Abstract
BACKGROUND
Extravasation is a critical step in cancer metastasis, in which adhesion of intravascular cancer cells to the vascular endothelial cells is controlled by cell surface adhesion molecules. The role of interleukin-17 (IL-17), insulin, and insulin-like growth factor 1 (IGF1) in adhesion of prostate cancer cells to the vascular endothelial cells is unknown, which is the subject of the present study.
METHODS
Human umbilical vein endothelial cells (HUVECs) and human prostate cancer cell lines (PC-3, DU-145, LNCaP, and C4-2B) were analyzed for expression of vascular cell adhesion molecule 1 (VCAM-1), integrins, and cluster of differentiation 44 (CD44) using flow cytometry and Western blot analysis. The effects of IL-17, insulin and IGF1 on VCAM-1 expression and adhesion of prostate cancer cells to HUVECs were examined. The interaction of VCAM-1 and CD44 was assessed using immunoprecipitation assays.
RESULTS
Insulin and IGF1 acted with IL-17 to increase VCAM-1 expression in HUVECs. PC-3, DU-145, LNCaP, and C4-2B cells expressed β1 integrin but not α4 integrin. CD44 was expressed by PC-3 and DU-145 cells but not by LNCaP or C4-2B cells. When HUVECs were treated with IL-17, insulin or IGF1, particularly with a combination of IL-17 and insulin (or IGF1), adhesion of PC-3 and DU-145 cells to HUVECs was significantly increased. In contrast, adhesion of LNCaP and C4-2B cells to HUVECs was not affected by treatment of HUVECs with IL-17 and/or insulin/IGF1. CD44 expressed in PC-3 cells physically bound to VCAM-1 expressed in HUVECs.
CONCLUSIONS
CD44-VCAM-1 interaction mediates the adhesion between prostate cancer cells and HUVECs. IL-17 and insulin/IGF1 enhance adhesion of prostate cancer cells to vascular endothelial cells through increasing VCAM-1 expression in the vascular endothelial cells. These findings suggest that IL-17 may act with insulin/IGF1 to promote prostate cancer metastasis.
Keywords: Prostate cancer metastasis, IL-17, Insulin, IGF1, VCAM-1
INTRODUCTION
More than 90% of deaths from cancer are caused by the metastases instead of the primary tumors [1]. Cancer metastasis is a process in which the secondary tumor sites are formed at locations distant to the primary site, including steps of stromal invasion and intravasation at the primary site, circulation in blood and lymph vessels, and extravasation and tumor formation at the distant site [2]. The number of cancer cells entering the systemic circulation (i.e., intravasation) daily can reach up to 4×106 per gram of primary tumor [3]. With a large number of cancer cells circulating intravascularly, the interactions between cancer cells and vascular endothelial cells play a key role in hematogenous cancer metastases to the distant sites of the body [4]. Several previous studies have shown that inflammatory cytokines cause adhesion of cancer cells to the activated vascular endothelium through inducing expression of adhesion molecules on the endothelium [5–7]. Colorectal cancer cells were observed to adhere to the vascular endothelium through binding to vascular cell adhesion molecule 1 (VCAM-1) in the presence of E-selectin [8]. It has been shown that cancer cells with expression of integrin α4β1 (also called very late antigen-4, VLA-4) favorably attached to bone marrow stromal cells that constitutively expressed VCAM-1, leading to bone metastasis [9]. Therefore, understanding the molecular mechanisms underlying the interactions between cancer cells and vascular endothelial cells is vital to our understanding of cancer cells extravasation during cancer metastasis.
Interleukin-17 (IL-17 or IL-17A) is an inflammatory cytokine [10]. When it binds to a heterodimer of IL-17RA/IL-17RC receptor complex, IL-17 is able to activate nuclear factor-κB (NF-κB) activator 1 (Act1) through SEFIR (similar expression to fibroblast growth factor genes, IL-17 receptors, and Toll-IL-1R) domains [11–15]. Activation of Act1 triggers lysine-63-linked ubiquitination of tumor necrosis factor receptor-associated factor 6 (TRAF6), leading to activation of transforming growth factor-β-activated kinase 1 (TAK1) and IκB kinase (IKK) complex, and finally resulting in activation of NF-κB pathway that induces transcription of a variety of cytokines, chemokines, and growth factor [16–19]. We have previously demonstrated that IL-17 promotes development of hormone-dependent and castration-resistant prostate cancer in mouse prostates [20,21]. However, the role of IL-17 in development of metastatic tumors has not been determined.
Insulin is a hormone produced by pancreas β cells. The abnormal high concentration of insulin (hyperinsulinemia) may circulate in the body of people with obesity and type 2 diabetes mellitus with insulin resistance. Insulin-like growth factor 1 (IGF1) is produced by liver when stimulated by insulin [22]. Two types of insulin receptors (IR-A and IR-B) can bind to either insulin or IGF1. The receptors of IGF1 also include a heterodimer of IR and IGF1 receptor (IGF1R). Both insulin and IGF1 have been found to induce VCAM-1 expression in the vascular endothelial cells [23,24]. IL-17 can also increase expression of adhesion molecules, including intercellular adhesion molecule-1 (ICAM-1), VCAM-1 and E-selectin in the endothelial cells [25,26]. It has been shown that insulin can augment tumor necrosis factor-α (TNF-α)-induced expression of VCAM-1 in the endothelial cells [27]. We have previously demonstrated that insulin and IGF1 can enhance IL-17-induced chemokine expression [17]. Therefore, we conducted the present study to investigate if insulin and IGF1 can enhance IL-17-induced VCAM-1 expression in human umbilical vein endothelial cells (HUVECs), and hence boost adhesion of prostate cancer cells to HUVECs.
MATERIALS AND METHODS
Cell culture
Human umbilical vein endothelial cells (HUVECs) were obtained from Life Technologies (Grand Island, NY, USA) and cultured in Medium 200 with Low Serum Growth Supplement and Gentamicin/Amphotericin B (Life Technologies, Grand Island, NY, USA). HUVECs of passages 5 – 9 were used in the experiments. Human prostate cancer cell lines PC-3, DU-145, and LNCaP were purchased from the American Type Culture Collection (Manassas, VA, USA) and C4-2B cell line was a gift from Dr. Leland WK Chung (Cedars-Sinai Medical Center, Los Angeles, CA, USA). PC-3 and DU-145 cells were cultured in Dulbecco’s Modified Eagle’s Medium (DMEM; Mediatech, Inc., Manassas, VA, USA) with 10% fetal bovine serum (FBS; Mediatech, Inc.) and 1% penicillin/streptomycin. C4-2B cells were cultured in Roswell Park Memorial Institute (RPMI)-1640 medium (Thermo Fisher Scientific, Inc., Waltham, MA, USA) with 10% FBS and 1% penicillin/streptomycin. LNCaP cells were cultured in T-medium (Life Technologies, Grand Island, NY, USA) with 5% FBS and 1% penicillin/streptomycin. The cells were cultured in a 5% CO2 humidified incubator at 37°C.
