Abstract
Seasonal variations in immunity are common in nature, and changes in day length are sufficient to trigger enhancement and suppression of immune function in many vertebrates. Drawing primarily on data from Siberian hamsters, this review describes formal and physiological aspects of the neuroendocrine regulation of seasonal changes in mammalian immunity. Photoperiod regulates immunity in a trait-specific manner, and seasonal changes in gonadal hormone secretion and thyroid hormone signaling all participate in seasonal immunomodulation. Photoperiod-driven changes in the hamster reproductive and immune systems are associated with changes in iodothyronine deiodinase-mediated thyroid hormone signaling, but photoperiod exerts opposite effects on the epigenetic regulation of reproductive neuroendocrine and lymphoid tissues. Photoperiodic changes in immunocompetence may explain a proportion of the annual variance in disease incidence and severity in nature, and provide a useful framework to help understand brain-immune interactions.
Keywords: hamster, reproduction, immune function, melatonin, thyroid hormones, epigenetic
1. Introduction: Seasonal biological rhythms are adaptations to a cyclic world
Biological clocks provide temporal organization for a wide range of physiological processes. In mammals, daily temporal organization of physiology and behavior is provided by entrainment of the circadian pacemaker (in the hypothalamic suprachiasmatic nucleus [SCN]) to the environmental light:dark cycle. This circadian pacemaker synchronizes countless metabolic and physiological processes to the environmental light-dark cycle, by resetting the period and phase of subsidiary central and peripheral oscillators on a daily basis (Butler & Silver, 2009). Seasonal time keeping in most mammals, on the other hand, is accomplished in large part by photoperiod-driven changes in the SCN-driven nocturnal pineal melatonin (MEL) rhythm. As day length changes over the course of the year, longer- and shorter-duration MEL signals are generated in short days (SD) of winter and in the long days (LD) of summer, respectively. Over intervals of many weeks, these changing MEL signals either entrain self-sustained circannual clocks (Hiebert et al., 2000) or directly induce seasonally-appropriate phenotypes (Goldman, 2001). The number and diversity of physiological processes driven by seasonal timekeeping mechanisms are beyond the scope of this review, but the functional significance and evolutionary importance of linking systems critical to survival and adaptation (e.g., reproduction, immune function) to seasonal time information is paramount. In periodic environments, biological clocks provide temporal coordination with, and anticipation of, predictable fluctuations in the environment (Pittendrigh, 1960; Bronson, 1989; Stevenson and Ball, 2011). Seasonal clocks also mediate temporal coordination of the internal milieu, ensuring temporal organization among countless physiological and biochemical processes (Aschoff, 1960).
The present review will highlight what is known about how changes in day length drive seasonal cycles of immunocompetence in mammals. The work will be selective rather than comprehensive, and will compare and contrast known mechanisms (formal, neural, endocrine) that have been established to be critical to the mediation of seasonal time information into the reproductive system, and then examine whether similar processes are at work in communicating time information into the immune system. Much of the laboratory-based research on mammalian immunological seasonality has used Siberian hamsters (Phodopus sungorus) as a model system, and the data discussed here will largely reflect this bias. We will examine insights gained into the phenomenology of immunological photoperiodism, and will review research that has examined immunological photoperiodism at both formal and physiological levels of analysis. The latter material will examine the respective contributions of several major neuroendocrine systems (e.g. pineal, gonadal, thyroid) towards the generation of seasonal cycles in immune function. Although short days increase circulating Cortisol concentrations in Siberian hamsters (Bilbo et al., 2002b; Weil et al., 2006), functional tests of the role of the hypothalamic-pituitary-adrenal axis in the mediation of photoperiod-induced changes in immunity in Siberian hamsters are limited in number (Demas et al., 2002), and will not be surveyed in the present review.
2. Seasonal rhythms in immune function are numerous and relevant to survival
One major function of the immune system is to defend the individual from the onslaught of foreign organisms attempting to colonize it (Janeway et al., 1999). The immune system is present almost everywhere in the body and can mobilize defensive responses to infection, injury, and tumor growth within minutes-to-days of invasion. Immunocompetence varies predictably over time. Over the lifespan, the immune system undergoes maturation and senescence (Shames, 2002; Linton & Thoman, 2001; McElhaney & Effros, 2009); however, the immune system also changes markedly over seasonal and circadian timescales (Nelson, 2004; Curtis et al., 2014).
A theoretical framework termed the “winter immunoenhancement hypothesis” has been proposed to explain the adaptive significance of seasonal changes in immunity (reviewed in Nelson, 2004). It contends that processes associated with maintaining or activating the immune system compete with somatic and reproductive development for access to a finite amount of energy (Sheldon & Verhulst, 1996; Zuk & Stoehr, 2002). When reproductive efforts are downregulated (in small rodents, this is typically during winter), energy may be redirected to the immune system. Not surprisingly, such seasonal modifications in immune function are most evident in organisms that evolved in highly seasonal environments and exhibit seasonal adaptations (e.g., seasonal reproduction, migration) that rely on time-of-year information for properly-timed initiation and termination. Unlike the reproductive system, however, the immune system of seasonal breeders does not exhibit an omnibus potentiation (e.g., spring breeding) or shutdown (e.g., winter anestrus) during any phase of the annual cycle. The absence of immune responsiveness during a fraction of the year could be fatal, just as indiscriminately robust immune function could lead to autoimmune disease. Thus, rather than oscillate between complete inhibition and maximal stimulation, the immune system exhibits a constellation of trait-specific enhancements and decrements at different times of year (Nelson, 2004).
Important to the winter immunoenhancement hypothesis is that engaging immune responses requires a biologically significant amount of energy. Components of the innate immune response (e.g., fever; discussed in detail below) are undoubtedly metabolically costly, with estimates of a 7–13% increase in energy production required for every 1°C increase in body temperature (Maier et al., 1994). Adaptive immune responses likewise require quantifiable amounts of additional energy. Demas et al. (1997) identified a 1–1.5°C increase in colonic temperature in mice mounting a primary antibody response to a novel antigen, along with a 25%–70% increase in oxygen consumption (depending on age). In peripubertal male Siberian hamsters, a simulated bacterial infection or inoculation with a novel antigen resulted in a transient delay in reproductive maturation in long days (Prendergast et al., 2004c), suggesting that activation of the immune system may redirect resources away from investment in reproduction at specific times of year.
If reproductive energy expenditures have been an important ultimate factor in the evolution of seasonal immune variations, then this might lead to the prediction that seasonal immunological plasticity might exhibit an opposite pattern in long-day breeding species and that it may be attenuated or absent in mammals that are not seasonal breeders. On this latter issue, Wistar rats, which do not exhibit reproductive responses to changes in photoperiod, nevertheless exhibited photoperiodic changes in multiple measures of immunity in the laboratory (Prendergast et al, 2007). Inbred strains of mice that are melatonin-proficient likewise exhibit modest changes in select aspects of immunity following photoperiod manipulations in the lab (Yellon & Tran, 2002). These data suggest that photoperiodic adjustments in immune function need not go hand-in-hand with photoperiodic reproductive responses. Seasonal modulation of immunity may constitute an adaptive response independently of energetic tradeoffs with reproductive and somatic development.
3. Phenomenology of immunological photoperiodism
Seasonal changes in illness and mortality exist in natural populations. For example, antibody production is markedly diminished during the winter in thirteen-lined ground squirrels (Citrellus tridecemlineatus; McKenna & Musacchia, 1968), and typhus infections in flying squirrels (Glaucomys volans) peak in the autumn and early winter months (Sonenshine et al., 1978). In wild cotton rats (Sigmodon hispidus), complex seasonal changes in cell-mediated and humoral immune function occur, with a general pattern of greater thymus and spleen masses, along with enhanced antibody responses in winter (Lochmiller et al., 1994). Other lines of evidence point to seasonal stress-induced immunocompromise in reproductively photoperiodic dasyurid marsupials (Antechinus sp.; Bradley et al., 1980; McDonald et al., 1981; McAllan & Dickman, 1986; McAllan et al., 2006). When male A. stuartii and A. flavipes come into seasonal breeding condition, rising plasma androgens cause corticosteroid-binding globulin concentrations to drop precipitously. The resulting elevation in plasma free plasma corticosteroid concentrations suppress immune function and lead to gastrointestinal hemorrhage, parasite colonization and death. Seasonal changes in day length have been argued to contribute to these phenomena (Demas & Nelson, 1996; Nelson, 2004).
Laboratory-based studies in deer mice and Syrian hamsters (Mesocricetus auratus) were among the first to establish that exposure to short day lengths increases spleen mass and numbers of lymphocytes and macrophages (Brainard et al., 1987,1988; Blom et al., 1994), and that short day-like melatonin treatments altered responsiveness of T and B cells to antigens (Champney et al., 1997). One of the first major functional consequences of photoperiodic adaptations in the immune system was reported by Nelson & Blom (1994), who showed that a carcinogen dose sufficient to induce squamous cell carcinomas in 89% of female deer mice (Peromyscus maniculatus) housed in long days (16L:8D) was completely ineffective in inducing tumors in mice housed in short photoperiods. The report went on to demonstrate that the effects of short days were independent of changes in ovarian steroid production, suggesting that photoperiod-driven changes in immune function and tumor suppression were not merely indirect consequences of photoperiodic changes in reproductive physiology, but were likely to reflect direct effects of day length on the immune system (Nelson & Blom, 1994).
Immune responses to photoperiod have been documented in numerous other rodents as well, including white-footed mice (Peromyscus leucopus; T cell-dependent inflammatory responses [delayed-type hypersensitivity (DTH) reactions] enhanced in short days; Pyter et al., 2005a), collared lemmings (Dicrostonyx groenlandicus; DTH enhanced in long and short days relative to extremely long [22 h light] days; Weil et al., 2006a), and Northern palm squirrels (Funambulus pennanti; hyposplenia under constant light conditions; Lahiri & Haldar, 2009; enhanced DTH and antibody responses, and attenuated proinflammatory cytokine production in short days; Ahmad & Haldar, 2012). Photoperiodic changes in functional immune assays are not evident in all seasonally-breeding species. Most notably, Syrian hamsters, which exhibit exceptionally robust reproductive responses to changes in day length, do not exhibit photoperiodic differences in DTH or antibody production (Zhou et al., 2002). On the other hand, even in some reproductively non-photoperiodic rodents, clear immunological responses to changes in day length have been reported (e.g., leukocyte subpopulations and LPS-induced sickness behaviors in Wistar rats [Rattus norvegicus]; Prendergast et al., 2007). Among inbred mouse (Mus musculus) strains, photoperiod-driven immunological plasticity may require the production of photoperiod-driven changes in melatonin production (see section 4.2), as C57 mice (which do not generate photoperiodic changes in melatonin production) fail to exhibit changes in immune function following transfer from long to short days, but C3H mice (which are melatonin-proficient) exhibit significant increases in the number of B and T lymphocytes, and in the numbers of helper, naïve, cytotoxic and activated (but not memory) T lymphocytes in short days (Yellon & Tran, 2002).
Studies by Nelson and colleagues established the role of day length in regulating the immune system of deer mice (e.g., Nelson & Blom, 1994; Demas & Nelson, 1996). This research group expanded studies of immunological photoperiodism to include other rodents, but with a special emphasis on Siberian hamsters (Phodopus sungorus), in part because of their robust photoperiodic reproductive responses. Scores of reports documenting photoperiodic changes in Siberian hamster immune function have since been published, establishing the Siberian hamster as a key model species for laboratory investigations of immunological photoperiodism.
Siberian hamsters are long-day breeders, and exhibit marked changes in constitutive measures of immunocompetence, innate immunity, adaptive immunity, and neural-immune interactions following experimental changes in day length in the laboratory (Table S1). A list of immunological phenotypes for which there is evidence of photoperiodic modulation in Siberian hamsters appears in Table S1. In lieu of addressing the mechanistic insights gained into each of these phenomena, this paper will focus on select immunological traits (leukocyte trafficking, delayed-type hypersensitivity reactions, and acute-phase responses to infection); such traits are representative of constitutive, adaptive and innate immune function.
