternatively, yoga may be less feasible for those with the greatest fatigue and self-reported cognitive problems. Of note, women who dropped out of the study represent a small percentage of the overall sample; the trial had excellent retention, with an attrition rate of less than 10%. In addition, because we did not assess objective measures of cognitive function, these data cannot address whether yoga benefits objective cognitive performance, another limitation. Although future trials that examine neuropsychological test performance would help to answer whether yoga also affects objective cognitive function, it is also important to note that better subjective cognitive function could substantially improve quality of life [9, 11].
Given the improved efficacy of cancer treatments, long-term health and quality of life following cancer is increasingly important. Breast cancer survivors often report and experience cognitive problems following cancer treatment, and perceived cognitive dysfunction may continue even after neuropsychological test performance improves [10]. These findings suggest that yoga can effectively reduce breast cancer survivors’ cognitive complaints, and prompt researchers to further explore mind-body and physical activity interventions for improving cancer-related cognitive problems.
Acknowledgements
We are grateful to Marcia Miller of Yoga on High who designed the breast cancer survivor yoga protocol. We also appreciate the helpful assistance of the Stress and Health Study lab staff and the Clinical Research Center nursing staff. Lastly, this work was supported by the Pelotonia Fellowship Program; any opinions, findings, and conclusions expressed in this material are those of the authors and do not necessarily reflect those of the Pelotonia Fellowship Program.
Sources of Funding: Work on this project was supported in part by grants R01 CA126857, K05 CA172296, UL1RR025755, and CA016058 from the National Institutes of Health, as well as an American Cancer Society Postdoctoral Fellowship Grant (121911-PF-12-040-01-CPPB) and Pelotonia Graduate and Postdoctoral Fellowships from the Ohio State University Comprehensive Cancer Center.
Footnotes
Conflict of Interest Statement: All authors declare no conflicts of interest.
References
- 1.Ahles TA, Root JC, Ryan EL. Cancer- and cancer treatment–associated cognitive change: an update on the state of the science. Journal of Clinical Oncology. 2012;30(30):3675–3686. doi: 10.1200/JCO.2012.43.0116. doi:10.1200/JCO.2012.43.0116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wefel JS, Schagen SB. Chemotherapy-Related Cognitive Dysfunction. Current Neurology and Neuroscience Reports. 2012;12(3):267–275. doi: 10.1007/s11910-012-0264-9. doi:10.1007/s11910-012-0264-9. [DOI] [PubMed] [Google Scholar]
- 3.Hodgson KD, Hutchinson AD, Wilson CJ, Nettelbeck T. A meta-analysis of the effects of chemotherapy on cognition in patients with cancer. Cancer Treatment Reviews. 2013;39(3):297–304. doi: 10.1016/j.ctrv.2012.11.001. doi:10.1016/j.ctrv.2012.11.001. [DOI] [PubMed] [Google Scholar]
- 4.Schilder CM, Seynaeve C, Beex LV, Boogerd W, Linn SC, Gundy CM, Schagen SB. Effects of tamoxifen and exemestane on cognitive functioning of postmenopausal patients with breast cancer: Results from the neuropsychological side study of the tamoxifen and exemestane adjuvant multinational trial. Journal of Clinical Oncology. 2010;28(8):1294–1300. doi: 10.1200/JCO.2008.21.3553. doi:10.1200/JCO.2008.21.3553. [DOI] [PubMed] [Google Scholar]
