Abstract
Animals inhabiting temperate and boreal latitudes experience marked seasonal changes in the quality of their environments and maximize reproductive success by phasing breeding activities with the most favorable time of year. Whereas the specific mechanisms driving seasonal changes in reproductive function vary across species, converging lines of evidence suggest gonadotropin-inhibitory hormone (GnIH) serves as a key component of the neuroendocrine circuitry driving seasonal changes in reproduction and sexual motivation in some species. In addition to anticipating environmental change through transduction of photoperiodic information and modifying reproductive state accordingly, GnIH is also positioned to regulate acute changes in reproductive status should unpredictable conditions manifest throughout the year. The present overview summarizes the role of GnIH in avian and mammalian seasonal breeding while considering the similarities and disparities that have emerged from broad investigations across reproductively photoperiodic species.
Keywords: GnIH, RFRP-3, photoperiod, melatonin, RFamide-related peptide
I. Introduction
The environment varies considerably throughout the seasons, with minimal food availability coinciding with maximal energetic demands during winter. Consequently, animals inhabiting temperate and boreal latitudes restrict breeding and other energetically costly activities to times of year when environmental conditions are optimal. Because day length (photoperiod) is the most reliable predictor of changing seasons, organisms use this signal to forecast local conditions and initiate adaptations well in advance of the appropriate time of year [10,23,27,30]. This strategy is complementary to responses to more proximate factors (e.g., temperature, calories, and more specific nutritional signals) [26,125], as it allows time for development of seasonal adaptations.
Day length is transduced into a pineal melatonin signal that subsequently acts on neural substrates controlling reproduction [15,30]. Long durations of nightly melatonin secretion are associated with short, winter-like days, whereas short melatonin durations accompany long, summer-like photoperiods. The final common pathway on which internal and external reproductively relevant stimuli converge to control reproduction is the gonadotropin-releasing hormone (GnRH) neuronal system [10]. GnRH neurons, in turn, stimulate pituitary gonadotropin secretion to appropriately regulate gametogenesis and sex steroid secretion.
The effects of photoperiod on reproductive functioning have been well established and, more recently, enormous progress has been made uncovering the neuroendocrine mechanisms by which photoperiodic and other environmental signals drive these reproductive changes [40,123,126]. Over the last several years, the RFamide (Arg-Phe-NH2) peptide, gonadotropin-inhibitory hormone [GnIH; also known as RFamide-related peptide (RFRP) in mammals] has emerged as an important component of the neural circuitry guiding seasonal changes in reproduction across avian and mammalian species (reviewed in [90,94,108,111]. Herein we review our current understanding of the role played by this neuropeptide in birds and mammals in seasonal breeding and consider the disparities observed among species.
II. Discovery and Functional Significance of GnIH
In the 1920s, Ernst Scharrer proposed the concept of “neurosecretion”, speculating that neurons transmit impulses as well as secrete chemical substances. Wolfgang Bargmann later established this fact through his studies of supraoptic and paraventricular neuronal secretion. The discovery of neurosecretion led to the creation of a new research field, neuroendocrinology. The recent identification of novel neurohormones that regulate reproductive physiology have broadened the horizons of the field, causing neuroendocrinologists to question long-held beliefs about the neural mechanisms governing reproductive function and incorporating these novel neuropetides into existing frameworks.
One such discovery was that of a hypothalamic neuropeptide, GnIH, in the avian brain, resulting from a search for novel neuropeptides regulating the release of pituitary hormones [105]. In quail, Tustui and colleagues found that GnIH acts directly on the pituitary to inhibit luteinizing hormone (LH) and follicle-stimulating hormone (FSH) release, providing the first demonstration of a hypothalamic neuropeptide inhibiting gonadotropin release in any vertebrate [105].
GnIH has a previously-unreported dodecapeptide structure, Ser-Ile-Lys-Pro-Ser-Ala-Tyr-Leu-Pro-Leu-Arg-Phe-NH2 (SIKPSAYLPLRFamide) [105]. The C-terminus of GnIH is identical to chicken LPLRFamide, the first reported RFamide peptide in vertebrates [25], which is likely to be a degraded fragment of GnIH (for reviews, see [102-104]. Subsequently, a cDNA encoding the precursor polypeptide for GnIH was identified in quail [85] and other avian species (for reviews, see [102-104]. The GnIH precursor encodes one GnIH and two GnIH-related peptides (GnIH-RP-1 and GnIH-RP-2) possessing an LPXRFamide (X = L or Q) motif at their C-termini in all avian species studied. GnIH was further isolated as a mature peptide in starlings [114] and zebra finches [100] and GnIH-RP-2 was also identified in quail [85] (Table 1). Converging lines of evidence over the past 14 years established that GnIH regulates avian reproduction by decreasing gonadotropin synthesis and release from gonadotropes in the anterior pituitary gland and/or acting on GnRH neurons in the hypothalamus via the GnIH receptor (GnIH-R; GPR147, also called Neuropeptide FF receptor 1 (NPFF1)) (reviewed in [102,104,108]).
Table 1.
Primary structures of avian and mammalian GnlHs [LPXRFamide (X = L or Q) peptides].
| Animal | Name | Sequence | Reference | |
|---|---|---|---|---|
| Birds | Quail | GnIH | SIKPSAYLPLRFa | Tsutsui et al. (2000) |
| GnIH-RP-1* | SLNFEEMKDWGSKNFMKVNTPTVNKVPNSVANLPLRFa | Satake et al. (2001) | ||
| GnIH-RP-2 | SSIQSLLNLPQRFa | Satake et al. (2001) | ||
| Chicken | GnIH* | SIRPSAYLPLRFa | Ikemoto et al. (2005) | |
| GnIH-RP-1* | SLNFEEMKDWGSKNFLKVNTPTVNKVPNSVANLPLRFa | Ikemoto et al. (2005) | ||
| GnIH-RP-2* | SSIQSLLNLPQRFa | Ikemoto et al. (2005) < | ||
| Sparrow | GnIH* | SIKPFSNLPLRFa | Osugi et al. (2004) | |
| GnIH-RP-1* | SLNFEEMEDWGSKDIIKMNPFTASKMPNSVANLPLRFa | Osugi et al. (2004) | ||
| GnIH-RP-2* | SPLVKGSSQSLLNLPQRFa | Osugi et al. (2004) | ||
| Starling | GnIH | SIKPFANLPLRFa | Ubuka et al. (2008) | |
| GnIH-RP-1* | SLNFDEMEDWGSKDIIKMNPFTVSKMPNSVANLPLRFa | Ubuka et al. (2008) | ||
| GnIH-RP-2* | GSSQSLLNLPQRFa | Ubuka et al. (2008) | ||
| Zebra finch | GnIH | SIKPFSNLPLRFa | Tobari et al. (2010) | |
| GnIH-RP-1* | SLNFEEMEDWRSKDIIKMNPFAASKMPNSVANLPLRFa | Tobari et al. (2010) | ||
| GnIH-RP-2* | SPLVKGSSQSLLNLPQRFa | Tobari et al. (2010) | ||
| Mammals | Human | RFRP-1 | MPHSFANLPLRFa | Ubuka et al. (2009) |
| RFRP-3 | VPNLPQRFa | Ubuka et al. (2009) | ||
| Macaque | RFRP-1* | MPHSVTNLPLRFa | Ubuka et al. (2009) | |
| RFRP-3 | SGRNMEVSLVRQVLNLPQRFa | Ubuka et al. (2009) | ||
| Bovine | RFRP-1 | SLTFEEVKDWAPKIKMNKPVVNKMPPSAANLPLRFa | Fukusumi et al. (2001) | |
| RFRP-3 | AMAHLPLRLGKNREDSLSRWVPNLPQRFa | Yoshida et al. (2003) | ||
| Ovine | RFRP-1* | SLTFEEVKDWGPKIKMNTPAVNKMPPSAANLPLRFa | Clarke et al. (2008) | |
| RFRP-3* | VPNLPQRFa | Clarke et al. (2008) | ||
| Rat | RFRP-1* | SVTFQELKDWGAKKDIKMSPAPANKVPHSAANLPLRFa | Ukena et al. (2002) | |
| RFRP-3 | ANMEAGTMSHFPSLPQRFa | Ukena et al. (2002) | ||
| Hamster (Siberian) | RFRP-1 | SPAPANKVPHSAANLPLRFa | Ubuka et al. (2012) | |
| RFRP-3 | TLSRVPSLPQRFa | Ubuka et al. (2012) | ||
| Hamster (Syrian) | RFRP-1* | SPAPANKVPHSAANLPLRFa | Kriegsfeld et al., (2006) | |
| RFRP-3* | ILSRVPSLPQRFa | Kriegsfeld et al., (2006) |
Putative peptides. The C-terminal LPXRFamide (X = L or Q) motifs are shown in bold.
Following the discovery of GnIH in birds, GnIH peptides possessing the C-terminal LPXRFamide (X = L or Q) motif have been further identified in a number of other vertebrates including mammals (for reviews, see [106-108]. In mammals, cDNAs that encode LPXRFamide peptides were initially investigated through a gene database search [39]. The GnIH precursor cDNAs identified from mammalian brains encode three GnIHs [also known as RFamide-related peptides (RFRPs)], RFRP-1, −2, and −3, in bovines and humans (for reviews, see [102-104]. RFRP-1 and −3 are LPXRFamide peptides, but RFRP-2 is not an LPXRFamide peptide. In rodents, GnIH cDNAs encode two peptides, RFRP-1 and −3 (for reviews, see [102-104]; rats [117], hamsters [113], bovines [28,128], monkeys [115] and humans [116]) (Table 1). RFRP-3 inhibits gonadotropin synthesis and/or release across all mammalian species investigated (e.g., [17,29,44,45,53,66,84,113]). In addition, RFRP-3 inhibits GnRH-stimulated gonadotropin synthesis in pituitary gonadotropes in mammals [84], indicating the potential for actions at the level of the pituitary. Thus, as in birds, the mammalian GnIHs, RFRP-1 and RFRP-3, appear to act as key neurohormones to inhibit gonadotropin secretion in several mammalian species.