In common with the reproductive system, the immune system can discriminate long from short photoperiods for the purposes of changing reaction norms and engaging adaptive responses. In concurrence with the winter immunoenhancement hypothesis, and consistent with an energetic tradeoff scenario, initial reports in deer mice and hamsters suggested that immune function was categorically enhanced following exposure to short winter photoperiods (Demas & Nelson, 1996; Yellon et al., 1999b). However, a substantial body of research that followed revealed that seasonal enhancement and suppression of immunity are trait- and species-specific. In Siberian hamsters, for example, memory B-cell mediated antibody responses are suppressed following adaptation to short winter photoperiods in the lab (Drazen et al., 2000), whereas memory T-cell dependent DTH responses are enhanced under short days (Bilbo et al., 2002b). In deer mice, lymphocyte mitosis is greater in short relative to long days (Demas & Nelson, 1996), whereas in Siberian hamsters the inverse relation exists (Prendergast et al., 2002b). As is evident in Table S1, adaptation to short photoperiods either enhances or attenuates the immune response, depending on the trait considered. Importantly, few of these changes in immunity compare in magnitude with the log-scale decreases typically observed in the hamster reproductive system following transfer from long to short days. Nevertheless, functionally-meaningful consequences arise from modest changes in the immune system. For example, long-day housed hamsters heal wounds two days faster than short-day housed hamsters (Kinsey et al., 2003), and a bacterial infection that is lethal to only 50% of hamsters housed in short days is lethal in ~90% of long-day housed hamsters (Prendergast et al., 2003a). Increases in the number of circulating leukocytes, increases in the magnitude of DTH responses (Bilbo et al., 2002b; Bilbo & Nelson, 2003; Prendergast et al., 2004a), and decreases in the behavioral, thermoregulatory and cytokine responses to bacterial infection (typically elicited via treatment with a bacterial lipopolysaccharide; LPS) are consistently observed in Siberian hamsters following transfer from long to short days (Bilbo et al., 2002a). In order, the above seasonal immunophenotypes reflect enumerative, adaptive, and innate aspects of immune function.
In Siberian hamsters, perhaps the most striking photoperiodic change in immunity is evident in the acute phase response (APR) to infection. The APR to infection reflects an organized constellation of changes in physiology, motivational state and behavior, that has evolved to facilitate fever during the early stages of infection and to conserve energy (Hart, 1988). Many of the symptoms presented during the APR are direct consequences of increases in central and peripheral proinflammatory cytokine production—principally IL1β, IL-6 and TNFα (Dantzer et al., 2008). First described in hamsters by Bilbo and colleagues (Bilbo et al., 2002a), several components of the APR to a simulated bacterial infection (LPS injection) are strikingly attenuated in hamsters that have adapted to short days (Fig. 1). The duration of LPS-induced fever is reduced by nearly 50% in SD, and the profound anorexic and anhedonic motivational states that occur during the days following LPS treatment are likewise reduced. LPS treatments typically suppress grooming and nest-building behaviors in rodents (Aubert et al., 2007), but the LPS-induced suppression of nest building is absent in SD hamsters (Prendergast et al., 2008). The combination of shorter fever duration, attenuated anorexia, and persistent nest building (a thermoregulatory behavior) results in a marked attenuation of body mass loss in SD relative to LD hamsters, and is likely an important adaptation which allows hamsters to mitigate the energetic costs of mounting an APR during the energetic landscape of winter (Bilbo et al., 2002a; Nelson, 2004). Attenuated APRs to pathogens are not restricted to LPS: sickness responses to gram-positive bacterial and viral mimetics are likewise attenuated in short-day housed hamsters (Baillie & Prendergast, 2008), suggesting an omnibus downregulation of innate inflammatory responses in winter. Whether immune responses to pathogens that are more prevalent at different times of year are differentially modulated by changes in day length is a provocative question, the answer to which would have important adaptive implications, however, little is known about seasonal cycles of disease in Siberian hamsters in nature.
Figure 1. Sickness responses to simulated bacterial infections are attenuated in short days.
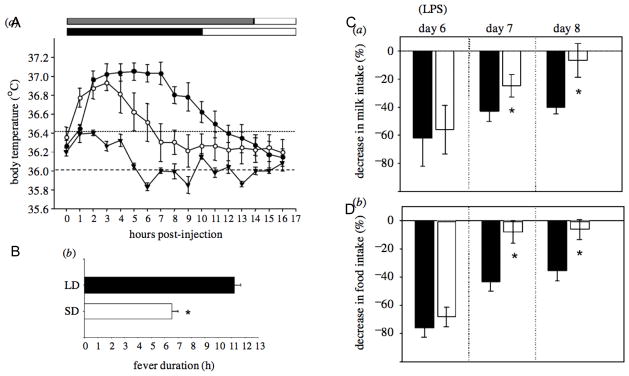
Sickness responses to LPS (25 μg) of male Siberian hamsters following 11 weeks of exposure to long (14L) or short (10L) photoperiods. (A) Mean ± SEM core body temperature during the first 16 h following LPS injections (shaded and unshaded bars along the figure top depict the scotophase and photophase, respectively, of the LD and SD photoperiods); LD: filled circles, SD: open circles, saline-injected controls: triangles. (B) Mean ± SEM duration of LPS-induced fever. (C) Mean ± SEM anhedonic response to LPS (assessed by change in consumption of sweetened condensed milk). (D) Mean ± SEM anorexic response to LPS (assessed by change in food intake). * P<0.05 vs. LD-LPS value. (Data from: Bilbo, S.D., Drazen, D.L, Quan, N., He, L., Nelson, R.J., Short day lengths attenuate the symptoms of infection in Siberian hamsters, Proc. Biol. Sci., 2002, 269[1490], 447–454, by permission of the Royal Society.)
Accompanying these decreases in motivational and behavioral components of the APR in SD hamsters are reductions in production of the cytokines IL-1β, IL-6 (Bilbo et al., 2001a) and TNFα (Prendergast et al., 2003a; Navara et al., 2007) by various lymphoid cells. Such decreases in cytokine production may be sufficient to yield the observed reductions in sickness responses to LPS, however, they do not appear to be necessary. When treated with identical doses of recombinant IL-1β, the magnitude and persistence of anorexia, anhedonia, cachexia, and the duration of fever were still greater in LD relative to SD hamsters (Wen & Prendergast, 2007). This outcome indicates that SDs attenuate the symptoms of infection not solely because cytokine production is reduced in SD, but also because the substrates upon which cytokines act to generate physiological and behavioral components of the APR are less responsive to cytokines in short days.
In summary, widespread changes occur in the hamster immune system following adaptation to short photoperiods. Some of the more striking changes manifest in the several-fold enhancement of DTH reactions in SD, and the attenuation of proinflammatory cytokine production and sickness behavior/physiology following innate immune stimulation in SD. The amplitude of the seasonal cycle in several measures of immunity encompasses a range that would be clinically diagnostic of an immunocompromised state (Lange et al., 1989; Gordin et al., 1994), yet hamsters exhibit such changes in the absence of co-morbid illness and in response to little more than a few hours’ change in day length.
4. Transduction of photoperiod cues
In order to engage a photoperiodic response— reproductive or immunological— an individual must detect the photoperiod in its environment. Understanding the mechanisms that permit internalization of day length cues has been a major goal of the study of seasonality (Prendergast, 2005). The reproductive neuroendocrine system has proven an attractive model system for dissecting the mechanisms by which animals measure seasonal time, in no small part due to the categorical changes in reproductive morphology and physiology that occur following manipulations of day length in the laboratory. Not surprisingly, most of what we know about how the brain constructs an internal representation of day length has been based on studies of reproductive physiology, with a strong bias in the literature towards mammals. Below, we selectively review formal and molecular mechanisms of photoperiod signal transduction in the reproductive neuroendocrine axis with an emphasis on models, pathways and signaling molecules that may be relevant to photoperiodic modulation of immune function.
4.1. Formal models of reproductive photoperiodism: relevance to immunity
The vast majority of laboratory investigations of photoperiodism use long (LD) or short (SD) day lengths that remain invariant for months at a time; and when photoperiod conditions are switched (e.g., hamsters are transferred from LD to SD), the decrease (4–8 h) in day length typically occurs in a single day. These resemble conditions that never occur in nature. Rather, in the field, photoperiod changes by no more than 6–7 minutes per day, even at extreme latitudes, and follows a sinusoidal annual pattern. The hamster reproductive neuroendocrine system requires many successive MEL signals in order to initiate a gonadal response, and importantly, the CNS processes these signals within the context of the signals that preceded them (“photoperiodic history”). Photoperiod / MEL signal-processing mechanisms are sensitive to the frequency of MEL signal occurrence and the direction of change in MEL signal length (Elliott et al., 1989; Gorman & Zucker, 1997; Prendergast et al., 1998). Both the induction of the winter reproductive phenotype and the timing of gonadal recrudescence in the spring are dependent on the processing of day length signals by this system (Prendergast et al., 2002a).
Until relatively recently, formal analyses of mammalian reproductive photoperiodism stressed a critical day length (CDL) concept: decreases in day length below the CDL (typically 13–14 h of light / day) triggered gonadal regression in autumn, whereas photoperiods above the CDL were permissive for gonadal development (reviewed in Lee & Gorman, 2000). It is now recognized that an individual’s history of photoperiod exposure markedly modifies the CDL, and prior exposure to longer or shorter photoperiods can determine the direction of the reproductive response to an intermediate-duration photoperiod. When preceded by longer day lengths, intermediate day lengths induce gonadal regression, whereas they stimulate gonadal growth when preceded by shorter days (Duncan et al., 1985; Hoffmann & lllnerova, 1986). Such “photoperiod history” effects within the reproductive neuroendocrine system modify the CDL and are of functional significance in the field, as they permit initiation of phenotypic changes not in response to absolute day length, but in response to smaller changes in day length. They also permit disambiguation of identical intermediate-duration day lengths that occur in late summer and early spring (Prendergast et al., 2000).
Whether a CDL concept is even relevant to immunological responses to photoperiod, and if so whether it exhibits similar history-dependent plasticity as is evident in the reproductive system, has been the subject of several studies. In the first report, juvenile hamsters were gestated in long (16 h light/day; 16L) or short (8L) days and raised in long, short or intermediate (14L) day lengths from birth. Intermediate-duration photoperiods inhibited gonadal regression in hamsters that were gestated in 16L, and accelerated gonadal growth among hamsters gestated in 8L, indicating that the reproductive neuroendocrine system is capable of comparing postnatal day length with day lengths experienced during gestation, and can initiate reproductive responses appropriate for the direction of change in day length (Prendergast et al., 2004a). In contrast to such divergent reproductive responses, across a broad array of immune assays (circulating leukocytes phenotypes, DTH reactions, antibody production) hamsters gestated in long and short day lengths exhibited comparable immune responses to 14L postnatally (Prendergast et al., 2004a). Because divergent reproductive responses imply that antecedent photoperiod information (acquired prenatally) impacts the interpretation of a contemporary, ambiguous day length (e.g., 14L), these results suggested that the immune system did not appear to have access to this information, or was unable to respond to it, highlighting a fundamental distinction between the retention of photoperiod information across the prenatal-postnatal interval by the reproductive and immune systems.
A later report also found limited evidence for retention of photoperiod history information in the immune system (Prendergast & Pyter, 2009). In this study, hamsters were raised from weaning in long (15L) or short (9L) photoperiods, and were then transferred to 1 of 7 photoperiods ranging from 9L to 15L, inclusive. A 13L photoperiod elicited gonadal regression in hamsters with a 15L history, whereas 11L was sufficient to trigger gonadal growth in hamsters with a 9L photoperiod history—illustrating the plasticity in the CDL for reproductive responses. In contrast, the CDL for photoperiodic enhancement and suppression of blood leukocyte concentrations was 13L, and was unaffected by photoperiod history, confirming earlier reports. However, in all experimental photoperiods, leukocyte concentrations were lower in hamsters with a 9L relative to a 15L photoperiod history. In one sense, this reflects a photoperiod history effect, but one that manifests itself quite differently in the immune system than in the reproductive system. In this case, photoperiod history imparted what may be interpreted as a hysteresis effect: altering the baseline values from which experimental photoperiods subsequently exerted their effects. Skin DTH reactions were also affected by photoperiod history, but also in a manner dissimilar to that observed in the reproductive system. In 15L hamsters, day lengths <12L enhanced DTH responses; in contrast, among 9L hamsters DTH was only marginally attenuated by increases in day length, and only in 15L (Prendergast & Pyter, 2009).
Taken together, studies of photoperiod history effects in the immune system suggest that prenatal-postnatal transmission of photoperiod history information does not appear to impact immune responses, but photoperiod history information acquired postnatally does so. In the reproductive system, photoperiod history effects shift the CDL. In the immune system, in contrast, photoperiod history effects appear to manifest either as a hysteresis effect (leukocyte concentrations), or as an overall dampening of responsiveness to change in photoperiod (skin DTH). Although reproductive and immunological responses to photoperiod share some formal similarities, the reproductive and immune systems appear to rely on prior photoperiod information in fundamentally different ways.
