Abstract
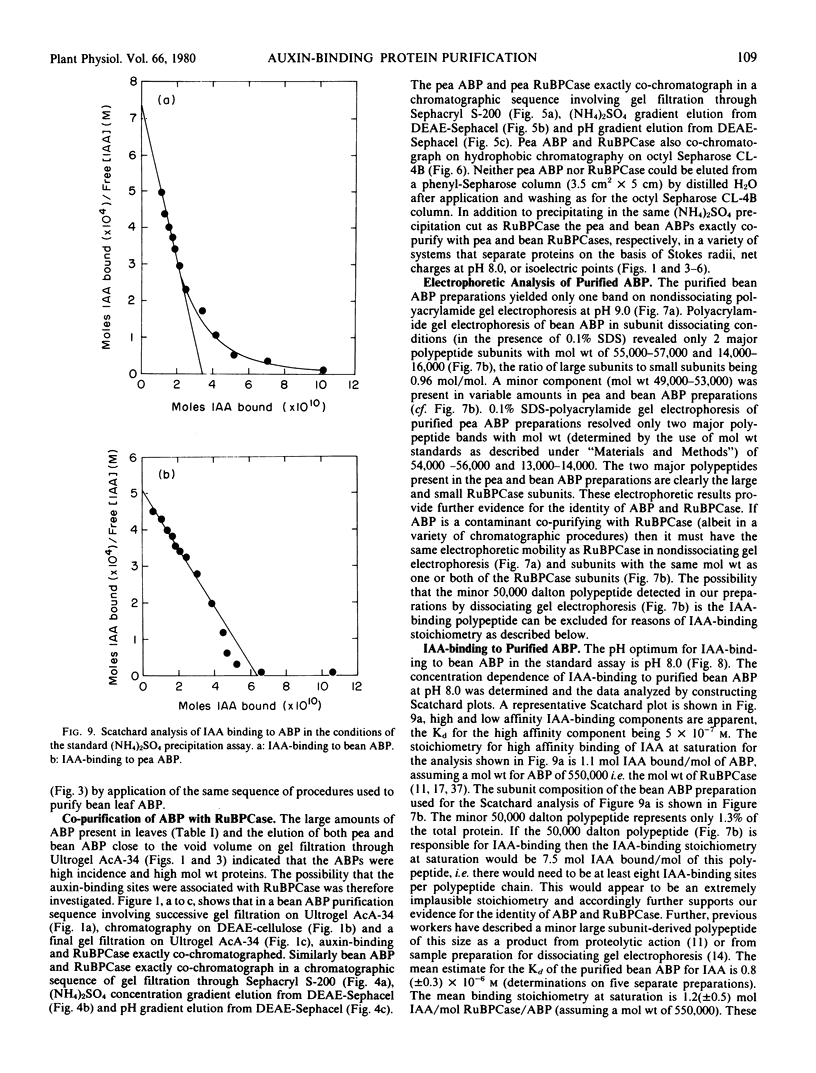
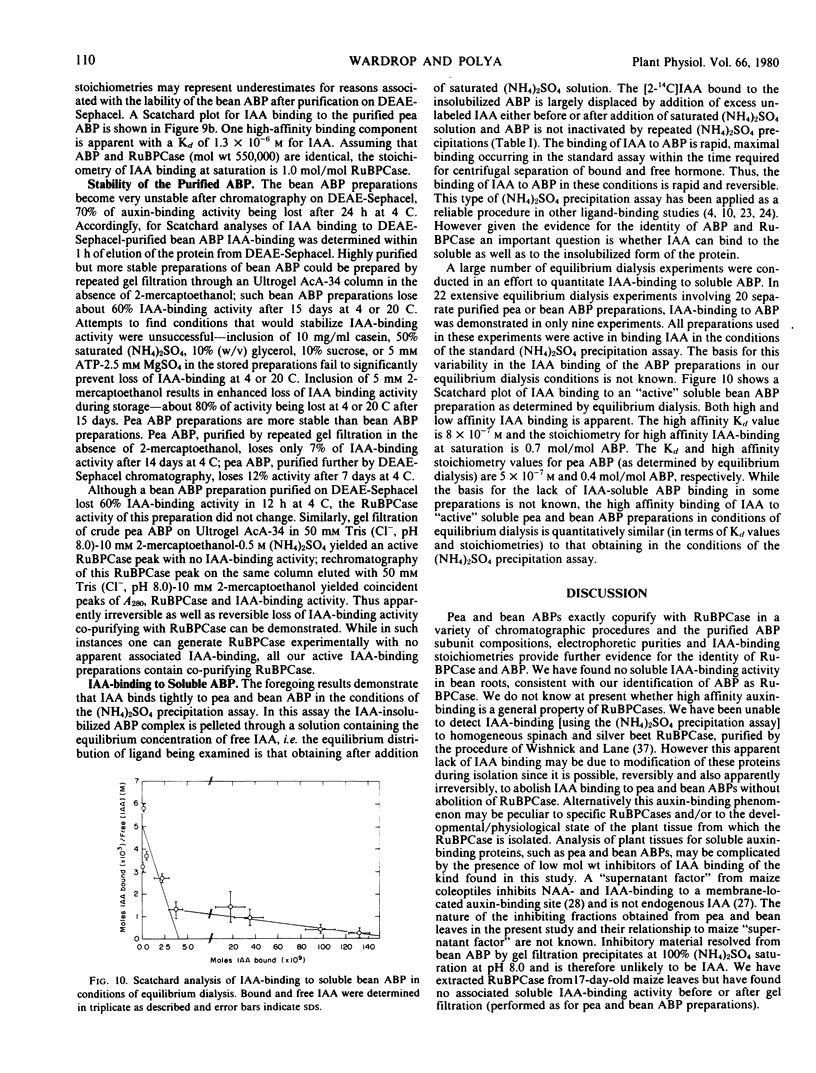
Soluble auxin-binding proteins (ABPs) were purified to constant specific activity from bean and pea leaves by a procedure involving (NH4)2SO4 fractionation, anion exchange chromatography and gel filtration. Pea and bean ABPs exactly co-purify with ribulose-1,5-bisphosphate carboxylase (RuBPCase) in a variety of chromatographic separation procedures. The subunit compositions, electrophoretic purities and indole-3-acetic acid (IAA)-binding stoichiometries of the purified ABPs provide further evidence for the identity of RuBPCase and ABP. Pea ABP and bean ABP have dissociation constants for IAA of 0.8 and 1.3 micromolar, respectively, as determined by an (NH4)2SO4 precipitation assay for IAA-binding to insolubilized ABP. IAA can bind to soluble bean and pea ABP (RuBPCase) as determined by equilibrium dialysis with affinities and stoichiometries similar to those determined for insolubilized ABP.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bideon G. M. Purification and characterization of a cyclic nucleotide-regulated 5'-nucleotidase from potatoe. Biochim Biophys Acta. 1975 Apr 19;384(2):443–457. doi: 10.1016/0005-2744(75)90045-5. [DOI] [PubMed] [Google Scholar]
- Cross J. W., Briggs W. R. Properties of a Solubilized Microsomal Auxin-binding Protein from Coleoptiles and Primary Leaves of Zea mays. Plant Physiol. 1978 Jul;62(1):152–157. doi: 10.1104/pp.62.1.152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doskeland S. O., Ueland P. M., Haga H. J. Factors affecting the binding of [3H]adenosine 3':5'-cyclic monophosphate to protein kinase from bovine adrenal cortex. Biochem J. 1977 Mar 1;161(3):653–665. doi: 10.1042/bj1610653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray J. C., Kerwick R. G. An immunological investigation of the structure and function of ribulose 1,5-bisphosphate carboxylase. Eur J Biochem. 1974 May 15;44(2):481–489. doi: 10.1111/j.1432-1033.1974.tb03506.x. [DOI] [PubMed] [Google Scholar]
- Henriques F., Park R. B. Identification of chloroplast membrane peptides with subunits of coupling factor and ribulose-1,5 diphosphate carboxylase. Arch Biochem Biophys. 1976 Oct;176(2):472–478. doi: 10.1016/0003-9861(76)90190-9. [DOI] [PubMed] [Google Scholar]
- Huner N. P., Macdowall F. D. Changes in the net charge and subunit properties of ribulose bisphosphate carboxylase--oxygenase during cold hardening of Puma rye. Can J Biochem. 1979 Feb;57(2):155–164. doi: 10.1139/o79-019. [DOI] [PubMed] [Google Scholar]
- Polya G. M., Bowman J. A. Ligand Specificity of a High Affinity Cytokinin-binding Protein. Plant Physiol. 1979 Sep;64(3):387–392. doi: 10.1104/pp.64.3.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M. Auxin-binding Sites of Maize Coleoptiles Are Localized on Membranes of the Endoplasmic Reticulum. Plant Physiol. 1977 Apr;59(4):594–599. doi: 10.1104/pp.59.4.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M., Dohrmann U. Characterization of naphthaleneacetic Acid binding to receptor sites on cellular membranes of maize coleoptile tissue. Plant Physiol. 1977 Mar;59(3):357–364. doi: 10.1104/pp.59.3.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ray P. M. Specificity of Auxin-binding Sites on Maize Coleoptile Membranes as Possible Receptor Sites for Auxin Action. Plant Physiol. 1977 Oct;60(4):585–591. doi: 10.1104/pp.60.4.585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy P., Biswas B. B. A receptor protein for indoleacetic acid from plant chromatin and its role in transcription. Biochem Biophys Res Commun. 1977 Feb 21;74(4):1597–1606. doi: 10.1016/0006-291x(77)90625-8. [DOI] [PubMed] [Google Scholar]
- Wardrop A. J., Polya G. M. Ligand Specificity of Bean Leaf Soluble Auxin-binding Protein. Plant Physiol. 1980 Jul;66(1):112–118. doi: 10.1104/pp.66.1.112. [DOI] [PMC free article] [PubMed] [Google Scholar]