- 5.Wefel JS, Lenzi R, Theriault RL, Davis RN, Meyers CA. The cognitive sequelae of standard-dose adjuvant chemotherapy in women with breast carcinoma. Cancer. 2004;100(11):2292–2299. doi: 10.1002/cncr.20272. doi:10.1002/cncr.20272. [DOI] [PubMed] [Google Scholar]
- 6.Jim HSL, Phillips KM, Chait S, Faul LA, Popa MA, Lee Y-H, Small BJ. Meta-Analysis of Cognitive Functioning in Breast Cancer Survivors Previously Treated With Standard-Dose Chemotherapy. Journal of Clinical Oncology. 2012;30(29):3578–3587. doi: 10.1200/JCO.2011.39.5640. doi:10.1200/JCO.2011.39.5640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pullens MJJ, De Vries J, Roukema JA. Subjective cognitive dysfunction in breast cancer patients: a systematic review. Psycho-Oncology. 2010;19(11):1127–1138. doi: 10.1002/pon.1673. doi:10.1002/pon.1673. [DOI] [PubMed] [Google Scholar]
- 8.Breckenridge LM, Bruns GL, Todd BL, Feuerstein M. Cognitive limitations associated with tamoxifen and aromatase inhibitors in employed breast cancer survivors. Psycho-Oncology. 2012;21(1):43–53. doi: 10.1002/pon.1860. doi:10.1002/pon.1860. [DOI] [PubMed] [Google Scholar]
- 9.Ganz PA, Kwan L, Castellon SA, Oppenheim A, Bower JE, Silverman DHS, Belin TR. Cognitive complaints after breast cancer treatments: examining the relationship with neuropsychological test performance. Jnci-Journal of the National Cancer Institute. 2013;105(11):791–801. doi: 10.1093/jnci/djt073. doi:10.1093/jnci/djt073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ribi K, Aldridge J, Phillips K-A, Thompson A, Harvey V, Thuerlimann B, Bernhard J. Subjective cognitive complaints one year after ceasing adjuvant endocrine treatment for early-stage breast cancer. British Journal of Cancer. 2012;106(10):1618–1625. doi: 10.1038/bjc.2012.156. doi:10.1038/bjc.2012.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reid-Arndt SA, Hsieh C, Perry MC. Neuropsychological functioning and quality of life during the first year after completing chemotherapy for breast cancer. Psycho-Oncology. 2010;19(5):535–544. doi: 10.1002/pon.1581. doi:10.1002/pon.1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Colcombe S, Kramer AF. Fitness effects on the cognitive function of older adults: A meta-analytic study. Psychological Science. 2003;14(2):125–130. doi: 10.1111/1467-9280.t01-1-01430. doi:10.1111/1467-9280.t01-1-01430. [DOI] [PubMed] [Google Scholar]
- 13.Weuve J, Kang J, Manson J, Breteler M, Ware J, Grodstein F. Physical activity, including walking, and cognitive function in older women. JAMA. 2004;292(12):1454–1461. doi: 10.1001/jama.292.12.1454. doi:10.1001/jama.292.12.1454. [DOI] [PubMed] [Google Scholar]
- 14.Jones LW, Courneya KS, Mackey JR, Muss HB, Pituskin EN, Scott JM, Haykowsky M. Cardiopulmonary function and age-related decline across the breast cancer survivorship continuum. Journal of Clinical Oncology. 2012;30(20):2530–2537. doi: 10.1200/JCO.2011.39.9014. doi:10.1200/JCO.2011.39.9014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwan ML, Sternfeld B, Ergas IJ, Timperi AW, Roh JM, Hong C-C, Kushi LH. Change in physical activity during active treatment in a prospective study of breast cancer survivors. Breast Cancer Research and Treatment. 2012;131(2):679–690. doi: 10.1007/s10549-011-1788-4. doi:10.1007/s10549-011-1788-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mishra SI, Scherer RW, Geigle PM, Berlanstein DR, Topaloglu O, Gotay CC, Snyder C. Exercise interventions on health-related quality of life for cancer survivors. Cochrane Database of Systematic Reviews. 