4.2. Melatonin and reproduction -physiological mechanisms
For the majority of traits investigated, the secretion of pineal MEL is required for mammalian photoperiodic responses to occur (reviewed in Bartness et al., 1993; Goldman, 2001). The light-entrainable circadian clock in the SCN drives the MEL secretory rhythm; circadian oscillators entrained to lights-off (‘dusk’) and lights-on (‘dawn’) regulate the onset and offset, respectively, of nocturnal MEL secretion via a multi-synaptic pathway (Illnerova, 1991; reviewed in Moore, 1996). Over the annual cycle, the duration of peak nocturnal MEL varies inversely with day length, and it is the duration of MEL secretion that communicates seasonal information to the reproductive system. Manipulations of reproductive condition can be achieved by pinealectomizing (PINx) animals and providing exogenous infusions of MEL centrally (Badura & Goldman, 1992) or peripherally (Carter & Goldman, 1983a,b). When MEL signals are provided to hamsters on a daily basis over an interval of several weeks, infusions ≥ 8 h duration per day inhibit reproductive development, whereas infusions ≤ 6 h per day are compatible with gonadal growth (Goldman, 2001). The information extracted from a melatonin-based day length signal by the reproductive neuroendocrine system is based entirely upon the duration of the signal. Neither the amplitude of the MEL signal nor the circadian phase during which it occurs has any bearing on its interpretation by systems that regulate reproductive condition (Goldman et al., 1984; Gorman, 2003; but see Gunduz & Stetson, 2001 for a challenge to this hypothesis). Gonadal recrudescence (redevelopment) occurs after 4–5 months of exposure to short days (Reiter, 1972); this is likely triggered by an interval timing mechanism that is associated with the development of refractoriness to inhibitory MEL signals (Bittman, 1978; Freeman & Zucker, 2001).
The neural pathways mediating seasonal reproductive responses to melatonin have been described in recent years. High-affinity MEL binding sites in the central nervous system and pituitary have been implicated in the effects of MEL on body mass and reproductive hormone secretion. Lesions of these brain regions also prevent photoinduced changes in reproductive physiology (Maywood & Hastings, 1995). Delivery of long-duration MEL infusions directly into the SCN, the thalamic paraventricular nuclei (PVt), or the nucleus reuniens nuclei (NRe) is sufficient to induce short-day-like changes in reproductive physiology (Dowell & Lynch, 1987; Hastings et al., 1988; Badura & Goldman, 1992; Freeman & Zucker, 2001).
4.3. Melatonin and immunity -physiological mechanisms
Insights into the dependence of photoperiodic time measurement on pineal MEL have been derived almost exclusively from studies of photoperiodic changes in reproductive physiology and adiposity (Bartness et al., 1993; Goldman, 2001). However, in the past decade a number of reports have examined whether photoperiod-like changes in immune function could be elicited in a predictable direction via manipulations of MEL signaling.
In hamsters housed in long days, pharmacological injections of MEL delivered daily 4 h before the onset of darkness (which lengthens the endogenous MEL profile, resembling a short day-like MEL signal) effectively mimicked the effects of short days on febrile responses to a simulated infection, suggesting that the duration of melatonin is sufficient in this regard (Bilbo and Nelson, 2002); a similar paradigm yielded comparable results on circulating leukocyte concentrations (Prendergast et al., 2003b). Pineal-dependence of adaptive immunity has also been inferred from an experiment in which surgical pinealectomy (PINx) blocked the short-day suppression of anti-SRBC antibody production (Yellon et al., 1999b). Lastly, PINx hamsters housed in short-days failed to exhibit the typical short-day attenuation in most acute-phase sickness responses to a simulated bacterial infection (cachexia, anorexia, suppression of nest building), demonstrating pineal-dependence of the innate immune system and major symptoms of the APR to bacterial infection (Wen et al., 2007).
Although chronic MEL implants, which result in continuously elevated plasma MEL, are far less effective in eliciting gonadal regression than a coherent daily MEL signal that is followed by a MEL-free interval (Gorman, 2003; Prendergast, 2010), such MEL implants have also been used to probe immunity. In female hamsters, chronic s.c. MEL implants failed to impart the short day phenotype in the innate immune response to bacterial infection. Indeed, chronic MEL implants blocked the typical abrogation of fever and lethargy responses to LPS treatment (Fenn et al., 2011). One interpretation of this outcome is that the immune system also appears to be less responsive to chronically-elevated MEL, and in common with reproductive targets of MEL, may require a MEL-free interval in order to maximally decode seasonal MEL signals and abstract time-of-year information.
4.4. Sites of MEL action in the immune response to photoperiod
The target tissues at which MEL acts to engage seasonal adaptations in the hamster immune system have not been fully identified. However, in other rodents, splenocytes, bone marrow cells, and lymphoid cells in the circulation all possess functional MEL receptors, and MEL treatments in vitro enhance or suppress immune responses in a cell- and antigen-specific manner (e.g., Pozo et al., 1997). In hamsters, MEL can inhibit or stimulate lymphocyte and splenocyte proliferation in vitro, depending on sex, age and photoperiod (Prendergast et al., 2001; 2002b). This is consistent with other reports in deer mice, in which exogenous MEL in vivo has been reported to enhance splenocyte proliferation in vitro, although MEL treatments did not inhibit primary immunoglobulin M antibody production (Demas & Nelson, 1998).
MEL-dependent photoperiodic changes in immune function may be mediated entirely by an action of MEL in the periphery. Alternatively, MEL may enact seasonal changes in immune function via pathways originating in the CNS. To examine this issue, Freeman and colleagues (2007) challenged PINx hamsters with MEL implants targeting a hypothalamic site (SCN) known to mediate effects of MEL on the reproductive response. Chronic MEL implants in the SCN induced a winter phenotype in the immune system: attenuating LPS-induced anorexia and cachexia. However, SCN MEL implants failed to induce a winter-like phenotype in blood leukocyte concentrations or in behavioral thermoregulatory (nest building) responses to LPS. In the same study, s.c. MEL implants (which deliver MEL to all target tissues, central and peripheral) induced winter-like changes in all behavioral and immunological traits. These results suggest that the MEL acts at the SCN to induce seasonal changes in neural-immune pathways that regulate some, but not all, behavioral responses to an inflammogen. Although the relevance of MEL activity at other subcortical sites towards behavioral immune responses has not been investigated, the existing data suggest that some anatomical overlap exists between neural substrates mediating the effects of MEL on the reproductive and immune systems, but that instantiation of the full winter immunophenotype requires MEL action at extra-SCN targets (Freeman et al., 2007).
Lastly, on a comparative note, pineal MEL secretion is clearly the predominant signal by which photoperiod information is transduced from the retina to the reproductive neuroendocrine system in mammals, and most photoperiodic responses in the immune system appear to be pineal-dependent. This highly canalized pathway may be the exception rather than the rule, however. In non-mammalian vertebrates (e.g., birds, fish and reptiles) for example, MEL is neither sufficient nor necessary for photoperiodic reproductive responses to occur (Borg, 2009; Juss et al. 1993; Follett et al 1985). It would not be surprising to identify, in non-mammalian vertebrates, a role for extra-pineal and/or extra-retinal systems in the transduction of photoperiod information into the immune system. Additional comparative work in non-mammalian vertebrates, identifying the phenomenology and mechanisms of seasonal immunomodulation, will provide essential insights into this conjecture.
5. Effects of seasonal changes in gonadal hormones on immunity
Photoperiodic changes in immunity are dependent on pineal MEL secretion (reviewed above). However, in addition to abolishing photoperiodic immune responses, PINx manipulations also abolish photoperiodic reproductive responses. Gonadal hormones are potent immunomodulators (Klein, 2000), and temporally, photoperiodic changes in immune function roughly parallel changes in reproductive function (Prendergast et al., 2002b; Yellon et al., 2005). Thus, the extent to which photoperiodic changes in immune function are merely a downstream consequence of the concurrent changes in reproductive physiology and hormone secretion bears consideration.
In hamsters, photoperiodic changes in skin inflammatory responses occur independently of changes in the reproductive system (Prendergast et al., 2005). In castrated hamsters, short-day suppression of lymphocyte proliferation persists (Prendergast et al., 2002b); and short-day inhibition of immunoglobulin G antibody responses likewise occurs normally in hamsters that are reproductively nonresponsive (and retain fully-developed gonads) under short days (Drazen et al., 2000), collectively suggesting gonadal hormone independence in these measures of immunity. In contrast, some in vitro measures of immune function have been reported to track gonadal status more closely (Prendergast et al., 2002b) and exhibit direct responsiveness to gonadal hormone treatments in vitro (Bilbo & Nelson, 2001).
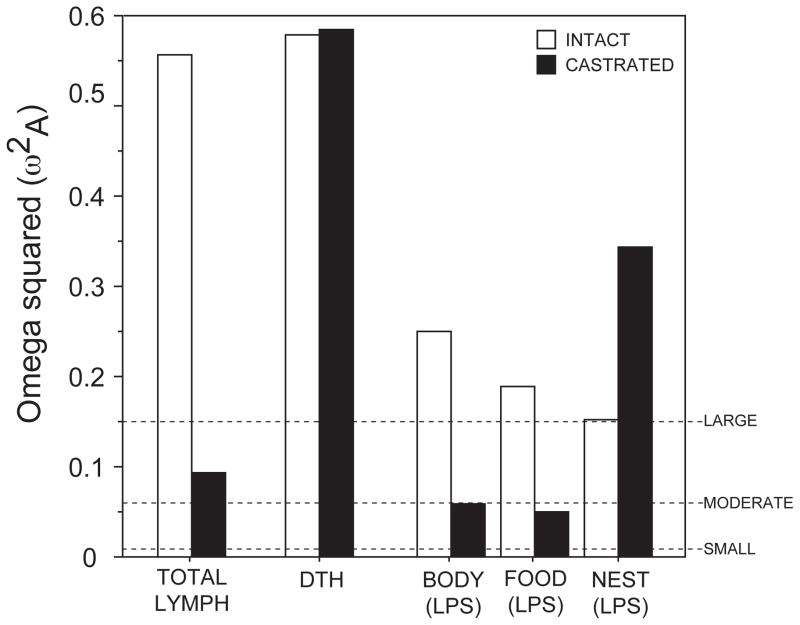
In one report, castrated and intact male Siberian hamsters were exposed to long or short days for ~ 3 months, and photoperiodic responses in multiple aspects of immunity (numerous lymphocyte subtypes, acute phase thermoregulatory, ingestive and somatic responses to LPS) were assessed. In gonad-intact hamsters, short days increased total and L-selectin (CD62L+) lymphocyte counts and CD3+ lymphocytes (i.e., T cells) in blood, and markedly attenuated sickness responses to LPS (Prendergast et al., 2008). Independent of photoperiod (i.e., in LD hamsters), castration alone increased total and CD62L+ lymphocyte and CD3+ T lymphocyte numbers and attenuated somatic and anorexic sickness responses, indicating that the removal of elevated gonadal hormone production was sufficient to mimic some aspects of the immunological response to short days. However, despite these effects of castration in long days, effects of photoperiod were still evident on blood lymphocyte concentrations and on sickness behaviors in castrated hamsters. In general, among castrated hamsters, the magnitude of most immunological responses to short days was diminished relative to those seen in gonad-intact hamsters, but were nevertheless present.
The magnitude of the effect of photoperiod on several aspects of immunity (constitutive measures, behavioral symptoms of innate immune activation, adaptive immune responses) in intact and castrated hamsters, as estimated via the omega square statistic, is depicted in Fig. 2. Note that, depending on the immunological trait, the effects of gonadectomy on immune responses to photoperiod varies markedly. The short-day phenotype in several measures of immunity can be partially instated via elimination of gonadal hormones alone, but for most immunological traits there exist pineal-dependent effects of photoperiod on immune function that do not depend on concurrent seasonal changes in gonadal hormone secretion.
Figure 2. Gonadal hormone-dependent and -independent effects of photoperiod on immunity in hamsters.
Effects of gonadectomy on the magnitude of the effect of photoperiod on several measures of immunity in male Siberian hamsters. TOTAL LYMPH = blood lymphocyte concentrations; DTH = peak delayed type hypersensitivity skin inflammatory responses; BODY (LPS) = peak decreases in body mass in response to LPS treatment; FOOD (LPS) = peak decreases in food intake in response to LPS treatment; NEST (LPS) = acute suppression of nest building behavior in response to LPS treatment. Adapted from Prendergast et al. (2008). Effect sizes are estimated using the omega square statistic which provides an estimate of the total dependent variance that can be accounted for by the independent variable in a given experimental population.
If changes in reproductive condition do not solely mediate photoperiodic changes in immunity, then what other signals might participate in mediating the effects of photoperiod into the immune system? We turn, once again, to the photoperiodic control of the reproductive system for clues.