Western blot analysis
Proteins of HUVECs, PC-3, DU-145, LNCaP and C4-2B cells were extracted using RIPA lysis buffer, which contains 50 mM sodium fluoride, 0.5% Igepal CA-630 (NP-40), 10 mM sodium phosphate, 150 mM sodium chloride, 25 mM Tris (pH 8.0), 1 mM phenylmethylsulfonyl fluoride, 2 mM ethylenediaminetetraacetic acid (EDTA), and 1.2 mM sodium vanadate. The concentration of protein was measured using Bio-Rad protein Assay Dye Reagent Concentrate (Bio-Rad Laboratories, Hercules, CA, USA) and BioTek ELx800 microplate reader (BioTek, Winooski, VT, USA). Approximately 80 μg of protein was loaded to 10% SDS-polyacrylamide gel electrophoresis and transferred to polyvinylidene difluoride membrane. 5% nonfat dry milk in TBST buffer (25 mM Tris-HCl, 125 mM sodium chloride and 0.1% Tween 20) was used to block the membrane. The membrane was incubated with primary antibodies at 4°C overnight, and then incubated with IRDye® 800CW- or IRDye® 680RD-conjugated secondary antibodies (LI-COR Biosciences, Lincoln, NE, USA) at room temperature for 1 hour (h). The results were scanned with an Odyssey Infrared Imager (LI-COR Biosciences). For loading control, the membrane was re-probed for glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The antibodies used included: rabbit anti-VCAM-1 and mouse anti-CD44 antibodies (Cell Signaling Technology, Danvers, MA, USA), and mouse anti-GAPDH antibodies (Millipore, Billerica, MA, USA).
Static adhesion assays
Approximately 1×105 HUVECs were seeded in each well of the 96-well plates. Two days later when the cells reached about 95% confluence, they were treated with 20 ng/ml recombinant human IL-17 (R&D Systems, Inc., Minneapolis, MN, USA), 50 ng/ml recombinant human insulin, or 50 ng/ml recombinant human IGF1 (Sigma Aldrich, Inc., St Louis, MO, USA), or a combination of IL-17 and insulin (or IGF1), for 24 h. Prostate cancer cells were stained with 0.8 μM calcein AM (Life Technologies, Grand Island, NY, USA) for 15 minutes at 37°C and then washed three times with complete medium, thus the stained live prostate cancer cells gave rise to intense green fluorescence. Next, prostate cancer cells (0.5×105 cells in 100-μl complete medium) were added onto HUVECs in the 96-well plates and incubated for 15 minutes at 37°C. After incubation, each well was gently washed three times with phosphate-buffered saline (PBS) to remove non-adherent prostate cancer cells. The adherent prostate cancer cells were visualized and photomicrographs were taken using an inverted fluorescence microscope with a digital camera (LEICA DMIRB, Leica Microsystems Inc., Buffalo Grove, IL, USA). The fluorescence intensity (representing the number of adherent prostate cancer cells) was measured with a microplate reader (FLUOstar Optima, BMG Labtech, Cary, NC, USA) at excitation/emission wavelengths of 495 nm/520 nm.
Flow cytometry analysis and fluorescence-activated cell sorting (FACS)
HUVECs, PC-3, DU-145, LNCaP and C4-2B cells were grown to confluence in 100-mm tissue culture dishes and harvested using an Enzyme-Free PBS-based Cell Dissociation Buffer (Life Technologies, Grand Island, NY, USA). Approximately 1× 106 cells were suspended in 100-μl FACS buffer (2% bovine serum albumin and 0.1% sodium azide in PBS). Fluorescein isothiocyanate (FITC)-conjugated mouse IgG1 (as isotype control) and mouse anti-human VCAM-1 antibodies (Ancell, Bayport, MN, USA) were added to HUVECs separately. Phycoerythrin (PE)-conjugated mouse IgG1 (as isotype control), mouse anti-human β1 integrin and α4 integrin antibodies (Ancell, Bayport, MN, USA), and mouse anti-human CD44 antibodies (Cell Signaling Technology, Danvers, MA, USA) were added to PC-3, DU-145, LNCaP, and C4-2B cells separately. The cells were incubated with the antibodies (1:50 dilution) on ice for 45 minutes, followed by washing with FACS buffer twice. Flow cytometry analysis was conducted using a BD LSRII analyzer and FACS was conducted using BD FACSAria (BD Biosciences, San Jose, CA, USA).
Immunoprecipitation (IP)
HUVECs were grown to 100% confluence in 100-mm dishes and then treated with 20 ng/ml IL-17 and 50 ng/ml IGF1 for 24 h or treated with PBS as control. Approximately 3×106 PC-3 or LNCaP cells were plated onto the confluent HUVECs for 30 minutes at 37°C. Non-adherent cancer cells were washed off with PBS. Next, HUVECs and adherent cancer cells were lysed in RIPA buffer. In a parallel set of groups, HUVECs and adherent cancer cells were first fixed with 2% formaldehyde at room temperature for 10 minutes according to a previous study [28], in order to preserve the binding of molecules between HUVECs and adherent cancer cells. After quenching with ice-cold 1.25 M glycine, HUVECs and adherent cancer cells were lysed in RIPA buffer. Mouse anti-CD44 antibodies (1 μg) or mouse isotype IgG control antibodies (1 μg) were added to the protein extract and incubated at 4°C for 1 h. Then, 10 μl of protein A Sepharose™ CL-4B beads (GE Healthcare, Waukesha, WI, USA) was added for incubation at 4°C overnight. After three washes with PBS, the immunoprecipitated protein was released from the beads by boiling and analyzed for VCAM-1 and CD44 using Western blot analysis.
Statistical analysis
The results were presented as mean ± standard deviation (SD) of three independent experiments (n = 3). Two-way analysis of variance (ANOVA) and one-way ANOVA and Tukey’s test were used to determine the statistical significance using GraphPad Prism® 5.0 (Graphpad Software, La Jolla, CA, USA). P < 0.05 was considered as statistically significant.
RESULTS
Insulin and IGF1 enhance IL-17-induced VCAM-1 expression
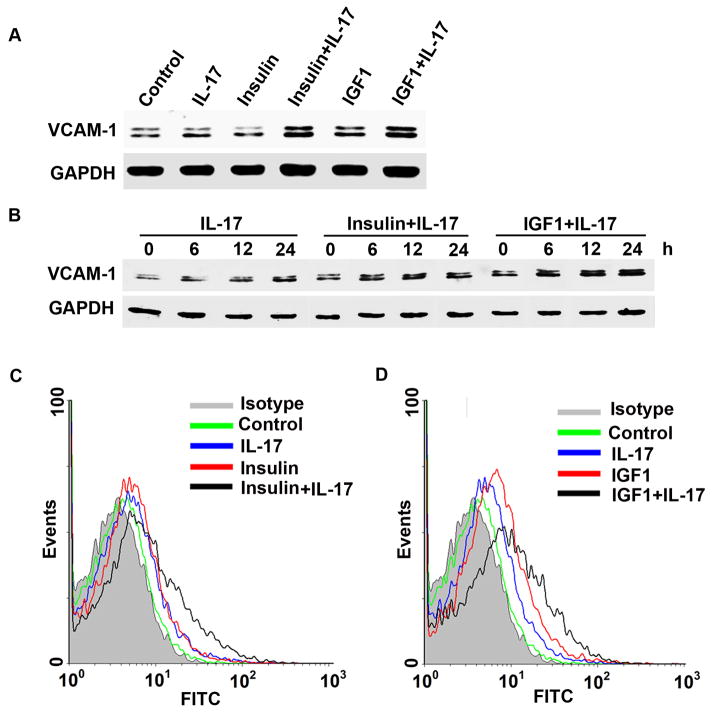
When treated with IL-17, insulin, or IGF1 alone for 24 h, VCAM-1 expression was only slightly increased in HUVECs (Fig. 1A), however, a combination of IL-17 and insulin (or IGF1) dramatically increased VCAM-1 expression (Fig. 1A). Expression of ICAM-1 and E-selectin was not affected (data not shown). Induction of VCAM-1 expression in HUVECs by IL-17 or the combination of IL-17 and insulin/IGF1 reached a peak level at 24 h (Fig. 1B). Extension of the treatment time to 48 h did not further increase the levels of VCAM-1 expression (data not shown). Flow cytometry analysis showed that the combination of IL-17 and insulin/IGF1 increased VCAM-1 expression on the surfaces of HUVECs to the levels higher than IL-17 or insulin/IGF1 alone (Fig. 1C and 1D).