GnIH is part of a large family of RFamide peptides (Figure 1) that began with the discovery of the cardioexcitatory neuropeptide containing the C-terminal Phe–Met–Arg–Phe–NH2 (FMRFamide) identified in the ganglia of the bi-valve mollusk, Macrocallista nimbosa [78]. This class of peptides has pronounced regulatory actions on a variety of physiological processes such as food intake, pain perception and endocrine activity [102,103,108]. Most related to the present overview, GnIH has emerged as a major regulator of the reproductive axis and the seasonal control of reproductive function across vertebrates.
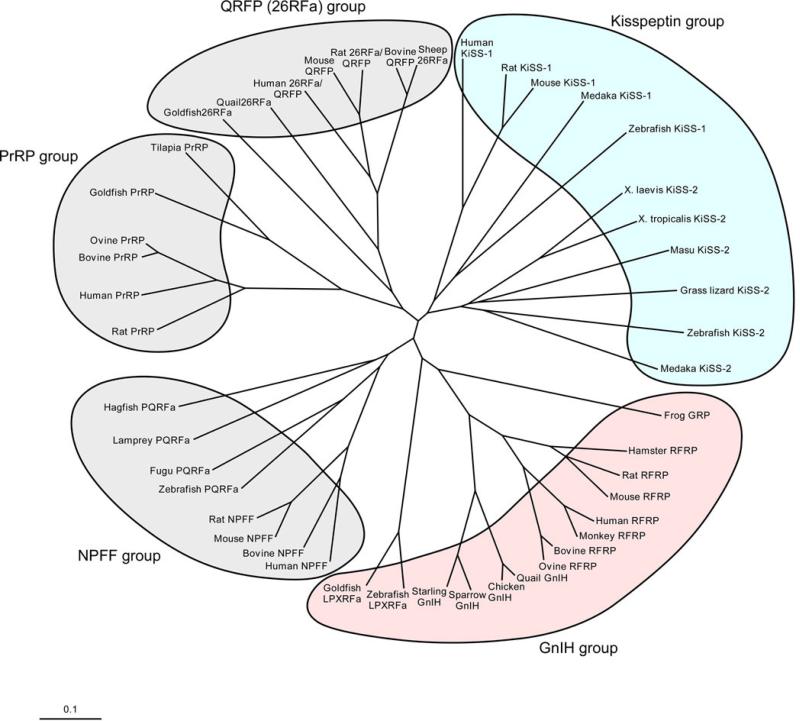
Figure 1.
Phylogenetic tree of the RFamide peptide family. Extensive studies over the past decade have demonstrated that vertebrate brains produce a variety of RFamide peptides. To date, five groups of the RFamide peptide family have been documented as follows: (1) NPFF group, (2) PRL-releasing peptide (PrRP) group, (3) GnIH group, (4) kisspeptin group, (5) pyroglutamylated RFamide peptide (QRFP)/26RFamide group. GnIH was discovered in the quail hypothalamus. GnIH inhibits gonadotropin synthesis and release in birds. GnIH orthologs sharing a common C-terminal LPXRFamide (X = L or Q) motif have been identified in other vertebrates from fish to humans. As in birds, mammalian and fish GnIH orthologs inhibit gonadotropin secretion. By contrast, the newly identified neuropeptide kisspeptin, encoded by the KiSS-1 gene, acts to stimulate the reproductive axis. Mammalian kisspeptin possesses a C-terminal RFamide or RYamide motif. The KiSS-1 gene has been identified in mammals, amphibians and fish. A second isoform of KiSS-1, designated KiSS-2, has also been identified in various vertebrates, but not birds, rodents and primate. Reviewed in [103,109].
III. Seasonal reproduction and GnIH in birds
III.a. Mechanisms driving seasonal changes in avian reproduction
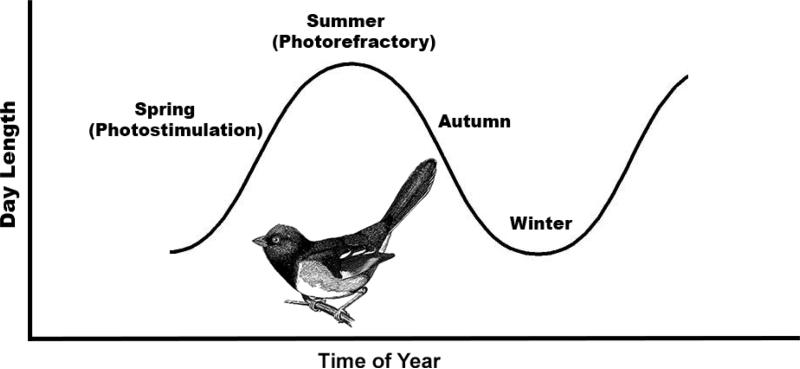
The majority of temperate-zone passeriformes exhibit dynamic seasonal changes in their reproductive activities. In most seasonally-breeding birds, the gonads rapidly develop in the spring in response to increasing day lengths, a process called photostimulation. The breeding season concludes when day lengths are still increasing, with birds becoming unresponsive, or photorefractory, to long days. Exposure to short day lengths is required for many passerine birds to regain photosensitivity whereby long day lengths can, again, act to stimulate the reproductive system the following year [23,124] (Figure 2).
Figure 2.
Annual timekeeping mechanism in most avian species. Exposure to short day lengths of winter sensitizes birds to the photostimulatory actions of increasing day lengths in spring. As day lengths continue to increase during the summer, animals become photorefractory, ignoring photoperiodic information and initiating a cascade of neuroendocrine events driving involution of the HPG axis. Reproduction ceases during the short days of winter while short days re-sensitize the reproductive axis to long day lengths the following spring.
In some birds, such as the Japanese quail, exposure to increasing day lengths over 11.5 hours results in rapid gonadal development. After about 3 months, when day lengths decrease below 14.5 hours, complete gonadal regression occurs in the wild [69]. However, if day lengths are artificially increased, a full return to reproductive maturity occurs. This phenomenon is called relative photorefractoriness because, even if the gonads have regressed under long day lengths, a subsequent increase in day length will initiate reproductive maturation without a short day length sensitization period [82].
Some species show characteristics of both absolute and relative photorefractoriness. Song sparrows [122] and house sparrows [22], for example, become photorefractory during exposure to long photoperiods, but the timing can vary widely among individuals. If the length of the long day is decreased slightly, gonadal regression occurs sooner and is more synchronous among individuals. Additionally, song sparrows can exhibit gonadal recrudescence without exposure to short photoperiods, a process required in absolutely photorefractory species [121].
III.b. GnIH and avian seasonality
Early investigations into the potential role of GnIH in guiding seasonal changes in reproduction in seasonally breeding birds explored whether or not predictable changes in GnIH immunoreactivity are observed across the seasons. For example, in house sparrows and song sparrows, GnIH-ir neurons are larger in birds at the termination of the breeding season than at other time of year, consistent with an inhibitory role of this neuropeptide in the regulation of seasonal breeding [6]. In contrast, in one study, differences in GnIH-ir cell number and size, as well as the synthesis of GnIH mRNA, were not observed between wild Australian zebra finches sampled during a breeding period in Southern Australia and a non-breeding period in central Australia [73]. The disparity between findings observed in sparrows and zebra finches might mirror differences in their reproductive strategy, with the former having distinct breeding seasons and the latter adopting a more opportunistic breeding strategy.
Because photoperiod is transduced into a melatonin signal whose duration is inversely proportional to day length, and melatonin administration inhibits reproductive activities in quail [35,70] and chicken [83], Ubuka et al. (2005) explored whether or not changes in the GnIH system of quail might be driven by melatonin. Removal of the pineal gland and eyes, the major sites of melatonin production in quail [118], decreased the expression of GnIH precursor mRNA and the content of GnIH peptide in the hypothalamus. Conversely, melatonin administration to these birds caused a dose-dependent increase in the expression of GnIH precursor mRNA and the production of GnIH peptide. This effect occurs, at least in part, through direct actions of melatonin on GnIH cells, with melatonin binding in the PVN and Mel1c, a melatonin receptor subtype, specifically expressed in GnIH-ir neurons [112].
Additional evidence for a direct action of melatonin on GnIH cells comes from in vitro studies demonstrating that GnIH administration dose-dependently increases GnIH release from hypothalamic blocks [16]. This being the case, one would expect melatonin production to exhibit a day-night pattern consistent with endogenous release rates. Indeed, GnIH release during the dark period is greater than that during the light period in hypothalamic blocks sampled from quail exposed to long-day photoperiods [16]. Consistent with this finding, plasma LH concentrations are lower during the dark period. Finally, GnIH release increases under short-day photoperiods, a time when the duration of nocturnal melatonin secretion increases. These results, combined with findings seen in quail, suggest that melatonin not only stimulates GnIH mRNA expression but also GnIH release [16].
III.c. Impact of social interactions on GnIH
To examine whether or not nest competition can alter GnIH to modulate reproductive functioning in the face of mating competition, Calisi et al. (2011) manipulated nesting opportunities for pairs of European starlings and examined changes in the GnIH system in successful and unsuccessful birds. Birds obtaining nest boxes had significantly fewer numbers of GnIH-ir cells than those without nest boxes in the beginning of breeding season, whereas the number of GnIH-ir cells were greater in birds with nest boxes than those without nest boxes in the middle of the breeding season. GnRH-ir cells, plasma testosterone and corticosterone did not vary with nest box ownership. These data suggest that GnIH may serve as a modulator of reproductive behavior, without gross modification of HPG axis functioning, in response to social environment [12].
More recently, Tobari et al. (2014) investigated the neurochemical means by which female presence alters reproductive axis function in male Japanese quail. They first found that norepinephrine (NE) release increases rapidly in the PVN of quail when viewing a female conspecific. Likewise, GnIH precursor mRNA expression increases in the PVN, with associated decreases in LH concentrations in plasma, when males viewed a female. The authors then established a link between these two events by showing that NE application stimulates GnIH release from diencephalic tissue blocks in vitro. Double-label immunohistochemistry revealed that GnIH neurons are innervated by noradrenergic fibers and immunohistochemistry combined with in situ hybridization demonstrated that GnIH neurons expressed α2A-adrenergic receptor mRNA. Together, these findings suggest that female presence increases NE release in the PVN and stimulates GnIH release, resulting in the suppression of LH release in this species [101].