7. Thyroid hormones seasonal timing
7.1 Thyroid hormones and reproductive photoperiodism
Research into neuroendocrine events downstream of MEL which participate in the regulation of the mammalian reproductive neuroendocrine axis system has recently focused on thyroid hormones. However, investigations of the role of thyroid hormones in the regulation of the reproductive axis in avian models dates back over 80 years, to research in mallard ducks (Anas platyrhynchos), which revealed striking light-dependent changes in thyroid hormone activity (Benoit & Aron, 1934) and inhibition of light-induced gonadal development following thyroidectomy (Benoit, 1936). This work was expanded upon three decades later in studies of Indian spotted munia (Uroloncha punctulata; Pandha & Thapliyal, 1964; Thapliyal & Pandha, 1965; 1967). In 1940, Voitkevitch (1940) described a relationship between thyroid and gonadal activity in European starlings (Sturnus vulgaris). Later, it was shown that gonadal maturation in starlings in long days is unaffected by thyroidectomy, but gonadal involution does not occur normally (Goldsmith and Nicholls, 1984). Rather, thyroidectomized starlings remain in a state of reproductive competence presumably indefinitely, and involution could only be induced via thyroxine treatment (Nicholls et al., 1988). However, starlings thyroidectomized on short days then transferred to long days showed robust gonadal growth (Wieselthier & van Tienhoven, 1972). Studies in Japanese quail (Coturnix coturnix) also indicated that thyroxine replacement restored gonadal growth (Follett & Nicholls, 1985). Wilson, Reinert and colleagues were among the first to use deiodinase inhibitors to probe the relative contributions of T3 and T4 during different stages of the seasonal reproductive cycle in photoperiodic avian species (Wilson, 2001). In tree sparrows (Spizella arborea) T4 was capable of driving gonadal growth, refractoriness and postnuptial moult, whereas T3 only stimulated gonadal growth, and only at specific concentrations (Reinert & Wilson, 1997; Wilson & Reinert, 2000). This group has argued that both T3 and T4 participate in the reproductive response to photoperiod and may play different roles in photostimulation and photorefractoriness (Reinert & Wilson, 1997; Wilson & Reinert, 2000).
Early work in mammals also indicated that thyroidectomy altered the timing of key seasonal reproductive events. The thyroid gland is required for the termination of the breeding season in ewes. Ewes that were thyroidectomized during the summer or early autumn failed to transition to anoestrus in early winter (Follett & Potts, 1990; Moenter et al., 1991; Webster et al., 1991). Moreover, thyroidectomized Soay rams exhibited a complete elimination of seasonal changes in testicular cycles (Parkinson & Follett, 1995). Thyroid hormones appear to act by regulating gonadotropin release from the pituitary gland, as thyroidectomized ewes transferred from long to short days failed to exhibit changes in luteinizing hormone concentrations (Dahl et al., 1994).
A remarkable advance in the understanding of how thyroid hormones participate in photoperiod signal transduction occurred in 2003, when Yoshimura and colleagues identified rapid and robust effects of abrupt changes in day length on the expression of iodothyronine deiodinase (DIO) enzymes in the quail mediobasal hypothalamus (Yoshimura et al., 2003). T4 is the primary output of the thyroid gland, but is essentially a prohormone for the biologically-active 3,5,3′-triiodothyronine (T3). Type II iodothyronine deiodinase (DIO2) is responsible for the deiodination of T4 into the active hormone, T3 (St. Germain et al., 2009). The majority of T4→T3 conversion occurs at the tissue-level and in a tissue-specific manner. Opposing DIO2 is the type III iodothyronine deiodinase (DIO3); DIO3 converts T4 into reverse T3 (rT3), which has little to no functional activity at the thyroid hormone receptor (St. Germain et al., 2009). In quail, transfer from short to long days rapidly upregulated the expression of TSH expression and DIO2 mRNA, which lead to increased T3 signaling (Yoshimura et al., 2003). Light-induced changes in DIO2 and DIO3 have been reported in other avian species, including great tits (Perfito et al., 2009), starlings (Bentley et al., 2013), and sparrows (Watanabe et al., 2007). Furthermore, many mammalian species have been shown to exhibit marked photoperiod-driven changes in DIO2 and DIO3 mRNA expression, including sheep (Hanon et al., 2008), Syrian hamsters (Yasuo et al., 2007) and Siberian hamsters (Watanabe et al., 2007); in all cases, the changes in mRNA expression precedes changes in gonadotrophin activity and gonadal status, suggesting a causal role.
In Siberian hamsters, photoperiod does not affect circulating T4 or T3 concentrations (O’Jile & Bartness, 1996; Prendergast et al., 2002a), but convergent evidence has accumulated indicating that the DIO enzymes may be key regulators of reproductive responses to photoperiod. Expression of DIO2 and DIO3 mRNA in and around the ependymal cell layer of the hypothalamus is exquisitely responsive to photoperiod. In hamsters, DIO3 expression is undetectable in long days, but is upregulated after exposure to just a few weeks of exposure to short days (Barrett et al., 2007; Watanabe et al., 2007); increased DIO3 expression reduces hypothalamic T3 production (Yoshimura, 2006; Barrett et al., 2007). In many rodent species, DIO2 expression is upregulated in long days in this region. A current model proposes that increases in DIO2 in long days amplifies T3 signaling and provokes gonadal growth via a TSHβ-dependent mechanism (Yasuo et al., 2010). Consistent with this model, treatment with T3 abolishes hamster reproductive responses to short days, effectively mimicking a long day phenotype by maintaining elevated levels of thyroid hormone signaling (Freeman et al., 2007; Barrett et al., 2007). Depending on species, hypothalamic expression of DIO2 mRNA, DIO3 mRNA, or both mRNAs is regulated by photoperiod (Yoshimura, 2006; Hut, 2011). Even in reproductively non-photoperiodic rodents (e.g. house mice, rats), hypothalamic expression of DIO2 is high in long days, and DIO3 is elevated in short days (Ono et al., 2008). The CNS targets at which MEL is necessary and sufficient to engage DIO2 and DIO3 responses in hamsters remain unidentified.
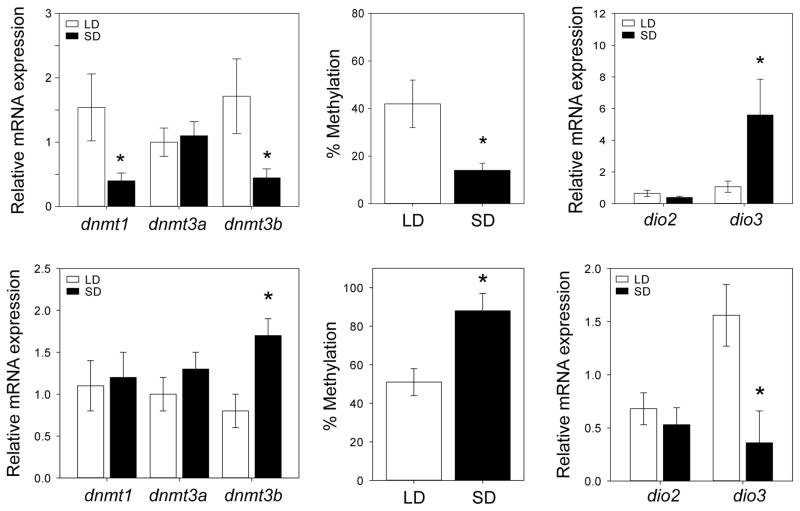
Recent work has indicated that epigenetic mechanisms participate in the photoperiod- and MEL-induced regulation of DIO3 mRNA (Stevenson & Prendergast, 2013). Following seasonal intervals of exposure to short days, the expression of DNA methyltransferases (DNMTs) is downregulated in the Siberian hamster hypothalamus, and hypothalamic DIO3 promoter-specific DNA methylation levels are substantially decreased. Exogenous MEL treatments mimicked the effects of short days on DNMT expression, DIO3 promoter methylation and DIO3 mRNA expression, indicating the sufficiency of MEL in initiating this epigenetic cascade— a sequence of events that would be poised to participate in the photoperiodic disinhibition of DIO3 mRNA expression in short days (Stevenson & Prendergast, 2013).
In sum, existing data implicate a mechanism of photoperiod signal transduction in the rodent reproductive system via MEL-driven changes in DIO2 expression and (in Siberian hamsters) epigenetic regulation of DIO3 expression, which results in SD photoperiodic inhibition of perihypothalamic T4 → T3 catabolism (Fig. 3). A number of important elements of this model remain to be critically evaluated, including the necessity and sufficiency of changes in DIO2 and/or DIO3 for inducing changes in gonadotrophin signaling in response to photoperiod manipulations.
Figure 3. Effects of photoperiod on epigenetic regulation of iodothyronine deiodinases in the hypothalamus and in leukocytes.
Effects of photoperiod on expression of (A,C) the DNA methyltransferases dnmtl, dnmt3a and dnmt3b; (B,D) dio3 proximal promoter methylation, and (C,E) dio2/dio3 mRNA expression in the hypothalamus (top row) and in leukocytes (bottom row) of Siberian hamsters. Adapted from Stevenson & Prendergast (2013) and Stevenson et al. (2014).
7.2 Thyroid hormones and immunological photoperiodism
In light of the proposed role of thyroid hormone signaling in the mediation of reproductive photoperiodism (Yoshimura, 2013), similar pathways may also participate in the gonadal hormone-independent photoperiodic control of immunity. Many studies document T3 as a potent regulator of the immune system. An exhaustive evaluation of this research area is beyond the scope of this review, however, relevant highlights include: evidence for the expression of thyroid hormone receptors (TRs) in lymphocytes, thymocytes & bone marrow (Segal & Ingbar, 1982; Foster et al., 1999); suppression of thymopoesis in TR-mutant mice during development (Arpin et al., 2000); reductions in B lineage cells in hypothyroid mice (Murphy et al., 1992), and the rescue thereof via T4 therapy (Montecino-Rodriguez, et al., 1996); T3 enhancement of T cell dependent DTH skin responses in euthyroid rats (Chandel & Chatterjee, 1989); inhibition of innate immune responses to Listeria infection in hypothyroid mice (Foster et al., 2000); and T3 enhancement of blood leukocyte concentrations and mitogen-induced splenocyte proliferation (Chatterjee & Chandel, 1983). T3-induced dendritic cell maturation facilitates naïve T cell proliferation and IFN-γ production (Mascanfroni et al., 2008); T3 also plays a key role in the cytoplasmic–nuclear shuttling of nuclear factor kβ on primed dendritic cells (Mascanfroni et al., 2010).
T4 involvement in immunity typically has been elaborated via hypothyroid and murine knockout models (reviewed in Dorshkind & Horseman, 2000). While instructive, these models are limited in that they involve completely abolishing T3 signaling, then re-introducing pharmacologically elevated, invariant T4 levels. Moreover, systemic T4 triggers GH secretion, and it may be unclear whether effects of global T4 manipulations are mediated through alterations in growth hormone and insulin-like growth factor -1 signaling (Spindler, et al., 1989).
Given that thyroid hormones are potent immunomodulators, the localized production of thyroid hormones in immune cells was evaluated as a possible mechanism for mediation of MEL-based photoperiod information into the immune system. Several lines of evidence suggest that thyroid hormones may indeed participate in this process. qPCR analyses indicated significantly lower DIO3 mRNA expression in peripheral blood leukocytes from SD compared to LD housed hamsters (Stevenson, et al., 2014; Fig. 3). This pattern of reduced DIO3 mRNA expression in short day leukocytes is evident at multiple time points, primarily during the light phase, and also in splenocytes (unpublished observations), and stands in contrast to the short-day-induced increase in DIO3 mRNA evident in the mediobasal hypothalamus of short-day hamsters (Watanabe et al., 2007; Barrett et al., 2007). In a functional assay, the conversion of T4 to T3 by lymphocytes in vitro was greater in lymphocytes collected from SD relative to LD hamsters, consistent with the predicted consequences of reduced DIO3 enzymatic activity in SD lymphocytes (Stevenson et al., 2014). Although the proportion of T3+ lymphocytes in the circulation did not differ between LD and SD hamsters, anti-T3 fluorescence was higher in the cytoplasmic region, and lower in the nuclear region, of SD lymphocytes, suggesting that changes in day length alter the amount of T3 signaling at nuclear vs. cytosolic thyroid hormone receptors. The functional significance of this photoperiodic shift in cellular compartmentalization of T3 is not presently clear but may play a role in lymphocyte maturation or activation (Mascanfroni et al., 2008; 2010).
In common with the reproductive neuroendocrine system, in the immune system photoperiodic regulation of DNA methylation has been associated with the aforementioned changes in DIO3 expression. DNA methyltransferase 3b (DNMT3b) mRNA expression was greater in leukocytes collected from SD compared to LD hamsters, and methylation-sensitive restriction enzyme assays indicated that DIO3 promoter methylation was greater in short day compared to long day leukocytes (Stevenson et al., 2014; Fig. 3). The phenotypes of leukocytes exhibiting photoperiodic DNMT3b and DIO3 responses have yet to be determined. However, photoperiodic regulation of DNMT3b in circulating leukocytes afford the potential photoperiod to enact broad changes in genomic DNA methylation in the immune system.
Photoperiod thus affects DIO3 mRNA, DNMT3b mRNA and DIO3 promoter methylation in both leukocytes and hypothalamic tissue, yet the effects of short days on these genomic changes occur in opposite directions in the two tissues. In the hypothalamus, long days are associated with increased DNMT3b mRNA expression, greater DNA methylation in the DIO3 promoter and reduced DIO3 mRNA expression; whereas in leukocytes, long days are associated with reduced DNMT3b, lower DIO3 promoter DNA methylation and greater DIO3 expression (Fig. 3). Photoperiodic changes in DIO expression afford tissue-specific regulation of T3 signaling against a background of seasonally-invariant T4 concentrations in the circulation.
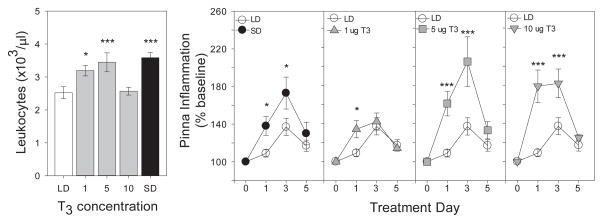
If short-day-induced decreases in DIO3 yield local, cell-specific increases in T3 that participate in photoperiodic changes in immune function, then one prediction would be that exogenous T3 treatment might bypass DIO2/DIO3-mediated T4 metabolism and mimic effects of short days on immunity, just as exogenous T3 treatment mimics the effects of long days on reproductive physiology (Barrett et al., 2007; Freeman et al., 2007). In a test of this hypothesis, exogenous T3 was shown to enhance blood leukocyte concentrations, and in a dose-dependent manner, to augment the magnitude of T cell-mediated adaptive skin immune responses (DTH; Stevenson et al., 2014; Fig. 4). These results indicate that increases in T3 signaling are sufficient to mimic the effects of short days on select aspects of constitutive and adaptive immunity. The extent to which exogenous T3 is also sufficient to mimic effects of short days on innate immunity and behavioral responses to infection is currently under investigation.
Figure 4. Thyroid hormone treatment mimics effects of short days on hamster immune function.
Effects of exogenous thyroid hormone treatments on (A) circulating leukocyte numbers and (B) Type IV skin inflammatory responses (DTH reactions) in male Siberian hamsters. Panel A depicts mean ± SEM blood leukocyte concentrations of Siberian hamsters housed in LD and injected s.c. daily with 1, 5, or 10 μg T3 or saline for 3 weeks; SD controls received daily saline injections. Panel B depicts mean ± SEM DTH skin inflammatory responses in hamsters treated with T3 or saline in LD for 6 weeks; DTH was elicited by cutaneous challenge with an antigen (DNFB) 1 week after antigen sensitization (SD controls received saline injections only). *P < 0.05, ***P < 0.005 vs. LD-saline value. Adapted from Stevenson et al. (2014).
Taken together, recent work suggests that epigenetic regulation of DIO3 expression and T3 signaling, in addition to potentially playing a central role in the transduction of photoperiod information in the reproductive neuroendocrine system, may also figure prominently in the mediation of photoperiod effects on select aspects of immune function (Fig. 5). A number of important questions remain to be answered about how photoperiod and MEL signals engage changes in leukocyte biology, including the leukocyte subtype(s) that are differentially expression DIO3, and whether MEL receptors are present in spleen, thymus, and T lymphocytes, as is the case in other rodents (cf. Carrillo-Vico et al., 2005).
Figure 5. Photoperiod and the epigenetic regulation of T3 signaling in the reproductive and immune systems.
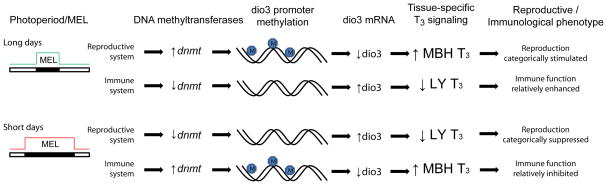
Schematic of pathways by which short photoperiods engage activation and inhibition of T3 signaling in tissues of the reproductive and immune systems. At the hypothalamic level of the HPG axis, exposure to LD is compatible with elevated levels of dnmt expression, increased dio3 promoter methylation, reduced levels of dio3 mRNA expression and increased thyroid hormone signaling; whereas exposure to SD decreases dnmt expression, permitting demethylation of the dio3 promoter, increases in dio3 mRNA expression and reductions in hypothalamic thyroid hormone signaling. In peripheral leukocytes, SD photoperiods exert opposite effects on epigenetic regulation of thyroid hormone signaling: in SDs, dnmt expression is relatively higher, maintaining higher levels of dio3 promoter methylation, lower levels of dio3 mRNA expression, and increases in T3 signaling in peripheral leukocytes; whereas in LD photoperiods, lymphocyte dnmt expression and dio3 promoter methylation are reduced, and dio3 mRNA expression is relatively higher, which is associated with reductions in leukocyte T3 production. Adapted from Stevenson & Prendergast (2013).
8. Conclusions
The proximate causes of numerous seasonal rhythms in health and immune function, in both the human and veterinary literature, remain incompletely explained. Seasonal variations in day length exert robust changes in diverse aspects of immune function. Seasonal changes in the capacity for immunosurveilliance, in the thresholds for immunological responses, and in the magnitude of immune responses to select pathogens and antigens may explain some of the seasonal variance in the epidemiology of disease in nature. Conversely, seasonality in the immune system may have evolved partly to anticipate seasonal changes in the variety of pathogens in the environment. In addition, from a basic regulatory biology perspective, understanding the mechanisms by which seasonal time information, first registered in the central nervous system, comes to impart changes in the immune system stands to identify novel endogenous pathways by which the brain communicates with the periphery.
Supplementary Material
Highlights.
Day length (DL) alters numerous aspects of immune function in rodents
Most effects of DL on immunity require pineal melatonin secretion
Gonadal hormone-independent effects of DL vary in magnitude for different traits
Epigenetic control of thyroxine metabolism may mediate effects of DL on immunity
Acknowledgments
This work was supported by NIH Grant AI-67406 from the National Institute for Allergy and Infectious Diseases. We thank George Bentley for comments on a draft of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahmad R, Haldar C. Immune responses to lipopolysaccharide challenge in a tropical rodent (Funambulus pennanti): photoperiod entrainment and sex differences. Stress. 2012;15:172–183. doi: 10.3109/10253890.2011.606515. [DOI] [PubMed] [Google Scholar]
- Arpin C, Pihlgren M, Fraichard A, Aubert D, Samarut J, Chassande O, Marvel J. Effects of T3R alpha 1 and T3R alpha 2 gene deletion on T and B lymphocyte development. J Immunol. 2000;164:152–160. doi: 10.4049/jimmunol.164.1.152. [DOI] [PubMed] [Google Scholar]
- Aschoff J. Exogenous and endogenous components in circadian rhythms. Cold Spring Harb Symp Quant Biol. 1960;25:11–18. doi: 10.1101/sqb.1960.025.01.004. [DOI] [PubMed] [Google Scholar]
- Ashley NT, Zhang N, Weil ZM, Magalang UJ, Nelson RJ. Photoperiod alters duration and intensity of non-REM sleep following immune challenge in Siberian hamsters (Phodopus sungorus). Chronobiol. Intl. 2012;29:683–692. doi: 10.3109/07420528.2012.682682. [DOI] [PubMed] [Google Scholar]
- Ashley NT, Walton JC, Haim A, Zhang N, Prince LA, Fruchey AM, Lieberman RA, Weil ZM, Magaland UJ, Nelson RJ. Sleep deprivation attenuates endotoxin induced cytokine gene expression independent of day length and circulating Cortisol in male Siberian hamsters (Phodopus sungorus) J Exp Biol. 2013;216:2581–2586. doi: 10.1242/jeb.083832. [DOI] [PubMed] [Google Scholar]
- Aubert A, Goodall G, Dantzer R, Gheusi G. Differential effects of lipopolysaccharide on pup retrieving and nest building in lactating mice. Brain Behav Immun. 1997;11:107–118. doi: 10.1006/brbi.1997.0485. [DOI] [PubMed] [Google Scholar]
- Badura LL, Goldman BD. Central sites mediating reproductive responses to melatonin in juvenile male Siberian hamsters. Brain Res. 1992;598:98–106. doi: 10.1016/0006-8993(92)90172-6. [DOI] [PubMed] [Google Scholar]
- Baillie SR, Prendergast BJ. Photoperiodic regulation of behavioral responses to bacterial and viral mimetics: a test of the winter immunoenhancement hypothesis. J Biol Rhythms. 2008;23:81–90. doi: 10.1177/0748730407311518. [DOI] [PubMed] [Google Scholar]
- Barrett P, Ebling FJ, Schuhler S, Wilson D, Ross AW, Warner A, Jethwa P, Boelen A, Visser TJ, Ozanne DM, Archer ZA, Mercer JG, Morgan PJ. Hypothalamic thyroid hormone catabolism acts as a gatekeeper for the seasonal control of body weight and reproduction. Endocrinology. 2007;148:3608–3617. doi: 10.1210/en.2007-0316. [DOI] [PubMed] [Google Scholar]
- Bartness TJ, Powers JB, Hastings MH, Bittman EL, Goldman BD. The timed infusion paradigm for melatonin delivery: what has it taught us about the melatonin signal, its reception and the photoperiodic control of seasonal responses? J Pineal Res. 1993;15:161–190. doi: 10.1111/j.1600-079x.1993.tb00903.x. [DOI] [PubMed] [Google Scholar]
- Benoit J, Aron M. Sur le conditionnement hormonique du developpement testiculaire, chez les oiseaux. Rksultats de la thyroidectomie, chez le Coq et le Canard. Compt Rend SOC Biol Paris. 1934;16:221–223. [Google Scholar]
- Benoit J. Role de la thyroide dans la gonado-stimulation par la lumiere artificielle chez le Canard domestique. Compt Rend Sac Biol Paris. 1936;123:243–246. [Google Scholar]
- Bentley GE, Tucker S, Chou H, Hau M, Perfito N. Testicular growth and regression are not correlated with dio2 expression in a wild male songbird, Sturnus vulgaris, exposed to natural changes in photoperiod. Endocrinology. 2013;154:1813–1819. doi: 10.1210/en.2013-1093. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Nelson RJ. Sex steroid hormones enhance immune function in male and female Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2001;280:R207–R213. doi: 10.1152/ajpregu.2001.280.1.R207. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Nelson RJ. Melatonin regulates energy balance and attenuates fever in Siberian hamsters. Endocrinology. 2002;143:2527–2533. doi: 10.1210/endo.143.7.8922. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Drazen DL, Quan N, He L, Nelson RJ. Short day lengths attenuate the symptoms of infection in Siberian hamsters. Proc Biol Sci. 2002a;269:447–454. doi: 10.1098/rspb.2001.1915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Dhabhar FS, Viswanathan K, Saul A, Yellon SM, Nelson RJ. Short day lengths augment stress-induced leukocyte trafficking and stress-induced enhancement of skin immune function. Proc Natl Acad Sci USA. 2002b;99:4067–4072. doi: 10.1073/pnas.062001899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilbo SD, Nelson RJ. Sex differences in photoperiodic and stress-induced enhancement of immune function in Siberian hamsters. Brain Behav Immun. 2003;17:462–472. doi: 10.1016/s0889-1591(03)00063-1. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Dhabhar FS, Viswanathan K, Saul A, Nelson RJ. Photoperiod affects the expression of sex and species differences in leukocyte number and leukocyte trafficking of congeneric hamsters. Psychoneuroendocrinology. 2003a;28:1027–1043. doi: 10.1016/s0306-4530(02)00122-1. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Hotchkiss AK, Chiavegatto S, Nelson RJ. Blunted stress responses in delayed type hypersensitivity in mice lacking the neuronal isoform of nitric oxide synthase. J Neuroimmunol. 2003b;140:41–48. doi: 10.1016/s0165-5728(03)00175-9. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Nelson RJ. Photoperiod influences the effects of exercise and food restriction on an antigen-specific immune response in Siberian hamsters. Endocrinology. 2004;145:556–564. doi: 10.1210/en.2003-1035. [DOI] [PubMed] [Google Scholar]
- Bittman EL. Hamster refractoriness: the role of insensitivity of pineal target tissues. Science. 1978;202:648–650. doi: 10.1126/science.568311. [DOI] [PubMed] [Google Scholar]
- Blom JMC, Gerber JM, Nelson RJ. Day length affects immune cell numbers in deer mice: Interactions with age, sex, and prenatal photoperiod. Am J Physiol. 1994;267:R596–R601. doi: 10.1152/ajpregu.1994.267.2.R596. [DOI] [PubMed] [Google Scholar]
- Borg B. Photoperiodism in fishes. In: Nelson RJ, Denlinger DL, editors. Photoperiodism: the biological calendar. Oxford University Press; Oxford: 2009. [Google Scholar]
- Bradley AJ, McDonald IR, Lee AK. Stress and mortality in a small marsupial (Antechinus stuartii Macleay) Gen Comp Endocrinol. 1980;40:188–200. doi: 10.1016/0016-6480(80)90122-7. [DOI] [PubMed] [Google Scholar]
- Brainard GC, Knobler RL, Podolin PL, Lavasa M, Lublin FD. Neuroimmunology: Modulation of the master immune system by photoperiod. Life Sci. 1987;40:1319–1326. doi: 10.1016/0024-3205(87)90589-3. [DOI] [PubMed] [Google Scholar]
- Brainard GC, Watson-Whitmeyer M, Knobler RL, Lublin FD. Neuroendocrine regulation of immune parameters: Photoperiod control of the spleen in Syrian hamsters. Ann NY Acad Sci. 1988;540:704–706. doi: 10.1111/j.1749-6632.1988.tb27219.x. [DOI] [PubMed] [Google Scholar]
- Bronson F. Mammalian Reproductive Biology. University of Chicago Press; Chicago: 1989. [Google Scholar]
- Butler MP, Silver R. Basis of robustness and resilience in the suprachiasmatic nucleus: individual neurons form nodes in circuits that cycle daily. J Biol Rhythms. 2009;24:340–352. doi: 10.1177/0748730409344800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrillo-Vico A, Guerrero JM, Lardone PJ, Reiter RJ. A review of the multiple actions of melatonin on the immune system. Endocrine. 2005;27:189–200. doi: 10.1385/ENDO:27:2:189. [DOI] [PubMed] [Google Scholar]
- Carter DS, Goldman BS. Antigonadal effects of timed melatonin infusion in pinealectomized male Djungarian hamsters: duration is the critical parameter. Endocrinolology. 1983a;113:1261–1267. doi: 10.1210/endo-113-4-1261. [DOI] [PubMed] [Google Scholar]
- Carter DS, Goldman BS. Progonadal role of the pineal in the Djungarian hamster: mediation by melatonin. Endocrinology. 1983b;113:1268–1273. doi: 10.1210/endo-113-4-1268. [DOI] [PubMed] [Google Scholar]
- Champney TH, Prado J, Youngblood T, Appel K, McMurray DN. Immune responsiveness of splenocytes after chronic daily melatonin administration in male Syrian hamsters. Immunol Lett. 1997;58:95–100. doi: 10.1016/s0165-2478(97)00039-4. [DOI] [PubMed] [Google Scholar]
- Chandel AS, Chatterjee S. Effect of thyroid hormones on delayed type hypersensitivity reaction. Indian J Exp Biol. 1989;27:408–411. [PubMed] [Google Scholar]
- Chatterjee S, Chandel AS. Immunomodulatory role of thyroid hormones: in vivo effect of thyroid hormones on the blastogenic response of lymphoid tissues. Acta Endocrinol (Copenh) 1983;103:95–100. doi: 10.1530/acta.0.1030095. [DOI] [PubMed] [Google Scholar]
- Curtis AM, Bellet MM, Sassone-Corsi P, O’Neill LA. Circadian clock proteins and immunity. Immunity. 2014;40:178–186. doi: 10.1016/j.immuni.2014.02.002. [DOI] [PubMed] [Google Scholar]
- Dahl GE, Evans NP, Moenter SM, Karsch FJ. The thyroid gland is required for reproductive neuroendocrine responses to photoperiod in the ewe. Endocrinology. 1994;135:10–15. doi: 10.1210/endo.135.1.8013340. [DOI] [PubMed] [Google Scholar]
- Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demas GE, Nelson RJ. Photoperiod and temperature interact to affect immune parameters in adult male deer mice (Peromyscus maniculatus) J Biol Rhythms. 1996;11:95–103. doi: 10.1177/074873049601100202. [DOI] [PubMed] [Google Scholar]
- Demas GE, Chefer V, Talan MI, Nelson RJ. Metabolic costs of mounting an antigen-stimulated immune response in adult and aged C57BL/6J mice. Am J Physiol. 1997;273:R1631–R1637. doi: 10.1152/ajpregu.1997.273.5.R1631. [DOI] [PubMed] [Google Scholar]
- Demas GE, Nelson RJ. Exogenous melatonin enhances cell-mediated, but not humoral, immune function in adult male deer mice (Peromyscus maniculatus) J Biol Rhythms. 1998;13:245–252. doi: 10.1177/074873098129000084. [DOI] [PubMed] [Google Scholar]
- Demas GE, Drazen DL, Jasnow AM, Bartness TJ, Nelson RJ. Sympathoadrenal system differentially affects photoperiodic changes in humoral immunity of Siberian hamsters. J Neuroendocrinol. 2002;14:29–35. doi: 10.1046/j.0007-1331.2001.00736.x. [DOI] [PubMed] [Google Scholar]
- Demas GE, Bartness TJ, Nelson RJ, Drazen DL. Photoperiod modulates the effects of norepinephrine on lymphocyte proliferation in Siberian hamsters. Am J Physiol Regul Comp Physiol. 2003;285:R873–R879. doi: 10.1152/ajpregu.00209.2003. [DOI] [PubMed] [Google Scholar]
- Dorshkind K, Horseman ND. The roles of prolactin, growth hormone, insulin-like growth factor-l, and thyroid hormones in lymphocyte development and function: insights from genetic models of hormone and hormone receptor deficiency. Endocr Rev. 2000;21:292–312. doi: 10.1210/edrv.21.3.0397. [DOI] [PubMed] [Google Scholar]
- Dowell SF, Lynch GR. Duration of the melatonin pulse in the hypothalamus controls testicular function in pinealectomized mice(Peromyscus leucopus) Biol Reprod. 1987;36:1095–1101. doi: 10.1095/biolreprod36.5.1095. [DOI] [PubMed] [Google Scholar]
- Duncan MJ, Goldman BD, Di Pinto MN, Stetson MH. Testicular function in the Djungarian hamster, Phodopus sungorus. Endocrinology. 1985;116:424–430. doi: 10.1210/endo-116-1-424. [DOI] [PubMed] [Google Scholar]
- Drazen DL, Kriegsfeld LJ, Schneider JE, Nelson RJ. Leptin, but not immune function, is linked to reproductive responsiveness to photoperiod. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1401–R1407. doi: 10.1152/ajpregu.2000.278.6.R1401. [DOI] [PubMed] [Google Scholar]
- Drazen DL, Trasy A, Nelson RJ. Photoperiod differentially affects energetics of immunity in pregnant and lactating Siberian hamsters (Phodopus sungorus) Can J Zool. 2003;81:1406–1413. [Google Scholar]
- Elliot JA, Bartness TJ, Goldman BD. Effect of melatonin infusion duration and frequency on gonad, lipid and body mass in pinealectomized male Siberian hamsters. J Biol Rhythms. 1989;4:439–455. doi: 10.1177/074873048900400404. [DOI] [PubMed] [Google Scholar]
- Fenn AM, Fonken LK, Nelson RJ. Sustained melatonin treatment blocks body mass, pelage, reproductive and fever responses to short day lengths in female Siberian hamsters. J Pineal Res. 2011;51:180–186. doi: 10.1111/j.1600-079X.2011.00874.x. [DOI] [PubMed] [Google Scholar]
- Follett BK, Nicholls TJ. Influences of thyroidectomy and thyroxine replacement on photoperiodically controlled reproduction in quail. J Endocrinol. 1985;107:211–221. doi: 10.1677/joe.0.1070211. [DOI] [PubMed] [Google Scholar]
- Follett BK, Potts C. Hypothyroidism affects reproductive refractoriness and the seasonal oestrous period in Welsh Mountain ewes. J Endocrinol. 1990;127:103–109. doi: 10.1677/joe.0.1270103. [DOI] [PubMed] [Google Scholar]
- Follett BK, Foster RG, Nichols TJ. Photoperiodism in birds. CIBA Foundation Symposia. 1985;117:93–105. doi: 10.1002/9780470720981.ch7. [DOI] [PubMed] [Google Scholar]
- Fonken LK, Morris JS, Nelson RJ. Early life experiences affect adult delayed-type hypersensitivity in short and long photoperiods. Chronobiol Intl. 2011;28:101–108. doi: 10.3109/07420528.2010.538818. [DOI] [PubMed] [Google Scholar]
- Foster MP, Montecino-Rodriguez E, Dorshkind K. Proliferation of bone marrow pro-B cells is dependent on stimulation by the pituitary/thyroid axis. J Immunol. 1999;163:5883–5890. [PubMed] [Google Scholar]
- Foster MP, Jensen ER, Montecino-Rodriguez E, Leathers H, Horseman N, Dorshkind K. Humoral and cell-mediated immunity in mice with genetic deficiencies of prolactin, growth hormone, insulin-like growth factor-I, and thyroid hormone. Clin Immunol. 2000;96:140–149. doi: 10.1006/clim.2000.4889. [DOI] [PubMed] [Google Scholar]
- Freeman DA, Zucker I. Refractoriness to melatonin occurs independently at multiple brain sites in Siberian hamsters. Proc Natl Acad Sci USA. 2001;98:6447–6452. doi: 10.1073/pnas.111140398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freeman DA, Kampf-Lassin A, Galang J, Wen JC, Prendergast BJ. Melatonin acts at the suprachiasmatic nucleus to attenuate behavioral symptoms of infection. Behav Neurosci. 2007;121:689–697. doi: 10.1037/0735-7044.121.4.689. [DOI] [PubMed] [Google Scholar]
- Freeman DA, Teubner BJ, Smith CD, Prendergast BJ. Exogenous T3 mimics long day lengths in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2007;292:R2368–R2372. doi: 10.1152/ajpregu.00713.2006. [DOI] [PubMed] [Google Scholar]
- Goldman BD. Mammalian photoperiodic system: formal properties and neuroendocrine mechanisms of photoperiodic time measurement. J Biol Rhythms. 2001;16:283–301. doi: 10.1177/074873001129001980. [DOI] [PubMed] [Google Scholar]
- Goldman BD, Darrow JM, Yoge L. Effects of timed melatonin infusions on reproductive development in the Djungarian hamster, Phodopus sungorus. Endocrinology. 1984;114:2074–2083. doi: 10.1210/endo-114-6-2074. [DOI] [PubMed] [Google Scholar]
- Goldsmith AR, Nicholls TJ. Thyroidectomy prevents the development of photorefractoriness and the associated rise in plasma prolactin in starlings. Gen Comp Endocrinol. 1984;54:256–263. doi: 10.1016/0016-6480(84)90179-5. [DOI] [PubMed] [Google Scholar]
- Gordin FM, Hartigan PM, Klimas NG, Zolla-Pazner SB, Simberkoff MS, Hamilton JD. Delayed-type hypersensitivity skin tests are an independent predictor of human immunodeficiency virus disease progression. Department of Veterans Affairs Cooperative Study Group. J Infect Dis. 1994;169:893–897. doi: 10.1093/infdis/169.4.893. [DOI] [PubMed] [Google Scholar]
- Gorman MR. Melatonin implants disrupt developmental synchrony regulated by flexible interval timers. J Neuroendocrinol. 2003;15:1084–1094. doi: 10.1046/j.1365-2826.2003.01104.x. [DOI] [PubMed] [Google Scholar]
- Gorman MR, Zucker I. Pattern of change in melatonin duration determines testicular responses in Siberian hamsters, Phodopus sungorus. Biol Reprod. 1997;56:668–673. doi: 10.1095/biolreprod56.3.668. [DOI] [PubMed] [Google Scholar]
- Gündüz B, Stetson MH. A test of the coincidence and duration models of melatonin action in Siberian hamsters: the effects of 1-hr melatonin infusions on testicular development in intact and pinealectomized prepubertal Phodopus sungorus. J Pineal Res. 2001;30:97–107. doi: 10.1034/j.1600-079x.2001.300205.x. [DOI] [PubMed] [Google Scholar]
- Hadley AR, Tran LT, Fagoaga OR, Nehlsen-Cannarella SL, Yellon SM. Sex differences in photoperiod control of antigen-specific primary and secondary humoral immunity in Siberian hamsters. J Neuroimmunol. 2002;128:39–48. doi: 10.1016/s0165-5728(02)00144-3. [DOI] [PubMed] [Google Scholar]
- Hanon EA, Lincoln GA, Fustin JM, Dardente H, Masson-Pevet M, Morgan PJ, Hazlerigg DG. Ancestral TSH mechanism signals summer in a photoperiodic mammal. Curr Biol. 2008;18:1147–1152. doi: 10.1016/j.cub.2008.06.076. [DOI] [PubMed] [Google Scholar]
- Hart BL. Biological basis of the behavior of sick animals. Neurosci Biobehav Rev. 1988;12:123–137. doi: 10.1016/s0149-7634(88)80004-6. [DOI] [PubMed] [Google Scholar]
- Hastings MH, Walker AP, Roberts AC, Herbert J. Intra-hypothalamic melatonin blocks photoperiodic responsiveness in the male Syrian hamster. Neuroscience. 1988;24:987–991. doi: 10.1016/0306-4522(88)90081-4. [DOI] [PubMed] [Google Scholar]
- Hiebert SM, Thomas EM, Lee TM, Pelz KM, Yellon SM, Zucker I. Photic entrainment of circannual rhythms in golden-mantled ground squirrels: role of the pineal gland. J Biol Rhythms. 2000;15:126–134. doi: 10.1177/074873040001500207. [DOI] [PubMed] [Google Scholar]
- Hoffman K, Illnerova H. Photoperiodic effects in the Djungarian hamster. Neuroendocrinol. 1986;43:317–321. doi: 10.1159/000124562. [DOI] [PubMed] [Google Scholar]
- Hut RA. Photoperiodism: shall EYA compare thee to a summer’s day? Curr Biol. 2011;21:R22–25. doi: 10.1016/j.cub.2010.11.060. [DOI] [PubMed] [Google Scholar]
- Illnerova I. The suprachiasmatic nucleus and rhythmic pineal melatonin production. In: Klein DC, Moore RY, Reppert SM, editors. Suprachiasmatic Nucleus: The Mind’s Clock. University Press; New York: 1991. pp. 197–216. [Google Scholar]
- Janeway CA, Jr, Travers P, Capra JD, Walport MJ. Immunobiology: The Immune System in Health and Disease. Garland Publishers; New York: 1999. [Google Scholar]
- Juss TS, Meddle SL, Servant RS, King VM. Melatonin and photoperiodic time measurement in Japanese quail (Coturnix coturnixjaponica) Proc R Soc B. 1993;254:21–28. doi: 10.1098/rspb.1993.0121. [DOI] [PubMed] [Google Scholar]
- Kinsey SG, Prendergast BJ, Nelson RJ. Photoperiod and stress affect wound healing in Siberian hamsters. Physiol Behav. 2003;78:205–211. doi: 10.1016/s0031-9384(02)00967-8. [DOI] [PubMed] [Google Scholar]
- Klein SL. The effects of hormones on sex differences in infection: from genes to behavior. Neurosci Biobehav Rev. 2000;24:627–638. doi: 10.1016/s0149-7634(00)00027-0. [DOI] [PubMed] [Google Scholar]
- Lahiri S, Haldar C. Response of melatonin receptor MT1 in spleen of a tropical Indian rodent, Funambulus pennanti, to natural solar insolation and different photoperiodic conditions. Chronobiol Intl. 2009;26:1559–1574. doi: 10.3109/07420520903540960. [DOI] [PubMed] [Google Scholar]
- Lange JM, de Wolf F, Goudsmit J. Markers for progression in HIV infection. AIDS. 1989;3:S153–160. doi: 10.1097/00002030-198901001-00023. [DOI] [PubMed] [Google Scholar]
- Lee TM, Gorman MR. Timing of reproduction by the integration of photoperiod and other seasonal signals. In: Wallen K, Schneider J, editors. Reproduction in Context. MIT Press; 2000. pp. 191–218. [Google Scholar]
- Linton P, Thoman ML. T cell senescence. Front Biosci. 2001;6:D248–D261. doi: 10.2741/linton. [DOI] [PubMed] [Google Scholar]
- Lochmiller RL, Vestey MR, McMurry ST. Temporal variation in humoral and cell-mediated immune response in a Sigmodon hispidus population. Ecology. 1994;75:236–245. [Google Scholar]
- Maier SF, Watkins LR, Fleshner M. Psychoneuroimmunology. The interface between behavior, brain, and immunity. Am Psychol. 1994;49:1004–1017. doi: 10.1037//0003-066x.49.12.1004. [DOI] [PubMed] [Google Scholar]
- Mascanfroni ID, Montesinos MM, Alamino VA, Susperreguy S, Nicola JP, Ilarregui JM, Masini-Repiso AM, Rabinovich GA, Pellizas CG. Nuclear factor (NF)-κB-dependent thyroid hormone receptor betal expression controls dendritic cell function via Akt signaling. J Biol Chem. 2010;285:9569–9582. doi: 10.1074/jbc.M109.071241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mascanfroni ID, Montesinos MM, Susperreguy S, Cervi L, llarregui JM, Ramseyer VD, Masini-Repiso AM, Targovnik HM, Rabinovich GA, Pellizas CG. Control of dendritic cell maturation and function by triiodothyronine. FASEB J. 2008;22:1032–1042. doi: 10.1096/fj.07-8652com. [DOI] [PubMed] [Google Scholar]
- Maywood ES, Hastings MH. Lesions of the iodomelatonin-binding sites of the mediobasal hypothalamus spare the lactoropic, but block the gonadotropic response of male Syrian hamsters to short photoperiod and to melatonin. Endocrinology. 1995;136:144–153. doi: 10.1210/endo.136.1.7828525. [DOI] [PubMed] [Google Scholar]
- McAllan BM, Dickman CR. The role of photoperiod in the timing of reproduction in the Dasyurid Marsupial Antechinus stuartii. Oecologia (Berl) 1986;68:259–264. doi: 10.1007/BF00384797. [DOI] [PubMed] [Google Scholar]
- McAllan BM, Crowther MS, Dickman CR. Photoperiod as a reproductive cue in the marsupial genus Antechinus: ecological and evolutionary consequences. Biol J Linn Soc. 2006;87:365–379. [Google Scholar]
- McDonald IR, Lee AK, Bradley AJ, Than KA. Endocrine changes in dasyurid marsupials with differing mortality patterns. Gen Comp Endocrinol. 1981;44:292–301. doi: 10.1016/0016-6480(81)90004-6. [DOI] [PubMed] [Google Scholar]
- McElhaney JE, Effros RB. Immunosenescence: what does it mean to health outcomes in older adults? Curr Opin Immunol. 2009;21:418–424. doi: 10.1016/j.coi.2009.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKenna JM, Musacchia XJ. Antibody formation in hibernating ground squirrels (Citrellus tridecemlineatus) Proc Soc Exp Biol Med. 1968;129:720–724. doi: 10.3181/00379727-129-33408. [DOI] [PubMed] [Google Scholar]
- Moenter SM, Woodfill CJI, Karsch FJ. Role of the thyroid gland in seasonal reproduction: thyroidectomy blocks seasonal suppression of reproductive neuroendocrine activity in ewes. Endocrinology. 1991;128:1337–1344. doi: 10.1210/endo-128-3-1337. [DOI] [PubMed] [Google Scholar]
- Montecino-Rodriguez E, Clark R, Johnson A, Collins L, Dorshkind K. Defective B cell development in Snell dwarf (dw/dw) mice can be corrected by thyroxine treatment. J Immunol. 1996;157:3334–3340. [PubMed] [Google Scholar]
- Moore RY. Neural control of the pineal gland. Behav Brain Res. 1996;73:125–130. doi: 10.1016/0166-4328(96)00083-6. [DOI] [PubMed] [Google Scholar]
- Murphy WJ, Durum SK, Anver MR, Longo DL. Immunologic and hematologic effects of neuroendocrine hormones. Studies on DW/J dwarfmice. J Immunol. 1992;148:3799–3805. [PubMed] [Google Scholar]
- Navara KJ, Trainor BC, Nelson RJ. Photoperiod alters macrophage responsiveness but not expression of Toll-like receptors in Siberian hamsters. Comp Biochem Physiol A Mol Integr Physiol. 2007;148:354–359. doi: 10.1016/j.cbpa.2007.05.019. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Blom JM. Photoperiodic effects on tumor development and immune function. J Biol Rhythms. 1994;9:233–249. doi: 10.1177/074873049400900305. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Demas GE. Seasonal changes in immune function. Q Rev Biol. 1996;71:511–548. doi: 10.1086/419555. [DOI] [PubMed] [Google Scholar]
- Nelson RJ. Seasonal immune function and sickness responses. Trends Immunol. 2004;25:187–192. doi: 10.1016/j.it.2004.02.001. [DOI] [PubMed] [Google Scholar]
- Nicholls TJ, Follett BK, Goldsmith AR, Pearson H. Possible homologies between photorefractoriness in sheep and birds: the effect of thyroidectomy on the length of the ewe’s breeding season. Reprod Nutr Develop. 1988;28:375–385. doi: 10.1051/rnd:19880304. [DOI] [PubMed] [Google Scholar]
- O’Jile JR, Bartness TJ. Effects of thyroxine on the photoperiodic control of energy balance and reproductive status in Siberian hamsters. Physiol Behav. 1996;52:267–270. doi: 10.1016/0031-9384(92)90269-8. [DOI] [PubMed] [Google Scholar]
- Ono H, Hoshino Y, Yasuo S, Watanabe M, Nakane Y, Murai A, Ebihara S, Korf HW, Yoshimura T. Involvement of thyrotropin in photoperiodic signal transduction in mice. Proc Natl Acad Sci USA. 2008;105:18238–18242. doi: 10.1073/pnas.0808952105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandha SK, Thapliyal JP. Effect of thyroidectomy upon the testis of Indian Spotted Munia, Uroloncha punctulata. Naturwissenschaften. 1964;51:202. [Google Scholar]
- Parkinson TJ, Follett BK. Thyroidectomy abolishes seasonal testicular cycles of Soay rams. Proc Biol Sci. 1995;259:1–6. doi: 10.1098/rspb.1995.0001. [DOI] [PubMed] [Google Scholar]
- Pawlak J, Golab M, Markowska M, Majewski P, Skwarlo-Sonta K. Photoperiod-related changes in hormonal and immune status of male Siberian hamsters, Phodopus sungorus. Comp Biochem Physiol A: Mol Integr Physiol. 2009;152:299–303. doi: 10.1016/j.cbpa.2008.10.016. [DOI] [PubMed] [Google Scholar]
- Perfito N, Jeong SY, Silverin B, Calisi RM, Bentley GE, Hau M. Anticipating spring: wild populations of great tits (Parus major) differ in expression of key genes for photoperiodic time measurement. PLoS One. 2012;7:e34997. doi: 10.1371/journal.pone.0034997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pittendrigh CS. Crcadian rhythms and the circadian organization of living organisms. Cold Spring Harb Symp Quant Biol. 1960;25:159–184. doi: 10.1101/sqb.1960.025.01.015. [DOI] [PubMed] [Google Scholar]
- Pozo D, Delgado M, Fernandez-Santos JM, Calvo JR, Gomariz RP, Martin-Lacave I, Ortiz GG, Guerrero JM. Expression of the Mel1a-melatonin receptor mRNA in T and B subsets of lymphocytes from rat thymus and spleen. FASEB J. 11:466–473. doi: 10.1096/fasebj.11.6.9194527. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ. Internalization of seasonal time. Horm Behav. 2005;48:503–511. doi: 10.1016/j.yhbeh.2005.05.013. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ. MT1 melatonin receptors mediate somatic, behavioral, and reproductive neuroendocrine responses to photoperiod and melatonin in Siberian hamsters. Endocrinology. 2010;151:714–721. doi: 10.1210/en.2009-0710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Zucker I, Yellon SM, Ringold DA, Gorman MR. Melatonin chimeras alter reproductive development and photorefractoriness in Siberian hamsters. J Biol Rhythms. 1998;13:518–531. doi: 10.1177/074873098129000345. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Gorman MR, Zucker I. Establishment and persistence of photoperiodic memory in hamsters. Proc Natl Acad Sci USA. 2000;97:5586–5591. doi: 10.1073/pnas.100098597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Yellon SM, Tran LT, Nelson RJ. Photoperiod modulates the inhibitory effect of in vitro melatonin on lymphocyte proliferation in female Siberian hamsters. J Biol Rhythms. 2001;16:224–233. doi: 10.1177/074873040101600305. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Mosinger B, Jr, Kolattukudy PE, Nelson RJ. Hypothalamic gene expression in reproductively photoresponsive and photorefractory Siberian hamsters. Proc Natl Acad Sci USA. 2002a;99:16291–16296. doi: 10.1073/pnas.232490799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Wynne-Edwards KE, Yellon SM, Nelson RJ. Photorefractoriness of immune function in male Siberian hamsters (Phodopus sungorus) JNeuroendocrinol. 2002b;14:318–329. doi: 10.1046/j.1365-2826.2002.00781.x. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Hotchkiss AK, Bilbo SD, Kinsey SG, Nelson RJ. Photoperiodic adjustments in immune function protect Siberian hamsters from lethal endotoxemia. J Biol Rhythms. 2003a;18:51–62. doi: 10.1177/0748730402239676. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Hotchkiss AK, Nelson RJ. Photoperiodic regulation of circulating leukocytes in juvenile Siberian hamsters: mediation by melatonin and testosterone. J Biol Rhythms. 2003b;18:473–480. doi: 10.1177/0748730403258486. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Bilbo SD, Nelson RJ. Photoperiod controls the induction, retention and retrieval of antigen-specific immunological memory. Am J Physiol Regul Integr Comp Physiol. 2004a;286:R54–R60. doi: 10.1152/ajpregu.00381.2003. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Bilbo SD, Dhabhar FS, Nelson RJ. Effects of photoperiod history on immune responses to intermediate day lengths in Siberian hamsters Phodopus sungorus. J Neuroimmunol. 2004b;149:31–93. doi: 10.1016/j.jneuroim.2003.12.006. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Hotchkiss AK, Bilbo SD, Nelson RJ. Peripubertal immune challenges attenuate reproductive development in male Siberian hamsters (Phodopus sungorus) Biol Reprod. 2004c;70:813–820. doi: 10.1095/biolreprod.103.023408. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Bilbo SD, Nelson RJ. Short day lengths enhance skin immune responses in gonadectomised Siberian hamsters. J Neuroendocrinol. 2005;17:18–21. doi: 10.1111/j.1365-2826.2005.01273.x. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Kampf-Lassin A, Yee JR, Galang J, McMaster N, Kay LM. Winter day lengths enhance T lymphocyte phenotypes, inhibit cytokine responses, and attenuate behavioral symptoms of infection in laboratory rats. Brain Behav Immun. 2007;21:1096–1108. doi: 10.1016/j.bbi.2007.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Baillie SR, Dhabhar FS. Gonadal hormone-dependent and -independent regulation of immune function by photoperiod in Siberian hamsters. Am J Physiol Regul Integr Comp Physiol. 2008;294:R384–392. doi: 10.1152/ajpregu.00551.2007. [DOI] [PubMed] [Google Scholar]
- Prendergast BJ, Pyter LM. Photoperiod history differentially impacts reproduction and immune function in adult Siberian hamsters. J Biol Rhythms. 2009;24:509–522. doi: 10.1177/0748730409349714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast BJ, Galang J, Kay LM, Pyter LM. Influence of the olfactory bulbs on blood leukocytes and behavioral responses to infection in Siberian hamsters. Brain Res. 2009;1268:48–57. doi: 10.1016/j.brainres.2009.01.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pyter LM, Neigh GN, Nelson RJ. Social environment modulates photoperiodic immune and reproductive responses in adult male white-footed mice (Peromyscus leucopus) Am J Physiol Regul Integr Comp Physiol. 2005a;288:R891–R896. doi: 10.1152/ajpregu.00680.2004. [DOI] [PubMed] [Google Scholar]
- Pyter LM, Samuelsson AR, Quan N, Nelson RJ. Photoperiod alters hypothalamic cytokine gene expression and sickness responses following immune challenge in female Siberian hamsters (Phodopus sungorus) Neuroscience. 2005b;131:779–784. doi: 10.1016/j.neuroscience.2004.11.046. [DOI] [PubMed] [Google Scholar]
- Reinert BD, Wilson FE. Effects of thyroxine (T4) or triiodothyronine (T3) replacement therapy on the programming of seasonal reproduction and postnuptial molt in thyroidectomized male American tree sparrows (Spizella arborea) exposed to long days. J Exp Zool. 1997;279:267–276. doi: 10.1002/(sici)1097-010x(19971101)279:4<367::aid-jez6>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Reiter RJ. Evidence for refractoriness of the pituitary-gonadal axis to the pineal gland in golden hamsters and its possible implications in annual reproductive rhythms. Anat Rec. 1972;173:365–371. doi: 10.1002/ar.1091730311. [DOI] [PubMed] [Google Scholar]
- Segal J, Ingbar SH. Specific binding sites for the triiodothyronine in the plasma membrane of rat thymocytes. Correlation with biochemical responses. J Clin Invest. 1982;70:919–926. doi: 10.1172/JCI110703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shames RS. Gender differences in the development and function of the immune system. J Adolesc Health. 2002;30:59–70. doi: 10.1016/s1054-139x(01)00382-2. [DOI] [PubMed] [Google Scholar]
- Sheldon BC, Verhulst S. Ecological immunology: costly parasite defences and trade-offs in evolutionary ecology. Trends Ecol Evol. 1996;11:317–321. doi: 10.1016/0169-5347(96)10039-2. [DOI] [PubMed] [Google Scholar]
- Sonenshine DE, Bozeman FM, Williams MS, Masiello SA, Chadwick DP, Stocks NI, Lauer DM, Elisberg BL. Epizootiology of epidemic typhus (Rickettsia prowazekii) in flying squirrels. Am J Trop Med Hyg. 1978;27:339–349. doi: 10.4269/ajtmh.1978.27.339. [DOI] [PubMed] [Google Scholar]
- Spindler SR, Crew MD, Nyborg JK. Thyroid hormone transcriptional regulatory region of the growth hormone gene. Endocr Res. 1989;15:475–493. doi: 10.3109/07435808909036349. [DOI] [PubMed] [Google Scholar]
- Stevenson TJ, Ball GF. Information theory and the neuropeptidergic regulation of seasonal reproduction in mammals and birds. Proc Biol Sci. 2011;278:2477–2485. doi: 10.1098/rspb.2010.2181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson TJ, Prendergast BJ. Reversible DNA methylation regulates seasonal photoperiodic time measurement. Proc Natl Acad Sci USA. 2013;110:16651–16656. doi: 10.1073/pnas.1310643110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson TJ, Onishi KG, Bradley SP, Prendergast BJ. Cell-autonomous iodothyronine deiodinase expression mediates seasonal plasticity in immune function. Brain Behav Immun. 2014;36:61–70. doi: 10.1016/j.bbi.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- St Germain DL, Galton VA, Hernandez A. Minireview: Defining the roles of the iodothyronine deiodinases: current concepts and challenges. Endocrinology. 2009;150:1097–1107. doi: 10.1210/en.2008-1588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapliyal JP, Pandha SK. Thyroid-gonad relationship in Spotted Munia, Uroloncha punctulata. J Exp Zool. 1965;158:253–262. doi: 10.1002/jez.1401580302. [DOI] [PubMed] [Google Scholar]
- Thapliyal JP, Pandha SK. The thyroid and the hypophysial gonadal axis in the female Spotted Munia, Uroloncha punctulata. Gen Comp Endocrinol. 1967;8:84–93. [Google Scholar]
- Voitkevich AA. Dependence of seasonal periodicity in gonadal changes on thyroid gland Sturnus vulgaris. L Dokl Akad Nauk SSSR. 1940;27:741–745. [Google Scholar]
- Watanabe T, Yamamura T, Watanabe M, Yasuo S, Nakao N, Dawson A, Ebihara S, Yoshimura T. Hypothalamic expression of thyroid hormone-activating and -inactivating enzyme genes in relation to photorefractoriness in birds and mammals. Am J Physiol Regul Integr Comp Physiol. 2007;292:R568–R572. doi: 10.1152/ajpregu.00521.2006. [DOI] [PubMed] [Google Scholar]
- Webster JR, Moenter SM, Barrell GK, Karsch FJ. Role of the thyroid gland in seasonal reproduction. II. Thyroxine allows a season specific suppression of gonadotropin secretion in sheep. Endocrinology. 1991;129:176–183. doi: 10.1210/endo-129-1-176. [DOI] [PubMed] [Google Scholar]
- Weil ZM, Martin LB, 2nd, Nelson RJ. Photoperiod differentially affects immune function and reproduction in collared lemmings (Dicrostonyx groenlandicus) J Biol Rhythms. 2006a;21:384–393. doi: 10.1177/0748730406292444. [DOI] [PubMed] [Google Scholar]
- Weil ZM, Pyter LM, Martin LB, Nelson RJ. Perinatal photoperiod organizes adult immune responses in Siberian hamsters (Phodopus sungorus) Am J Physiol Reg Integr Comp Physiol. 2006b;290:R1714–R1719. doi: 10.1152/ajpregu.00869.2005. [DOI] [PubMed] [Google Scholar]
- Wen JC, Prendergast BJ. Photoperiodic regulation of behavioral responsiveness to proinflammatory cytokines. Physiol Behav. 2007;90:717–725. doi: 10.1016/j.physbeh.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen JC, Dhabhar FS, Prendergast BJ. Pineal-dependent and -independent effects of photoperiod on immune function in Siberian hamsters (Phodopus sungorus) Horm Behav. 2007;51:31–39. doi: 10.1016/j.yhbeh.2006.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieselthier AS, van Tienhoven A. The effect of thyroidectomy on testicular size and on the photorefractory period in the starling (Sturnus vulgaris L.) J Exp Zool. 1972;179:331–338. doi: 10.1002/jez.1401790306. [DOI] [PubMed] [Google Scholar]
- Wilson FE. A test of the hypothesis that T3 is the ‘seasonality’ thyroid hormone in American tree sparrows (Spizella arborea); intracerebro-ventricular infusion of iopanoic acid, an inhibitor of T3 synthesis and degradation. J Comp Physiol B. 2001;171:113–119. doi: 10.1007/s003600000155. [DOI] [PubMed] [Google Scholar]
- Wilson FE, Reinert BD. Thyroid hormone acts centrally to programme photostimulated male American tree sparrows (Spizella arborea) for vernal and autumnal components of seasonality. J Neuroendocrinol. 2000;12:87–95. doi: 10.1046/j.1365-2826.2000.00437.x. [DOI] [PubMed] [Google Scholar]
- Workman JL, DeWitt SJ, Fonken LK, Nelson RJ. Environmental enrichment enhances delayed-type hypersensitivity in both short- and long-day Siberian hamsters. Physiol Behav. 2010;19:638–643. doi: 10.1016/j.physbeh.2010.01.029. [DOI] [PubMed] [Google Scholar]
- Yasuo S, Yoshimura T, Ebihara S, Korf HW. Temporal dynamics of type 2 deiodinase expression after melatonin injections in Syrian hamsters. Endocrinology. 2007;148:4385–4392. doi: 10.1210/en.2007-0497. [DOI] [PubMed] [Google Scholar]
- Yasuo S, Yoshimura T, Ebihara S, Korf HW. Photoperiodic control of TSH-β expression in the mammalian pars tuberalis has different impact on the induction and suppression of the hypothalamo-hypopysial gonadal axis. J Neuroendocrinol. 2010;22:43–50. doi: 10.1111/j.1365-2826.2009.01936.x. [DOI] [PubMed] [Google Scholar]
- Yellon SM, Tran LT. Photoperiod, reproduction, and immunity in select strains of inbred mice. J Biol Rhythms. 2002;17:65–75. doi: 10.1177/074873002129002348. [DOI] [PubMed] [Google Scholar]
- Yellon SM, Kim K, Hadley AR, Tran LT. Time course and role of the pineal gland in photoperiod control of innate immune cell functions in male Siberian hamsters. J Neuroimmunol. 2005;161:137–144. doi: 10.1016/j.jneuroim.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Yellon SM, Fogoaga OR, Nehlsen-Cannarella SL. Influence of photoperiod on immune cell functions in the male Siberian hamster. Am J Physiol. 1999a;276:R97–R102. doi: 10.1152/ajpregu.1999.276.1.R97. [DOI] [PubMed] [Google Scholar]
- Yellon SM, Teasley LA, Fagoaga OR, Nguyen HC, Truong HN, Nehlsen-Cannarella L. Role of photoperiod and the pineal gland in T cell-dependent humoral immune reactivity in the Siberian hamster. J Pineal Res. 1999b;27:243–248. doi: 10.1111/j.1600-079x.1999.tb00622.x. [DOI] [PubMed] [Google Scholar]
- Yoshimura T, Yasuo S, Watanabe M, ligo M, Yamamura T, Hirunagi K, Ebihara S. Light induced hormone conversion of T4 to T3 regulates photoperiodic response of gonads in birds. Nature. 2003;426:178–181. doi: 10.1038/nature02117. [DOI] [PubMed] [Google Scholar]
- Yoshimura T. Molecular mechanism of the photoperiodic response of gonads in birds and mammals. Comp Biochem Physiol A, Mol Integr Physiol. 2006;144:345–350. doi: 10.1016/j.cbpa.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Yoshimura T. Thyroid hormone and seasonal regulation of reproduction. Front Neuroendocrinol. 2013;34:157–166. doi: 10.1016/j.yfrne.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Zhou S, Cagampang FR, Stirland JA, Loudon AS, Hopkins SJ. Different photoperiods affect proliferation of lymphocytes but not expression of cellular, humoral, or innate immunity in hamsters. J Biol Rhythms. 2002;17:392–405. doi: 10.1177/074873002237133. [DOI] [PubMed] [Google Scholar]
- Zuk M, Stoehr AM. Immune defense and host life history. Am Nat. 2002;160:S9–S22. doi: 10.1086/342131. [DOI] [PubMed] [Google Scholar]
- Zysling DA, Demas GE. Metabolic stress suppresses humoral immune function in long-day, but not short-day, Siberian hamsters (Phodopus sungorus) J Comp Physiol B. 2007;177:339–347. doi: 10.1007/s00360-006-0133-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.