Abstract
Background
We sought to determine whether survivors of standard risk ALL (SR-ALL) treated without cranial radiation have increased risk for obesity by assessing changes in body mass index (BMI) during and after treatment; identifying contributing patient and treatment factors; comparing rates of overweight/obese to national health data.
Procedure
Eligibility for this retrospective cohort study included 1) previous enrollment on legacy therapy trials CCG1922 or CCG1952; 2) continuous first remission; and 3) age at follow-up evaluation of 6-16.99 years. Height and weight from diagnosis, consolidation, start of maintenance, last cycle of maintenance, and off-therapy were analyzed.
Results
The 269 subjects were a median age of 3.5 years at diagnosis and 13.3 years at follow-up. BMI% significantly increased from induction to consolidation (+17.6 ± 1.6%), start of maintenance to end-of-treatment (+3.3 ± 1.6%) and decreased from end-of-treatment to follow-up (−3.5 ± 1.6%,). Higher BMI% at follow-up was associated with higher BMI% at diagnosis (p < 0.0001), but not age at diagnosis, gender, or race. Patients previously randomized to dexamethasone had a stronger association between BMI% at diagnosis and BMI% at follow-up than those who received prednisone (p=0.0005). At follow-up, 39% of survivors were overweight or obese; the relative risk of overweight/obese was 1.028 (p=0.738) compared to the general population.
Conclusions
Our study of patients with SR-ALL found a significant increase in BMI% largely during the first month of therapy that is greater with dexamethasone than prednisone. However, after therapy, there was no increased risk of overweight/obese BMI compared to non-cancer peers.
Keywords: Acute lymphoblastic leukemia, Children, Cancer Survivors, Body Mass Index, Obesity
Introduction
The vast majority of children with standard risk (SR) B-precursor acute lymphoblastic leukemia (ALL) will become long term survivors, with an overall 5-year survival rate approaching 95%. [1] Unfortunately, the expanding population of ALL survivors is at increased risk for multiple late complications of therapy including cardiomyopathy, obesity, peripheral neuropathy, osteopenia, and neurocognitive impairment. [2] Obesity is of particular concern due to known associations with insulin resistance, cardiovascular health, and health-related quality of life. [3]
While ALL survivors have been reported to have a high prevalence of obesity [4-7], it is not clear whether these rates are higher than expected compared to our increasingly overweight society. Past studies have not done comprehensive comparisons to healthy peers or national health data. Another key question is whether patients treated with modern therapy without cranial radiation are still at risk. Most past studies included patients who received cranial radiation and/or older treatment regimens encompassing multiple decades. [4-11] Some early studies linked weight gain to cranial radiation presumably mediated by altered pituitary function. [4-5,7] There are also conflicting reports regarding obesity in studies conducted outside of the United States [12-17], possibly due to decreased corticosteroid exposure during maintenance in Berlin-Frankfurt-Munster protocols.
There is a critical need to study a representative group of SR-ALL survivors treated with modern therapy without cranial radiation to determine whether they develop higher than expected rates of obesity. This study provides a unique opportunity to study a large population and addresses several limitations of past studies. Our aims were to 1) assess changes in body mass index (BMI) over the course of treatment and follow-up; 2) identify patient and treatment factors contributing to BMI changes, including previous corticosteroid exposure; 3) determine if rates of overweight and obesity were greater than those in non-cancer peers. We hypothesized that treatment for SR-ALL on contemporary treatment regimens without cranial radiation is associated with an increase in BMI on therapy that persists after therapy and that dexamethasone exposure confers a greater risk for obesity than prednisone.
Methods
Study Design
This is a retrospective longitudinal cohort study of patients with SR-ALL enrolled on a long-term outcomes study (ALTE02C2) at 22 selected Children’s Oncology Group (COG) sites who previously enrolled in two legacy COG trials for SR-ALL. The primary aim of ALTE02C2 was to measure neurocognitive outcomes, with those results previously reported. [18-19] Subjects also underwent height, weight, and blood pressure (BP) measurement. Eligibility included continuous first remission without history of central nervous system leukemia or cranial radiation, age 6-16.99 years at evaluation, and no history of pre-existing developmental disorder or very low birth weight.
The first legacy study (CCG 1922) randomized patients to prednisone or dexamethasone, and between oral or intravenous 6-mercaptopurine in a 2 × 2 factorial design. [20] The second legacy study (CCG 1952) randomized patients to 6-mercaptopurine or thioguanine, and between intrathecal methotrexate or triples in a 2 × 2 factorial design. [21-22] Table I shows details of the regimens. On both, males received an additional year of maintenance therapy.