III.d. Interactions between the GnIH system and the stress axis
Given the unpredictability of local conditions during the breeding season, animals maintain phenotypic plasticity and cease reproduction temporarily if local conditions are unfavorable [125]. Because adverse conditions activate the stress axis to transiently inhibit reproduction, Calisi et al. (2008) examined whether or not GnIH might participate in stress-induced reproductive suppression in house sparrows. Birds exposed to capture-handling stress exhibited significantly more GnIH-ir neurons in spring and increased GnIH cellular activation in both spring and fall relative to control animals. These data suggest that the GnIH participates in short-term changes in reproductive function when animals experience unpredictable, stressful conditions [13]. These inhibitory effects are likely mediated through direct actions in GnIH neurons via glucocorticoid receptors (GR); GR mRNA is expressed in GnIH neurons in quail and 24 hours of treatment with corticosterone (CORT) increases GnIH mRNA expression in quail diencephalon [97].
III.e. Implications for Gonadal GnIH in seasonal reproductive function
In addition to acting on GnIH cells in the brain to guide seasonal changes in reproduction, melatonin also appears to act directly on the gonads to regulate local GnIH activity. European starling testes express mRNA for GnIH, GnIH-R and the melatonin receptors, Mel1b and Mel1c [64]. GnIH and GnIH-R expression levels in the testes are relatively low during the breeding season and melatonin administration significantly increases GnIH mRNA. Additionally, melatonin and GnIH administration decrease testosterone release from gonadotropin-stimulated testes in vitro. Together, these results suggest that local inhibition of sex steroid secretion is regulated seasonally in starling testis by melatonin-induced alterations in gonadal GnIH [64].
As indicated previously, stress acts, at least in part, through direct actions of CORT on GnIH cells in the brain. More recent findings suggest that CORT may also act directly at the level of the gonads to alter reproductive function via changes in gonadal GnIH. In European starlings, CORT and metabolic stress decrease testosterone and estradiol secretion from gonadotropin-stimulated testes and ovaries in culture [65]. This effect is season specific; prior to the breeding season, the testes and ovaries respond to CORT and metabolic stress by significantly decreasing gonadal steroid release. During the breeding season, however, the testes do not respond to these stressors, whereas the ovaries respond to CORT with reductions in gonadal steroid production. Interestingly, whereas CORT, but not metabolic stress, up-regulates GnIH in the testes, metabolic stress, but not CORT, up-regulates GnIH in the ovaries [65], suggesting sex differences in the regulation of gonadal GnIH and the mechanisms by which different stressors impact gonadal functioning.
IV. Seasonal reproduction and GnIH in mammals
IV.a. Mechanisms driving seasonal changes in mammalian reproduction
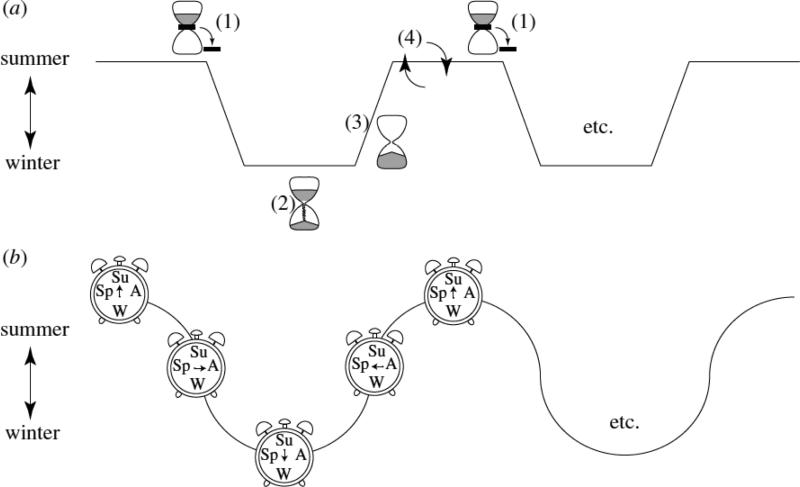
To synchronize breeding and other aspects of their physiology and behavior with the annual geophysical cycle, mammals use one of two mechanisms (Figure 3; reviewed in [30,52,72]). The first mechanism, typically observed in large long-lived mammals, employs an endogenous circannual clock with a period of approximately a year, even in organisms deprived of annual time cues. When animals are exposed to the natural environment, circannual rhythms adopt the period of the sidereal year through entrainment to the annual photoperiodic cycle [46,54,127,130]. The second type of rhythm, typically employed by small short-lived mammals, is similar to that described previously in birds, incorporating alternating periods of photosensitivity and photorefractoriness. In contrast to birds, however, photoperiodic mammals inhibit reproduction when day lengths fall below a species-specific minimum in the fall. A few months after reproductive cessation, as spring approaches, animals become refractory to short day lengths and restore reproductive functioning. To break refractoriness, these mammals require exposure to long day lengths.
Figure 3.
Annual timekeeping mechanisms. (a) Interval timer of the Siberian hamster. (1) Decreasing day lengths trigger the interval timer/induce the winter phenotype. (2) The timer runs to completion. (3) Refractoriness/spontaneous reversion to the spring phenotype. (4) Prolonged exposure to long day lengths breaks refractoriness/resets the interval timer. (b) Circannual clock of the golden-mantled ground squirrel. Successive oscillations between the summer and winter phenotypes are driven by an endogenous clock rather than exogenous factors. (from [72] with permission).
IV.b. GnIH and mammalian seasonality
As in birds, the striking seasonal changes in gonadal activity ultimately result from reduced stimulation of the pituitary by GnRH leading to low concentrations of circulating gonadal steroid hormones. One might expect that the reduction in circulating gonadal steroids relax feedback inhibition on the HPG axis. However, such a homeostatic mechanism of control would not allow the maintenance of reproductive quiescence, necessitating appropriate seasonal changes in the potency of negative feedback regulation. Such a mechanism ensures that gonadotropins maintain the ability to stimulate gametogenesis during the breeding season, even in the face of high levels of gonadal steroid hormone secretion, whereas, during the non-breeding season, even low concentrations of gonadal steroids are sufficient to suppress gonadotropin secretion, ensuring that reproductive capacity is held in check [55,99,110].
Because increased negative feedback of gonadal steroids is required for seasonal reproductive quiescence, initial studies searched for neural loci in which melatonin signaling might alter sex steroid receptor numbers or affinity. In Syrian hamsters, the DMH emerged as an important target as it expressed both sex steroid and melatonin receptors and was essential for SD- or melatonin-induced reproductive regression [4,58,62,63]. These findings pointed to the possibility that melatonin signaling is decoded in the DMH, possibly through GnIH neurons, to alter negative feedback across the seasons. However, despite being necessary for reproductive regression in Syrian hamsters, the DMH is not essential for enhanced gonadal steroid negative feedback following SD exposure [43]. Although these findings do not rule out a role for the DMH as a component of the circuitry guiding seasonal changes in reproductive function, they do indicate that melatonin acts upstream of the DMH, a circuit described further below.
Early studies exploring the effects of photoperiod on GnIH expression in Syrian and Siberian hamsters first explored whether or not GnIH might exhibit changes predictably associated with lay length or melatonin administration. Contrary to expectation, extended exposure to inhibitory day lengths leads to suppression of GnIH immunostaining and mRNA [59,81] as well as decreased fiber density and projections to GnRH cells [59,113]. Similar results are seen following several weeks of melatonin administration to long-day animals and pinealectomy prevents the suppression of GnIH in short days [81,113]. Analogous seasonal patterns of GnIH expression have been observed in European [90] and Turkish [74] hamsters as well as the semi-desert rodent, Jerboa [42]. In male hamsters, the short-day-induced reduction in GnIH is not the result of low circulating gonadal steroid concentrations; castration of long-day hamsters or providing testosterone implants to short-day hamsters does not alter the pattern of GnIH protein or mRNA [59,81,113].
Because the pars tuberalis (PT) of the anterior pituitary has the highest density of melatonin receptors across species [7,61,93,119,120] and has been importantly implicated in the control of seasonal breeding [21,36,67,68], more recent research has focused on this critical site and the possibility that GnIH is altered secondary to the actions of melatonin on this neural locus. In hamsters, melatonin acts on the pars tuberalis to inhibit thyroid-stimulating hormone (TSH) synthesis which, in turn, acts on tanycytes in the hypothalamic ependymal layer to alter deiodinase activity and, consequently, the conversion of local thyroxine (T4) to triiodothyronine (T3) [5,36,80,98]. As spring approaches and melatonin duration is decreased, increased T3 acts on downstream circuitry to guide the transition from the winter to summer breeding phenotype. If GnIH is downstream of this mechanism, then exposure of photoinhibited hamsters to TSH or T3 should lead to increased GnIH expression. Indeed, chronic central administration of TSH or T3 to SD hamsters for several weeks restores the LD pattern of GnIH, implicating GnIH as part of this established melatonin-mediated neurochemical circuit [38,50].
Despite these findings being consistent with a role for GnIH in seasonal breeding, they are at odds with a simple model of control in which GnIH is low during breeding and elevated during reproductive quiescence. One possibility is that hamsters require enhanced GnIH expression to suppress GnRH during the initial period of regression, whereas this level of inhibition is not necessary in hamsters with a fully regressed reproductive axis and low testosterone concentrations. Another possibility is that sex and timing of administration can impact the response of the reproductive axis to GnIH [1,113]. For example, whereas GnIH suppresses gonadotropin secretion in ovariectomized female hamsters treated in late afternoon [53], GnIH stimulates GnRH and gonadotropin secretion in male Syrian hamsters [1]. Additionally, reproductive function can be restored in short-day-housed male Syrian hamsters by infusion of GnIH [1]. In male Siberian hamsters, GnIH suppresses HPG axis function in long-day, reproductive-competent animals but stimulates gonadotropin secretion in short-day reproductively-quiescent animals [113]. Thus, future studies examining the pattern of GnIH expression throughout the development of reproductive quiescence across a number of species and in both sexes, in addition to molecular approaches that directly target GnIH function/expression are necessary to fully understand the complex role of this peptide in seasonal breeding.
In sheep and goats, GnIH neurons are prominent in the premammillary hypothalamus [41,94,96]. In sheep, GnIH mRNA and protein, and GnIH projections upon GnRH neurons, are increased in the hypothalamus at the time of reproductive quiescence during long-days relative to animals in non-breeding condition held in short days [20,96]. In goats, GnIH cell numbers are higher in the follicle phase of the ovulatory cycle during breeding season relative to short-day, anestrus females [41]. As in hamsters, photoperiodic changes in GnIH are not a result of altered sex steroids in sheep [96]. In another long-day breeder, the brushtail possum, GnIH cell numbers were increased during the summer, non-breeding season relative the breeding season in females [37]. Thus, in long (i.e., hamsters) and short (i.e., sheep, possum) day breeders, the photoperiodic regulation of GnIH is highly conserved and the downstream targets of the GnIH system diverged to accommodate long and short-day breeding strategies.
IV.c. Implications for Gonadal GnIH in seasonal reproductive function
Although not specifically investigated in a seasonal context in mammals, it is likely that GnIH acts locally in the gonads to drive seasonal changes in gonadal functioning. In Syrian hamsters and mice, for example, the gonads express GnIH and GPR147 [2,91,129]. In hamster testis, GnIH is expressed in spermatocytes and in round to early elongated spermatids whereas GPR147 protein is seen in myoid cells in all stages of spermatogenesis, pachytene spermatocytes, maturation division spermatocytes, and in round and late elongated spermatids. In mice, testicular GnIH synthesis is increased during reproductive senescence and may contribute to the decline in testicular GnRH activity seen at this time [2]. Finally, in mice, GnIH and GPR147 are present in ovarian granulosa cells of healthy and antral follicles during proestrus and estrus and in the luteal cells during diestrus [91], suggesting participation in follicular development and atresia.
IV.d. Interactions between the GnIH system and the stress axis
As in birds, stress has pronounced inhibitory actions on the HPG axis of mammals and likely contributes to short-term inhibition of reproduction when conditions become temporarily unfavorable. To explore whether or not stress suppresses reproductive functioning through stimulation of the GnIH system, Kirby et al. (2009) investigated the impact of acute immobilization stress on GnIH mRNA and protein levels in adult male rats. Following this stressor, GnIH mRNA and protein levels increased rapidly following stress, returning to baseline 24 hours later [48]. As seen in birds, glucocorticoids appears to act directly on GnIH cells expressing GR, pointing to an evolutionarily conserved mechanism of control in rodents and birds. However, the role of the GnIH system in suppressing reproduction during times of stress may not be common across all species as stress does not affect GnIH peptide or mRNA expression in sheep [71].
More recently, Son et al. (2014) used a GnIH-expressing neuronal cell line, rHypoE-23 cells derived from rat hypothalamus to further explore the impact of glucocorticoids on the GnIH system [97]. rHypoE-23 cells express GR mRNA and increase GnIH mRNA expression in response to corticosterone treatment. To determine the specific means by which corticosterone acts to increase GnIH expression, the authors characterized the promoter activity of the rat GnIH gene. DNA deletion analysis revealed a CORT-responsive region upstream of the GnIH precursor coding region that included two GC response elements (GREs). Mutation of one these GREs abolished CORT responsiveness. These results provide a putative molecular basis for transcriptional activation of GnIH under stress by CORT and GR [97].
V. GnIH as a Fuel Detector
In environments where energy availability fluctuates, animals use photoperiod to phase breeding with anticipated times of maximal food availability [10]. Should food become scarce during the breeding season, reproduction is temporarily inhibited [9,87]. Food deprivation and other metabolic challenges inhibit reproductive axis functioning and sexual motivation [8,11,86,89,92]. Several lines of evidence suggest that GnIH might act to relay metabolic information to the HPG axis and neural circuits guiding sexual motivation. First, GnIH injections stimulate food intake across most species, including mice, rats and non-human primates [19,44], although one recent report indicates that direct amygdalar injections decrease food intake in mice [51]. Additionally, GnIH neurons are activated by food restriction in hamsters and infusion of GnIH inhibits sexual motivation in this species [49,75]. In male and female Obese (Ob) mice, GnIH mRNA levels are lower than wild-type animals with normal body compositions, suggesting reduced stimulation of feeding circuits when energy storage is maximized [77]. Finally, the inhibition of LH normally accompanying food restriction is reduced in mice lacking GPR147 [57]. Together, these findings suggest that GnIH might participate in fine-tuning the precise timing of seasonal breeding by relaying metabolic state to reproductive and motivational circuits when energy availability is uncertain.
VI. Regulation of Seasonal Reproduction by Kisspeptin
Although not the focus of this review, another neuropeptide in the RFamide family, kisspeptin, deserves brief consideration as several lines of evidence suggest that kisspeptin works in conjunction with GnIH to guide seasonal changes in reproduction across species (reviewed in [90]). Kisspeptin received substantial attention by reproductive biologists following the discovery that a loss-of-function mutations in the kisspeptin receptor, GRP54, gene are associated with a failure to attain puberty and infertility in rodents and humans [24,47,76,88]. Given the pronounced stimulatory role of kisspeptin in puberty and adult reproductive status, this peptide represented an attractive candidate system responsible for controlling seasonal changes in reproduction.
The gene for kisspeptin, KiSS1, has been identified in most vertebrates, including mammals, amphibians, and fish – but not birds (reviewed in [104]). The role of kisspeptin in seasonal breeding has principally been explored in Syrian and Siberian hamsters [3,33,34,60,79] and sheep [14,18,31,32,56,95,96]. Across species, kisspeptin is generally elevated during the breeding season and suppressed following exposure to inhibitory photoperiods or appropriately timed melatonin administration. Likewise, infusion of kisspeptin can restore reproductive function to reproductively quiescent hamsters and sheep [3,14]. Together with the aforementioned overview of the participation of RFRP-3 in mammalian seasonality, these findings point to an important role for kisspeptin in the regulation of seasonal breeding.
VI. Conclusions and Considerations
The combined descriptive, correlational and empirical evidence presented herein suggests a key role for GnIH in avian and mammalian seasonal breeding. In birds and mammals, GnIH mRNA and peptide expression exhibit pronounced seasonal changes associated with alterations in reproductive status. Additionally, in birds melatonin can act directly on GnIH cells to alter GnIH expression in a manner consistent with a role in phasing seasonal breeding. However, there is no clear evidence, as of yet, that GnIH is involved in the downregulation of GnRH synthesis or release in birds refractory to long day lengths. It is more likely that GnIH acts within the breeding season to modulate physiology and behavior during substages of this critical component of the annual cycle. In mammals, melatonin-responsive systems upstream of GnIH cells (e.g., pars tuberalis) likely participate in seasonal changes in GnIH expression. Whether GnIH, in turn, acts to alter reproductive axis activity to guide changes in reproductive status associated with refractoriness to long or short days requires direct empirical investigation. The observations that administration of GnIH hastens changes in reproductive status in mammals provide indirect evidence for this possibility, but whether GnIH participates in photorefractory transitions under unmanipulated conditions requires further study.
In addition to acting on the hypothalamus to alter HPG axis functioning, GnIH also operates locally within gonadal tissues to appropriately modulate testicular and ovarian functioning. Finally, the observations that GnIH expression is markedly impacted by stress and social interactions places GnIH in a position to further guide more transient changes in reproductive functioning should unfavorable breeding conditions manifest, temporarily, during the breeding season. A full understanding of the specific role of GnIH in seasonal breeding requires more exacting approaches presently hampered by the limited availability of experimental tools in seasonally-breeding species. By developing species-specific molecular/genetic approaches, for example, that allow precise, systematic manipulation of GnIH cells and their targets, further insight can be gained into the specific functional role of this neuropeptide. In this regard, exciting opportunities exist for researchers interested in understanding the neural circuits and neuroendocrine mechanisms driving vertebrate seasonal breeding.
Highlights.
GnIH acts as a central component of the reproductive axis across vertebrate species.
GnIH plays a key role in seasonal breeding among birds and mammals.
Transient changes in reproductive function are also mediated by GnIH.
Table 2.
Seasonal and melatonin-induced changes in GnIH/RFRP-3 in birds and mammals
| Species | GnIH/RFRP-3 | References |
|---|---|---|
| Birds: | ||
| House Sparrows (Passer domesticus) | Larger GnIH-ir cells during non-breeding season | [5] |
| Stress increases GnIH-ir cell numbers in spring | [8] | |
| Zebra Finches (Taeniopygia guttata) | No Change in GnIH-ir cell size, number of GnIH mRNA | [48] |
| Japanese Quail (Coturnix coturnix japonica) | Increased GnIH mRNA and GnIH peptide with melatonin administration | [74,80] |
| Female presence stimulates GnIH release | [64] | |
| European Starling (Sturnus vulgaris) | Reduced GnIH-ir numbers in breeding males | [7] |
| Increased testicular GnIH with melatonin administration | [41] | |
| Mammals: | ||
| Syrian Hamsters (Mesocricetus Auratus) | RFRP-3 peptide and mRNA reduced in short days or by melatonin | [38,51] |
| GnIH stimulates GnRH and gonadotropin secretion in LD males | [1] | |
| Siberian Hamsters (Phodopus Sungorus) | RFRP-3 peptide and mRNA reduced in short days or by melatonin | [51,75] |
| GnIH inhibits LH secretion in LD animals and stimulates LH in SD males | [75] | |
| Sheep (Ovis Aries) | GnIH mRNA, protein and projections to GnRH cells increased in SD ewes | [12,59] |
| Goats (Capra aegagrus) | Higher during follicular portion of ovulatory cycle than anestrus females | [25] |
Acknowledgments
Grant support: Supported by NIH R01 HD-050470 and NSF IOS-1257638 (to L.J.K.) and Grants-in-Aid for Scientific Research from the Ministry of Education, Science and Culture, Japan (22132004 and 22227002 to K.T.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure Statement:
The authors have nothing to disclose.