2012;(8):CD007566. doi: 10.1002/14651858.CD007566.pub2. doi:10.1002/14651858.CD007566.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bower JE, Garet D, Sternlieb B, Ganz PA, Irwin MR, Olmstead R, Greendale G. Yoga for persistent fatigue in breast cancer survivors. Cancer. 2012;118(15):3766–3775. doi: 10.1002/cncr.26702. doi:10.1002/cncr.26702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cohen L, Warneke C, Fouladi RT, Rodriguez MA, Chaoul-Reich A. Psychological adjustment and sleep quality in a randomized trial of the effects of a Tibetan yoga intervention in patients with lymphoma. Cancer. 2004;100(10):2253–2260. doi: 10.1002/cncr.20236. doi:10.1002/cncr.20236. [DOI] [PubMed] [Google Scholar]
- 19.Kiecolt-Glaser JK, Bennett JM, Andridge R, Peng J, Shapiro CL, Malarkey WB, Glaser R. Yoga’s impact on inflammation, mood, and fatigue in breast cancer survivors: A randomized controlled trial. Journal of Clinical Oncology. 2014 doi: 10.1200/JCO.2013.51.8860. JCO.2013.51.8860. doi:10.1200/JCO.2013.51.8860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gothe N, Pontifex MB, Hillman C, McAuley E. The acute effects of yoga on executive function. Journal of Physical Activity and Health. 2012. Retrieved from http://yogafordepression.com/wp-content/uploads/images/The-Acute-Effects-of-Yoga-on-Executive-Function.pdf. [DOI] [PubMed]
- 21.Ganz PA, Bower JE, Kwan L, Castellon SA, Silverman DHS, Geist C, Cole SW. Does tumor necrosis factor-alpha (TNF-α) play a role in post-chemotherapy cerebral dysfunction? Brain, Behavior, and Immunity. 2013;30:S99–S108. doi: 10.1016/j.bbi.2012.07.015. Supplement. doi:10.1016/j.bbi.2012.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kesler S, Janelsins M, Koovakkattu D, Palesh O, Mustian K, Morrow G, Dhabhar FS. Reduced hippocampal volume and verbal memory performance associated with interleukin-6 and tumor necrosis factor-alpha levels in chemotherapy-treated breast cancer survivors. Brain, Behavior, and Immunity. 2013;30:S109–S116. doi: 10.1016/j.bbi.2012.05.017. Supplement. doi:10.1016/j.bbi.2012.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miller AH, Ancoli-Israel S, Bower JE, Capuron L, Irwin MR. Neuroendocrine-immune mechanisms of behavioral comorbidities in patients with cancer. Journal of Clinical Oncology. 2008;26(6):971–982. doi: 10.1200/JCO.2007.10.7805. doi:10.1200/JCO.2007.10.7805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cramer H, Lange S, Klose P, Paul A, Dobos G. Yoga for breast cancer patients and survivors: a systematic review and meta-analysis. BMC Cancer. 2012;12(1):412. doi: 10.1186/1471-2407-12-412. doi:10.1186/1471-2407-12-412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Harder H, Parlour L, Jenkins V. Randomised controlled trials of yoga interventions for women with breast cancer: a systematic literature review. Supportive Care in Cancer. 2012;20(12):3055–3064. doi: 10.1007/s00520-012-1611-8. doi:10.1007/s00520-012-1611-8. [DOI] [PubMed] [Google Scholar]
- 26.Sadja J, Mills PJ. Effects of yoga interventions on fatigue in cancer patients and survivors: A systematic review of randomized controlled trials. EXPLORE: The Journal of Science and Healing. 2013;9(4):232–243. doi: 10.1016/j.explore.2013.04.005. doi:10.1016/j.explore.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Buffart LM, Uffelen J. G. van, Riphagen II, Brug J, Mechelen W. van, Brown WJ, Chinapaw MJ. Physical and psychosocial benefits of yoga in cancer patients and survivors, a systematic review and meta-analysis of randomized controlled trials. BMC Cancer. 2012;12(1):559. doi: 10.1186/1471-2407-12-559. doi:10.1186/1471-2407-12-559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stanton AL, Bernaards CA, Ganz PA. The BCPT symptom scales: A measure of physical symptoms for women diagnosed with or at risk for breast cancer. Journal of the National Cancer Institute. 2005;97(6):448–456. doi: 10.1093/jnci/dji069. doi:10.1093/jnci/dji069. [DOI] [PubMed] [Google Scholar]