Fig. 1.
VCAM-1 expression in HUVECs was induced by IL-17 and insulin/IGF1. A: Western blot analysis of VCAM-1 expression in HUVECs treated with IL-17, insulin, and IGF1, alone or in combination, for 24 h. B: Western blot analysis of VCAM-1 expression in HUVECs treated with IL-17, insulin, and IGF1, alone or in combination, for 6, 12, and 24 h. C–D: Flow cytometry analysis of VCAM-1 surface expression in HUVECs treated with IL-17, insulin, and IGF1, alone or in combination, for 24 h.
Prostate cancer cells differentially express integrins and CD44
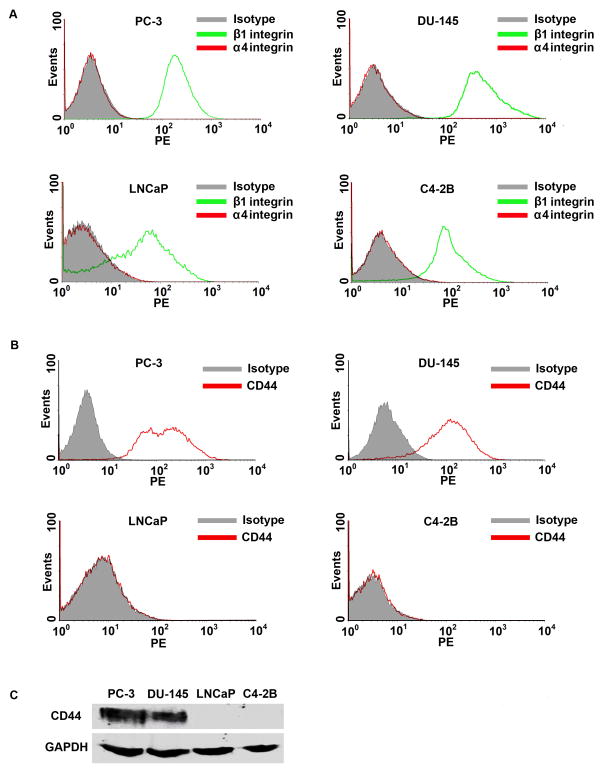
The conventional ligand of VCAM-1 is very late antigen-4 (VLA-4), which is made up by α4 and β1 integrins. Flow cytometry analysis showed that PC-3, DU-145, LNCaP, and C4-2B cells expressed β1 integrin, but not α4 integrin (Fig. 2A). This finding indicates that there is no intact VLA-4 ligand in PC-3, DU-145, LNCaP, and C4-2B cells to bind to VCAM-1 expressed in HUVECs. PC-3, DU-145, LNCaP, and C4-2B cells did not express VCAM-1, nor did HUVECs express CD44 (data not shown). Since it has been reported that CD44 can physically interact with VCAM-1 [29], we assessed CD44 expression in the prostate cancer cells. Flow cytometry analysis showed that more than 95% of PC-3 and DU-145 cells expressed high levels of CD44 on the cell surfaces (Fig. 2B), whereas LNCaP and C4-2B cells did not express CD44 at any detectable levels (Fig. 2B). Western blot analysis confirmed the results from flow cytometry analysis (Fig. 2C).
Fig. 2.
Expression of adhesion molecules in prostate cancer cells. A–B: Flow cytometry analysis of α4 integrin, β1 integrin, and CD44 expression in PC-3, DU-145, LNCaP, and C4-2B cells. C: Western blot analysis of CD44 expression in PC-3, DU-145, LNCaP, and C4-2B cells. Equal loading of proteins was confirmed by re-probing GAPDH.
Combination of IL-17 and insulin/IGF1 treatment enhances adhesion of PC-3 and DU-145 cells to HUVECs
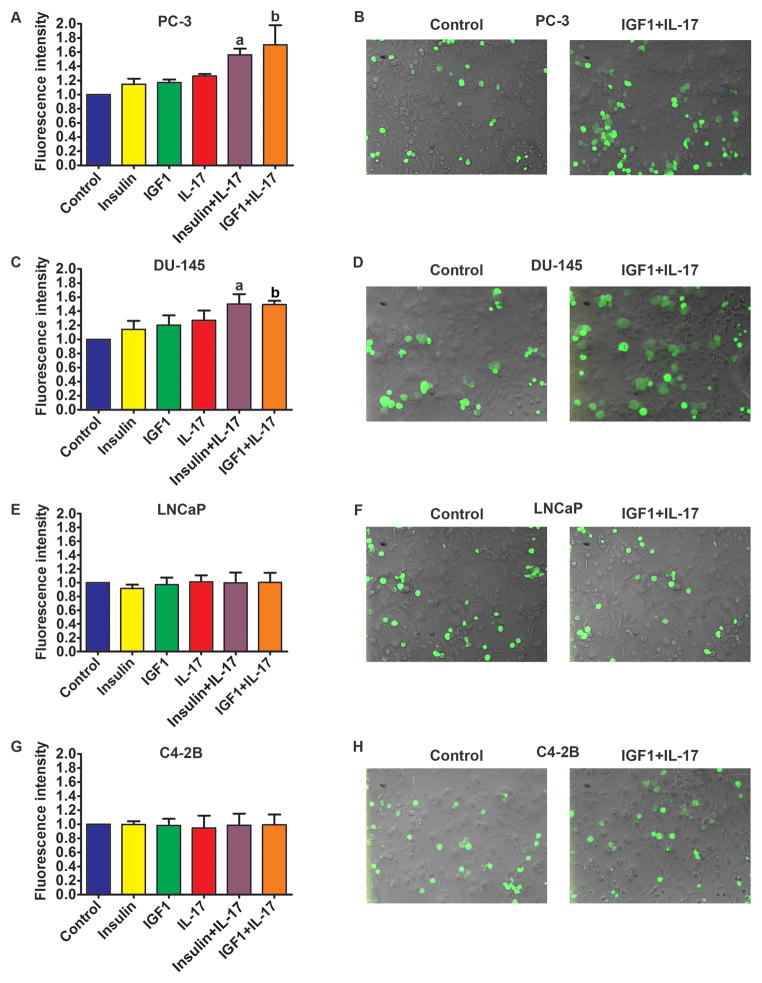
In assays of static adhesion within 15 minutes of time, few PC-3 cells adhered to the untreated HUVECs (Fig. 3A and 3B, the control group). When HUVECs were treated with insulin, IGF1, and IL-17 alone for 24 h prior to addition of PC-3 cells, there were slightly more PC-3 cells adhered to HUVECs, which was statistically insignificant (Fig. 3A). In contrast, when HUVECs were treated with a combination of IL-17 and insulin/IGF1 for 24 h prior to addition of PC-3 cells, the number of PC-3 cells adhered to HUVECs was significantly increased compared to the control group or any group treated with IL-17 or insulin/IGF1 alone (Fig. 3A and 3B, P < 0.05). Similarly, the combination of IL-17 and insulin/IGF1 also significantly increased the adhesion of DU-145 cells to HUVECs (Fig. 3C and 3D, P < 0.05). In contrast, when HUVECs were treated with IL-17, insulin, and IGF1, either alone or in combination, there was no increase in adhesion between LNCaP cells and HUVECs (Fig. 3E and 3F) or between C4-2B cells and HUVECs (Fig. 3G and 3H).