References
- 1.Ancel C, Bentsen AH, Sebert ME, Tena-Sempere M, Mikkelsen JD, Simonneaux V. Stimulatory effect of RFRP-3 on the gonadotrophic axis in the male Syrian hamster: the exception proves the rule. Endocrinology. 2012;153:1352–63. doi: 10.1210/en.2011-1622. [DOI] [PubMed] [Google Scholar]
- 2.Anjum S, Krishna A, Sridaran R, Tsutsui K. Localization of gonadotropin-releasing hormone (GnRH), gonadotropin-inhibitory hormone (GnIH), kisspeptin and GnRH receptor and their possible roles in testicular activities from birth to senescence in mice. Journal of experimental zoology. Part A, Ecological genetics and physiology. 2012;317:630–44. doi: 10.1002/jez.1765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ansel L, Bentsen AH, Ancel C, Bolborea M, Klosen P, Mikkelsen JD, Simonneaux V. Peripheral kisspeptin reverses short photoperiod-induced gonadal regression in Syrian hamsters by promoting GNRH release. Reproduction. 2011;142:417–25. doi: 10.1530/REP-10-0313. [DOI] [PubMed] [Google Scholar]
- 4.Bae HH, Mangels RA, Cho BS, Dark J, Yellon SM, Zucker I. Ventromedial hypothalamic mediation of photoperiodic gonadal responses in male Syrian hamsters. Journal of biological rhythms. 1999;14:391–401. doi: 10.1177/074873099129000795. [DOI] [PubMed] [Google Scholar]
- 5.Barrett P, Ebling FJ, Schuhler S, Wilson D, Ross AW, Warner A, Jethwa P, Boelen A, Visser TJ, Ozanne DM, Archer ZA, Mercer JG, Morgan PJ. Hypothalamic thy- roid hormone catabolism acts as a gatekeeper for the seasonal control of body weight and reproduction. Endocrinology. 2007;148:3608–3617. doi: 10.1210/en.2007-0316. [DOI] [PubMed] [Google Scholar]
- 6.Bentley GE, Perfito N, Ukena K, Tsutsui K, Wingfield JC. Gonadotropin-inhibitory peptide in song sparrows (Melospiza melodia) in different reproductive conditions, and in house sparrows (Passer domesticus) relative to chicken-gonadotropin-releasing hormone. Journal of neuroendocrinology. 2003;15:794–802. doi: 10.1046/j.1365-2826.2003.01062.x. [DOI] [PubMed] [Google Scholar]
- 7.Bittman EL, Weaver DR. The distribution of melatonin binding sites in neuroendocrine tissues of the ewe. Biology of reproduction. 1990;43:986–93. doi: 10.1095/biolreprod43.6.986. [DOI] [PubMed] [Google Scholar]
- 8.Bronson FH. Effect of food manipulation on the GnRH-LH-estradiol axis of young female rats. The American journal of physiology. 1988;254:R616–21. doi: 10.1152/ajpregu.1988.254.4.R616. [DOI] [PubMed] [Google Scholar]
- 9.Bronson FH. Food-restricted, prepubertal, female rats: rapid recovery of luteinizing hormone pulsing with excess food, and full recovery of pubertal development with gonadotropin-releasing hormone. Endocrinology. 1986;118:2483–7. doi: 10.1210/endo-118-6-2483. [DOI] [PubMed] [Google Scholar]
- 10.Bronson FH. Mammalian reproductive biology. University of Chicago Press; Chicago, IL: 1989. [Google Scholar]
- 11.Bronson FH, Marsteller FA. Effect of short-term food deprivation on reproduction in female mice. Biology of reproduction. 1985;33:660–7. doi: 10.1095/biolreprod33.3.660. [DOI] [PubMed] [Google Scholar]
- 12.Calisi RM, Diaz-Munoz SL, Wingfield JC, Bentley GE. Social and breeding status are associated with the expression of GnIH. Genes, brain, and behavior. 2011;10:557–64. doi: 10.1111/j.1601-183X.2011.00693.x. [DOI] [PubMed] [Google Scholar]
- 13.Calisi RM, Rizzo NO, Bentley GE. Seasonal differences in hypothalamic EGR-1 and GnIH expression following capture-handling stress in house sparrows (Passer domesticus). General and comparative endocrinology. 2008;157:283–7. doi: 10.1016/j.ygcen.2008.05.010. [DOI] [PubMed] [Google Scholar]
- 14.Caraty A, Smith JT, Lomet D, Ben Said S, Morrissey A, Cognie J, Doughton B, Baril G, Briant C, Clarke IJ. Kisspeptin synchronizes preovulatory surges in cyclical ewes and causes ovulation in seasonally acyclic ewes. Endocrinology. 2007;148:5258–67. doi: 10.1210/en.2007-0554. [DOI] [PubMed] [Google Scholar]
- 15.Carter DS, Goldman BD. Antigonadal effects of timed melatonin infusion in pinealectomized male Djungarian hamsters (Phodopus sungorus sungorus): duration is the critical parameter. Endocrinology. 1983;113:1261–7. doi: 10.1210/endo-113-4-1261. [DOI] [PubMed] [Google Scholar]
- 16.Chowdhury VS, Yamamoto K, Ubuka T, Bentley GE, Hattori A, Tsutsui K. Melatonin stimulates the release of gonadotropin-inhibitory hormone by the avian hypothalamus. Endocrinology. 2010;151:271–80. doi: 10.1210/en.2009-0908. [DOI] [PubMed] [Google Scholar]
- 17.Clarke IJ, Sari IP, Qi Y, Smith JT, Parkington HC, Ubuka T, Iqbal J, Li Q, Tilbrook A, Morgan K, Pawson AJ, Tsutsui K, Millar RP, Bentley GE. Potent action of RFamide-related peptide-3 on pituitary gonadotropes indicative of a hypophysiotropic role in the negative regulation of gonadotropin secretion. Endocrinology. 2008;149:5811–21. doi: 10.1210/en.2008-0575. [DOI] [PubMed] [Google Scholar]
- 18.Clarke IJ, Smith JT, Caraty A, Goodman RL, Lehman MN. Kisspeptin and seasonality in sheep. Peptides. 2009;30:154–63. doi: 10.1016/j.peptides.2008.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Clarke IJ, Smith JT, Henry BA, Oldfield BJ, Stefanidis A, Millar RP, Sari IP, Chng K, Fabre-Nys C, Caraty A, Ang BT, Chan L, Fraley GS. Gonadotropin-inhibitory hormone is a hypothalamic peptide that provides a molecular switch between reproduction and feeding. Neuroendocrinology. 2012;95:305–16. doi: 10.1159/000332822. [DOI] [PubMed] [Google Scholar]
- 20.Dardente H, Birnie M, Lincoln GA, Hazlerigg DG. RFamide-related peptide and its cognate receptor in the sheep: cDNA cloning, mRNA distribution in the hypothalamus and the effect of photoperiod. Journal of neuroendocrinology. 2008;20:1252–9. doi: 10.1111/j.1365-2826.2008.01784.x. [DOI] [PubMed] [Google Scholar]
- 21.Dardente H, Wyse CA, Birnie MJ, Dupre SM, Loudon AS, Lincoln GA, Hazlerigg DG. A molecular switch for photoperiod responsiveness in mammals. Current biology : CB. 2010;20:2193–8. doi: 10.1016/j.cub.2010.10.048. [DOI] [PubMed] [Google Scholar]
- 22.Dawson A. Photoperiodic control of the termination of breeding and the induction of moult in House sparrows Passer domesticus. Ibis. 1998;140:35–40. [Google Scholar]
- 23.Dawson A, King VM, Bentley GE, Ball GF. Photoperiodic control of seasonality in birds. Journal of biological rhythms. 2001;16:365–80. doi: 10.1177/074873001129002079. [DOI] [PubMed] [Google Scholar]
- 24.de Roux N, Genin E, Carel JC, Matsuda F, Chaussain JL, Milgrom E. Hypogonadotropic hypogonadism due to loss of function of the KiSS1-derived peptide receptor GPR54. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:10972–6. doi: 10.1073/pnas.1834399100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dockray GJ, Reeve JR, Jr., Shively J, Gayton RJ, Barnard CS. A novel active pentapeptide from chicken brain identified by antibodies to FMRFamide. Nature. 1983;305:328–30. doi: 10.1038/305328a0. [DOI] [PubMed] [Google Scholar]
- 26.Farner DS, Follett BK. Reproductive periodicity in birds. In: Barrington EJW, editor. Hormones and Evolution. Academic Press; New York: 1979. pp. 829–872. [Google Scholar]
- 27.Follett BK. Birds. Marshall's Physiology of Reproduction. In: Lamming GE, editor. 4th Edition Vol. 1. Churchill-Livingstone; New York: 1984. pp. 283–350. [Google Scholar]
- 28.Fukusumi S, Habata Y, Yoshida H, Iijima N, Kawamata Y, Hosoya M, Fujii R, Hinuma S, Kitada C, Shintani Y, Suenaga M, Onda H, Nishimura O, Tanaka M, Ibata Y, Fujino M. Characteristics and distribution of endogenous RFamide-related peptide-1. Biochimica et biophysica acta. 2001;1540:221–32. doi: 10.1016/s0167-4889(01)00135-5. [DOI] [PubMed] [Google Scholar]
- 29.Gibson EM, Humber SA, Jain S, Williams WP, 3rd, Zhao S, Bentley GE, Tsutsui K, Kriegsfeld LJ. Alterations in RFamide-related peptide expression are coordinated with the preovulatory luteinizing hormone surge. Endocrinology. 2008;149:4958–69. doi: 10.1210/en.2008-0316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goldman BD. Mammalian photoperiodic system: formal properties and neuroendocrine mechanisms of photoperiodic time measurement. Journal of biological rhythms. 2001;16:283–301. doi: 10.1177/074873001129001980. [DOI] [PubMed] [Google Scholar]
- 31.Goodman RL, Coolen LM, Lehman MN. A role for neurokinin B in pulsatile GnRH secretion in the ewe. Neuroendocrinology. 2014;99:18–32. doi: 10.1159/000355285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Goodman RL, Jansen HT, Billings HJ, Coolen LM, Lehman MN. Neural systems mediating seasonal breeding in the ewe. Journal of neuroendocrinology. 2010;22:674–81. doi: 10.1111/j.1365-2826.2010.02014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Greives TJ, Kriegsfeld LJ, Demas GE. Exogenous kisspeptin does not alter photoperiod-induced gonadal regression in Siberian hamsters (Phodopus sungorus). General and comparative endocrinology. 2008;156:552–8. doi: 10.1016/j.ygcen.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Greives TJ, Mason AO, Scotti MA, Levine J, Ketterson ED, Kriegsfeld LJ, Demas GE. Environmental control of kisspeptin: implications for seasonal reproduction. Endocrinology. 2007;148:1158–66. doi: 10.1210/en.2006-1249. [DOI] [PubMed] [Google Scholar]
- 35.Guyomarc'h C, Lumineau S, Vivien-Roels B, Richard J, Deregnaucourt S. Effect of melatonin supplementation on the sexual development in European quail (Coturnix coturnix). Behavioural processes. 2001;53:121–130. doi: 10.1016/s0376-6357(01)00133-4. [DOI] [PubMed] [Google Scholar]
- 36.Hanon EA, Lincoln GA, Fustin JM, Dardente H, Masson-Pevet M, Morgan PJ, Hazlerigg DG. Ancestral TSH mechanism signals summer in a photoperiodic mammal. Current biology : CB. 2008;18:1147–52. doi: 10.1016/j.cub.2008.06.076. [DOI] [PubMed] [Google Scholar]
- 37.Harbid AA, McLeod BJ, Caraty A, Anderson GM. Seasonal changes in RFamide-related peptide-3 neurons in the hypothalamus of a seasonally breeding marsupial species, the brushtail possum (Trichosurus vulpecula). The Journal of comparative neurology. 2013;521:3030–41. doi: 10.1002/cne.23328. [DOI] [PubMed] [Google Scholar]
- 38.Henson JR, Carter SN, Freeman DA. Exogenous T(3) elicits long day-like alterations in testis size and the RFamides Kisspeptin and gonadotropin-inhibitory hormone in short-day Siberian hamsters. Journal of biological rhythms. 2013;28:193–200. doi: 10.1177/0748730413487974. [DOI] [PubMed] [Google Scholar]
- 39.Hinuma S, Shintani Y, Fukusumi S, Iijima N, Matsumoto Y, Hosoya M, Fujii R, Watanabe T, Kikuchi K, Terao Y, Yano T, Yamamoto T, Kawamata Y, Habata Y, Asada M, Kitada C, Kurokawa T, Onda H, Nishimura O, Tanaka M, Ibata Y, Fujino M. New neuropeptides containing carboxy-terminal RFamide and their receptor in mammals. Nature cell biology. 2000;2:703–8. doi: 10.1038/35036326. [DOI] [PubMed] [Google Scholar]
- 40.Ikegami K, Yoshimura T. Seasonal time measurement during reproduction. The Journal of reproduction and development. 2013;59:327–33. doi: 10.1262/jrd.2013-035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Jafarzadeh Shirazi MR, Zamiri MJ, Salehi MS, Moradi S, Tamadon A, Namavar MR, Akhlaghi A, Tsutsui K, Caraty A. Differential Expression of RFamide-Related Peptide, a Mammalian Gonadotrophin-Inhibitory Hormone Orthologue, and Kisspeptin in the Hypothalamus of Abadeh Ecotype Does During Breeding and Anoestrous Seasons. Journal of neuroendocrinology. 2014;26:186–194. doi: 10.1111/jne.12137. [DOI] [PubMed] [Google Scholar]
- 42.Janati A, Talbi R, Klosen P, Mikkelsen JD, Magoul R, Simonneaux V, El Ouezzani S. Distribution and seasonal variation in hypothalamic RF-amide peptides in a semi-desert rodent, the jerboa. Journal of neuroendocrinology. 2013;25:402–11. doi: 10.1111/jne.12015. [DOI] [PubMed] [Google Scholar]
- 43.Jarjisian SG, Piekarski DJ, Place NJ, Driscoll JR, Paxton EG, Kriegsfeld LJ, Zucker I. Dorsomedial hypothalamic lesions block Syrian hamster testicular regression in short day lengths without diminishing increased testosterone negative-feedback sensitivity. Biology of reproduction. 2013;89:23. doi: 10.1095/biolreprod.113.109587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson MA, Tsutsui K, Fraley GS. Rat RFamide-related peptide-3 stimulates GH secretion, inhibits LH secretion, and has variable effects on sex behavior in the adult male rat. Hormones and behavior. 2007;51:171–80. doi: 10.1016/j.yhbeh.2006.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kadokawa H, Shibata M, Tanaka Y, Kojima T, Matsumoto K, Oshima K, Yamamoto N. Bovine C-terminal octapeptide of RFamide-related peptide-3 suppresses luteinizing hormone (LH) secretion from the pituitary as well as pulsatile LH secretion in bovines. Domestic animal endocrinology. 2009;36:219–24. doi: 10.1016/j.domaniend.2009.02.001. [DOI] [PubMed] [Google Scholar]
- 46.Karsch FJ, Bittman EL, Foster DL, Goodman RL, Legan SJ, Robinson JE. Neuroendocrine basis of seasonal reproduction. Recent Prog Horm Res. 1984;40:185–232. doi: 10.1016/b978-0-12-571140-1.50010-4. [DOI] [PubMed] [Google Scholar]
- 47.Kauffman AS, Clifton DK, Steiner RA. Emerging ideas about kisspeptin-GPR54 signaling in the neuroendocrine regulation of reproduction. Trends Neurosci. 2007;30:504–11. doi: 10.1016/j.tins.2007.08.001. [DOI] [PubMed] [Google Scholar]
- 48.Kirby ED, Geraghty AC, Ubuka T, Bentley GE, Kaufer D. Stress increases putative gonadotropin inhibitory hormone and decreases luteinizing hormone in male rats. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:11324–9. doi: 10.1073/pnas.0901176106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Klingerman CM, Williams WP, 3rd, Simberlund J, Brahme N, Prasad A, Schneider JE, Kriegsfeld LJ. Food Restriction-Induced Changes in Gonadotropin-Inhibiting Hormone Cells are Associated with Changes in Sexual Motivation and Food Hoarding, but not Sexual Performance and Food Intake. Frontiers in endocrinology. 2011;2:101. doi: 10.3389/fendo.2011.00101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Klosen P, Sebert ME, Rasri K, Laran-Chich MP, Simonneaux V. TSH restores a summer phenotype in photoinhibited mammals via the RF-amides RFRP3 and kisspeptin. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27:2677–86. doi: 10.1096/fj.13-229559. [DOI] [PubMed] [Google Scholar]
- 51.Kovacs A, Laszlo K, Galosi R, Ollmann T, Peczely L, Zagoracz O, Bencze N, Lenard L. Intraamygdaloid microinjection of RFamide-related peptide-3 decreases food intake in rats. Brain research bulletin. 2014;107:61–8. doi: 10.1016/j.brainresbull.2014.07.002. [DOI] [PubMed] [Google Scholar]
- 52.Kriegsfeld LJ, Bittman EL. Photoperiodism and Reproduction in Mammals. In: Nelson RJ, Denlinger DL, Somers DE, editors. Photoperiodism: The Biological Calendar. Oxford University Press; USA, New York: 2010. [Google Scholar]
- 53.Kriegsfeld LJ, Mei DF, Bentley GE, Ubuka T, Mason AO, Inoue K, Ukena K, Tsutsui K, Silver R. Identification and characterization of a gonadotropin-inhibitory system in the brains of mammals. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:2410–5. doi: 10.1073/pnas.0511003103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Lee TM, Zucker I. Suprachiasmatic nucleus and photic entrainment of circannual rhythms in ground squirrels. Journal of biological rhythms. 1991;6:315–30. doi: 10.1177/074873049100600403. [DOI] [PubMed] [Google Scholar]
- 55.Legan SJ, Karsch FJ, Foster DL. The endocrin control of seasonal reproductive function in the ewe: a marked change in response to the negative feedback action of estradiol on luteinizing hormone secretion. Endocrinology. 1977;101:818–24. doi: 10.1210/endo-101-3-818. [DOI] [PubMed] [Google Scholar]
- 56.Lehman MN, Ladha Z, Coolen LM, Hileman SM, Connors JM, Goodman RL. Neuronal plasticity and seasonal reproduction in sheep. The European journal of neuroscience. 2010;32:2152–64. doi: 10.1111/j.1460-9568.2010.07530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Leon S, Garcia-Galiano D, Ruiz-Pino F, Barroso A, Manfredi-Lozano M, Romero-Ruiz A, Roa J, Vazquez MJ, Gaytan F, Blomenrohr M, van Duin M, Pinilla L, Tena-Sempere M. Physiological roles of gonadotropin-inhibitory hormone signaling in the control of mammalian reproductive axis: studies in the NPFF1 receptor null mouse. Endocrinology. 2014;155:2953–65. doi: 10.1210/en.2014-1030. [DOI] [PubMed] [Google Scholar]
- 58.Lewis D, Freeman DA, Dark J, Wynne-Edwards KE, Zucker I. Photoperiodic control of oestrous cycles in Syrian hamsters: mediation by the mediobasal hypothalamus. Journal of neuroendocrinology. 2002;14:294–9. doi: 10.1046/j.1365-2826.2002.00779.x. [DOI] [PubMed] [Google Scholar]
- 59.Mason AO, Duffy S, Zhao S, Ubuka T, Bentley GE, Tsutsui K, Silver R, Kriegsfeld LJ. Photoperiod and reproductive condition are associated with changes in RFamide-related peptide (RFRP) expression in Syrian hamsters (Mesocricetus auratus). Journal of biological rhythms. 2010;25:176–85. doi: 10.1177/0748730410368821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mason AO, Greives TJ, Scotti MA, Levine J, Frommeyer S, Ketterson ED, Demas GE, Kriegsfeld LJ. Suppression of kisspeptin expression and gonadotropic axis sensitivity following exposure to inhibitory day lengths in female Siberian hamsters. Hormones and behavior. 2007;52:492–8. doi: 10.1016/j.yhbeh.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Masson-Pevet M, Gauer F. Seasonality and melatonin receptors in the pars tuberalis in some long day breeders. Biological signals. 1994;3:63–70. doi: 10.1159/000109527. [DOI] [PubMed] [Google Scholar]
- 62.Maywood ES, Bittman EL, Hastings MH. Lesions of the melatonin- and androgen-responsive tissue of the dorsomedial nucleus of the hypothalamus block the gonadal response of male Syrian hamsters to programmed infusions of melatonin. Biology of reproduction. 1996;54:470–7. doi: 10.1095/biolreprod54.2.470. [DOI] [PubMed] [Google Scholar]
- 63.Maywood ES, Hastings MH. Lesions of the iodomelatonin-binding sites of the mediobasal hypothalamus spare the lactotropic, but block the gonadotropic response of male Syrian hamsters to short photoperiod and to melatonin. Endocrinology. 1995;136:144–53. doi: 10.1210/endo.136.1.7828525. [DOI] [PubMed] [Google Scholar]
- 64.McGuire NL, Kangas K, Bentley GE. Effects of melatonin on peripheral reproductive function: regulation of testicular GnIH and testosterone. Endocrinology. 2011;152:3461–70. doi: 10.1210/en.2011-1053. [DOI] [PubMed] [Google Scholar]
- 65.McGuire NL, Koh A, Bentley GE. The direct response of the gonads to cues of stress in a temperate songbird species is season-dependent. PeerJ. 2013;1:e139. doi: 10.7717/peerj.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Murakami M, Matsuzaki T, Iwasa T, Yasui T, Irahara M, Osugi T, Tsutsui K. Hypophysiotropic role of RFamide-related peptide-3 in the inhibition of LH secretion in female rats. The Journal of endocrinology. 2008;199:105–12. doi: 10.1677/JOE-08-0197. [DOI] [PubMed] [Google Scholar]
- 67.Nakane Y, Yoshimura T. Universality and diversity in the signal transduction pathway that regulates seasonal reproduction in vertebrates. Frontiers in neuroscience. 2014;8:115. doi: 10.3389/fnins.2014.00115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Nakao N, Ono H, Yoshimura T. Thyroid hormones and seasonal reproductive neuroendocrine interactions. Reproduction. 2008;136:1–8. doi: 10.1530/REP-08-0041. [DOI] [PubMed] [Google Scholar]
- 69.Nicholls TJ, Goldsmith AR, Dawson A. Photorefractoriness in birds and comparison with mammals. Physiological reviews. 1988;68:133–76. doi: 10.1152/physrev.1988.68.1.133. [DOI] [PubMed] [Google Scholar]
- 70.Ohta M, Kadota C, Konishi H. A role of melatonin in the initial stage of photoperiodism in the Japanese quail. Biology of reproduction. 1989;40:935–41. doi: 10.1095/biolreprod40.5.935. [DOI] [PubMed] [Google Scholar]
- 71.Papargiris MM, Rivalland ET, Clarke IJ, Smith JT, Pereira A, Tilbrook AJ. Evidence that RF-amide related peptide-3 is not a mediator of the inhibitory effects of psychosocial stress on gonadotrophin secretion in ovariectomised ewes. Journal of neuroendocrinology. 2011;23:208–15. doi: 10.1111/j.1365-2826.2010.02094.x. [DOI] [PubMed] [Google Scholar]
- 72.Paul MJ, Zucker I, Schwartz WJ. Tracking the seasons: The internal calendars of vertebrates. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2008;363:341–346. doi: 10.1098/rstb.2007.2143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Perfito N, Zann R, Ubuka T, Bentley G, Hau M. Potential roles for GNIH and GNRH-II in reproductive axis regulation of an opportunistically breeding songbird. General and comparative endocrinology. 2011;173:20–6. doi: 10.1016/j.ygcen.2011.04.016. [DOI] [PubMed] [Google Scholar]
- 74.Piekarski DJ, Jarjisian SG, Perez L, Ahmad H, Dhawan N, Zucker I, Kriegsfeld LJ. Effects of Pinealectomy and Short Day Lengths on Reproduction and Neuronal RFRP-3, Kisspeptin, and GnRH in Female Turkish Hamsters. Journal of biological rhythms. 2014;29:181–191. doi: 10.1177/0748730414532423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Piekarski DJ, Zhao S, Jennings KJ, Iwasa T, Legan SJ, Mikkelsen JD, Tsutsui K, Kriegsfeld LJ. Gonadotropin-inhibitory hormone reduces sexual motivation but not lordosis behavior in female Syrian hamsters (Mesocricetus auratus). Hormones and behavior. 2013;64:501–10. doi: 10.1016/j.yhbeh.2013.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Pinilla L, Aguilar E, Dieguez C, Millar RP, Tena-Sempere M. Kisspeptins and reproduction: physiological roles and regulatory mechanisms. Physiological reviews. 2012;92:1235–316. doi: 10.1152/physrev.00037.2010. [DOI] [PubMed] [Google Scholar]
- 77.Poling MC, Shieh MP, Munaganuru N, Luo E, Kauffman AS. Examination of the Influence of Leptin and Acute Metabolic Challenge on RFRP-3 Neurons of Mice in Development and Adulthood. Neuroendocrinology. 2014 doi: 10.1159/000369276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Price DA, Greenberg MJ. Structure of a molluscan cardioexcitatory neuropeptide. Science. 1977;197:670–1. doi: 10.1126/science.877582. [DOI] [PubMed] [Google Scholar]
- 79.Revel FG, Saboureau M, Masson-Pevet M, Pevet P, Mikkelsen JD, Simonneaux V. Kisspeptin mediates the photoperiodic control of reproduction in hamsters. Current biology : CB. 2006;16:1730–5. doi: 10.1016/j.cub.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 80.Revel FG, Saboureau M, Pevet P, Mikkelsen JD, Simonneaux V. Melatonin regulates type 2 deiodinase gene expression in the Syrian hamster. Endocrinology. 2006;147:4680–7. doi: 10.1210/en.2006-0606. [DOI] [PubMed] [Google Scholar]
- 81.Revel FG, Saboureau M, Pevet P, Simonneaux V, Mikkelsen JD. RFamide-related peptide gene is a melatonin-driven photoperiodic gene. Endocrinology. 2008;149:902–12. doi: 10.1210/en.2007-0848. [DOI] [PubMed] [Google Scholar]
- 82.Robinson JE, Follett BK. Photoperiodism in Japanese quail: the termination of seasonal breeding by photorefractoriness. Proceedings of the Royal Society of London. Series B, Containing papers of a Biological character. Royal Society. 1982;215:95–116. doi: 10.1098/rspb.1982.0030. [DOI] [PubMed] [Google Scholar]
- 83.Rozenboim I, Aharony T, Yahav S. The effect of melatonin administration on circulating plasma luteinizing hormone concentration in castrated White Leghorn roosters. Poultry science. 2002;81:1354–9. doi: 10.1093/ps/81.9.1354. [DOI] [PubMed] [Google Scholar]
- 84.Sari IP, Rao A, Smith JT, Tilbrook AJ, Clarke IJ. Effect of RF-amide-related peptide-3 on luteinizing hormone and follicle-stimulating hormone synthesis and secretion in ovine pituitary gonadotropes. Endocrinology. 2009;150:5549–56. doi: 10.1210/en.2009-0775. [DOI] [PubMed] [Google Scholar]
- 85.Satake H, Hisada M, Kawada T, Minakata H, Ukena K, Tsutsui K. Characterization of a cDNA encoding a novel avian hypothalamic neuropeptide exerting an inhibitory effect on gonadotropin release. The Biochemical journal. 2001;354:379–85. doi: 10.1042/0264-6021:3540379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Schneider JE, Wade GN. Availability of metabolic fuels controls estrous cyclicity of Syrian hamsters. Science. 1989;244:1326–8. doi: 10.1126/science.2734610. [DOI] [PubMed] [Google Scholar]
- 87.Schneider JE, Wise JD, Benton NA, Brozek JM, Keen-Rhinehart E. When do we eat? Ingestive behavior, survival, and reproductive success. Hormones and behavior. 2013;64:702–28. doi: 10.1016/j.yhbeh.2013.07.005. [DOI] [PubMed] [Google Scholar]
- 88.Seminara SB, Messager S, Chatzidaki EE, Thresher RR, Acierno JS, Jr., Shagoury JK, Bo-Abbas Y, Kuohung W, Schwinof KM, Hendrick AG, Zahn D, Dixon J, Kaiser UB, Slaugenhaupt SA, Gusella JF, O'Rahilly S, Carlton MB, Crowley WF, Jr., Aparicio SA, Colledge WH. The GPR54 gene as a regulator of puberty. N Engl J Med. 2003;349:1614–27. doi: 10.1056/NEJMoa035322. [DOI] [PubMed] [Google Scholar]
- 89.Shahab M, Zaman W, Bashir K, Arslan M. Fasting-induced suppression of hypothalamic-pituitary-gonadal axis in the adult rhesus monkey: evidence for involvement of excitatory amino acid neurotransmitters. Life sciences. 1997;61:1293–300. doi: 10.1016/s0024-3205(97)00674-7. [DOI] [PubMed] [Google Scholar]
- 90.Simonneaux V, Ancel C, Poirel VJ, Gauer F. Kisspeptins and RFRP-3 Act in Concert to Synchronize Rodent Reproduction with Seasons. Frontiers in neuroscience. 2013;7:22. doi: 10.3389/fnins.2013.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Singh P, Krishna A, Sridaran R, Tsutsui K. Immunohistochemical localization of GnRH and RFamide-related peptide-3 in the ovaries of mice during the estrous cycle. Journal of molecular histology. 2011;42:371–81. doi: 10.1007/s10735-011-9340-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Sisk CL, Bronson FH. Effects of food restriction and restoration on gonadotropin and growth hormone secretion in immature male rats. Biology of reproduction. 1986;35:554–61. doi: 10.1095/biolreprod35.3.554. [DOI] [PubMed] [Google Scholar]
- 93.Skene DJ, Masson-Pevet M, Pevet P. Characterization of melatonin binding sites in the pars tuberalis of the European hamster. Journal of neuroendocrinology. 1992;4:189–92. doi: 10.1111/j.1365-2826.1992.tb00158.x. [DOI] [PubMed] [Google Scholar]
- 94.Smith JT. The role of kisspeptin and gonadotropin inhibitory hormone in the seasonal regulation of reproduction in sheep. Domestic animal endocrinology. 2012;43:75–84. doi: 10.1016/j.domaniend.2011.11.003. [DOI] [PubMed] [Google Scholar]
- 95.Smith JT, Clay CM, Caraty A, Clarke IJ. KiSS-1 messenger ribonucleic acid expression in the hypothalamus of the ewe is regulated by sex steroids and season. Endocrinology. 2007;148:1150–7. doi: 10.1210/en.2006-1435. [DOI] [PubMed] [Google Scholar]
- 96.Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, Bateman K, Goodman RL, Tilbrook AJ, Ubuka T, Bentley GE, Clarke IJ, Lehman MN. Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology. 2008;149:5770–82. doi: 10.1210/en.2008-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Son YL, Ubuka T, Narihiro M, Fukuda Y, Hasunuma I, Yamamoto K, Belsham DD, Tsutsui K. Molecular basis for the activation of gonadotropin-inhibitory hormone gene transcription by corticosterone. Endocrinology. 2014;155:1817–26. doi: 10.1210/en.2013-2076. [DOI] [PubMed] [Google Scholar]
- 98.Stevenson TJ, Prendergast BJ. Reversible DNA methylation regulates seasonal photoperiodic time measurement. Proceedings of the National Academy of Sciences of the United States of America. 2013;110:16651–6. doi: 10.1073/pnas.1310643110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tamarkin L, Hutchison JS, Goldman BD. Regulation of serum gonadotropins by photoperiod and testicular hormone in the Syrian hamster. Endocrinology. 1976;99:1528–33. doi: 10.1210/endo-99-6-1528. [DOI] [PubMed] [Google Scholar]
- 100.Tobari Y, Iijima N, Tsunekawa K, Osugi T, Okanoya K, Tsutsui K, Ozawa H. Identification of gonadotropin-inhibitory hormone in the zebra finch (Taeniopygia guttata): Peptide isolation, cDNA cloning and brain distribution. Peptides. 2010;31:816–26. doi: 10.1016/j.peptides.2010.01.015. [DOI] [PubMed] [Google Scholar]
- 101.Tobari Y, Son YL, Ubuka T, Hasegawa Y, Tsutsui K. A new pathway mediating social effects on the endocrine system: female presence acting via norepinephrine release stimulates gonadotropin-inhibitory hormone in the paraventricular nucleus and suppresses luteinizing hormone in quail. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2014;34:9803–11. doi: 10.1523/JNEUROSCI.3706-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Tsutsui K. A new key neurohormone controlling reproduction, gonadotropin-inhibitory hormone (GnIH): Biosynthesis, mode of action and functional significance. Progress in neurobiology. 2009;88:76–88. doi: 10.1016/j.pneurobio.2009.02.003. [DOI] [PubMed] [Google Scholar]
- 103.Tsutsui K, Bentley GE, Bedecarrats G, Osugi T, Ubuka T, Kriegsfeld LJ. Gonadotropin-inhibitory hormone (GnIH) and its control of central and peripheral reproductive function. Frontiers in neuroendocrinology. 2010;31:284–95. doi: 10.1016/j.yfrne.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 104.Tsutsui K, Bentley GE, Kriegsfeld LJ, Osugi T, Seong JY, Vaudry H. Discovery and evolutionary history of gonadotrophin-inhibitory hormone and kisspeptin: new key neuropeptides controlling reproduction. Journal of neuroendocrinology. 2010;22:716–27. doi: 10.1111/j.1365-2826.2010.02018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Tsutsui K, Saigoh E, Ukena K, Teranishi H, Fujisawa Y, Kikuchi M, Ishii S, Sharp PJ. A novel avian hypothalamic peptide inhibiting gonadotropin release. Biochemical and biophysical research communications. 2000;275:661–7. doi: 10.1006/bbrc.2000.3350. [DOI] [PubMed] [Google Scholar]
- 106.Tsutsui K, Ubuka T. Gonadotropin-inhibitory Hormone. In: Kastin AJ, Vaudry H, editors. Handbook of biologically active peptides. Section on brain peptides. Academic Press; London: 2012. pp. 802–11. [Google Scholar]
- 107.Tsutsui K, Ubuka T, Bentley GE, Kriegsfeld LJ. Gonadotropin-inhibitory hormone (GnIH): discovery, progress and prospect. General and comparative endocrinology. 2012;177:305–14. doi: 10.1016/j.ygcen.2012.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tsutsui K, Ubuka T, Bentley GE, Kriegsfeld LJ. Review: regulatory mechanisms of gonadotropin-inhibitory hormone (GnIH) synthesis and release in photoperiodic animals. Frontiers in neuroscience. 2013;7:60. doi: 10.3389/fnins.2013.00060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Tsutsui K, Ukena K. Hypothalamic LPXRF-amide peptides in vertebrates: identification, localization and hypophysiotropic activity. Peptides. 2006;27:1121–9. doi: 10.1016/j.peptides.2005.06.036. [DOI] [PubMed] [Google Scholar]
- 110.Turek FW. The interaction of the photoperiod and testosterone in regulating serum gonadotropin levels in castrated male hamsters. Endocrinology. 1977;101:1210–5. doi: 10.1210/endo-101-4-1210. [DOI] [PubMed] [Google Scholar]
- 111.Ubuka T, Bentley GE, Tsutsui K. Neuroendocrine regulation of gonadotropin secretion in seasonally breeding birds. Frontiers in neuroscience. 2013;7:38. doi: 10.3389/fnins.2013.00038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ubuka T, Bentley GE, Ukena K, Wingfield JC, Tsutsui K. Melatonin induces the expression of gonadotropin-inhibitory hormone in the avian brain. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:3052–7. doi: 10.1073/pnas.0403840102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ubuka T, Inoue K, Fukuda Y, Mizuno T, Ukena K, Kriegsfeld LJ, Tsutsui K. Identification, expression, and physiological functions of Siberian hamster gonadotropin-inhibitory hormone. Endocrinology. 2012;153:373–85. doi: 10.1210/en.2011-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Ubuka T, Kim S, Huang YC, Reid J, Jiang J, Osugi T, Chowdhury VS, Tsutsui K, Bentley GE. Gonadotropin-inhibitory hormone neurons interact directly with gonadotropin-releasing hormone-I and -II neurons in European starling brain. Endocrinology. 2008;149:268–78. doi: 10.1210/en.2007-0983. [DOI] [PubMed] [Google Scholar]
- 115.Ubuka T, Lai H, Kitani M, Suzuuchi A, Pham V, Cadigan PA, Wang A, Chowdhury VS, Tsutsui K, Bentley GE. Gonadotropin-inhibitory hormone identification, cDNA cloning, and distribution in rhesus macaque brain. The Journal of comparative neurology. 2009;517:841–55. doi: 10.1002/cne.22191. [DOI] [PubMed] [Google Scholar]
- 116.Ubuka T, Morgan K, Pawson AJ, Osugi T, Chowdhury VS, Minakata H, Tsutsui K, Millar RP, Bentley GE. Identification of human GnIH homologs, RFRP-1 and RFRP-3, and the cognate receptor, GPR147 in the human hypothalamic pituitary axis. PloS one. 2009;4:e8400. doi: 10.1371/journal.pone.0008400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Ukena K, Iwakoshi E, Minakata H, Tsutsui K. A novel rat hypothalamic RFamide-related peptide identified by immunoaffinity chromatography and mass spectrometry. FEBS letters. 2002;512:255–8. doi: 10.1016/s0014-5793(02)02275-5. [DOI] [PubMed] [Google Scholar]
- 118.Underwood H, Binkley S, Siopes T, Mosher K. Melatonin rhythms in the eyes, pineal bodies, and blood of Japanese quail (Coturnix coturnix japonica). General and comparative endocrinology. 1984;56:70–81. doi: 10.1016/0016-6480(84)90063-7. [DOI] [PubMed] [Google Scholar]
- 119.Weaver DR, Provencio I, Carlson LL, Reppert SM. Melatonin receptors and signal transduction in photorefractory Siberian hamsters (Phodopus sungorus). Endocrinology. 1991;128:1086–92. doi: 10.1210/endo-128-2-1086. [DOI] [PubMed] [Google Scholar]
- 120.Williams LM, Morgan PJ, Hastings MH, Lawson W, Davidson G, Howell HE. Melatonin Receptor Sites in the Syrian Hamster Brain and Pituitary. Localization and Characterization Using [|]lodomelatonin*. Journal of neuroendocrinology. 1989;1:315–20. doi: 10.1111/j.1365-2826.1989.tb00122.x. [DOI] [PubMed] [Google Scholar]
- 121.Wingfield JC. Control of testicular cycles in the song sparrow, Melospiza melodia melodia: interaction of photoperiod and an endogenous program? General and comparative endocrinology. 1993;92:388–401. doi: 10.1006/gcen.1993.1176. [DOI] [PubMed] [Google Scholar]
- 122.Wingfield JC. Environmental and endocrine control of reproduction: an ecological approach. In: Mikami SI, Wada M, editors. Avian Endocrinology: Environmental and Ecological Aspects. Japanese Scientific Societies Press; Springer-Verlag, Tokyo: [Google Scholar]
- 123.Wingfield JC. Organization of vertebrate annual cycles: implications for control mechanisms. Philosophical transactions of the Royal Society of London. Series B, Biological sciences. 2008;363:425–41. doi: 10.1098/rstb.2007.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wingfield JC, Farner DS. Control of seasonal reproduction in temperate-zone birds. In: Reiter RJ, Follett BK, editors. Progress in reproductive biology. Karger; New York: 1980. pp. 62–101. [Google Scholar]
- 125.Wingfield JC, Maney DL, Breuner CW, Jacobs JD, Lynn S, Ramenofsky M, Richardson RD. Ecological bases of hormone–behavior interactions: the “Emergency Life History Stage”. Am. Zool. 1998;38:191–206. [Google Scholar]
- 126.Wood S, Loudon A. Clocks for all seasons: unwinding the roles and mechanisms of circadian and interval timers in the hypothalamus and pituitary. The Journal of endocrinology. 2014;222:R39–59. doi: 10.1530/JOE-14-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Woodfill CJ, Wayne NL, Moenter SM, Karsch FJ. Photoperiodic synchronization of a circannual reproductive rhythm in sheep: identification of season-specific time cues. Biology of reproduction. 1994;50:965–76. doi: 10.1095/biolreprod50.4.965. [DOI] [PubMed] [Google Scholar]
- 128.Yoshida H, Habata Y, Hosoya M, Kawamata Y, Kitada C, Hinuma S. Molecular properties of endogenous RFamide-related peptide-3 and its interaction with receptors. Biochimica et biophysica acta. 2003;1593:151–7. doi: 10.1016/s0167-4889(02)00389-0. [DOI] [PubMed] [Google Scholar]
- 129.Zhao S, Zhu E, Yang C, Bentley GE, Tsutsui K, Kriegsfeld LJ. RFamide-related peptide and messenger ribonucleic acid expression in mammalian testis: association with the spermatogenic cycle. Endocrinology. 2010;151:617–27. doi: 10.1210/en.2009-0978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Zucker I, Lee TM, Dark J. The suprachiasmatic nucleus and the annual rhythm of mam- mals. In: Klein DC, Reppert SM, editors. Suprachiasmatic Nucleus: The Mind's Clock. Academic Press; New York: 1991. pp. 246–260. [Google Scholar]