- 29.Servaes P, Verhagen CAHHVM, Bleijenberg G. Relations between fatigue, neuropsychological functioning, and physical activity after treatment for breast carcinoma. Cancer. 2002;95(9):2017–2026. doi: 10.1002/cncr.10891. doi:10.1002/cncr.10891. [DOI] [PubMed] [Google Scholar]
- 30.Radloff LS. The CES-D scale A self-report depression scale for research in the general population. Applied psychological measurement. 1977;1(3):385–401. [Google Scholar]
- 31.Beck AT, Epstein N, Brown G, Steer RA. An inventory for measuring clinical anxiety: Psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56(6):893–897. doi: 10.1037//0022-006x.56.6.893. doi:10.1037/0022-006X.56.6.893. [DOI] [PubMed] [Google Scholar]
- 32.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Research. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. doi:10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 33.Ware JE, Sherbourne CD. The MOS 36-item short-form health survey (SF-36): I. Conceptual framework and item selection. Medical Care. 1992;30(6):473–483. doi:10.1097/00005650-199206000-00002. [PubMed] [Google Scholar]
- 34.Stewart AL, Mills KM, King AC, Haskell WL, Gillis D, Ritter PL. CHAMPS physical activity questionnaire for older adults: Outcomes for interventions. Medicine & Science in Sports & Exercise. 2001;33(7):1126–1141. doi: 10.1097/00005768-200107000-00010. doi:10.1097/00005768-200107000-00010. [DOI] [PubMed] [Google Scholar]
- 35.Harada ND, Chiu V, King AC, Stewart AL. An evaluation of three self-report physical activity instruments for older adults. Medicine & Science in Sports & Exercise. 2001;33(6):962–970. doi: 10.1097/00005768-200106000-00016. doi:10.1097/00005768-200106000-00016. [DOI] [PubMed] [Google Scholar]
- 36.Courneya KS, Conner M, Rhodes RE. Effects of different measurement scales on the variability and predictive validity of the “two-component” model of the theory of planned behavior in the exercise domain. Psychology & Health. 2006;21(5):557–570. doi:10.1080/14768320500422857. [Google Scholar]
- 37.Hillman CH, Erickson KI, Kramer AF. Be smart, exercise your heart: exercise effects on brain and cognition. Nature Reviews Neuroscience. 2008;9(1):58–65. doi: 10.1038/nrn2298. doi:10.1038/nrn2298. [DOI] [PubMed] [Google Scholar]
- 38.Schagen SB, Das E, Vermeulen I. Information about chemotherapy-associated cognitive problems contributes to cognitive problems in cancer patients. Psycho-Oncology. 2012;21(10):1132–1135. doi: 10.1002/pon.2011. doi:10.1002/pon.2011. [DOI] [PubMed] [Google Scholar]
- 39.Chiesa A, Calati R, Serretti A. Does mindfulness training improve cognitive abilities? A systematic review of neuropsychological findings. Clinical psychology review. 2011;31(3):449–464. doi: 10.1016/j.cpr.2010.11.003. doi:10.1016/j.cpr.2010.11.003. [DOI] [PubMed] [Google Scholar]
- 40.Ganz PA, Kwan L, Stanton AL, Bower JE, Belin TR. Physical and psychosocial recovery in the year after primary treatment of breast cancer. Journal of Clinical Oncology. 2011;29(9):1101–1109. doi: 10.1200/JCO.2010.28.8043. doi:10.1200/JCO.2010.28.8043. [DOI] [PMC free article] [PubMed] [Google Scholar]