Fig. 3.
Adhesion of prostate cancer cells to HUVECs. A, C, E, and G: Quantification of green fluorescence-labelled prostate cancer cells adhered to HUVECs within 15 minutes. HUVECs were treated with IL-17, insulin, and IGF1, alone or in combination, for 24 h prior to addition of prostate cancer cells. Fluorescence intensity was proportional to the number of prostate cancer cells adhered to HUVECs. The fluorescence intensity of the control group was arbitrarily designated as “1”, so the other groups were normalized with a formula: the fluorescence intensity of the treated group = the recorded fluorescence intensity of the treated group ÷ the recorded fluorescence intensity of the control group. Data represent means ± standard deviations of three independent experiments (n = 3). a, P < 0.05 compared to the control, insulin alone and IL-17 alone treatment groups; b, P < 0.05 compared to the control, IGF1 alone and IL-17 alone treatment groups. B, D, F, and H: representative photomicrographs of the adhered prostate cancer cells labelled with green fluorescence. HUVECs were not labelled and laid in the background beneath the green cells.
CD44-VCAM-1 interaction mediates the adhesion between prostate cancer cells and HUVECs
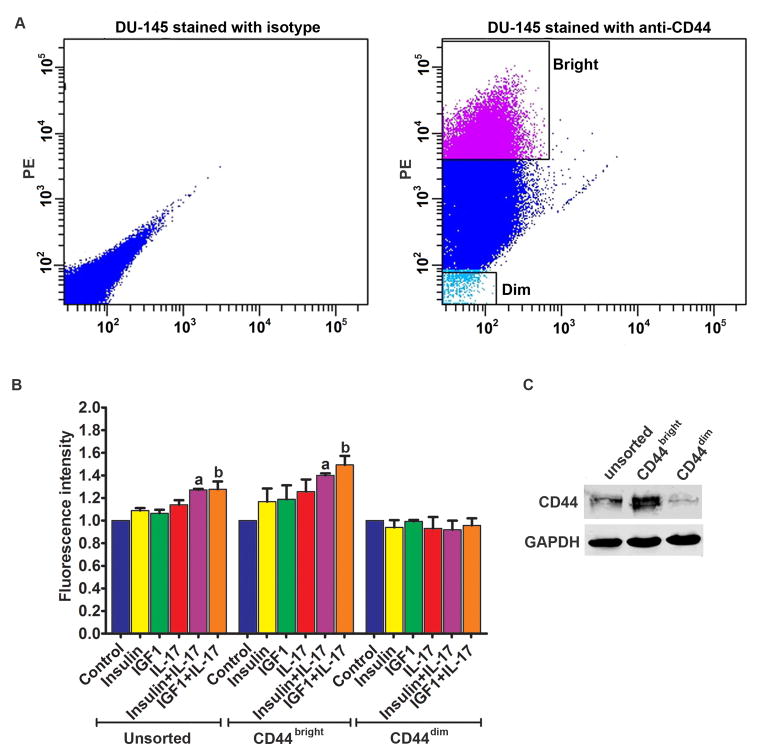
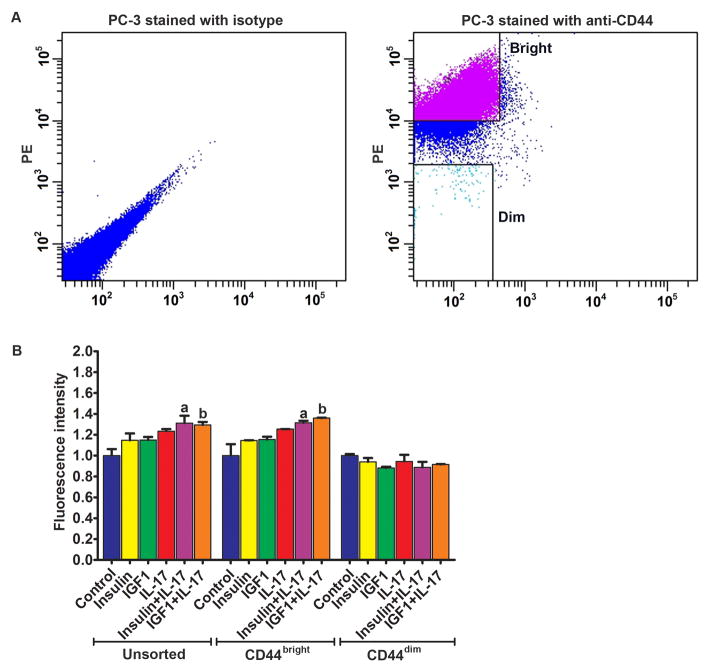
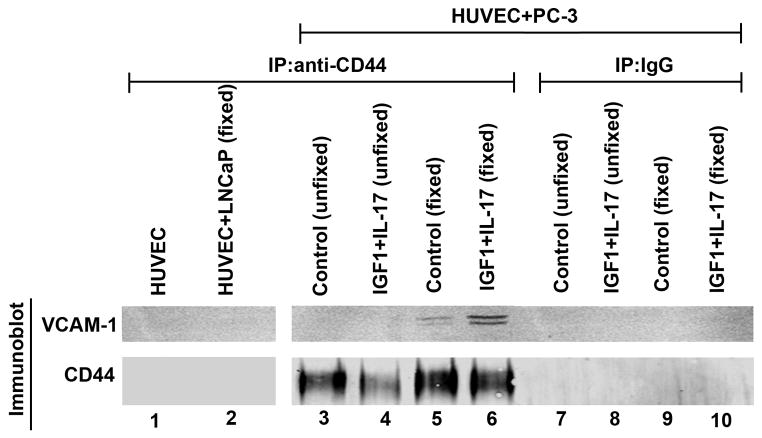
DU-145 cells were sorted into CD44bright and CD44dim populations using FACS (Fig. 4A). When HUVECs were treated with the combination of IL-17 and insulin/IGF1, there were significantly more CD44bright DU-145 cells adhered to HUVECs, compared to the unsorted DU-145 cells (Fig. 4B). However, the adhesion of CD44dim DU-145 cells to HUVECs was not increased by IL-17 and/or insulin/IGF1 treatment (Fig. 4B). Western blot analysis confirmed that CD44bright DU-145 cells expressed higher levels of CD44 than the unsorted DU-145 cells, whereas CD44dim DU-145 cells expressed little CD44 (Fig. 4C). Similarly, PC-3 cells were sorted into CD44bright and CD44dim populations using FACS (Fig. 5A). When HUVECs were treated with the combination of IL-17 and insulin/IGF1, there were significantly more CD44bright PC-3 cells adhered to HUVECs, compared to the HUVECs treated with IL-17 or insulin/IGF1 alone (Fig. 5B). However, there was no statistical difference between CD44bright and the unsorted PC-3 cells. In contrast, the adhesion of CD44dim PC-3 cells to HUVECs was not increased by IL-17 and/or insulin/IGF1 treatment (Fig. 5B). Since the adhesion between prostate cancer cells and HUVECs appeared to be dependent on expression of CD44 that has been shown to physically interact with VCAM-1 [29], we checked if CD44 binds to VCAM-1 when prostate cancer PC-3 cells adhered to HUVECs. We used three different negative controls: first, HUVECs alone control; as HUVECs expressed VCAM-1 but no CD44, anti-CD44 IP did not pull down VCAM-1 or CD44 (Fig. 6, lane 1); second, addition of LNCaP cells to HUVECs; as LNCaP cells expressed no CD44, anti-CD44 IP did not pull down VCAM-1 or CD44 (Fig. 6, lane 2); and third, IP with isotype IgG; as the non-specific IgG did not pull down CD44, VCAM-1 was not pulled down, either (Fig. 6, lanes 7–10). We initially used anti-CD44 antibodies to immunoprecipitate the CD44-VCAM-1 complex when PC-3 cells were added onto HUVECs that were not treated (control group) or treated with IL-17 and IGF1 to increase VCAM-1 expression, without fixation using 2% formaldehyde. To our surprise, we did not pull down any VCAM-1 in either the control group or the IL-17 and IGF1 treated group, though CD44 was pulled down (Fig. 6, lanes 3–4). We suspected that the CD44-VCAM-1 interaction might be transient or weak, hence could not remain stable during the protein extraction procedure. Therefore, we adopted a previously reported technique to cross-link the protein interaction using 2% formaldehyde [28]. We found that anti-CD44 antibodies pulled down VCAM-1 in both the control and IL-17/IGF1 treated groups (Fig. 6, lanes 5–6). The amount of VCAM-1 protein pulled down was consistent to the levels of expression (Fig. 1A).
Fig. 4.
Adhesion of the unsorted, CD44bright, and CD44dim DU-145 cells to HUVECs. A: Left panel, flow cytometry analysis of DU-145 cells stained with isotype control IgG; right panel, a representative dot plot shows how CD44bright and CD44dim DU-145 cells were sorted using FACS. B: Quantification of green fluorescence-labelled prostate cancer cells adhered to HUVECs within 15 minutes. HUVECs were treated with IL-17, insulin, and IGF1, alone or in combination, for 24 h prior to addition of prostate cancer cells. Fluorescence intensity was proportional to the number of prostate cancer cells adhered to HUVECs. The fluorescence intensity of the control group was arbitrarily designated as “1”, so the other groups were normalized with a formula: the fluorescence intensity of the treated group = the recorded fluorescence intensity of the treated group ÷ the recorded fluorescence intensity of the control group. Data represent means ± standard deviations of three independent experiments (n = 3). In the unsorted DU-145 cells panel, a indicates P < 0.05 compared to the control, insulin alone and IL-17 alone treatment groups; b indicates P < 0.05 compared to the control, IGF1 alone and IL-17 alone treatment groups. In the CD44bright DU-145 cells panel, a and b indicate P < 0.05 compared to the corresponding single treatment and combined treatment groups within the CD44bright DU-145 cells panel, and between the CD44bright DU-145 cells panel and the unsorted DU-145 cells panel, as well as between the CD44bright DU-145 cells panel and the CD44dim DU-145 cells panel. C: Western blot analysis of CD44 expression in the unsorted, CD44bright, and CD44dim DU-145 cells. Equal loading of proteins was confirmed by re-probing GAPDH.
Fig. 5.
Adhesion of the unsorted, CD44bright, and CD44dim PC-3 cells to HUVECs. A: Left panel, flow cytometry analysis of PC-3 cells stained with isotype control IgG; right panel, a representative dot plot shows how CD44bright and CD44dim PC-3 cells were sorted using FACS. B: Quantification of green fluorescence-labelled prostate cancer cells adhered to HUVECs within 15 minutes. HUVECs were treated with IL-17, insulin, and IGF1, alone or in combination, for 24 h prior to addition of prostate cancer cells. Fluorescence intensity was proportional to the number of prostate cancer cells adhered to HUVECs. The fluorescence intensity of the control group was arbitrarily designated as “1”, so the other groups were normalized with a formula: the fluorescence intensity of the treated group = the recorded fluorescence intensity of the treated group ÷ the recorded fluorescence intensity of the control group. Data represent means ± standard deviations of three independent experiments (n = 3). In the unsorted PC-3 cells panel, a indicates P < 0.05 compared to the control, insulin alone and IL-17 alone treatment groups; b indicates P < 0.05 compared to the control, IGF1 alone and IL-17 alone treatment groups. In the CD44bright PC-3 cells panel, a and b indicate P < 0.05 compared to the corresponding single treatment and combined treatment groups within the CD44bright PC-3 cells panel, and between the CD44bright PC-3 cells panel and the CD44dim PC-3 cells panel.
Fig. 6.
CD44 expressed in prostate cancer cells binds to VCAM-1 expressed in HUVECs. Proteins were extracted from HUVECs alone and HUVECs with addition of LNCaP or PC-3 cells with or without cross-linking using 2% formaldehyde fixation. Immunoprecipitation (IP) was performed using isotype control mouse IgG or mouse anti-CD44 antibodies. Immunobot (Western blot) was performed using anti-VCAM-1 or anti-CD44 antibodies. Where indicated (including the HUVEC + LNCaP group), HUVECs were treated with IL-17 and IGF1 for 24 h. Unfixed, adherent PC-3 cells and HUVECs were harvested for protein extraction without fixation. Fixed, adherent PC-3 cells and HUVECs were harvested for protein extraction after fixation with 2% formaldehyde for 10 minutes.
DISCUSSION
It has been reported that IL-17 can activate NF-κB pathway [19], which is responsible for synthesis of VCAM-1 in the vascular endothelial cells [30]. In the present study, we showed that insulin and IGF1 were able to enhance IL-17-induced VCAM-1 expression in HUVECs. Of note, VCAM-1 is a glycoprotein with two different splice isoforms, namely, VCAM-1a (full-length with Mr ~ 90–95 kDa) and VCAM-1b (lacking exon 5 with Mr ~80–83 kDa) [31]. We observed that IL-17 single treatment slightly increased VCAM-1b but not VCAM-1a, while insulin single treatment slightly decreased VCAM-1a but slightly increased VCAM-1b, yet the combination of IL-17 and insulin treatment dramatically increased both VCAM-1a and VCAM-1b (Fig. 1A). The exact molecular mechanism that caused the different effects of IL-17 or insulin single treatment is not clear. Our previous study has demonstrated that insulin and IGF1 are able to enhance IL-17-induced expression of proinflammatory chemokines and cytokines [17]. We have shown that the underlying mechanism involves inhibition of glycogen synthase kinase 3 β (GSK3B) by Akt. Akt is activated by insulin and IGF1 through their receptor-activated phosphatidylinositol 3-kinase (PI3K). GSK3B phosphorylates CAAT enhancer binding protein β (C/EBPβ) and inhibits C/EBPβ’s transcriptional function that is responsible for IL-17-induced gene expression [17,32]. Insulin and IGF1 can activate PI3K/Akt to phosphorylate GSK3B at serine 9 and inhibit GSK3B activity, and consequently increase C/EBPβ function to enhance IL-17-induced gene expression. Recently, we have demonstrated that insulin and IGF1 can also activate PI3K/Akt to phosphorylate GSK3A at serine 21 and inhibit GSK3A activity, and consequently enhance IL-17-induced gene expression [33]. We believe this mechanism may also be true for the enhanced expression of VCAM-1 in HUVECs treated with IL-17 and insulin/IGF1. IL-17 alone did not dramatically increase VCAM-1 expression to enhance adhesion because GSK3 negatively inhibits IL-17 signaling as demonstrated by previous studies [17,33,34]. In the presence of IGF1 or insulin, GSK3 function is inhibited by PI3K/Akt activated by IGF1 or insulin [17,33], thus VCAM-1 expression and subsequent adhesion are enhanced by IL-17. We have shown that the synergy between IL-17 and insulin/IGF1 can be blocked by melatonin that inhibits Akt [17] or by a new pan-Akt inhibitor AZD5363 [33]. Therefore, it is potentially possible to use melatonin or AZD5363 to manipulate the cross-talk between IL-17 and insulin/IGF1 signaling pathways for preventive and therapeutic purposes.
VCAM-1 expression on the surface of endothelial cells contributes to leukocyte capture via binding to VLA-4 (α4β1 integrin) expressed on the surface of leukocytes [35,36]. In the present study, we showed that the static adhesion of PC-3 and DU-145 cells to HUVECs was increased when HUVECs were treated with IL-17 and insulin/IGF1, which may be due to increased expression of VCAM-1 on the treated HUVECs. However, PC-3 and DU-145 cells did not express VLA-4, indicating that these prostate cancer cells cannot adhere to the endothelial cells through VLA-4-VCAM-1 interaction. It has been reported that VCAM-1 may bind to CD44 [4,29]. Therefore, we checked CD44 expression in the prostate cancer cells. We found that PC-3 and DU-145 cells expressed CD44 on their surface, whereas LNCaP and C4-2B cells did not express CD44. CD44 is a cell surface adhesion molecule and its main ligand is hyaluronic acid (HA) [37]. However, HA expression on HUVECs is usually not induced by inflammatory cytokines such as tumor necrosis factor-α (TNF-α), IL-1β, lipopolysaccharide, or interferon γ [38]. Thus, CD44-HA interaction may not be able to explain the increased adhesion of PC-3 and DU-145 cells to HUVECs. P-selectin, L-selectin and E-selectin have been reported to bind to CD44 and facilitate capture of colon cancer cells and leukocytes on the vascular endothelial cells [39,40]. However, we did not detect any E-selectin expression in the HUVECs used in our study, thus E-selectin is unlikely to play any role in the adhesion of prostate cancer cells to HUVECs. We believe that the adhesion of prostate cancer cells to HUVECs is mediated through CD44-VCAM-1 interaction, based on the following evidence: first, only prostate cancer cells that express CD44 (PC-3 and DU-145 cells) adhered to HUVECs, particularly when VCAM-1 expression was enhanced by the treatment with IL-17 and insulin/IGF1, whereas the prostate cancer cells that do not express CD44 (LNCaP and C4-2B cells) did not adhere to HUVECs even after the treatment with IL-17 and insulin/IGF1; second, the sorted CD44dim populations of PC-3 and DU-145 cells no longer adhered to HUVECs, due to reduced or lack of CD44 expression; and third, CD44 expressed in PC-3 cells physically bound to VCAM-1 expressed in HUVECs under the static adhesion condition.
It is of significance to identify CD44-VCAM-1 interaction that mediates adhesion of prostate cancer cells to the vascular endothelial cells, as CD44 is usually expressed in the stem cell-like prostate and breast cancer cells that circulate intravascularly and eventually metastasize to distant organs [41–44]. Thus, prostate cancer stem cells may adhere to the vascular endothelium through CD44-VCAM-1 interaction. Previously, Draffin et al. have shown that CD44 is able to facilitate the adherence of metastatic prostate and breast cancer cells to bone marrow endothelial cells via binding to HA [45]. When CD44 expression was down-regulated by miR34-a, prostate cancer regeneration and metastasis was inhibited [46]. In the present study, we provided evidence to support that CD44-VCAM-1 interaction may also contribute to the adhesion of prostate cancer cells to the vascular endothelium. Of particular interest, the adhesion is enhanced by IL-17 and insulin/IGF1 due to increased VCAM-1 expression in the vascular endothelial cells. This may be relevant to the increased risks of metastasis and mortality in obese men with prostate cancer. It has been found that obese men have a 3.6-fold increase in risk of prostate cancer metastasis and a 2.6-fold increased risk of prostate cancer-specific mortality, compared to prostate cancer patients with normal body mass index [47]. Another recent study found that overweight and obese men were threefold and fivefold more likely to develop metastases than normal weight men [48]. It is well known that obese people have increased serum levels of insulin and IGF1 [49] as well as IL-17 [50]. Thus, we speculate that, due to increased levels of IL-17 and insulin/IGF1, VCAM-1 expression is increased in the vascular endothelial cells in obese men with prostate cancer, which facilitates adhesion of the stem cell-like circulating tumor cells through CD44-VCAM-1 interaction and subsequently promotes extravasation and metastasis of prostate cancer. Further studies are required to validate this speculation.
CONCLUSIONS
CD44-VCAM-1 interaction mediates the adhesion between prostate cancer cells and HUVECs. IL-17 and insulin/IGF1 enhance adhesion of prostate cancer cells to vascular endothelial cells through increasing VCAM-1 expression in the vascular endothelial cells. These findings suggest that IL-17 may act with insulin/IGF1 to promote prostate cancer metastasis.
Acknowledgments
Grant sponsor: National Institute of General Medical Sciences; Grant number: P20GM103518; National Cancer Institute; Grant number: R01CA174714; Grant sponsor: Department of Defense; Grant numbers: W81XWH-14-1-0050, W81XWH-14-1-0149, and W81XWH-14-1-0458; Grant sponsor: The Developmental Fund of Tulane Cancer Center (TCC) and Louisiana Cancer Research Consortium (LCRC) Fund.
The authors thank Mary Price from Tulane Cancer Center and Louisiana Cancer Research Consortium FACS Core for flow cytometry analysis. Jiandong Mei is a visiting scholar at Tulane University School of Medicine sponsored by the China Scholarship Council (201406240145).
ABBREVIATIONS
- CD44
cluster of differentiation 44
- C/EBPβ
CAAT enhancer binding protein β
- FACS
fluorescence-activated cell sorting
- FBS
fetal bovine serum
- GAPDH
glyceraldehyde 3-phosphate dehydrogenase
- GSK3
glycogen synthase kinase
- HA
hyaluronic acid
- HUVECs
human umbilical vein endothelial cells
- ICAM-1
intercellular adhesion molecule-1
- IGF1
insulin-like growth factor 1
- IL-17
interleukin-17
- IP
immunoprecipitation
- NF-κB
nuclear factor-κB
- PBS
phosphate-buffered saline
- VCAM-1
vascular cell adhesion molecule-1
- VLA-4
very late antigen-4
Footnotes
The content of this article is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Defense.
References
- 1.Entschladen F, Drell TLt, Lang K, Joseph J, Zaenker KS. Tumour-cell migration, invasion, and metastasis: navigation by neurotransmitters. Lancet Oncol. 2004;5(4):254–258. doi: 10.1016/S1470-2045(04)01431-7. [DOI] [PubMed] [Google Scholar]
- 2.Orr FW, Wang HH, Lafrenie RM, Scherbarth S, Nance DM. Interactions between cancer cells and the endothelium in metastasis. J Pathol. 2000;190(3):310–329. doi: 10.1002/(SICI)1096-9896(200002)190:3<310::AID-PATH525>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 3.Wong SY, Hynes RO. Lymphatic or hematogenous dissemination: how does a metastatic tumor cell decide? Cell Cycle. 2006;5(8):812–817. doi: 10.4161/cc.5.8.2646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Miles FL, Pruitt FL, van Golen KL, Cooper CR. Stepping out of the flow: capillary extravasation in cancer metastasis. Clin Exp Metastasis. 2008;25(4):305–324. doi: 10.1007/s10585-007-9098-2. [DOI] [PubMed] [Google Scholar]
- 5.Fidler IJ. The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer. 2003;3(6):453–458. doi: 10.1038/nrc1098. [DOI] [PubMed] [Google Scholar]
- 6.Scherbarth S, Orr FW. Intravital videomicroscopic evidence for regulation of metastasis by the hepatic microvasculature: effects of interleukin-1alpha on metastasis and the location of B16F1 melanoma cell arrest. Cancer Res. 1997;57(18):4105–4110. [PubMed] [Google Scholar]
- 7.Huh SJ, Liang S, Sharma A, Dong C, Robertson GP. Transiently entrapped circulating tumor cells interact with neutrophils to facilitate lung metastasis development. Cancer Res. 2010;70(14):6071–6082. doi: 10.1158/0008-5472.CAN-09-4442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Auguste P, Fallavollita L, Wang N, Burnier J, Bikfalvi A, Brodt P. The host inflammatory response promotes liver metastasis by increasing tumor cell arrest and extravasation. Am J Pathol. 2007;170(5):1781–1792. doi: 10.2353/ajpath.2007.060886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matsuura N, Puzon-McLaughlin W, Irie A, Morikawa Y, Kakudo K, Takada Y. Induction of experimental bone metastasis in mice by transfection of integrin alpha 4 beta 1 into tumor cells. Am J Pathol. 1996;148(1):55–61. [PMC free article] [PubMed] [Google Scholar]
- 10.Kolls JK, Linden A. Interleukin-17 family members and inflammation. Immunity. 2004;21(4):467–476. doi: 10.1016/j.immuni.2004.08.018. [DOI] [PubMed] [Google Scholar]
- 11.Novatchkova M, Leibbrandt A, Werzowa J, Neubuser A, Eisenhaber F. The STIR-domain superfamily in signal transduction, development and immunity. Trends in biochemical sciences. 2003;28(5):226–229. doi: 10.1016/S0968-0004(03)00067-7. [DOI] [PubMed] [Google Scholar]
- 12.Chang SH, Park H, Dong C. Act1 adaptor protein is an immediate and essential signaling component of interleukin-17 receptor. J Biol Chem. 2006;281(47):35603–35607. doi: 10.1074/jbc.C600256200. [DOI] [PubMed] [Google Scholar]
- 13.Qian Y, Liu C, Hartupee J, Altuntas CZ, Gulen MF, Jane-Wit D, Xiao J, Lu Y, Giltiay N, Liu J, Kordula T, Zhang QW, Vallance B, Swaidani S, Aronica M, Tuohy VK, Hamilton T, Li X. The adaptor Act1 is required for interleukin 17-dependent signaling associated with autoimmune and inflammatory disease. Nature immunology. 2007;8(3):247–256. doi: 10.1038/ni1439. [DOI] [PubMed] [Google Scholar]
- 14.Maitra A, Shen F, Hanel W, Mossman K, Tocker J, Swart D, Gaffen SL. Distinct functional motifs within the IL-17 receptor regulate signal transduction and target gene expression. Proc Natl Acad Sci U S A. 2007;104(18):7506–7511. doi: 10.1073/pnas.0611589104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ho AW, Shen F, Conti HR, Patel N, Childs EE, Peterson AC, Hernandez-Santos N, Kolls JK, Kane LP, Ouyang W, Gaffen SL. IL-17RC is required for immune signaling via an extended SEF/IL-17R signaling domain in the cytoplasmic tail. J Immunol. 2010;185(2):1063–1070. doi: 10.4049/jimmunol.0903739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Liu C, Qian W, Qian Y, Giltiay NV, Lu Y, Swaidani S, Misra S, Deng L, Chen ZJ, Li X. Act1, a U-box E3 ubiquitin ligase for IL-17 signaling. Science signaling. 2009;2(92):ra63. doi: 10.1126/scisignal.2000382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ge D, Dauchy RT, Liu S, Zhang Q, Mao L, Dauchy EM, Blask DE, Hill SM, Rowan BG, Brainard GC, Hanifin JP, Cecil KS, Xiong Z, Myers L, You Z. Insulin and IGF1 enhance IL-17-induced chemokine expression through a GSK3B-dependent mechanism: a new target for melatonin’s anti-inflammatory action. Journal of pineal research. 2013;55(4):377–387. doi: 10.1111/jpi.12084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhu S, Pan W, Song X, Liu Y, Shao X, Tang Y, Liang D, He D, Wang H, Liu W, Shi Y, Harley JB, Shen N, Qian Y. The microRNA miR-23b suppresses IL-17-associated autoimmune inflammation by targeting TAB2, TAB3 and IKK-alpha. Nat Med. 2012;18(7):1077–1086. doi: 10.1038/nm.2815. [DOI] [PubMed] [Google Scholar]
- 19.Hwang SY, Kim JY, Kim KW, Park MK, Moon Y, Kim WU, Kim HY. IL-17 induces production of IL-6 and IL-8 in rheumatoid arthritis synovial fibroblasts via NF-kappaB- and PI3-kinase/Akt-dependent pathways. Arthritis research & therapy. 2004;6(2):R120–128. doi: 10.1186/ar1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zhang Q, Liu S, Ge D, Xue Y, Xiong Z, Abdel-Mageed AB, Myers L, Hill SM, Rowan BG, Sartor O, Melamed J, Chen Z, You Z. Interleukin-17 promotes formation and growth of prostate adenocarcinoma in mouse models. Cancer Res. 2012;72(10):2589–2599. doi: 10.1158/0008-5472.CAN-11-3795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang Q, Liu S, Xiong Z, Wang AR, Myers L, Melamed J, Tang WW, You Z. Interleukin-17 promotes development of castration-resistant prostate cancer potentially through creating an immunotolerant and pro-angiogenic tumor microenvironment. The Prostate. 2014;74(8):869–879. doi: 10.1002/pros.22805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baxter RC, Bryson JM, Turtle JR. Somatogenic receptors of rat liver: regulation by insulin. Endocrinology. 1980;107(4):1176–1181. doi: 10.1210/endo-107-4-1176. [DOI] [PubMed] [Google Scholar]
- 23.Madonna R, Pandolfi A, Massaro M, Consoli A, De Caterina R. Insulin enhances vascular cell adhesion molecule-1 expression in human cultured endothelial cells through a pro-atherogenic pathway mediated by p38 mitogen-activated protein-kinase. Diabetologia. 2004;47(3):532–536. doi: 10.1007/s00125-004-1330-x. [DOI] [PubMed] [Google Scholar]
- 24.Balaram SK, Agrawal DK, Allen RT, Kuszynski CA, Edwards JD. Cell adhesion molecules and insulin-like growth factor-1 in vascular disease. Journal of vascular surgery. 1997;25(5):866–876. doi: 10.1016/s0741-5214(97)70216-7. [DOI] [PubMed] [Google Scholar]
- 25.Roussel L, Houle F, Chan C, Yao Y, Berube J, Olivenstein R, Martin JG, Huot J, Hamid Q, Ferri L, Rousseau S. IL-17 promotes p38 MAPK-dependent endothelial activation enhancing neutrophil recruitment to sites of inflammation. J Immunol. 2010;184(8):4531–4537. doi: 10.4049/jimmunol.0903162. [DOI] [PubMed] [Google Scholar]
- 26.Xing X, Yang J, Yang X, Wei Y, Zhu L, Gao D, Li M. IL-17A induces endothelial inflammation in systemic sclerosis via the ERK signaling pathway. PLoS One. 2013;8(12):e85032. doi: 10.1371/journal.pone.0085032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mackesy DZ, Goalstone ML. Insulin augments tumor necrosis factor-alpha stimulated expression of vascular cell adhesion molecule-1 in vascular endothelial cells. J Inflamm (Lond) 2011;8:34. doi: 10.1186/1476-9255-8-34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klockenbusch C, Kast J. Optimization of formaldehyde cross-linking for protein interaction analysis of non-tagged integrin beta1. Journal of biomedicine & biotechnology. 2010;2010:927585. doi: 10.1155/2010/927585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wang PC, Weng CC, Hou YS, Jian SF, Fang KT, Hou MF, Cheng KH. Activation of VCAM-1 and its associated molecule CD44 leads to increased malignant potential of breast cancer cells. Int J Mol Sci. 2014;15(3):3560–3579. doi: 10.3390/ijms15033560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen CC, Rosenbloom CL, Anderson DC, Manning AM. Selective inhibition of E-selectin, vascular cell adhesion molecule-1, and intercellular adhesion molecule-1 expression by inhibitors of I kappa B-alpha phosphorylation. J Immunol. 1995;155(7):3538–3545. [PubMed] [Google Scholar]
- 31.Montes-Sanchez D, Ventura JL, Mitre I, Frias S, Michan L, Espejel-Nunez A, Vadillo-Ortega F, Zentella A. Glycosylated VCAM-1 isoforms revealed in 2D western blots of HUVECs treated with tumoral soluble factors of breast cancer cells. BMC Chem Biol. 2009;9:7. doi: 10.1186/1472-6769-9-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ruddy MJ, Wong GC, Liu XK, Yamamoto H, Kasayama S, Kirkwood KL, Gaffen SL. Functional cooperation between interleukin-17 and tumor necrosis factor-alpha is mediated by CCAAT/enhancer-binding protein family members. J Biol Chem. 2004;279(4):2559–2567. doi: 10.1074/jbc.M308809200. [DOI] [PubMed] [Google Scholar]
- 33.Chen C, Zhang Q, Liu S, Lambrechts M, Qu Y, You Z. AZD5363 Inhibits Inflammatory Synergy between Interleukin-17 and Insulin/Insulin-Like Growth Factor 1. Front Oncol. 2014;4:343. doi: 10.3389/fonc.2014.00343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shen F, Li N, Gade P, Kalvakolanu DV, Weibley T, Doble B, Woodgett JR, Wood TD, Gaffen SL. IL-17 receptor signaling inhibits C/EBPbeta by sequential phosphorylation of the regulatory 2 domain. Sci Signal. 2009;2(59):ra8. doi: 10.1126/scisignal.2000066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Galkina E, Ley K. Immune and inflammatory mechanisms of atherosclerosis. Annu Rev Immunol. 2009;27:165–197. doi: 10.1146/annurev.immunol.021908.132620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alon R, Kassner PD, Carr MW, Finger EB, Hemler ME, Springer TA. The integrin VLA-4 supports tethering and rolling in flow on VCAM-1. J Cell Biol. 1995;128(6):1243–1253. doi: 10.1083/jcb.128.6.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Goodison S, Urquidi V, Tarin D. CD44 cell adhesion molecules. Molecular pathology : MP. 1999;52(4):189–196. doi: 10.1136/mp.52.4.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mohamadzadeh M, DeGrendele H, Arizpe H, Estess P, Siegelman M. Proinflammatory stimuli regulate endothelial hyaluronan expression and CD44/HA-dependent primary adhesion. J Clin Invest. 1998;101(1):97–108. doi: 10.1172/JCI1604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Thomas SN, Zhu F, Schnaar RL, Alves CS, Konstantopoulos K. Carcinoembryonic antigen and CD44 variant isoforms cooperate to mediate colon carcinoma cell adhesion to E- and L-selectin in shear flow. J Biol Chem. 2008;283(23):15647–15655. doi: 10.1074/jbc.M800543200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dimitroff CJ, Lee JY, Rafii S, Fuhlbrigge RC, Sackstein R. CD44 is a major E-selectin ligand on human hematopoietic progenitor cells. J Cell Biol. 2001;153(6):1277–1286. doi: 10.1083/jcb.153.6.1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A. 2003;100(7):3983–3988. doi: 10.1073/pnas.0530291100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Collins AT, Berry PA, Hyde C, Stower MJ, Maitland NJ. Prospective identification of tumorigenic prostate cancer stem cells. Cancer Res. 2005;65(23):10946–10951. doi: 10.1158/0008-5472.CAN-05-2018. [DOI] [PubMed] [Google Scholar]
- 43.Patrawala L, Calhoun T, Schneider-Broussard R, Li H, Bhatia B, Tang S, Reilly JG, Chandra D, Zhou J, Claypool K, Coghlan L, Tang DG. Highly purified CD44+ prostate cancer cells from xenograft human tumors are enriched in tumorigenic and metastatic progenitor cells. Oncogene. 2006;25(12):1696–1708. doi: 10.1038/sj.onc.1209327. [DOI] [PubMed] [Google Scholar]
- 44.Hurt EM, Kawasaki BT, Klarmann GJ, Thomas SB, Farrar WL. CD44+ CD24(−) prostate cells are early cancer progenitor/stem cells that provide a model for patients with poor prognosis. Br J Cancer. 2008;98(4):756–765. doi: 10.1038/sj.bjc.6604242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Draffin JE, McFarlane S, Hill A, Johnston PG, Waugh DJ. CD44 potentiates the adherence of metastatic prostate and breast cancer cells to bone marrow endothelial cells. Cancer Res. 2004;64(16):5702–5711. doi: 10.1158/0008-5472.CAN-04-0389. [DOI] [PubMed] [Google Scholar]
- 46.Liu C, Kelnar K, Liu B, Chen X, Calhoun-Davis T, Li H, Patrawala L, Yan H, Jeter C, Honorio S, Wiggins JF, Bader AG, Fagin R, Brown D, Tang DG. The microRNA miR-34a inhibits prostate cancer stem cells and metastasis by directly repressing CD44. Nat Med. 2011;17(2):211–215. doi: 10.1038/nm.2284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gong Z, Agalliu I, Lin DW, Stanford JL, Kristal AR. Obesity is associated with increased risks of prostate cancer metastasis and death after initial cancer diagnosis in middle-aged men. Cancer. 2007;109(6):1192–1202. doi: 10.1002/cncr.22534. [DOI] [PubMed] [Google Scholar]
- 48.Keto CJ, Aronson WJ, Terris MK, Presti JC, Kane CJ, Amling CL, Freedland SJ. Obesity is associated with castration-resistant disease and metastasis in men treated with androgen deprivation therapy after radical prostatectomy: results from the SEARCH database. BJU Int. 2012;110(4):492–498. doi: 10.1111/j.1464-410X.2011.10754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cohen DH, LeRoith D. Obesity, type 2 diabetes, and cancer: the insulin and IGF connection. Endocr Relat Cancer. 2012;19(5):F27–45. doi: 10.1530/ERC-11-0374. [DOI] [PubMed] [Google Scholar]
- 50.Sumarac-Dumanovic M, Stevanovic D, Ljubic A, Jorga J, Simic M, Stamenkovic-Pejkovic D, Starcevic V, Trajkovic V, Micic D. Increased activity of interleukin-23/interleukin-17 proinflammatory axis in obese women. Int J Obes (Lond) 2009;33(1):151–156. doi: 10.1038/ijo.2008.216. [DOI] [PubMed] [Google Scholar]