Abstract
Seasonal reproduction is a common adaptive strategy among mammals that allows for breeding to occur at times of the year when it is most advantageous for the subsequent survival and growth of offspring. A major mechanism responsible for seasonal reproduction is a striking increase in the responsiveness of gonadotropin-releasing hormone (GnRH) neurons to the negative feedback effects of estradiol. The neural and neuroendocrine circuitry responsible for mammalian seasonal reproduction has been primarily studied in three animal models: the sheep, and two species of hamsters. In this review, we first describe the afferent signals, neural circuitry and transmitters/peptides responsible for seasonal reproductive transitions in sheep, and then compare these mechanisms with those derived from studies in hamsters. The results suggest common principles as well as differences in the role of specific brain nuclei and neuropeptides, including that of kisspeptin cells of the hypothalamic arcuate nucleus, in regulating seasonal reproduction among mammals.
Keywords: GnRH, neuroendocrine, seasonality, photoperiod, kisspeptin, dopamine, thyroid hormone
1. Introduction
Seasonal reproduction has been described as “nature’s contraceptive”, a naturally occurring mechanism to induce a reversible pattern of fertility and infertility in adult mammals (1). The ability of the adult reproductive system to be turned on and off has a distinct selective advantage, by helping to predict a time of year for breeding that ensures young are born when environmental conditions favor the energetic demands of lactation and survival of offspring (2–4). Although annual variations in reproductive function are common among animals living in regions with harsh winter seasons, the fundamental neuroendocrine changes responsible have been studied in only three species of mammals: sheep (Ovis aries), Syrian (or Golden) hamsters (Mesocricetus auratus), and Siberian (or Djungarian) hamsters (Phodopus sungorus). One major advantage of sheep is that hypophysial portal blood samples can be collected from unanesthetized animals so that secretion of GnRH can be directly assessed during different seasons. Moreover because of their large size, sheep have been an ideal model to study changes in episodic LH responsible for seasonal breeding and to map the underlying alterations in the neural circuitry in the hypothalamus controlling GnRH secretion.
Sheep show distinct annual patterns of reproductive activity: in females, these are comprised of periods of ovarian activity and ovulation, termed the breeding season, which alternate with periods of ovarian quiescence and anovulation, referred to as anestrus (2). These seasonal variations in reproductive activity at an ovarian level are the direct result of changes in the brain, and result from two complimentary alterations in the control of hypothalamic function, specifically in the regulation of gonadotropin-releasing hormone (GnRH) secretion. The first is a dramatic increase in the responsiveness to the negative-feedback actions of estradiol (E2) that peaks during anestrus, diminishing as the breeding season begins (6,7). The second is a steroid-independent inhibition of GnRH that is seen in ovariectomized (OVX) animals (3). However, of the two, the increase in E2 negative feedback is accepted as the main mechanism responsible for seasonal reproduction and, thus, has been a key point of interest, leading to development and implementation of the ovine model.
In this review, we will focus primarily on the seasonal regulation of reproduction in sheep, the afferent pathways by which the external environment times seasonal reproductive transitions, and the neural circuitry ultimately responsible for changes in the negative feedback responsiveness in GnRH secretion. In addition, we will compare the basic principles responsible for neuroendocrine control of seasonal reproduction in sheep with recent findings from Syrian and Siberian hamsters. This comparison reveals both similarities and differences in the functional circuitry mediating mammalian seasonal reproduction as well as in the roles of specific neurotransmitters and neuropeptides.
Finally, it should be noted that in addition to sheep and hamsters, studies in birds, such as the Japanese quail, have been extremely valuable in elucidating the mechanisms underlying seasonal reproduction, some of which differ fundamentally from those uncovered in mammals. The present review focuses specifically on mammalian models of seasonal reproduction, while other papers in this Special Issue will cover avian models.
2. Photoperiodic and circannual control of reproduction in sheep
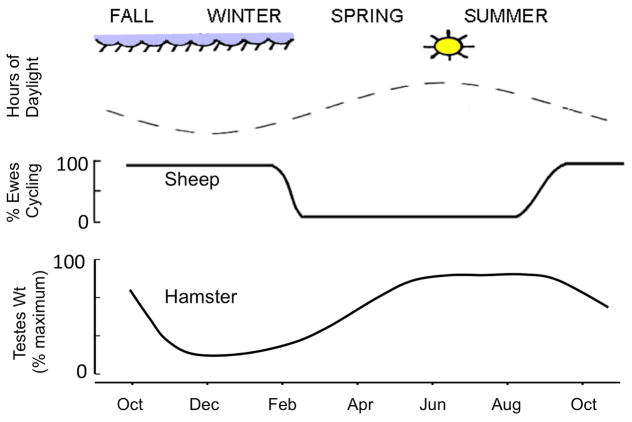
In seasonal mammals, a key environmental signal responsible for the timing of reproductive transitions is daylength or photoperiod (1). In female sheep, the short daylengths of fall and winter are stimulatory to the reproductive neuroendocrine axis leading to ovulatory cycles and fertility; by contrast, the long daylengths of spring and summer, are inhibitory to the reproductive axis in female sheep and produce an anovulatory state (9,10). Photoperiodic information is conveyed to the reproductive neuroendocrine system by way of a neurohormonal pathway that starts with retinal projections to the suprachiasmatic nucleus (SCN), and thence by way of a multi-synaptic circuit that includes neurons in the paraventricular nucleus, intermediolateral spinal cord and superior cervical ganglion, terminating in the innervation of the pineal gland. Seminal studies in sheep and other mammals in the 1980s showed the prominent role of pineal melatonin as an essential signal conveying photoperiodic information to the hypothalamus and rest of the brain (3). Melatonin is normally synthesized and secreted by the pineal gland in a diurnal fashion, with a dramatic increase occurring during the nighttime that returns to near undetectable levels in the morning (3). In sheep, the duration of the nighttime elevation in melatonin serves as an internal code of the external photoperiod, with long duration melatonin coding for short (stimulatory) daylengths, and short duration melatonin coding long (inhibitory) daylengths (4). In species that are “long day” breeders such as hamsters (see Section 4), the roles of these long and short duration melatonin signals are reversed (Fig. 1), with long duration (short day) signals being inhibitory to the HPG axis and short duration (long day) signals being stimulatory (5).
Figure 1.
Comparison of seasonal reproductive patterns in sheep and hamsters. Changes in fertility are controlled by variations in the duration of daylight throughout the year (top panel), but photoperiod has different effects in these species. Short days stimulate reproductive function in sheep, so all ewes have ovulatory cycles during the fall and winter, but become anovulatory in the spring and summer (middle panel). In contrast, reproductive activity in hamsters (illustrated as changes in size of the testes in the bottom panel) is stimulated by long days; thus hamsters are fertile in the spring and summer and reproductive inactive in the fall and winter.
In sheep, melatonin does not directly drive seasonal changes in reproduction but rather appears to synchronize an endogenous circannual rhythm (12–14). This was first demonstrated in long-term studies of pinealectomized female sheep, which show free-running circannual rhythms of reproductive activity and inactivity (6), that can be synchronized by once-yearly, 90-day blocks of melatonin infusions (7). Thus synchronization of the circannual rhythm by melatonin is analogous to the ability of light to entrain circadian rhythms in the SCN and other tissues (8). Moreover, like the entrainment of circadian rhythms, melatonin appears to be most effective during a specific phase of the circannual rhythm: from the summer solstice to the fall equinox (7).
While the physiological actions of melatonin are now well described in sheep, the sites of action of this indolamine remain controversial. The highest concentration of melatonin receptors in sheep, as in other species (9, 10), is in the pars tuberalis (PT) (11), but specific binding sites are also evident in the premammillary region (PMR) of the hypothalamus (11, 12). Early studies using local administration of melatonin via microimplants provided strong evidence that the PMR (12), not the PT (13), was an important site at which melatonin acted to advance the onset of the breeding season. However, the results of more recent studies have pointed to the PT as a critical site of action, at least for the transition to anestrus.
Recent work on the PT grew out of earlier reports demonstrating that thyroid hormones (T3 and T4) are critical for the transition into anestrus – thyroidectomized sheep remain in a persistent breeding season state (14) and T4 replacement is able to restore seasonal reproductive transitions (15). Interestingly, thyroid hormones are required for the transition from the breeding season to anestrous, but not for the reverse, that is the subsequent transition from the breeding season back to anestrous. These early studies also concluded that circulating T4 is likely a permissive signal since constant low levels are able to reinstate reproductive transitions in sheep (15). However, more recent work has provided strong evidence for the hypothesis that photoperiod-controlled changes in the local conversion of T4 to T3 in the brain are important for the effects of long days (16). Moreover, these changes appear to be due to actions of melatonin in the PT (16, 17), although tests of this hypothesis using melatonin administration have yet to be done in sheep.
According to this hypothesis, which is discussed in detail elsewhere (9, 18), a long-day melatonin pattern increases expression of the transcription factor, eyes absent 3 (Eya3), in the PT (19, 20). Eya3 is a circadian-controlled gene with a peak in expression occurring about 12 hrs after melatonin secretion increases with darkness. The amplitude of this peak increases if melatonin concentrations are low (i.e., as is the case with a long-day melatonin pattern), which stimulates expression of TSHβ (19). TSH then diffuses into nearby neural tissue which contains the TSH receptor (16), stimulates the expression of type 2 deiodinase (Dio2) (16), the enzyme that converts T4 to T3, and T3 then acts to induce neural changes that shut down episodic GnRH secretion. Long-day photoperiod has the opposite effect on type 3 diodinase (Dio3), although this action may not be mediated by TSH (17). Since Dio3 inactivates T4, a decrease in its activity would complement the increase in Dio2. While this is an attractive hypothesis for which there is now significant support, key questions remain, such as whether it applies to the transition to the breeding season and where and how T3 acts to produce its effects.
It is interesting to note that because T3 plays a major role in neuronal plasticity, including effects on neurogenesis (21), one possible mechanism for its action to cause the seasonal increase in responsiveness to E2 negative feedback during anestrus (see below) involves morphological rearrangements in the neural circuitry responsible for conveying this influence onto GnRH neurons. Indeed, seasonal plasticity and synaptic rearrangements have been described at several levels within the neuroendocrine pathways mediating E2 negative feedback in anestrous (22). Examples of this are discussed in more detail in the next section.
3. Neural pathways responsible for seasonal breeding in sheep
There is now a great deal of information on the neural circuitry within the hypothalamus of female sheep responsible for E2-negative feedback during anestrus. As noted in the introduction, seasonal reproductive transitions in the female sheep are a direct result of striking changes in the ability of E2 to inhibit GnRH; specifically, during anestrus, E2 gains the ability to dramatically suppress the frequency of GnRH and LH pulses (2, 28, 29). Because of this increased responsiveness to E2 inhibition, even the low levels of E2 present during anestrus strongly inhibit GnRH activity, preventing the rise in LH necessary for the late follicular-phase peak in E2 that induces the GnRH/LH surge that triggers ovulation (23). Even though ovulation is blocked during anestrus due to increased E2 negative feedback, the surge mechanism is still intact, and exogenous E2 can induce a positive feedback response and produce a GnRH/LH surge (2). Thus E2 negative, but not positive, feedback is under seasonal control, and an initial approach to uncovering the neuroendocrine changes responsible for seasonal breeding in sheep has focused on understanding how this negative feedback influence is conveyed to GnRH neurons.
Because GnRH neurons lack the alpha isoform of the estrogen receptor (24), the isoform responsible for negative feedback influence of E2 on GnRH secretion (25), much work has focused on identifying the afferent circuitry by which E2 exerts its inhibitory effects on GnRH/LH pulses during anestrus. Early pharmacological studies found that dopamine (DA) D2 receptor antagonists, administered either peripherally or intracerebrally, block the ability of E2 to inhibit GnRH/LH pulses during anestrus in female sheep (26). This implication of the involvement of DA in seasonal breeding, led to the discovery of a key set of dopaminergic neurons within the retrochiasmatic area (RCh), known as the A15 group, that are now believed to play a critical role in seasonal regulation of GnRH secretion (32–37). Several lines of evidence have since supported this hypothesis. First, lesions that include A15 neurons block E2-negative feedback during anestrus in ewes (27); while direct stimulation of A15 neurons inhibits GnRH/LH pulse frequency regardless of season (28). Second, multi-unit activity of the A15 group is decreased prior to GnRH/LH pulse production (28); and increased in the presence of E2 during anestrus (29) when pulses are inhibited. Finally, neural activity studies have shown that E2 increases tyrosine hydroxylase (TH), the rate-limiting enzyme for DA synthesis, (30) and Fos expression (31) in A15 neurons during anestrus but, importantly, not during the breeding season.
However, like GnRH neurons, A15 DA cells do not express ER-alpha; thus, the regulation of A15 DA cells by E2 must be indirect, meaning that estrogen-responsive afferents must be a key part of this circuitry. Studies using local administration of E2 have provided strong evidence identifying two primary areas of estrogen-responsive cells that are activated during estradiol negative feedback, specifically during anestrus: the ventromedial preoptic area (vmPOA) and the retrochiasmatic area (RCh) (31, 39–41). ER-alpha positive cells in both regions provide input to the A15 region (23) and vmPOA and RCh fibers directly contact TH-positive dendrites and somas of the A15 (23). Pharmacological and anatomical studies suggest that this input may be either glutamatergic and/or GABAergic, and E2 stimulates glutamate and inhibits GABA release in the A15, respectively (32). Moreover, there are seasonal changes in the number of glutamatergic, but not GABAergic, input to A15; A15 dopaminergic somas and dendrites receive more glutamatergic inputs during anestrus than the breeding season (32). This difference complements the increased number of total synaptic inputs onto A15 dendrites seen during anestrus (33), as well as an increase in A15 dendritic length and bifurcations (33). Thus, seasonal plasticity in the number of glutamatergic inputs to A15 may account in part for the increased activity of A15 neurons, and the inhibition of GnRH pulse frequency, during anestrus.
The next logical question is what are the projections and pathways by which A15 neurons inhibit GnRH pulse frequency in anestrus? In sheep, a majority of GnRH cell bodies are located in POA, with smaller populations of GnRH neurons being found both in the anterior hypothalamic area (AHA) and the mediobasal hypothalamus (MBH). However, tract-tracing studies have confirmed that the primary projections of A15 neurons are caudally toward the MBH, not rostrally toward the POA or AHA (23). Moreover, dopaminergic terminals have been shown to be in direct axo-axonic contact with GnRH terminals in the sheep median eminence (ME) during breeding season (34). Likewise, retrograde tracers injected into the ME labeled cells in the anterior hypothalamic area in the region of the A15 (35). These findings suggest that DA released by A15 neurons may directly inhibit GnRH pulses by acting at the level of GnRH axons within the median eminence; however, although D2 dopamine receptors are present in ovine GnRH neurons (36), their trafficking and presence in GnRH axons and terminals within the median eminence is unclear.
Although some GnRH cells may receive direct A15 contacts, recent evidence suggest that A15-mediated regulation of GnRH secretion may occur via an alternative, multi-synaptic pathway with the arcuate nucleus (ARC) at its center. A subpopulation of neurons expressing kisspeptin located in the ARC is an ideal candidate for mediating DA actions on GnRH secretion. Kisspeptin is a key stimulator of GnRH secretion and like A15 cells, has been implicated in seasonal breeding in sheep (37) as well as other species (see below). ARC Kiss cells are unique in that they co-express two other peptides that have also been linked to GnRH regulation, neurokinin B (NKB) and dynorphin (38). The aptly named KNDy cells have become a center of focus for the neuroendocrine regulation of reproduction (39).
Several studies have supported the hypothesis that ARC KNDy neurons are critical to seasonal breeding. The number of kisspeptin peptide (Fig. 2A) and mRNA-containing cells decreases in the ARC during anestrus, largely due to a stronger inhibition by E2 (37). Furthermore there are parallel decreases in the number of kisspeptin-positive inputs to GnRH cells within the MBH in anestrus ewes compared to breeding season (Fig. 2B) (37). This decrease in stimulatory inputs to GnRH neurons may allow for increased inhibition of GnRH pulses by other inputs. In addition, recent studies have found that: i) KNDy cells, using dynorphin as a marker, co-express the D2 dopamine receptor (D2R), the subtype responsible for the seasonal inhibition of GnRH pulses (26) (40); ii) that this co-expression changes with season, with about 80% of KNDy cells colocalizing D2R in anestrus compared to 40% in the breeding season and iii) that intracerebroventricular infusion of a kisspeptin antagonist completely blocks the ability of D2R antagonists to stimulate GnRH pulse frequency during anestrous (36). Thus, it can be inferred that A15 dopamine neurons projecting into the ARC act to inhibit kisspeptin neurons resulting in the inhibition of GnRH secretion during anestrus.
Figure 2.
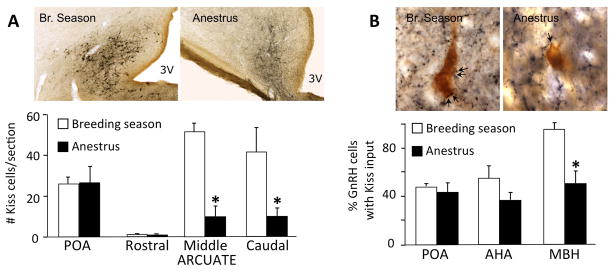
Seasonal changes in number of ARC kisspeptin cells (A) and their inputs to GnRH neurons (B). A: The number of kisspeptin-immunoreactive (Kiss) neurons in the middle and caudal ARC is decreased during anestrous (* p<.001). Images show examples of sections through the caudal ARC immunostained for kisspeptin in ewes perfused during either the breeding season or anestrus. Ewes were ovariectomized and implanted with E2 (OVX+E) to control for seasonal variation in endogenous steroid levels. Note that the number of kisspeptin cells in the POA, another major kisspeptin population (76), does not change seasonally. Bar = 100 μm. B: The percentage of GnRH cells in the MBH that receive one or more kisspeptin (Kiss) close contacts was less in anestrous than in breeding season ewes (* p<.001); GnRH cells in the POA or anterior hypothalamic area (AHA) showed no significant seasonal differences. In addition, GnRH cells in the MBH and AHA had significantly fewer Kiss contacts per cell in anestrous than in the breeding season (data not shown). Images show examples of dual immunoperoxidase stained sections in which close contacts (e.g., arrows) are seen between kisspeptin terminals (blue-black) and GnRH somas (brown) in the MBH. Bar = 15 μm. (A and B modified from ref. 48)
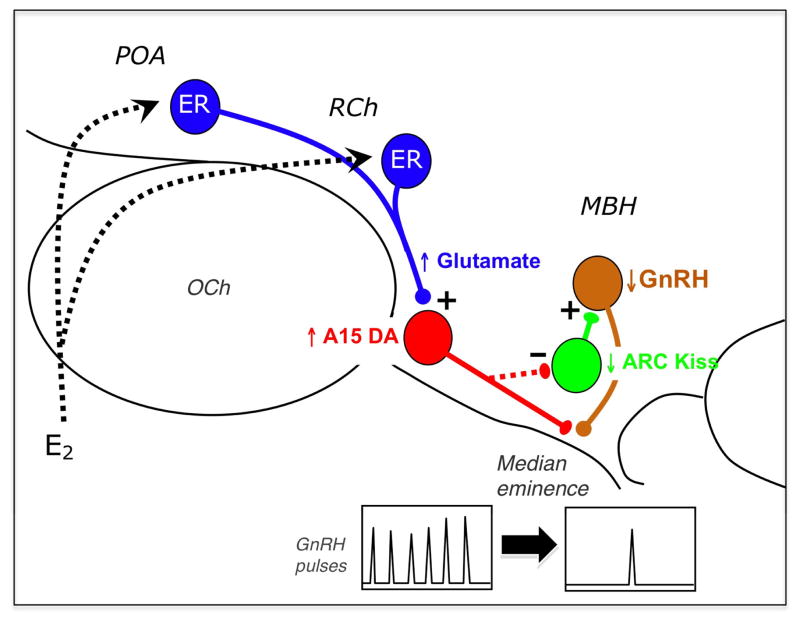
Taken together, we propose the following model (Fig. 3) for the neural circuitry responsible for E2 negative feedback in anestrous ewes. During anestrous, E2 acts upon ER-alpha (ER) containing cells in the vmPOA and RCh. These neurons, in turn, simulate A15 dopamine cells using glutamate as a transmitter. Activated A15 cells project caudally to the mediobasal hypothalamus (MBH), where dopamine is released. Dopamine acts upon GnRH neurons either directly, via axo-axonic contacts in the median eminence, and/or indirectly, via kisspeptin cells in the arcuate nucleus, to inhibit GnRH pulse frequency.
Figure 3.
Schematic depiction of the neural circuitry in the hypothalamus regulating seasonal control of estradiol (E2) negative feedback. E2 acts upon ER-alpha containing cells (blue) in the ventromedial preoptic area (POA) and retrochiasmatic area (RCh); these neurons, in turn, stimulate A15 dopamine cells (red) via glutamatergic inputs. A15 dopamine cells project caudally to the mediobasal hypothalamus (MBH) and inhibit GnRH cells (brown) either directly at the level of their terminals in the median eminence, and/or indirectly via kisspeptin (Kiss) cells (green) in the arcuate nucleus. Seasonal plasticity has been demonstrated at several sites in this circuitry including that of glutamatergic contacts onto A15 neurons (42); the dendritic morphology of dopaminergic A15 neurons (43); and the number of kisspeptin inputs to GnRH neurons in the MBH (48). Such morphological changes may result in a functional “reconnection” of this circuitry during anestrus, and underlie the ability of E2 to inhibit GnRH pulse frequency at this time of year. OCh = optic chiasm. (Modified from ref. 27).
Although there is good evidence that ARC KNDy neurons are a key part of this circuitry in the anestrous ewe, several questions remain. The decrease in kisspeptin within the KNDy population during anestrous may be due to increased DA release from A15 terminals; however the possibility that this effect may simply be due to decreased expression of D2R, and/or changes in other inputs, has yet to be determined. In our original study, dynorphin was chosen as the KNDy cell marker because, unlike kisspeptin, its expression is believed to remain constant between seasons (36); circumstantial evidence suggests that NKB may also be under seasonal regulation (41) but this needs to be directly tested. Furthermore, despite their expression of D2R, it remains to be demonstrated whether ARC KNDy neurons receive direct contact from A15 neurons, or if there is seasonal plasticity in this portion of the circuitry.
Although the diagram presented in Fig. 3 summarizes the pathways by which E2 ultimately inhibits GnRH and LH pulse frequency during anestrous, it does not address the question of what changes in this pathway might be responsible for the increased responsiveness to E2 seen during anestrus. As noted above, seasonal neuroplasticity has been observed at multiple levels in this circuitry (22). These include increases during anestrus in the total number of synapses (33), as well as specific glutamatergic inputs (32), onto A15 dopamine neurons; increased dendritic length of dopamine A15 neurons (33); increases in the number of kisspeptin inputs onto GnRH neurons in the MBH (37) as well as earlier EM observations of decreased synaptic inputs onto GnRH neurons in the POA (42). Neural plasticity at the level of both ARC and GnRH neurons has long been associated with reproductive function, both development (43) and adulthood (54, 55), consistent with the possibility that synaptic and dendritic plasticity in this circuit may also play an important role in seasonal reproduction.
4. Comparison with neural mechanisms controlling seasonality in hamsters
In addition to sheep, the neuroendocrine mechanisms controlling seasonal breeding have been extensively studies in two other mammals: Syrian (or Golden) and Siberian (or Djungarian) hamsters. It is thus of interest to compare neuroendocrine control of seasonality among these three species. In many respects they are very similar (3, 5, 18): 1) they all use photoperiod as the primary environmental cue to time reproduction, 2) the duration of melatonin secretion from the pineal gland is the key endocrine signal linking external photoperiod to the HPG axis, 3) the neural pathway by which photoperiod controls melatonin secretion appears to be the same, 4) both steroid-dependent and steroid-independent inhibition of gonadotropin secretion can be demonstrated in all three species (3, 44–46), and 5) earlier data pointed to intrahypothalamic sites of action for melatonin in hamsters (47–51), while more recent data supports the hypothesis that melatonin acts to stimulate conversion of T4 to T3 in the hypothalamus via TSH production in the PT at the transition from short to long photoperiod (18, 52–54).
Although this broad perspective identified many similarities among these three mammals, a more detailed examination reveals some minor variations. First, steroid-independent inhibition of gonadotropin secretion plays a much more important role in seasonal breeding in hamsters than in ewes (3, 45, 55), a difference that correlates with the much greater suppression of gonadal function by inhibitory photoperiod in hamsters (55). Second, the intrahypothalamic sites of action of melatonin varies among these three; as noted above melatonin appears to act in the PMR in ewes, while sites of melatonin binding (10) and results of studies using local administration of melatonin or lesions indicate that this indoleamine acts in the dorsomedial hypothalamus (DMH) and SCN in Syrian (47, 48) and Siberian (49–51) hamsters, respectively. Finally, while changes in both Dio2 and Dio3 have been implicated in the response to long days in ewes (16, 17), hamsters appear to be more selective. Thus, long days induced an increase in Dio2 in Syrian hamsters (53, 56, 57), but expression of Dio3 was undetectable in either photoperiod (18). In contrast, changes in Dio3 appear to be important in Siberian hamsters (58, 59), while inconsistent responses were observed in expression of Dio2 (58–60). Readers interested in a more detailed discussion of these topics are referred to other papers on seasonality in hamsters in this Special Issue.
There are also some major differences among these species in seasonal reproductive function. One might expect that these mechanisms would be more closely related in the two species of hamsters than between them and sheep, and this is true for two major aspects of seasonality. First, hamsters are long-day breeders in that they are reproductively active during the long days of spring and summer, while sheep are fertile during the short days of fall and winter (Fig. 1) (5). This difference undoubtedly reflects the duration of pregnancy, which is a few weeks in hamsters and approximately five months in sheep (3). Since seasonal reproductive patterns most likely evolved to ensure young are born in the spring when environmental conditions favor their survival, the differences in durations of pregnancy required the season of fertility, and thus the effects of photoperiod, to differ between hamsters and sheep. The second major difference is that sheep can become photorefractory to both long and short daylength (61, 62), while hamsters can only become photorefractory to short days (5). Thus if male hamsters are maintained on continuous short day length they will first be infertile, but after several months they will undergo testicular recrudescence and then remain reproductively active as long as they are maintained on this “inhibitory” photoperiod (5). In contrast, under similar circumstances, sheep will continue to show fluctuations in reproductive activity of about a year, a phenomenon that reflects the endogenous circannual rhythm in reproduction discussed above (7, 63, 64). It has been suggested that photorefractoriness to short days allows the reproductive axis of male hamsters to begin activity in anticipation of long days so that mature sperm, which take several weeks to produce, are available at the right time of year (5). It is unclear why hamsters have not developed endogenous circannual rhythms similar to those seen in sheep, but one possibility is that this has been precluded by their relatively short life span.
Given these differences between sheep and hamsters, one might expect that the neural mechanisms driving seasonal differences in GnRH secretion would be similar in Syrian and Siberian hamsters, and differ significantly from those in sheep and this is true for the role of A15 DA neurons because there is no evidence that this system plays a role in seasonality in hamsters. However, when one examines the role of kisspeptin, marked differences are evident between Syrian and Siberian hamsters, with the former more closely resembling sheep. Specifically there is strong evidence that changes in kisspeptin are critical for seasonal changes in fertility in male Syrian hamsters (18, 65). Thus, as in the ewe, kisspeptin expression is elevated during stimulatory photoperiod (66, 67) and administration of kisspeptin can restore fertility in hamsters maintained on inhibitory photoperiod (66, 68). In the hamster, as in the sheep, there are two major populations of kisspeptin cells, one in the ARC and the other in the preoptic region (76); in hamsters, the latter population is specifically localized in the anteroventral periventricular nucleus (AVPV). Although kisspeptin expression increases in both the ARC and AVPV the former is most likely critical for seasonality because it is controlled by melatonin (66, 67) and its expression is not altered by testosterone administration to photosuppressed hamsters (66, 67). In contrast, kisspeptin expression in the AVPV can be induced by testosterone administration to such hamsters and is not affected by pinealectomy (67). Thus the increase in kisspeptin in the AVPV during the breeding season is most likely an effect, rather than a cause, of the reactivation of the hypothalamo-pituitary-testicular axis in Syrian hamsters.
When kisspeptin expression is examined in Siberian hamsters a much different picture emerges: kisspeptin expression in the ARC is inhibited during stimulatory photoperiod, while expression in the AVPV is elevated (69, 70). However, the latter can be explained by the seasonal differences in testosterone because testosterone administration to photosuppressed hamsters increases kisspeptin expression in the AVPV as it does in Syrian hamsters (71). The seasonal changes in the ARC may also reflect changes in endogenous testosterone because this steroid is an inhibitor of kisspeptin expression in the ARC of Siberian hamsters (71), as it is in other species. Finally, exogenous kisspeptin infusion was unable to restore fertility to Siberian hamsters exposed to inhibitory photoperiod (72). This lack of effect might reflect a loss of responsiveness produced by continuous kisspeptin administration as seen in some other species (73), but this seems unlikely because intermittent administration (once/day) was also ineffective in Siberian hamsters (72). It is interesting to note in this regard that constant infusion of kisspeptin was more effective than intermittent (twice/day) injection in inducing ovulation in anestrous ewes (74), and that continuous icv administration of this peptide (6 nmoles/day) to Syrian hamsters exposed to short days stimulated testicular growth (66). On the other hand, a similar dose of kisspeptin (10 nmoles/day) failed to stimulate testicular growth in Syrian hamsters when given sc (68). Taken together, the results from studies in Siberian hamsters support the conclusion that kisspeptin does not play an important role in driving seasonal reproductive function in this species, and instead seasonal changes in kisspeptin expression reflect corresponding changes in circulating testosterone concentrations.
If kisspeptin is not responsible for seasonal changes in reproduction in Siberian hamsters, what system is? Early data raised the possibility that glutamatergic neurons might be involved (75), but more recent work has focused on an interesting alternative: RFamide-related peptide-3 (RFRP-3) (65). RFRP-3, one mammalian analogue of avian gonadotropin-inhibitory hormone, is found primarily in neurons located in the DMH and adjacent structures, and inhibits LH secretion, most likely by suppressing GnRH secretion, under most circumstances (18, 65). Surprisingly given its inhibitory actions, RFRP3 expression (mRNA and protein) is increased by stimulatory photoperiod in both Siberian and Syrian hamsters (76–78). These changes in expression are driven by melatonin (76, 78) and not influenced by castration of reproductively-active hamsters to decrease testosterone or elevating testosterone with exogenous treatment in hamsters whose reproductive activity was inhibited by photoperiod (76, 77). In parallel with this positive correlation with reproductive function, exogenous RFRP-3 stimulates LH secretion in both species of hamsters if administered during inhibitory photoperiod (78, 79); in contrast, during stimulatory photoperiod RFRP-3 inhibits LH secretion in Siberian males (78), but stimulates it in Syrian males (79). These data raise the possibility that long-day photoperiod stimulates release of RPRP-3 which in turn activates the hypothalamo-pituitary-testicular axis in male hamsters. This hypothesis is supported by the report that prolonged icv administration of RFRP-3 can induce testicular growth in Syrian hamsters (79). Interestingly this treatment also increased the number of kisspeptin-positive cells in the ARC (79) so that RFRP-3 may act via kisspeptin neurons in these hamsters. However, this connection is unlikely to be important in Siberian hamsters because expression of these two peptides can be experimental disassociated (80). Moreover, as discussed above, kisspeptin probably plays little, or no role, in photoperiod-induced changes in reproduction in these hamsters.
5. Conclusions
In summary, ARC kisspeptin neurons play a key role in the photoperiodic control of GnRH secretion in sheep and Syrian hamsters (Fig. 4), but long days has opposite effects in these two species. Thus in the ewe, long days increase the activity of glutamatergic neurons that innervate A15 DA neurons. DA released from these neurons in turn inhibits the activity of ARC kisspeptin neurons, thus inhibiting GnRH and LH secretion and inducing infertility. In Syrian hamsters, long days stimulate release of RFRP-3 from neurons in the dorsomedial hypothalamus, which increases the activity of ARC kisspeptin neurons (presumably by suppressing an unidentified inhibitory neural system because RFRP-3 acts via an inhibitory G protein). The increase in kisspeptin then stimulates GnRH secretion to cause testicular recrudescence and the resulting increase in testosterone stimulates kisspeptin expression in the AVPV. In contrast, kisspeptin appears to play no role in mediating the effects of photoperiod in male Siberian hamsters. The initial response of this species to long days is similar to that of Syrian hamsters: increased release of RFRP-3. Thereafter the pathway diverges with RFRP-3 suppressing an unknown inhibitory system to stimulate GnRH secretion possibly via glutamatergic neurons. The increase in GnRH ultimately leads to increased testosterone, which, in turn, acts to stimulate kisspeptin expression in the AVPV and inhibit this peptide in the ARC.
Figure 4.
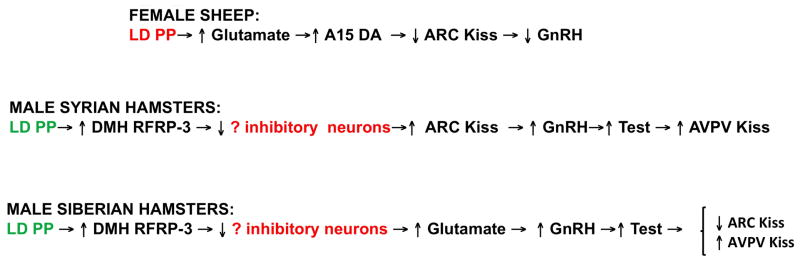
Comparison of the neural systems mediating the effects of long days on GnRH secretion in sheep, Syrian and Siberian hamsters. Note that long days (LD PP) are inhibitory in sheep, but stimulatory in hamsters. In sheep and Syrian hamsters, changes in ARC kisspeptin play a central role in the photoperiodic control of GnRH release, but different afferent inputs are involved in these two species. In contrast, kisspeptin does not appear to be important for seasonal changes in GnRH secretion in Siberian hamsters; rather the increase in circulating testosterone drives the changes observed in kisspeptin expression. See text for further details.
If one focuses on the sheep, there are clearly important gaps in our understanding of the mechanisms by which photoperiod controls GnRH secretion. One such gap is the link between melatonin action and changes in the neural circuitry mediating E2 negative feedback. Thus, it is not clear whether the effects of melatonin in the PT can account for both transitions between breeding and anestrous seasons. Early data with melatonin microimplants suggests not (13), but expression of both Dio2 and Dio3 changed during prolonged exposure to either long day or short day photoperiod (17), although these alterations did not correlate well with changes in reproductive function. Similarly the sites of thyroid action at the transition to anestrus (81) are not entirely consistent with the current model for induction of type 2 deioidinase, and the specific actions of T3 remain largely unknown. Other important questions relate to the neural circuitry mediating E2 negative feedback in anestrus, including: 1) the identity of the estrogen-responsive and glutamatergic and GABAergic neurons, 2) whether A15 neurons project directly to KNDy neurons, and 3) the specific changes in this circuit that account for transitions between breeding season and anestrus. Although many questions remain, much is now known about the actions of melatonin and the neural circuitry underlying seasonal breeding in the ewe that allows for the design of experimental approaches to address these questions.
Highlights.
Seasonal reproduction is mediated by gonadotropin-releasing hormone (GnRH) neurons
Increased responsiveness of GnRH cells to estradiol negative feedback is a key factor
Arcuate kisspeptin neurons also play an important role in seasonal reproduction
Similarities and differences exist between sheep and hamsters in central mechanisms
Acknowledgments
We thank our many students and colleagues who are responsible for much of the work and concepts described in this review (see original citations). This work was supported by a grant from the NIH (RO1 HD017864).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lincoln GA, Short RV. Seasonal breeding: nature’s contraceptive. Recent progress in hormone research. 1980;36:1–52. doi: 10.1016/b978-0-12-571136-4.50007-3. [DOI] [PubMed] [Google Scholar]
- 2.Karsch FJ, Goodman RL, Legan SJ. Feedback basis of seasonal breeding: test of an hypothesis. Journal of reproduction and fertility. 1980;58(2):521–35. doi: 10.1530/jrf.0.0580521. [DOI] [PubMed] [Google Scholar]
- 3.Karsch FJ, Bittman EL, Foster DL, Goodman RL, Legan SJ, Robinson JE. Neuroendocrine basis of seasonal reproduction. Recent progress in hormone research. 1984;40:185–232. doi: 10.1016/b978-0-12-571140-1.50010-4. [DOI] [PubMed] [Google Scholar]
- 4.Malpaux B, Viguie C, Skinner DC, Thiery JC, Chemineau P. Control of the circannual rhythm of reproduction by melatonin in the ewe. Brain research bulletin. 1997;44(4):431–8. doi: 10.1016/s0361-9230(97)00223-2. [DOI] [PubMed] [Google Scholar]
- 5.Malpaux B. Seasonal Regulation of Reproduction in Mammals. In: Neill JD, editor. Knobil and NMeill’s Physiology of Reproduction. 3. Amsterdam: Elsvier; 2006. pp. 2231–81. [Google Scholar]
- 6.Bittman EL, Karsch FJ, Hopkins JW. Role of the pineal gland in ovine photoperiodism: regulation of seasonal breeding and negative feedback effects of estradiol upon luteinizing hormone secretion. Endocrinology. 1983;113(1):329–36. doi: 10.1210/endo-113-1-329. [DOI] [PubMed] [Google Scholar]
- 7.Woodfill CJ, Wayne NL, Moenter SM, Karsch FJ. Photoperiodic synchronization of a circannual reproductive rhythm in sheep: identification of season-specific time cues. Biology of reproduction. 1994;50(4):965–76. doi: 10.1095/biolreprod50.4.965. [DOI] [PubMed] [Google Scholar]
- 8.van Esseveldt KE, Lehman MN, Boer GJ. The suprachiasmatic nucleus and the circadian time-keeping system revisited. Brain research Brain research reviews. 2000;33(1):34–77. doi: 10.1016/s0165-0173(00)00025-4. [DOI] [PubMed] [Google Scholar]
- 9.Hazlerigg DG, Simonneaux V. Seasonal Regulation of Reproduction in Mammals. In: Plant TM, Zeleznik A, editors. Knobil and Neill’s Physiology of Reproduction. 4. Amsterdam: Elsevier; 2014. In Press. [Google Scholar]
- 10.Weaver DR, Rivkees SA, Reppert SM. Localization and characterization of melatonin receptors in rodent brain by in vitro autoradiography. The Journal of neuroscience : the official journal of the Society for Neuroscience. 1989;9(7):2581–90. doi: 10.1523/JNEUROSCI.09-07-02581.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Reviers MM, Ravault JP, Tillet Y, Pelletier J. Melatonin binding sites in the sheep pars tuberalis. Neuroscience letters. 1989;100(1–3):89–93. doi: 10.1016/0304-3940(89)90665-4. [DOI] [PubMed] [Google Scholar]
- 12.Malpaux B, Daveau A, Maurice-Mandon F, Duarte G, Chemineau P. Evidence that melatonin acts in the premammillary hypothalamic area to control reproduction in the ewe: presence of binding sites and stimulation of luteinizing hormone secretion by in situ microimplant delivery. Endocrinology. 1998;139(4):1508–16. doi: 10.1210/endo.139.4.5879. [DOI] [PubMed] [Google Scholar]
- 13.Malpaux B, Skinner DC, Maurice F. The ovine pars tuberalis does not appear to be targeted by melatonin to modulate luteinizing hormone secretion, but may be important for prolactin release. Journal of neuroendocrinology. 1995;7(3):199–206. doi: 10.1111/j.1365-2826.1995.tb00748.x. [DOI] [PubMed] [Google Scholar]
- 14.Dahl GE, Evans NP, Moenter SM, Karsch FJ. The thyroid gland is required for reproductive neuroendocrine responses to photoperiod in the ewe. Endocrinology. 1994;135(1):10–5. doi: 10.1210/endo.135.1.8013340. [DOI] [PubMed] [Google Scholar]
- 15.Dahl GE, Evans NP, Thrun LA, Karsch FJ. Thyroxine is permissive to seasonal transitions in reproductive neuroendocrine activity in the ewe. Biology of reproduction. 1995;52(3):690–6. doi: 10.1095/biolreprod52.3.690. [DOI] [PubMed] [Google Scholar]
- 16.Hanon EA, Lincoln GA, Fustin JM, Dardente H, Masson-Pevet M, Morgan PJ, et al. Ancestral TSH mechanism signals summer in a photoperiodic mammal. Current biology : CB. 2008;18(15):1147–52. doi: 10.1016/j.cub.2008.06.076. [DOI] [PubMed] [Google Scholar]
- 17.Saenz de Miera C, Hanon EA, Dardente H, Birnie M, Simonneaux V, Lincoln GA, et al. Circannual variation in thyroid hormone deiodinases in a short-day breeder. Journal of neuroendocrinology. 2013;25(4):412–21. doi: 10.1111/jne.12013. [DOI] [PubMed] [Google Scholar]
- 18.Revel FG, Masson-Pevet M, Pevet P, Mikkelsen JD, Simonneaux V. Melatonin controls seasonal breeding by a network of hypothalamic targets. Neuroendocrinology. 2009;90(1):1–14. doi: 10.1159/000219588. [DOI] [PubMed] [Google Scholar]
- 19.Dardente H, Wyse CA, Birnie MJ, Dupre SM, Loudon AS, Lincoln GA, et al. A molecular switch for photoperiod responsiveness in mammals. Current biology : CB. 2010;20(24):2193–8. doi: 10.1016/j.cub.2010.10.048. [DOI] [PubMed] [Google Scholar]
- 20.Dupre SM, Miedzinska K, Duval CV, Yu L, Goodman RL, Lincoln GA, et al. Identification of Eya3 and TAC1 as long-day signals in the sheep pituitary. Current biology : CB. 2010;20(9):829–35. doi: 10.1016/j.cub.2010.02.066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Thompson CC, Potter GB. Thyroid hormone action in neural development. Cerebral cortex. 2000;10(10):939–45. doi: 10.1093/cercor/10.10.939. [DOI] [PubMed] [Google Scholar]
- 22.Lehman MN, Ladha Z, Coolen LM, Hileman SM, Connors JM, Goodman RL. Neuronal plasticity and seasonal reproduction in sheep. The European journal of neuroscience. 2010;32(12):2152–64. doi: 10.1111/j.1460-9568.2010.07530.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Goodman RL, Jansen HT, Billings HJ, Coolen LM, Lehman MN. Neural systems mediating seasonal breeding in the ewe. Journal of neuroendocrinology. 2010;22(7):674–81. doi: 10.1111/j.1365-2826.2010.02014.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lehman MN, Ebling FJ, Moenter SM, Karsch FJ. Distribution of estrogen receptor-immunoreactive cells in the sheep brain. Endocrinology. 1993;133(2):876–86. doi: 10.1210/endo.133.2.8344223. [DOI] [PubMed] [Google Scholar]
- 25.Hardy SL, Anderson GM, Valent M, Connors JM, Goodman RL. Evidence that estrogen receptor alpha, but not beta, mediates seasonal changes in the response of the ovine retrochiasmatic area to estradiol. Biology of reproduction. 2003;68(3):846–52. doi: 10.1095/biolreprod.102.010215. [DOI] [PubMed] [Google Scholar]
- 26.Meyer SL, Goodman RL. Neurotransmitters involved in mediating the steroid-dependent suppression of pulsatile luteinizing hormone secretion in anestrous ewes: effects of receptor antagonists. Endocrinology. 1985;116(5):2054–61. doi: 10.1210/endo-116-5-2054. [DOI] [PubMed] [Google Scholar]
- 27.Havern RL, Whisnant CS, Goodman RL. Dopaminergic structures in the ovine hypothalamus mediating estradiol negative feedback in anestrous ewes. Endocrinology. 1994;134(4):1905–14. doi: 10.1210/endo.134.4.7907976. [DOI] [PubMed] [Google Scholar]
- 28.Martin GB, Thiery JC. Hypothalamic multiunit activity and LH secretion in conscious sheep. Experimental brain research. 1987;67(3):469–78. doi: 10.1007/BF00247280. [DOI] [PubMed] [Google Scholar]
- 29.Goodman RL, Thiery JC, Delaleu B, Malpaux B. Estradiol increases multiunit electrical activity in the A15 area of ewes exposed to inhibitory photoperiods. Biology of reproduction. 2000;63(5):1352–7. doi: 10.1095/biolreprod63.5.1352. [DOI] [PubMed] [Google Scholar]
- 30.Gayrard V, Malpaux B, Tillet Y, Thiery JC. Estradiol increases tyrosine hydroxylase activity of the A15 nucleus dopaminergic neurons during long days in the ewe. Biology of reproduction. 1994;50(5):1168–77. doi: 10.1095/biolreprod50.5.1168. [DOI] [PubMed] [Google Scholar]
- 31.Lehman MN, Durham DM, Jansen HT, Adrian B, Goodman RL. Dopaminergic A14/A15 neurons are activated during estradiol negative feedback in anestrous, but not breeding season, ewes. Endocrinology. 1996;137(10):4443–50. doi: 10.1210/endo.137.10.8828506. [DOI] [PubMed] [Google Scholar]
- 32.Singh SR, Hileman SM, Connors JM, McManus CJ, Coolen LM, Lehman MN, et al. Estradiol negative feedback regulation by glutamatergic afferents to A15 dopaminergic neurons: variation with season. Endocrinology. 2009;150(10):4663–71. doi: 10.1210/en.2009-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Adams VL, Goodman RL, Salm AK, Coolen LM, Karsch FJ, Lehman MN. Morphological plasticity in the neural circuitry responsible for seasonal breeding in the ewe. Endocrinology. 2006;147(10):4843–51. doi: 10.1210/en.2006-0408. [DOI] [PubMed] [Google Scholar]
- 34.Kuljis RO, Advis JP. Immunocytochemical and physiological evidence of a synapse between dopamine- and luteinizing hormone releasing hormone-containing neurons in the ewe median eminence. Endocrinology. 1989;124(3):1579–81. doi: 10.1210/endo-124-3-1579. [DOI] [PubMed] [Google Scholar]
- 35.Jansen HT, Hileman SM, Kuehl DE, Lubbers LS, Jackson GL, Lehman MN. A subset of estrogen receptor-containing neurons project to the median eminence in the ewe. J Neuroendocrinol. 1996;8(12):921–927. doi: 10.1111/j.1365-2826.1996.tb00822.x. [DOI] [PubMed] [Google Scholar]
- 36.Goodman RL, Maltby MJ, Millar RP, