Abstract
Drugs that target the folate synthesis pathway have a long history of effectiveness against a variety of pathogens. As antimalarials, the antifolates were safe and well tolerated, but resistance emerged quickly and has persisted even with decreased drug pressure. The primary determinants of resistance in Plasmodium falciparum are well-described point mutations in the enzymes dihydropteroate synthase (DHPS) and dihydrofolate reductase (DHFR) targeted by the combination sulfadoxine–pyrimethamine (SP). Recent work has highlighted the contributions of additional parasite adaptation to antifolate resistance. In fact, the evolution of antifolate-resistant parasites is multifaceted and complex. Gene amplification of the first enzyme in the parasite folate synthesis pathway, GTP-cyclohydrolase (GCH1) is strongly associated with resistant parasites and potentially contributes to persistence of resistant parasites. Further understanding of how parasites adjust flux through the folate pathway is important to the further development of alternative agents targeting this crucial synthesis pathway.
Keywords: malaria, Plasmodium falciparum, antifolates, pyrimethamine, sulfadoxine, GTP-cyclohydrolase (GCH1)
Introduction
Owing to the central importance of the folate-synthesis pathway to cell growth and division, drugs targeting this key pathway have played an important role against tumor cells as well as various pathogens.1 This review will provide an overview of antifolates used to treat and prevent infection with the most lethal of the human malaria parasites, Plasmodium falciparum. We will discuss the point mutations in target enzymes known to confer drug resistance and present that, beyond these well-characterized point mutations, malaria antifolate resistance has added complexities. Specifically, changes in other components of the folate-synthesis and folate-salvage pathways alter folate metabolic flux, potentially enabling the development of resistance. We propose that these adaptations also contribute to the persistence of resistant parasites, even with dramatically decreased antifolate drug pressure.
Over the past 10 years, morbidity and mortality due to malaria has decreased dramatically. Various factors have contributed to this decreased burden of disease: effective treatment regimens in the form of artemisinin-based combination therapy (ACT), barriers to transmission such as insecticide treated bed nets, indoor residual spraying, and improved diagnostics allowing for earlier appropriate treatment.1 In actuality, despite the overall decline in malaria infection, there are localized regions with persistently high clinical attack rates.2 In addition, development of drug resistance by the parasite and insecticide resistance of the mosquito threaten recent progress and decrease hope for a further decline in malaria burden.
Sulfadoxine and pyrimethamine (SP) are used in combination to target two enzymes in the parasite folate-synthesis pathway. As resistance to choloroquine (CQ) increased, SP was increasingly used as a safe and inexpensive alternative for the treatment of malaria. Increased use, however, led to increased parasite SP resistance. For this reason, alternative agents, mainly ACT, have replaced SP as first-line treatment regimens for approximately the past 10 years.3,4 Though not an official treatment recommendation, SP is still used for treatment. In addition, SP is used in endemic areas as part of intermittent preventive treatment (IPT) in children and pregnant women or seasonal chemoprophylaxis (SMC) in children. However, increasing levels of resistance threaten even specialized uses of this once effective drug combination.
Molecular survey of the folate-biosynthesis pathway
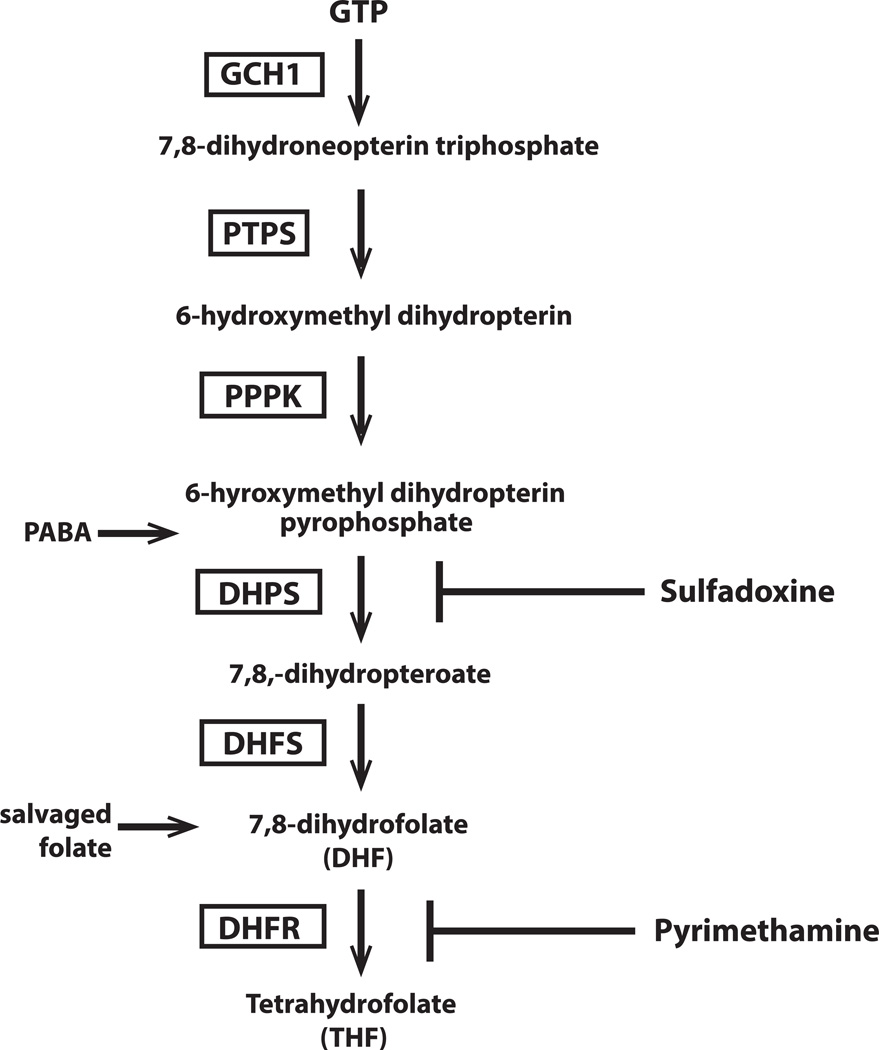
Folate derivatives are essential for DNA replication and protein synthesis and, thus, cell survival. Folates are one-carbon donors in purine biosynthesis and also act as cofactors in methionine production.5 Malaria parasites are able to synthesize folate de novo as well as scavenge it from the environment. GTP-cyclohydrolase 1 (GCH1) is the first enzyme in the folate-biosynthesis pathway (Fig.1) and is responsible for a ring-expansion reaction, converting GTP to the pteridine biosynthetic precursor, dihydroneopterin triphosphate.6 GCH1 is also present in the mammalian host, where it instead catalyzes the first reaction in the production of tetrahydrobiopterin, a cofactor important in the production of neurotransmitters and nitric oxide and in the catabolism of phenylalanine. Humans are unable to synthesize folate de novo owing to the absence of several key enzymes in the folate synthesis pathway and must therefore get the necessary folate through diet. Thus, drugs targeting this pathway play an important role against many pathogens.
Figure 1.
Folate biosynthesis in P. falciparum. GTP–cyclohydrolase 1 is the first enzyme in the folate biosynthesis pathway and has been found to exhibit extensive copy number variation (CNV). Enzymes in the folate pathway are boxed and substrates in plain text. GCH1, GTP-cyclohydrolase; PTPS, pyruvolytetrahydropterin synthase; PPPK, hydroxymethyldihydropterin pyrophosphokinase; DHPS, dihydropteroate synthase; DHFS, dihydrofolate synthase; DHFR, dihydrofolate reductase. Inhibitors of folate biosynthesis are shown at the right of the pathway; sulfadoxine (SDX), pyrimethamine (PYR). Para-aminobenzoic acid (PABA) enters as a substrate for DHPS. Salvaged folate can also enter the pathway upstream of DHFR.
Dihydropteroate synthase (DHPS), the third enzyme in the parasite folate-synthesis pathway, is responsible for combining pteridine with para-amino benzoic acid (pABA) to form dihydropteroate. The antimalarial sulfadoxine is a structural analog of pABA and is therefore able to inhibit DHPS.8 However, since Plasmodium also has the ability to bypass the de novo pathway and scavenge premade folate from its environment, many parasite isolates can survive in the presence of high concentrations of sulfadoxine if supplied with sufficient exogenous folate. Nonetheless, DHPS is essential, as parasites with a non-functional dhps are not viable.9
Dihydrofolate synthase (DHFS) catalyzes the final reaction in the de novo pathway producing dihydrofolate, which can then be converted into folate derivatives by three enzymes: dihydrofolate reductase (DHFR), serine hydroxymethyltransferase (SHMT), and thymidylate synthase (TS). Whereas the enzymes upstream of DHFR are involved only in de novo synthesis, DHFR is involved in both folate salvage and de novo synthesis, making this enzyme a potent drug target. DHFR is expressed as a bifunctional protein with thymidylate synthase (TS) and has been shown to inhibit the translation of its own mRNA.10 DHFR–TS is responsible for the synthesis of tetrahydrofolate and the recycling of dihydrofolate resulting from the synthesis of the nucleotide thymidine monophosphate (dTMP). Inhibition of DHFR with pyrimethamine (a structural analogue of dihydrofolate) halts the production of folate derivatives made from both exogenous and de novo folate.
While Plasmodium parasites can obtain folate both through exogenous uptake and de novo synthesis, the relative importance of each pathway is unclear. The predominant folate derivative circulating in the mammalian host is 5-CH3-THF, which represents 80–90% of the total folate pool.11 However, this form of folate was found to be a poor substrate for the two Plasmodium folate transporters.12 Nonetheless, both in vitro and in vivo studies have shown that high levels of exogenous folate can antagonize the effect of antifolate drug therapy.13,14 When the folate-synthesis pathway is compromised, parasites upregulate their salvage pathway to obtain necessary folate.9 The contribution of scavenging and de novo synthesis to the parasite folate pool is complex, with many contributing factors including parasite genetic background and concentration of folate (and/or antifolates) in the surrounding environment. This complexity must be considered when manipulations of the folate pathway are attempted, both experimentally and therapeutically.
General genetic changes that underlie drug resistance in P. falciparum
Single-nucleotide polymorphisms (SNPs) and copy-number variation (CNV) are two types of DNA alterations that can contribute to parasite drug resistance and subsequent treatment failure. Point mutations in enzyme targets and transporter pumps are the most commonly identified genetic alterations correlated with drug failure.15 Consequently, there is extensive documentation of resistance alleles and their associated drug resistance phenotypes as described above for dhps, dhfr and resistance to SP.16,17 In fact, point mutations were thought to be the primary factor responsible for conferring drug resistance in malaria.
Copy-number changes were long overlooked, owing to difficulty in detecting amplification events. However, improved techniques that can accurately assess copy-number changes have brought a greater appreciation to the role that gene amplification plays in altering phenotypes.18 The amplified pfmdr locus on chromosome 5, encoding the P. falciparum multidrug resistance protein, was the first and most intensely studied example of CNV.19–21 Analyses of field isolates21,22 and cultured parasites23 support a link between increased pfmdr copy number and mefloquine resistance. For Pfmdr, there is a complex relationship of CNV and point mutations that contribute to drug resistance in the parasite. CNV has more recently been implicated in altering resistance phenotypes to other antimalarials, such as piperaquine24 and halofantrine,25 as well as changing erythrocyte-invasion properties.26 Hence, CNV is now seen as a major contributor to genetic and phenotypic variation in P. falciparum, and, in particular, appears to be one mechanism that the parasite utilizes to upregulate expression of certain genes, including the first step of the folate-synthesis pathway, GCH1.27
History of antifolate use against malaria and the development of resistance
Resistance to antifolates was first noted in South America and Southeast Asia as early as the 1970s, a short time after they were introduced.15 Now, in both locations, mutations conferring high-level resistance to antifolates are fixed in the parasite population. In Africa, antifolate resistance was noted later: the early 1980s in East Africa and the late 1980s for West Africa. Monitoring of mutations conferring SP resistance is ongoing, and it appears that the degree of SP resistance is increasing in areas of Africa with ongoing use.28
Point mutations that confer resistance to antifolates arise in a stepwise fashion, and accumulation of these point mutations leads to increasing levels of SP resistance in vivo.15 In general, the mutation S108N in dhfr is the first to appear in SP-exposed parasites, and is followed by accumulation of additional mutations in both target enzymes DHFR and DHPS with ongoing drug pressure. In total, there are five well-characterized mutations––two in dhps (A437G, K540E) and three in dhfr (N51I, C59R, S108N).29 These five mutations are now widespread in Africa.28 In areas of previous heavy antifolate use in Southeast Asia and South America, a majority of parasites also harbor a fourth mutation in dhfr (I164L) that confers the highest level of resistance to SP. The presence of this allele in Africa has been debated, but recent reports suggest a gradual increase of high-level dhfr resistant genotypes, including the I164L quadruple-mutant allele.2,30 In this way, the underlying mechanisms of antifolate resistance was thought to be well understood.
Copy-number variation of GTP-cyclohydrolase and drug resistance
CNV of gch1, which encodes the enzyme that catalyzes the first step in the folate-biosynthesis pathway, was documented in several studies using different methods,17,31–33 and was hypothesized to serve a compensatory role for mutations in the SP-targeted downstream enzymes, DHPS and DHFR. Though debated, the mutations in these enzymes that confer resistance to SP are also thought to be deleterious for folate-synthesis reactions. Thus, increased flux through the pathway, starting with increased products from GCH1, would provide more substrate and thus facilitate folate synthesis in this setting.17
The link between gch1 CNV and resistance-associated point mutations in dhfr was first explored in detail by comparing the parasite population genetics of two countries with contrasting histories of antifolate drug pressure.31 In Thailand, where antifolates were used as a first–line treatment from 1970–1980, dhps and dhfr mutations are prevalent, and 72% of Thai parasites were found to carry more than one copy of gch1. In contrast, in Laos, where antifolates were rarely used until 2006, low frequencies of dhps and dhfr mutations were identified, and almost all parasites studied (98%) carried only one copy of gch1. Additionally, parasites harboring the dhfr 164L mutation, which confers a high level of pyrimethamine resistance, had a statistically significantly higher gch1 copy number than parasites carrying the wild-type dhfr 164I allele,31 suggesting that increased gch1 may increase or allow for increased drug resistance.
The above epidemiologic study was subsequently supported by experimental data in which gch1 overexpression in cultured parasites increased resistance to pyrimethamine in parasite genetic backgrounds that contained none or few mutations in dhfr and dhps.34 The observed increase in resistance to pyrimethamine is likely a result of increased flux through the pathway brought about by GCH1 overexpression. Notably, while the increase in resistance was fairly modest and may not lead to clinical failure of pyrimethamine, the increase in flux through the pathway is likely sufficient to facilitate the establishment and fixation of resistant dhfr/dhps alleles within a population of circulating parasites by compensating for the fitness disadvantage conferred by less efficient DHFR and/or DHPS enzymes.
Increased GCH1 was not universally beneficial to parasite survival. While increased gch1 expression enhanced pyrimethamine resistance in some parasite lines, there was a significant decrease in resistance when gch1 was overexpressed at high levels in parasite lines, with a marked decrease in efficiency of downstream enzymes due to a combination of point mutations and drug pressure.34 It is possible that one of the folate intermediates that accumulates owing to slowed downstream reactions is directly toxic to the parasites or inhibits another essential enzyme, as has been described with antifolate-treated bacteria35 Taken together, these experiments point to the fact that parasite adaptations to antifolates should balance between acquiring the necessary folate for survival and minimizing the harmful effects of metabolic build-up.
Evolution of dhfr mutations and gch1 CNV
The various pressures on a parasite population leading to the emergence of drug resistance can be difficult to quantify and assess. Trajectory analysis attempts to do this by combing molecular and phylogenetic data to predict the potential paths to the development of drug resistance. In the case of antifolate resistance, key mutations in the target enzymes, dhps and dhfr, must result in decreased drug affinity while maintaining the enzyme’s primary function in sustaining parasite growth.36,37 Previous trajectory-analysis studies, using data from yeast or bacterial surrogate systems, attempted to measure the balance of these two forces by quantitatively tracking growth in the presence (resistance) or absence (fitness) of drug.
Initial studies mapped potential pathways for the development of the highly resistant quadruple-mutant dhfr.38 The favored trajectory echoed what was seen in field surveys that the S108A mutation occurs first, followed by N51I and/or C59R, with I164L being the last or, in some cases, the penultimate mutation in dhfr.38 These studies also proposed that, if dhfr mutations alone were the sole factor driving the evolution of acquiring a quadruple-mutant DHFR, this allele would only occur at very high drug-selection pressure. There have been conflicting studies regarding the potential cost, as measured by slower growth rate, associated with the specific mutations in dhfr and dhps that confer resistance to SP, but, in general, there appears to be some loss in fitness as parasites acquire increasing numbers of dhfr mutations. However, there does not appear to be a consistent or linear relationship with increasing levels of drug resistance.37
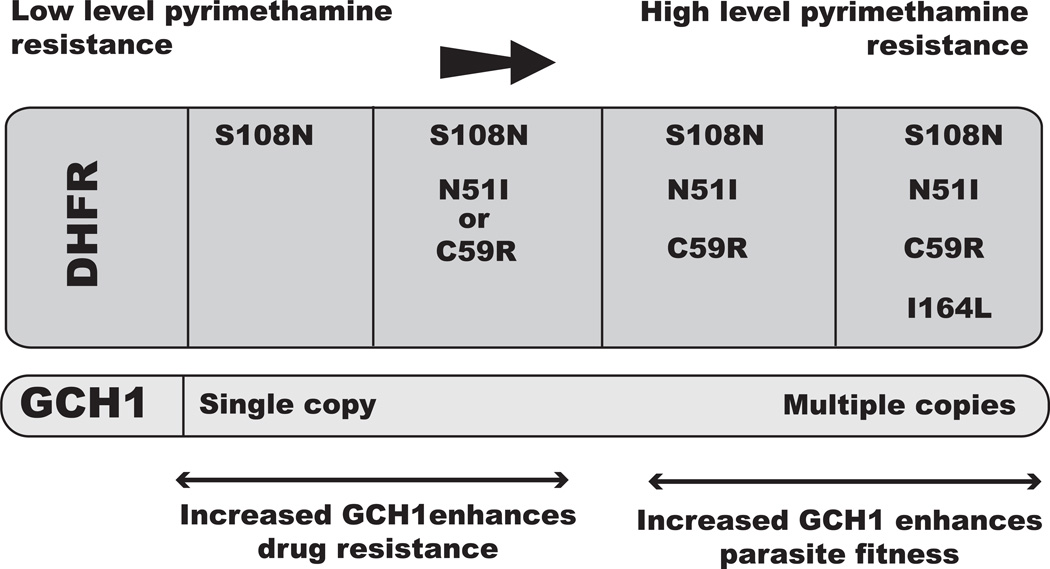
More recent trajectory analysis proposed that increased gch1 copy number facilitates the development of highly resistant parasites and, in fact, plays two important roles in the acquisition of resistance: by increasing resistance and by optimizing parasite fitness. This study utilized a bacterial surrogate system similar to that previously described, in which they expressed P. falciparum dhfr and gch1 to examine the roles that dhfr mutations and increased gch1 have on both parasite fitness and resistance.39 They found that gch1 overexpression increased resistance levels to pyrimethamine early in the dhfr mutational trajectory, when dhfr had no or few mutations. However, later on in the evolutionary trajectory, when more mutations arose in dhfr, gch1 no longer provided increased drug resistance (over that already conferred by the dhfr mutations). Rather, in the parasite harboring a fully resistant dhfr allele, gch1 overexpression instead provided a fitness advantage in the absence of drug pressure. This relationship between point mutations in dhfr and gch1 copy number is summarized in Figure 2.
Figure 2.
The relationship between GCH1 and DHFR in the evolution of antifolate resistance in P. falciparum. Successive point mutations in dhfr are shown to form the single S108N mutation to the completely resistant quadruple-mutant allele with the I164L mutation. Increasing gch1 copy number is seen in field samples of parasites that have the highly resistant quadruple-mutant allele of dhfr. The proposed role of increased GCH1 was derived from studies using manipulated parasite lines in the laboratory and bacterial models expressing Plasmodium dhfr and gch1. At low-level pyrimethamine resistance, increased GCH1 correlates with a shift to a more resistant parasite, and at higher levels of resistance, increased GCH1 correlates with increased fitness of parasites harboring the quadruple-mutant DHFR in the absence of drug pressure. Most studies that have been done focus on DHFR and pyrimethamine, thus DHPS was not included in this figure.
This study concluded that gch1 was co-opted from a role in providing drug resistance to a role in increasing fitness in the evolution of dhfr mutations.39 They propose that increased GCH1 acts as an adaptation to compensate for less-fit DHFR and DHPS enzymes, thus enabling antifolate drug–resistant parasites to compete with wild-type drug-sensitive parasites. While formal growth-rate studies should be done, in the field it has been observed that parasites harboring gch1 amplification and resistant DHFR and DHPS persist in the absence of strong antifolate drug pressure.31 This is contrary to what has been seen with CQ-resistant parasites, where a distinct fitness disadvantage has led to replacement of CQ-resistant parasites by parasites carrying the wild-type Pfcrt allele in parasite populations after drug selection with CQ is removed.40 We propose that other concurrent parasite adaptations to antifolate resistance enable the maintenance of resistant DHFR and DHPS with low drug pressure, and that this should be considered when testing future compounds that target the parasite folate-synthesis pathway.
Targeting the folate pathway
Owing to the previous success of antifolate compounds, there is broad interest in developing new inhibitors to both old and new enzyme targets in the folate pathway. Given the sequence divergence between human and parasite GCH1 and the important role GCH1 plays in regulating flux through the folate pathway, it is worthwhile to consider developing GCH1 inhibitors. However, despite GCH1’s importance in the folate pathway, DHFR may remain a better drug target, owing to its essential role in both de novo and salvage folate pathways. Currently, preclinical trials are being conducted on compound P218, a dhfr inhibitor that targets both the wild-type and quadruple-mutant dhfr.41 It will be important to examine how gch1 CNV and other parasite adaptations that alter flux through the folate pathway affect the development of resistance with new agents such as P218. As SP currently plays an important role in intermittent preventive treatment during pregnancy, it will be important to test whether any new compound is comparable in efficacy and safety to pyrimethamine.
Towards a better understanding of the folate pathway and its inhibitors
The work described here was informed by the decades of research on the folate pathway in Plasmodium, as well as model organisms. We now have an understanding of the genetic basis of resistance, the chemical binding of antifolates to their targets, and transcriptional changes and adaptations following treatment. While changes at the DNA sequence level are readily detectable and often associated with drug resistance, adaptations in metabolic flux can also contribute to the development of resistance but are not assessed by traditional screens. Recently published work traced the parasite’s development of resistance to a t-RNA synthetase inhibitor and identified alterations in amino acid homeostasis that preceded the genetic changes in the cytoplasmic prolyl-tRNA synthetase gene that later developed and conferred resistance to the inhibitor.42 We propose a similar set of events in the development of antifolate resistance.
Looking specifically at the folate pathway, there are a small but growing number of metabolomic studies looking at changes in macromolecules following drug exposure. In Escherichia coli, metabolomic analysis revealed that the dhfr inhibitor trimethoprim not only inhibited DHFR but also decreased enzymatic activity of another folate-related enzyme owing to the accumulation of DHFR’s substrate, dihydrofolate.35 Another metabolomics study in Mycobacterium tuberculosis determined the effect of the antifolate para-aminosalicyclic acid (PAS) on cellular metabolism. PAS was long thought to be an inhibitor of DHPS, but quantitative analysis of flux through the pathway revealed that PAS is actually metabolized by DHPS, and the resulting products poison downstream reactions and lead to growth inhibition.43 Breakthrough discoveries such as these only came about by closely monitoring metabolites, and highlight the importance of including such studies to uncover mechanisms of resistance. Similar follow-up metabolite analyses in P. falciparum are necessary to conclusively show that gch1 overexpression increases flux through the folate pathway. Such metabolite studies are possible but technically challenging owing to the many oxidation states of folate metabolites.35 Metabolomic studies in P. falciparum are becoming more common and sophisticated and it is likely that in the coming years it will be possible to quantitatively look at the effects of inhibition of the folate pathway directly and better understand how gch1 CNV evolved to contributes to antifolate resistance.
Current status and future of antifolate use for malaria
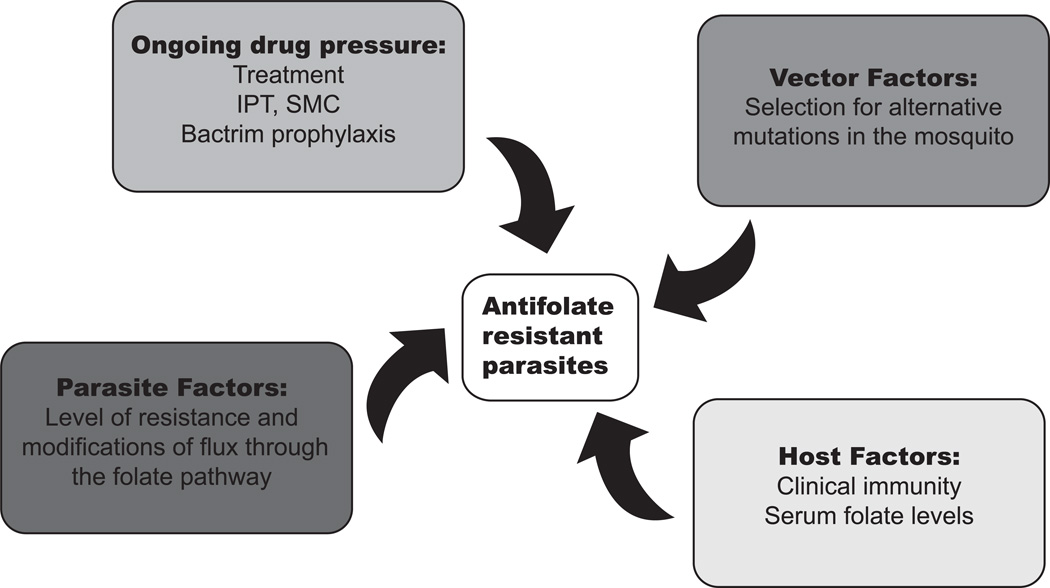
Even as use of SP for malaria treatment and prevention falls dramatically, surveys of circulating parasites in field settings demonstrate the continued presence of resistant haplotypes.2,30,44 A number of factors are likely to play roles in the maintenance of an SP-resistant parasite population, a partial list of which is included in Figure 3. This review focused on parasite factors, though other factors are also important in determining the level of resistance in the field.
Figure 3.
Pressures that contribute to the development and persistence of antifolate-resistant parasites. This figure highlights the complex factors that contribute to maintaining drug-resistant parasites from selection pressures in the mosquito vector to the human host, as well as the influence of varied ongoing drug pressure and potential parasite adaptions. SMC, seasonal malaria chemoprevention; IPT, intermittent preventative therapy.
Parasites experience varied pressures as they progress through their complex lifecycles. Immune pressure from the host is important with all infections and, for all antimalarials, it has long been recognized that host immunity plays an important role in the clinical success of an antimalarial, even in the face of rising drug resistance.45 Host factors specific to the antifolates include host nutritional and folate status, as mentioned above. Ongoing drug pressure is also multi-factorial and varies with region. The World Health Organization (WHO) still recommends preventative treatment of vulnerable populations (children and pregnant women) in areas of high transmission. SP is the mainstay of IPT for pregnant women, targeting the specific malarial syndrome of pregnancy-associated malaria.1 IPT has been shown to decrease maternal anemia and infant mortality, even with rising SP resistance. Similarly, SP is partnered with amiodaquine for SMC, where up to four doses of this drug combination can be given to children during the transmission season.28 The contribution of Bactrim (sulfamethoxazole/trimethoprim) prophylaxis given to those with advanced HIV to antifolate resistance in malaria has been debated, but it is likely to have some effect on circulating parasites in certain areas.46 The impact of the parasite sexual stage taken up by the mosquito and the mosquito vector itself on the maintenance and spread of resistant parasites is just beginning to be investigated. For example, recent studies identified a fitness disadvantage of chloroquine-resistant parasites in the mosquito vector.47 Specific to the antifolates, when mosquitoes collected in an area with known SP resistance were analyzed, the parasites found in the vector carried alternative dhfr mutations.48 The vector stage and the role that vector control plays in controlling the spread of resistant parasites is an interesting area for further investigation.
Key questions remain
We present a more complex series of events in the development of antifolate resistance in the malaria parasite beyond the well-characterized dhps and dhfr point mutations. However, many key questions remain. To date, there has not been a survey of gch1 copy number in parasites of African origin, and this would be informative for understanding the extent and trends in resistance where SP is still used. As techniques are improving for the determination of CNV, this should be feasible from field samples. In addition, the parasite’s ability to maintain a balance of flux through the folate-synthesis pathway should be explored as discussed above. Similar to what is described in drug-tolerant bacteria, modulations of metabolism in response to drug pressure can be important in enabling the development of drug resistance. Once we have a better understanding of folate-pathway homeostasis, identifying and targeting how the parasite adapts to perturbations in the pathway should follow.
Conclusions
Artemisinin has been the cornerstone of effective therapy, and its continued success is in jeopardy, necessitating the development of effective and durable antimalarials. SP had been important in treatment of malaria, but its use was limited to prevention and only in Africa, which had lower levels of pyrimethamine resistance compared to Southeast Asia or South America. The folate-synthesis pathway may remain a viable drug target; however, an appreciation of the adaptations the malaria parasites make to maintain folate homeostasis should be part of the evaluation of any novel antifolate candidate antimalarial.
References
- 1.WHO World Malaria Report
- 2.Mbogo GW, Nankoberanyi S, Tukwasibwe S, Baliraine FN, Nsobya SL, et al. Temporal changes in prevalence of molecular markers mediating antimalarial drug resistance in a high malaria transmission setting in Uganda. Am J Trop Med Hyg. 2014;91:54–61. doi: 10.4269/ajtmh.13-0647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Müller IB, Hyde JE. Folate metabolism in human malaria parasites--75 years on. Mol Biochem Parasitol. 2013;188:63–77. doi: 10.1016/j.molbiopara.2013.02.008. [DOI] [PubMed] [Google Scholar]
- 4.Salcedo-Sora JE, Ward SA. The folate metabolic network of Falciparum malaria. Mol Biochem Parasitol. 2013;188:51–62. doi: 10.1016/j.molbiopara.2013.02.003. [DOI] [PubMed] [Google Scholar]
- 5.Hyde JE. Exploring the folate pathway in Plasmodium falciparum. Acta Trop. 2005;94:191–206. doi: 10.1016/j.actatropica.2005.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hossain T, Rosenberg I, Selhub J, Kishore G, Beachy R, et al. Enhancement of folates in plants through metabolic engineering. Proc Natl Acad Sci U S A. 2004;101:5158–5163. doi: 10.1073/pnas.0401342101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nzila A, Ward SA, Marsh K, Sims PF, Hyde JE. Comparative folate metabolism in humans and malaria parasites (part I): pointers for malaria treatment from cancer chemotherapy. Trends Parasitol. 2005;21:292–298. doi: 10.1016/j.pt.2005.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nzila AM, Mberu EK, Sulo J, Dayo H, Winstanley PA, et al. Towards an understanding of the mechanism of pyrimethamine-sulfadoxine resistance in Plasmodium falciparum: genotyping of dihydrofolate reductase and dihydropteroate synthase of Kenyan parasites. Antimicrob Agents Chemother. 2000;44:991–996. doi: 10.1128/aac.44.4.991-996.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wang P, Wang Q, Aspinall TV, Sims PF, Hyde JE. Transfection studies to explore essential folate metabolism and antifolate drug synergy in the human malaria parasite Plasmodium falciparum. Mol Microbiol. 2004;51:1425–1438. doi: 10.1111/j.1365-2958.2003.03915.x. [DOI] [PubMed] [Google Scholar]
- 10.Zhang K, Rathod PK. Divergent regulation of dihydrofolate reductase between malaria parasite and human host. Science. 2002;296:545–547. doi: 10.1126/science.1068274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Belz S, Nau H. Determination of folate patterns in mouse plasma, erythrocytes, and embryos by HPLC coupled with a microbiological assay. Anal Biochem. 1998;265:157–166. doi: 10.1006/abio.1998.2865. [DOI] [PubMed] [Google Scholar]
- 12.Salcedo-Sora JE, Ochong E, Beveridge S, Johnson D, Nzila A, et al. The molecular basis of folate salvage in Plasmodium falciparum: characterization of two folate transporters. J Biol Chem. 2011;286:44659–44668. doi: 10.1074/jbc.M111.286054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Nduati E, Diriye A, Ommeh S, Mwai L, Kiara S, et al. Effect of folate derivatives on the activity of antifolate drugs used against malaria and cancer. Parasitol Res. 2008;102:1227–1234. doi: 10.1007/s00436-008-0897-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Carter JY, Loolpapit MP, Lema OE, Tome JL, Nagelkerke NJ, et al. Reduction of the efficacy of antifolate antimalarial therapy by folic acid supplementation. Am J Trop Med Hyg. 2005;73:166–170. [PubMed] [Google Scholar]
- 15.Le Bras J, Durand R. The mechanisms of resistance to antimalarial drugs in Plasmodium falciparum. Fundam Clin Pharmacol. 2003;17:147–153. doi: 10.1046/j.1472-8206.2003.00164.x. [DOI] [PubMed] [Google Scholar]
- 16.Bacon DJ, Tang D, Salas C, Roncal N, Lucas C, et al. Effects of point mutations in Plasmodium falciparum dihydrofolate reductase and dihydropterate synthase genes on clinical outcomes and in vitro susceptibility to sulfadoxine and pyrimethamine. PLoS One. 2009;4:e6762. doi: 10.1371/journal.pone.0006762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kidgell C, Volkman SK, Daily J, Borevitz JO, Plouffe D, et al. A systematic map of genetic variation in Plasmodium falciparum. PLoS Pathog. 2006;2:e57. doi: 10.1371/journal.ppat.0020057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Anderson TJ, Patel J, Ferdig MT. Gene copy number and malaria biology. Trends Parasitol. 2009;25:336–343. doi: 10.1016/j.pt.2009.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Duraisingh MT, Cowman AF. Contribution of the pfmdr1 gene to antimalarial drug-resistance. Acta Trop. 2005;94:181–190. doi: 10.1016/j.actatropica.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Myrick A, Munasinghe A, Patankar S, Wirth DF. Mapping of the Plasmodium falciparum multidrug resistance gene 5'-upstream region, and evidence of induction of transcript levels by antimalarial drugs in chloroquine sensitive parasites. Mol Microbiol. 2003;49:671–683. doi: 10.1046/j.1365-2958.2003.03597.x. [DOI] [PubMed] [Google Scholar]
- 21.Wilson CM, Serrano AE, Wasley A, Bogenschutz MP, Shankar AH, et al. Amplification of a gene related to mammalian mdr genes in drug-resistant Plasmodium falciparum. Science. 1989;244:1184–1186. doi: 10.1126/science.2658061. [DOI] [PubMed] [Google Scholar]
- 22.Price RN, Uhlemann AC, Brockman A, McGready R, Ashley E, et al. Mefloquine resistance in Plasmodium falciparum and increased pfmdr1 gene copy number. Lancet. 2004;364:438–447. doi: 10.1016/S0140-6736(04)16767-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Preechapornkul P, Imwong M, Chotivanich K, Pongtavornpinyo W, Dondorp AM, et al. Plasmodium falciparum pfmdr1 amplification, mefloquine resistance, and parasite fitness. Antimicrob Agents Chemother. 2009;53:1509–1515. doi: 10.1128/AAC.00241-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Eastman RT, Dharia NV, Winzeler EA, Fidock DA. Piperaquine resistance is associated with a copy number variation on chromosome 5 in drug-pressured Plasmodium falciparum parasites. Antimicrob Agents Chemother. 2011;55:3908–3916. doi: 10.1128/AAC.01793-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Van Tyne D, Park DJ, Schaffner SF, Neafsey DE, Angelino E, et al. Identification and functional validation of the novel antimalarial resistance locus PF10_0355 in Plasmodium falciparum. PLoS Genet. 2011;7:e1001383. doi: 10.1371/journal.pgen.1001383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Triglia T, Duraisingh MT, Good RT, Cowman AF. Reticulocyte-binding protein homologue 1 is required for sialic acid-dependent invasion into human erythrocytes by Plasmodium falciparum. Mol Microbiol. 2005;55:162–174. doi: 10.1111/j.1365-2958.2004.04388.x. [DOI] [PubMed] [Google Scholar]
- 27.Estivill X, Armengol L. Copy number variants and common disorders: filling the gaps and exploring complexity in genome-wide association studies. PLoS Genet. 2007;3:1787–1799. doi: 10.1371/journal.pgen.0030190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Venkatesan M, Alifrangis M, Roper C, Plowe CV. Monitoring antifolate resistance in intermittent preventive therapy for malaria. Trends Parasitol. 2013;29:497–504. doi: 10.1016/j.pt.2013.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sridaran S, McClintock SK, Syphard LM, Herman KM, Barnwell JW, et al. Anti-folate drug resistance in Africa: meta-analysis of reported dihydrofolate reductase (dhfr) and dihydropteroate synthase (dhps) mutant genotype frequencies in African Plasmodium falciparum parasite populations. Malar J. 2010;9:247. doi: 10.1186/1475-2875-9-247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gesase S, Gosling RD, Hashim R, Ord R, Naidoo I, et al. High resistance of Plasmodium falciparum to sulphadoxine/pyrimethamine in northern Tanzania and the emergence of dhps resistance mutation at Codon 581. PLoS One. 2009;4:e4569. doi: 10.1371/journal.pone.0004569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Nair S, Miller B, Barends M, Jaidee A, Patel J, et al. Adaptive copy number evolution in malaria parasites. PLoS Genet. 2008;4:e1000243. doi: 10.1371/journal.pgen.1000243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jiang H, Yi M, Mu J, Zhang L, Ivens A, et al. Detection of genome-wide polymorphisms in the AT-rich Plasmodium falciparum genome using a high-density microarray. BMC Genomics. 2008;9:398. doi: 10.1186/1471-2164-9-398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Ribacke U, Mok BW, Wirta V, Normark J, Lundeberg J, et al. Genome wide gene amplifications and deletions in Plasmodium falciparum. Mol Biochem Parasitol. 2007;155:33–44. doi: 10.1016/j.molbiopara.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 34.Heinberg A, Siu E, Stern C, Lawrence EA, Ferdig MT, et al. Direct evidence for the adaptive role of copy number variation on antifolate susceptibility in Plasmodium falciparum. Mol Microbiol. 2013;88:702–712. doi: 10.1111/mmi.12162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Kwon YK, Lu W, Melamud E, Khanam N, Bognar A, et al. A domino effect in antifolate drug action in Escherichia coli. Nat Chem Biol. 2008;4:602–608. doi: 10.1038/nchembio.108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brown KM, Costanzo MS, Xu W, Roy S, Lozovsky ER, et al. Compensatory mutations restore fitness during the evolution of dihydrofolate reductase. Mol Biol Evol. 2010;27:2682–2690. doi: 10.1093/molbev/msq160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Costanzo MS, Brown KM, Hartl DL. Fitness trade-offs in the evolution of dihydrofolate reductase and drug resistance in Plasmodium falciparum. PLoS One. 2011;6:e19636. doi: 10.1371/journal.pone.0019636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lozovsky ER, Chookajorn T, Brown KM, Imwong M, Shaw PJ, et al. Stepwise acquisition of pyrimethamine resistance in the malaria parasite. Proc Natl Acad Sci U S A. 2009;106:12025–12030. doi: 10.1073/pnas.0905922106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kümpornsin K, Modchang C, Heinberg A, Ekland EH, Jirawatcharadech P, et al. Origin of Robustness in Generating Drug-Resistant Malaria Parasites. Mol Biol Evol. 2014 doi: 10.1093/molbev/msu140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Laufer MK, Thesing PC, Eddington ND, Masonga R, Dzinjalamala FK, et al. Return of chloroquine antimalarial efficacy in Malawi. N Engl J Med. 2006;355:1959–1966. doi: 10.1056/NEJMoa062032. [DOI] [PubMed] [Google Scholar]
- 41.Yuthavong Y, Tarnchompoo B, Vilaivan T, Chitnumsub P, Kamchonwongpaisan S, et al. Malarial dihydrofolate reductase as a paradigm for drug development against a resistance-compromised target. Proc Natl Acad Sci U S A. 2012;109:16823–16828. doi: 10.1073/pnas.1204556109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Herman JD, Rice DP, Ribacke U, Silterra J, Deik AA, et al. A genomic and evolutionary approach reveals non-genetic drug resistance in malaria. Genome Biol. 2014;15:511. doi: 10.1186/s13059-014-0511-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chakraborty S, Gruber T, Barry CE, Boshoff HI, Rhee KY. Para-aminosalicylic acid acts as an alternative substrate of folate metabolism in Mycobacterium tuberculosis. Science. 2013;339:88–91. doi: 10.1126/science.1228980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Vembar SS, Scherf A, Siegel TN. Noncoding RNAs as emerging regulators of Plasmodium falciparum virulence gene expression. Curr Opin Microbiol. 2014;20C:153–161. doi: 10.1016/j.mib.2014.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wellems TE, Plowe CV. Chloroquine-resistant malaria. J Infect Dis. 2001;184:770–776. doi: 10.1086/322858. [DOI] [PubMed] [Google Scholar]
- 46.Bwakura-Dangarembizi M, Kendall L, Bakeera-Kitaka S, Nahirya-Ntege P, Keishanyu R, et al. A Randomized Trial of Prolonged Co-trimoxazole in HIV-Infected Children in Africa. New England Journal of Medicine. 2014;370:41–53. doi: 10.1056/NEJMoa1214901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mharakurwa S, Sialumano M, Liu K, Scott A, Thuma P. Selection for chloroquine-sensitive Plasmodium falciparum by wild Anopheles arabiensis in Southern Zambia. Malar J. 2013;12:453. doi: 10.1186/1475-2875-12-453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Mharakurwa S, Kumwenda T, Mkulama MA, Musapa M, Chishimba S, et al. Malaria antifolate resistance with contrasting Plasmodium falciparum dihydrofolate reductase (DHFR) polymorphisms in humans and Anopheles mosquitoes. Proc Natl Acad Sci U S A. 2011;108:18796–18801. doi: 10.1073/pnas.1116162108. [DOI] [PMC free article] [PubMed] [Google Scholar]