Table I. Therapy Comparison for Legacy Treatment Studies, CCG1922 and CCG1952.
| CCG 1922 | CCG1952 | ||||
|---|---|---|---|---|---|
| Phase of Therapy |
Time from Diagnosis |
Chemotherapy | Time from Diagnosis |
Chemotherapy | |
| Induction (Time Point 1) |
0 weeks | PRED (40mg/m2/d × 28d) VCR L-ASP IT MTX |
DEX (6mg/m2/d × 28d) VCR L-ASP IT MTX |
0 weeks | PRED (40mg/m2/d × 28d) VCR L-ASP IT MTX |
| Consolidation (Time Point 2) |
1 month | PRED (40mg/m2/d × 10d) VCR Oral or IV MP Oral MTX IT MTX |
DEX (6mg/m2/d × 10d) VCR Oral or IV MP Oral MTX IT MTX |
1 month | PRED (10d taper) VCR Oral MP or TG IT MTX or ITT |
| Interim Maintenance |
NA | NA | PRED (40 mg/m2/d × 10d) VCR Oral MTX Oral MP or TG |
||
| Delayed Intensification |
VCR DEX (10mg/m2/d × 21d + 7d taper) L-ASP DOX CPM TG ARA-C IT MTX |
VCR DEX (10mg/m2/d × 14d) L-ASP DOX CPM TG ARA-C IT MTX or ITT |
|||
| Interim Maintenance II |
NA | Repeat Interim Maintenance |
|||
| Delayed Intensification II |
NA | Repeat Delayed Intensification |
|||
| Maintenance (Time Point 3) |
6 months | PRED (40mg/m2/d × 5d/mo) VCR Oral MP Oral MTX IT MTX |
DEX (6mg/m2/d × 5d/mo) VCR Oral MP Oral MTX IT MTX |
10 months | PRED (40mg/m2/d × 5d/mo) VCR Oral MP or TG Oral MTX IT MTX or ITT |
| End of Therapy (Time Point 4) |
Males 38 months Females 26 months |
Males 38 months Females 26 months |
|||
| Follow-Up (Time Point 5) |
Approximately 5-14 years | ||||
Abbreviations: PRED, prednisone; VCR, vincristine; L-ASP, L-asparaginase; IT MTX, intrathecal methotrexate; DEX, dexamethasone; MTX, methotrexate; MP, mercaptopurine; TG, thioguanine; ITT, intrathecal triples; DOX, doxorubicin; CPM, cyclophosphamide; ARA-C, cytarabine
The institutional review board of each participating center and the Oregon Health & Science University approved the current study. Informed consent, and assent when indicated, was obtained for all participants.
Data Collection
Data from CCG 1922/1952 included: demographics; randomization; weight and height at diagnosis, consolidation (4 weeks after diagnosis), first maintenance cycle (6-10 months after diagnosis), and last maintenance cycle, a surrogate for end of treatment (26 (girls) to 38 (boys) months after diagnosis). Data from ALTE02C2 included: weight, height and BP. All height and weight data were obtained from routine clinical assessment at the time of the scheduled study visits.
Statistical Considerations
BMI and BP were the major outcomes. BMI was calculated from height and weight data and converted to gender- and age- specific BMI percentiles (BMI%) using the Centers for Disease Control and Prevention’s Year 2000 growth charts for patients 2-20 years. [23] Weight percentiles were used as surrogates for measurements prior to age 24 months as BMI is not standardized. Systolic and diastolic BPs were converted to height-, age- and gender- adjusted z-scores using the National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents guidelines for children 1-17 years. [24]
Baseline characteristics and outcomes were described. For BMI data, the primary outcomes were repeated measures of BMI% at five time points. Univariate analyses assessed BMI% at diagnosis, age, gender, race and corticosteroid exposure with BMI% at follow-up. Subjects were categorized as either prednisone- or dexamethasone-exposed based on the corticosteroid received for the majority of therapy. Multivariate analysis was used to study the adjusted association of BMI% at diagnosis with BMI% at follow-up after controlling for corticosteroid exposure. Longitudinal regression models were employed to study the change in BMI% over time, where the time effect on BMI%, unadjusted and adjusted, was assessed after controlling for the factors described above.
BMI% was classified into four clinical categories: underweight for < 5%, normal weight for 5 to < 85%, overweight for 85 to < 95% and obese for 95% and greater. [25] The proportion of subjects overweight/obese at follow-up was compared to the National Health and Nutrition Examination Survey (NHANES) data collected in 2005-2006 [26-27] using ages 7 to 17 years, and the corresponding age, race and gender adjusted relative risk was computed using a log-binomial regression model. The years 2005-2006 were selected as the majority of subjects underwent follow-up evaluation then.
For BP data, primary outcomes were systolic and diastolic z-scores. Univariate analyses assessed BMI% at diagnosis, BMI% at follow-up, gender, race and corticosteroid exposure with each BP outcome. All factors were included as independent variables in a multivariate analysis of their adjusted association with each outcome.
Analyses were performed using SAS software, v9.2. A p-value <0.05 was considered significant except in the corticosteroid comparison where the p-value threshold was <0.01 after a Bonferroni correction for multiple comparisons.
Results
Subject Characteristics
Subjects were enrolled on CCG 1922 and1952 from 1993-2000 and evaluated on ALTE02C2 from 2004-2008. Among 483 eligible patients from CCG1922/1952 at 22 ALTE02C2 sites, 269 (56%) enrolled and met criteria for analysis. Reasons for non-participation in ALTE02C2 were not collected. Participants were not statistically different from non-participants for age at diagnosis, time since diagnosis, gender, or randomization. [18-19] Subject characteristics are listed in Table II. There was a slight male predominance; the majority was non-Hispanic white and had prednisone as the corticosteroid exposure. The median age was 3.5 years at diagnosis and 13.3 years at follow-up.
Table II. Study Group Characteristics (N=269).
| Characteristic | |
|---|---|
| Gender, N(%) | |
| Male | 146 (54.3) |
| Female | 123 (45.7) |
| Race/Ethnicity, N(%) | |
| Non-Hispanic White | 214 (82.3) |
| Hispanic | 21 (8.1) |
| Non-Hispanic Black | 6 (2.3) |
| Other | 19 (7.3) |
| Unknown | 9 (3.3) |
| Age at Cancer Diagnosis, Years | |
| Mean (SD) | 4.0 (1.8) |
| Median (Range) | 3.5 (1.0 - 9.8) |
| Age at Follow-up, Years | |
| Mean (SD) | 13.1 (2.4) |
| Median (Range) | 13.3 (7.5 – 16.99) |
| Time from Diagnosis to Follow-up, Years | |
| Mean (SD) | 9.1 (2.1) |
| Median (Range) | 9.1 (4.8 – 13.7) |
| Treatment Protocol, N(%) | |
| CCG-1922 | 93 (34.6) |
| CCG-1952 | 176 (65.4) |
| Corticosteroid Exposure N(%) | |
| Dexamethasone | 52 (19.3) |
| Prednisone | 217 (80.7) |
BMI Analyses
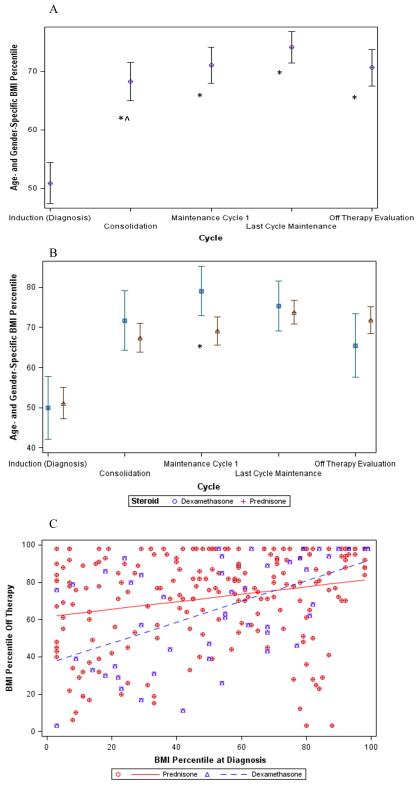
The BMI% trend for the entire cohort is shown in Figure 1A. BMI% increased significantly between diagnosis and consolidation (+17.4 ± 2.3%, p<0.0001), but BMI% changes between subsequent time points were not significantly different. When the time effect was adjusted for age at diagnosis, gender, race and corticosteroid exposure, there were significant differences in BMI% between consolidation and induction (+17.6 ± 1.6%, p<0.0001), end of treatment and start of maintenance (estimate +3.3 ± 1.6%, p=0.038) and follow-up and end of treatment (−3.5 ± 1.6%, p=0.030). As shown in Figure 1B, the difference in BMI% between dexamethasone and prednisone is statistically different only at the start of maintenance (79.1% ± 3.2% vs 69.1 ± 1.8%, p=0.01).
Figure 1.
BMI Percentile During and After Treatment: (A) Unadjusted BMI% trend for the entire cohort; * denotes p<0.05 when compared to diagnosis, ^ denotes p<0.05 when compared to the previous time point; (B) Corticosteroid comparison for the cohort; * denotes p<0.01 for the specified time point; (C) Interaction between BMI% at diagnosis and follow-up by corticosteroid; stronger association in dexamethasone (p<0.05).
By univariate analysis, BMI% at diagnosis was negatively associated with the change in BMI% at follow-up from diagnosis (p<0.0001). Age at diagnosis, gender, and race were not significant predictors of change in BMI% (Table III). By multivariate analysis, a significant interaction was observed between BMI% at diagnosis and corticosteroid exposure (Figure 1C) such that patients who received dexamethasone have a stronger association between BMI% at diagnosis and BMI% at follow-up than those who received prednisone (p=0.005).
Table III. Predictors of Change in BMI Percentile from Diagnosis to Follow-Up.
| Univariate Analysis | ||||
|---|---|---|---|---|
| Variable | Estimate | 95% CI | P-value | |
| Age at Diagnosis | 0.061 | −2.179 | 2.300 | 0.958 |
| Gender (Male vs. Female) |
−0.254 | −8.336 | 7.828 | 0.951 |
| Race (Non-white vs. White) |
8.811 | −1.775 | 19.397 | 0.102 |
| Steroid (Dexamethasone vs. Prednisone) |
−7.789 | −17.951 | 2.373 | 0.132 |
| BMI percentile at diagnosis | −0.738 | −0.842 | −0.633 | <0.0001 |
| Multivariate Analysis | ||||
|---|---|---|---|---|
| Variable | Estimate | 95% CI | P-value | |
| BMI percentile at diagnosis | −0.798 | −0.911 | −0.685 | <0.0001 |
| Corticosteroid (Dexamethasone vs. Prednisone) |
−25.204 | −41.184 | −9.225 | 0.002 |
| Interaction of BMI percentile at diagnosis and corticosteroid |
0.357 | 0.086 | 0.627 | 0.010 |
We compared the frequency of therapy toxicities with the potential to impact future BMI% between overweight/obese and underweight/normal subjects. We found no significant increase in reported toxicities of blood/coagulation, gastrointestinal, liver, and pancreas between the groups.
We assessed changes in underweight/normal and overweight/obese status over time. Approximately 64% of subjects retained their status from diagnosis, whether underweight/normal or overweight/obese, at follow-up. When status changed in either direction, the majority (53%) changed by the end of treatment and maintained that status at follow-up.
Comparison to NHANES population data
At follow-up, 39% of survivors were overweight/obese (Table IV). Compared to the NHANES population and adjusting for gender, race and age, the relative risk of overweight/obese in survivors at follow-up was 1.028 (p=0.738). Subjects were significantly less likely to be overweight/obese than non-cancer peers at diagnosis (RR 0.359, p<0.0001), a time when they are acutely ill. There were no other significant differences between subjects and non-cancer peers until the first maintenance cycle (6 months after diagnosis) when the relative risk of overweight/obesity in subjects exposed to dexamethasone was 1.3 (p=0.034). Elevated risk was not observed in any other subgroups or at subsequent time points, including the follow up time point. Multivariate analysis for the obese group showed no elevated risk at the follow-up time point.
Table IV. BMI Distribution by Clinical Category and Study Time Point.
| Time Point |
Underweight N(%) |
Normal N(%) |
Overweight N(%) |
Obese N(%) |
|---|---|---|---|---|
| 1 | 13 (4.87%) | 217 (81.27%) | 21 (7.87%) | 16 (5.99%) |
| 2 | 10 (3.73%) | 160 (59.70%) | 44 (16.42%) | 54 (20.15%) |
| 3 | 1 (0.38%) | 149 (56.02%) | 66 (24.81%) | 50 (18.8%) |
| 4 | 2 (0.76%) | 156 (59.09%) | 53 (20.08%) | 53 (20.08%) |
| 5 | 3 (1.18%) | 152 (59.84%) | 46 (18.11%) | 53 (20.87%) |
Blood Pressure Analyses
At follow-up, data from 249 and 248 subjects were available for systolic and diastolic z-scores, respectively. Systolic z-score (0.515 ± 0.099) was elevated versus population norms, whereas diastolic z-score (0.081 ± 0.058) was equivalent. By univariate analysis, BMI% at follow-up was significantly associated with systolic z-score (p=0.036) but not diastolic z-score (p=0.718). By multivariate analysis, no association was detected between systolic or diastolic z-score and the factors: age at diagnosis, BMI% at diagnosis, BMI% at follow-up, gender, race, and corticosteroid exposure.
Discussion
This study addresses the need to assess the risk of overweight and obesity in long term survivors of SR-ALL treated with modern therapy without cranial radiation, compared to that expected from national health data. In this retrospective longitudinal study, we described the pattern of change in BMI over the course of treatment and at follow-up, analyzed factors contributing to BMI changes, and compared the risk of overweight and obese in survivors of SR-ALL to the non-cancer population, adjusting for age, gender, and race. We found that there is a significant increase in BMI% from diagnosis to a follow-up time point. This increase largely reflects weight gained during the first month of therapy and is greater in those who received dexamethasone rather than prednisone. The greatest predictor of BMI% at follow-up was BMI% at diagnosis, particularly accentuated in those who received dexamethasone rather than prednisone. We did not observe an increased risk of overweight and obesity in patients with SR-ALL at follow-up compared to non-cancer peers. In contrast to previous reports, we did not find that BMI% at follow-up was associated with blood pressure, gender (despite males receiving more corticosteroids than females), or age at diagnosis. [4-11]
No previously available studies assess the risk of obesity in a large cohort of patients with SR-ALL treated on modern treatment protocols that avoid cranial radiation. When compared to HR, the SR-ALL population is more homogeneous with most patients completing therapy before the onset of puberty, therefore reducing potential confounding. There is only one longitudinal study in a SR-ALL cohort treated without cranial radiation using a chemotherapy regimen similar to the patients in our study. [17] In this cohort of 56 Saudi Arabian survivors, authors found the prevalence of overweight and obesity was similar to the general Saudi Arabian population. Two studies have published longitudinal combined SR and HR data in patients treated without cranial radiation. [14, 16] While both authors found increased weight in long-term survivors, they report disparate results regarding timing of weight gain.
In contrast to the meta-analysis by Zhang et al [28], we do not conclude that survivors of SR-ALL are more overweight/obese than non-cancer peers. This may be because Zhang et al included adolescents and individuals who received cranial radiation, while our analysis did not. Cranial radiation can affect pituitary function and decreased growth hormone levels are associated with insulin and obesity.[3] In addition, there would not be agreement that a BMI z-score of 0.83, which the authors state corresponds to the 80th percentile, represents a BMI that is significantly higher than the reference population. The World Health Organization defines overweight as a z-score greater than 1 standard deviation (SD) and obese as a z-score greater than 2 SD.[29] Greater than the 85th and 95th percentiles of BMI for age, respectively, are accepted consensus definitions of overweight and obese.[30] Finally, reference population percentiles in the Zhang et al. article do not reflect the actual distribution of the current population, as NHANES data suggest that approximately 40% of the general population has a BMI percentile greater than the 85th percentile rather than the expected 15%. [26]
We would agree that the prevalence of elevated BMI is high, but it is similar to the general pediatric population. Given the impact of weight on other known late effects such as cardiomyopathy, neuropathy, and joint injury from avascular necrosis [2], it is still vital to aggressively address the high prevalence in this specific population to limit future morbidity and mortality. Although BMI is a valid and important measure, other aspects of body composition such as lean muscle mass or fat mass [12] may impact morbidity and mortality beyond BMI and remain an important area for future study.
Our study had the ability to compare patients previously randomized to dexamethasone or prednisone without confounding from cranial radiation. Previous studies have identified prednisone dose equivalents as a factor in BMI change. [6] In our cohort, prednisone dose equivalents calculated using the historic conversion were almost equal by gender between the 2 corticosteroid groups; however, data suggests that historic conversions may underestimate the true potency of dexamethasone. [31-33] The higher BMI% during therapy in patients exposed to dexamethasone further supports this assertion. Moreover, we observed an interaction between corticosteroid preparation and BMI% at diagnosis, such that there is a higher follow-up BMI% in patients treated with dexamethasone who had higher BMI% at diagnosis.
Treatment with glucocorticoids has been implicated in the physiology of adiposity and there is data that dexamethasone may act more potently than prednisone. Studies have found higher leptin levels in survivors of childhood ALL, with cranial radiation and female gender identified as risk factors. [34-36] Wallace et al observed increases in BMI and serum leptin and decreases in sex hormone binding globulin after 5 weeks of glucocorticoid administration and concluded that dexamethasone was significantly more active in altering lipid metabolism parameters than prednisolone. [37] Kiess showed that serum leptin levels were high in otherwise healthy obese children and these levels further increased after a single dose dexamethasone suppression test. [38] Our results indicate that dexamethasone is associated with greater BMI increases during therapy, but not at follow-up.
This study has several strengths that overcome limitations of previous reports. First, this population is entirely patients with NCI SR-ALL treated using strategies similar to current therapies without cranial irradiation. Second, we were able to take advantage of a previous therapeutic randomization to study the association of BMI and corticosteroid formulation. Finally, this was a multi-site study. Therefore, it is less likely that our results are confounded by geographic variation in overweight/obesity rates.
Our results must be interpreted in the setting of potential limitations. We do not know the reason for non-participation on ALTE02C2. However, as that study was primarily a neurocognitive study, reasons for non-participation were unlikely to be related to concerns about assessing body composition and likely did not introduce significant bias into the analysis. Second, the corticosteroid comparison is imperfect because the prednisone group received dexamethasone during delayed intensification. However, the majority of corticosteroid therapy is given continuously during 28 days of induction and monthly 5-day pulses during 2-3 years of maintenance when patients received exclusively dexamethasone or prednisone. A final weakness is the lack of ethnic diversity, with non-Hispanic white overrepresented as compared to the US population. However, our analyses adjusted for race, as well as age and gender.
While our study suggests that corticosteroid preparation is a contributing factor to long term increase in BMI, further studies are needed to understand the physiologic mechanism. Moreover, this is not a factor that is easily modified as dexamethasone improves leukemia outcomes. [20] The current COG trial for children with newly diagnosed SR-ALL (AALL0932) includes a longitudinal assessment of peripheral neuropathy by trained therapists and will help determine if BMI is associated with severity of neuropathy, presumably mediated by reduced physical activity. The dramatic rise in BMI early in the ALL treatment course suggests that any interventions in diet or physical activity would be best timed during therapy. As we look forward to more than 90% of these patients becoming long-term survivors, we must make every effort to reduce the morbidity and mortality associated with treatment of ALL.
Abbreviations Key
- ALL
Acute Lymphoblastic Leukemia
- BMI
Body Mass Index
- BMI%
Body Mass Index Percentile
- BP
Blood Pressure
- CCG
Children’s Cancer Group
- COG
Children’s Oncology Group
- NCI
National Cancer Institute
- NHANES
National Health and Nutrition Examination Survey
- SR
Standard Risk
Footnotes
Conflict of Interest Statement
The authors have no conflict of interest to report.
References
- 1.Hunger SP, Lu X, Devidas M, Camitta BM, Gaynon PS, Winick NJ, Reaman GH, Carroll WL. Improved Survival for Children and Adolescents with Acute Lymphoblastic Leukemia between 1990 and 2005: A Report from the Children’s Oncology Group. J Clin Oncol. 2012;30(14):1663–1669. doi: 10.1200/JCO.2011.37.8018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ness KK, Armenian SH, Kadan-Lottick N, Gurney JG. Adverse Effects of Treatment in Childhood Acute Lymphoblastic Leukemia: General Overview and Implications for Long-Term Cardiac Health. Expert Rev Hematol. 2011;4(2):185–197. doi: 10.1586/ehm.11.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pulgaron ER. Childhood Obesity: A Review of Increased Risk for Physical and Psychological Comorbidities. Clin Ther. 2013;35(1):18–32. doi: 10.1016/j.clinthera.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sklar CA, Mertens AC, Walter A, Mitchell D, Nesbit ME, O’Leary M, Hutchinson R, Meadows AT, Robison LL. Changes in Body Mass Index and Prevalence of Overweight in Survivors of Childhood Acute Lymphoblastic Leukemia: Role of Cranial Irradiation. Med Pediatr Oncol. 2000;35:91–95. doi: 10.1002/1096-911x(200008)35:2<91::aid-mpo1>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 5.Oeffinger KC, Mertens AC, Sklar CA, Yasui Y, Fears T, Stovall M, Vik TA, Inshkp PD, Robison LL. Obesity in Adult Survivors of Childhood Acute Lymphoblastic Leukemia: A Report from the Childhood Cancer Survivor Study. J Clin Oncol. 2003;21(7):1359–1365. doi: 10.1200/JCO.2003.06.131. [DOI] [PubMed] [Google Scholar]
- 6.Chow EJ, Pihoker C, Hunt K, Wilkinson K, Friedman DL. Obesity and Hypertension Among Children After Treatment for Acute Lymphoblastic Leukemia. Cancer. 2007;110(10):2313–2320. doi: 10.1002/cncr.23050. [DOI] [PubMed] [Google Scholar]
- 7.Garmey EG, Liu Q, Sklar C, Meacham LR, Mertens AC, Stovall MA, Yasui Y, Robison LL, Oeffinger KC. Longitudinal Changes in Obesity and Body Mass Index Among Adult Survivors of Childhood Acute Lymphoblastic Leukemia: A Report from the Childhood Cancer Survivor Study. J Clin Oncol. 2008;26(28):4639–4645. doi: 10.1200/JCO.2008.16.3527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Razzouk BI, Rose SR, Hongeng S, Wallace D, Smeltzer MP, Zacher M, Pui CH, Hudson MM. Obesity in Survivors of Childhood Acute Lymphoblastic Leukemia and Lymphoma. J Clin Oncol. 2007;25(10):1183–1189. doi: 10.1200/JCO.2006.07.8709. [DOI] [PubMed] [Google Scholar]
- 9.Gofman I, Ducore J. Risk Factors for the Development of Obesity in Children Surviving ALL and NHL. J Pediatr Hematol Oncol. 2009;31(2):101–107. doi: 10.1097/MPH.0b013e31818c0120. [DOI] [PubMed] [Google Scholar]
- 10.Esbenshade AJ, Simmons JH, Koyama T, Koehler E, Whitlock JA, Friedman DL. Body Mass Index and Blood Pressure Changes Over the Course of Treatment of Pediatric Acute Lymphoblastic Leukemia. Pediatr Blood Cancer. 2011;56:372–378. doi: 10.1002/pbc.22782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Love E, Schneiderman JE, Stephens D, Lee S, Barron M, Tsangaris E, Urbach S, Staneland P, Greenberg M, Nathan PC. A Cross-Sectional Study of Overweight in Pediatric Survivors of Acute Lymphoblastic Leukemia (ALL) Pediatr Blood Cancer. 2011;57(7):1204–1209. doi: 10.1002/pbc.23010. [DOI] [PubMed] [Google Scholar]
- 12.Jarfelt M, Lannering B, Bosaeus I, Johannsson G, Bjarnason R. Body composition in Young Adult Survivors of Childhood Acute Lymphoblastic Leukaemia. Eur J Endocrinol. 2005;153:81–89. doi: 10.1530/eje.1.01931. [DOI] [PubMed] [Google Scholar]
- 13.Papadia C, Naves LA, Costa SSS, Vaz JAR, Domingues L, Augusto Casulari L. Incidence of Obesity Does Not Appear to Be Increased after Treatment of Acute Lymphoblastic Leukemia in Brazilian Children: Role of Leptin, Insulin, and IGF-1. Horm Res. 2007;68:164–170. doi: 10.1159/000100781. [DOI] [PubMed] [Google Scholar]
- 14.Asner S, Ammann RA, Ozsahin H, Beck-Popovic M, von der Weid NX. Obesity in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia. Pediatr Blood Cancer. 2008;51:118–122. doi: 10.1002/pbc.21496. [DOI] [PubMed] [Google Scholar]
- 15.Veringa SJE, van Dulmen-den Broeder E, Kaspers GJL, Veening MA. Blood Pressure and Body Composition in Long-Term Survivors of Childhood Acute Lymphoblastic Leukemia. Pediatr Blood Cancer. 2012;58(2):278–82. doi: 10.1002/pbc.23251. [DOI] [PubMed] [Google Scholar]
- 16.Breene RAL, Williams FM, Hartle J, Gattens M, Acerini CL, Murray MJ. Auxological Changes in UK Survivors of Childhood Acute Lymphoblastic Leukemia Treated Without Cranial Irradiation. Br J Cancer. 2011;104:746–749. doi: 10.1038/bjc.2011.16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Aldhafiri F, Al-Nasser A, Al-Sugair A, Al-Mutairi H, Young D, Reilly JJ. Obesity and Metabolic Syndrome in Adolescent Survivors of Standard Risk Childhood Acute Lymphoblastic Leukemia in Saudi Arabia. Pediatr Blood Cancer. 2012;59:133–137. doi: 10.1002/pbc.24012. [DOI] [PubMed] [Google Scholar]
- 18.Kadan-Lottick NS, Brouwers P, Breiger D, Kaleita T, Dziura J, Liu H, Chen L, Nicoletti M, Stork L, Bostrom B, Neglia JP. A Comparison of Neurocognitive Functioning in Children Previously Randomized to Dexamethasone or Prednisone in the Treatment of Childhood Acute Lymphoblastic Leukemia. Blood. 2009;114(9):1746–1752. doi: 10.1182/blood-2008-12-186502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kadan-Lottick NS, Brouwers P, Breiger D, Kaleita T, Dziura J, Northrup V, Chen L, Nicoletti M, Bostrom B, Stork L, Neglia JP. Comparison of Neurocognitive Functioning in Children Previously Randomly Assigned to Intrathecal Methotrexate Compared with Triple Intrathecal Therapy for the Treatment of Childhood Acute Lymphoblastic Leukemia. J Clin Oncol. 2009;27(35):5986–5992. doi: 10.1200/JCO.2009.23.5408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bostrom BC, Sensel MR, Sather HN, Gaynon PS, La MK, Johnston K, Erdmann GR, Gold S, Heerema NA, Hutchison RJ, Provisor AJ, Trigg ME, Childrens Cancer Group Dexamethasone Versus Prednisone and Daily Oral Versus Weekly Intravenous Mercaptopurine for Patients with Standard-Risk Acute Lymphoblastic Leukemia: A Report from the Children’s Cancer Group. Blood. 2003;101:3809–3817. doi: 10.1182/blood-2002-08-2454. [DOI] [PubMed] [Google Scholar]
- 21.Matloub Y, Lindemulder S, Gaynon PS, Sather H, La M, Broxson E, Yanofsky R, Hutchison R, Heerema NA, Nachman J, Blake M, Wells LM, Sorrell AD, Masterson M, Kelleher JF, Stork LC, Childrens Oncology Group Intrathecal Triple Therapy Decreases Central Nervous System Relapse but Fails to Improve Event-Free Survival When Compared with Intrathecal Methotrexate: Results of the Children’s Cancer Group (CCG) 1952 Study for Standard-Risk Acute Lymphoblastic Leukemia, Reported by the Children’s Oncology Group. Blood. 2006;108(4):1165–1173. doi: 10.1182/blood-2005-12-011809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stork LC, Matloub Y, Broxson E, La M, Yanofsky R, Sather H, Hutchinson R, Herrema NA, Sorrell AD, Masterson M, Bleyer A, Gaynon PS. Oral 6-Mercaptopurine Versus Oral 6-Thioguanine and Veno-occlusive Disease in Children with Standard-Risk Acute Lymphoblastic Leukemia: Report of the Children’s Oncology Group CCG-1952 Clinical Trial. Blood. 2010;115(14):2740–2748. doi: 10.1182/blood-2009-07-230656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuczmarski RJ, Ogden CL, Guo SS, Grummer-Strawn LM, Flegal KM, Mei Z, Wei R, Curtin LR, Roche AF, Johnson CL. 2000 CDC Growth Charts for the United States: Methods and Development. Vital and Health Statistics 11. 2002;246:1–166. [PubMed] [Google Scholar]
- 24.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents The Fourth Report on the Diagnosis, Evaluation, and Treatment of High Blood Pressure in Children and Adolescents. Pediatrics. 2004;114(2 suppl):555–576. 4th Report. [PubMed] [Google Scholar]
- 25.Barlow SE, the Expert Committee Expert Committee Recommendations Regarding the Prevention, Assessment, and Treatment of Child and Adolescent Overweight and Obesity: Summary Report. Pediatrics. 2007;120(Supplement):S164–S192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 26.Ogden CL, Carroll MD, Flegal KM. High Body Mass Index for Age Among US Children and Adolescents, 2003-2006. JAMA. 2008;299(20):2401–2405. doi: 10.1001/jama.299.20.2401. [DOI] [PubMed] [Google Scholar]
- 27.Centers for Disease Control and Prevention [Accessed January 27];National Health and Nutrition Examination Survey 2005-2006 raw data. 2012 NHANES 2005-2006 home page: http://www.cdc.gov/nchs/nhanes/nhanes2005-2006/nhanes05_06.htm. Published November 2007.
- 28.Zhang FF, Kelly MJ, Saltzman E, Must A, Roberts SB, Parsons SK. Obesity in Pediatric ALL Survivors: A Meta-Analysis. Pediatrics. 2014;133:e704–715. doi: 10.1542/peds.2013-3332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.De Onis M, Onyango AW, Borghi E, Siyam A, Nishida C, Siekmann J. Development of a WHO Growth Reference for School-Aged Children and Adolescents. Bull World Health Organ. 2007;85:660–667. doi: 10.2471/BLT.07.043497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barlow SE, Expert Committee Expert Committee Recommendations Regarding the Prevention, Assessment, and Treatment of Child and Adolescent Overweight and Obesity: Summary Report. Pediatrics. 2007;120:S164–192. doi: 10.1542/peds.2007-2329C. [DOI] [PubMed] [Google Scholar]
- 31.Cantrill HL, Walterman SR, Plamberg PR, Zink HA, Becker B. In vitro Determination of Relative Corticosteroid Potency. J Clin Endocrinol Metab. 1975;40:1073–1077. doi: 10.1210/jcem-40-6-1073. [DOI] [PubMed] [Google Scholar]
- 32.Liddle GW. Clinical Pharmacology of the Anti-Inflammatory Steroids. Clinical Pharmacology and Therapeutics. 1962;2:615–635. doi: 10.1002/cpt196125615. [DOI] [PubMed] [Google Scholar]
- 33.Meikle AW, Tyler FH. Potency and Duration of Action of Glucocorticoids: Effects of Hydrocortisone, Prednisone and Dexamethasone on Human Pituitary-Adrenal Function. Am J Med. 1977;63:200–207. doi: 10.1016/0002-9343(77)90233-9. [DOI] [PubMed] [Google Scholar]
- 34.Kohler JA, Moon RJ, Wright S, Willows E, Davies JH. Increased Adiposity and Altered Adipocyte Function in Female Survivors of Childhood Acute Lymphoblastic Leukaemia Treated Without Cranial Radiation. Horm Res Paediatr. 2011;75:433–440. doi: 10.1159/000324412. [DOI] [PubMed] [Google Scholar]
- 35.Tonorezos ES, Vega GL, Sklar CA, Chou JF, Moskowitz CS, Mo Q, Church TS, Ross R, Janiszewski PM, Oeffinger KC. Adipokines, Body Fatness, and Insulin Resistance Among Survivors of Childhood Leukemia. Pediatr Blood Cancer. 2012;58(1):31–36. doi: 10.1002/pbc.22964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Karaman S, Ercan O, Yildiz I, Bolayirli M, Celkan T, Apak H, Ozkan A, Onal H, Canbolat A. Late Effects of Childhood ALL Treatment on Body Mass Index and Serum Leptin Levels. J Pediatr Endocrinol Metab. 2010;23(7):669–674. doi: 10.1515/jpem.2010.23.7.669. [DOI] [PubMed] [Google Scholar]
- 37.Wallace AM, Tucker P, Williams DM, Hughes IA, Ahmed SF. Short-term Effects of Prednisolone and Dexamethasone on Circulating Concentrations of Leptin and Sex Hormone-Binding Globulin in Children Being Treated for Acute Lymphoblastic Leukaemia. Clin Endocrinol. 2003;58:770–776. doi: 10.1046/j.1365-2265.2003.01790.x. [DOI] [PubMed] [Google Scholar]
- 38.Kiess W, Englaro P, Hanitsch S, Rascher W, Attanasio A, Blum WF. High Leptin Concentrations in Serum of Very Obese Children are Further Stimulated by Dexamethasone. Horm Metab Res. 1996;28(12):708–710. doi: 10.1055/s-2007-979883. [DOI] [PubMed] [Google Scholar]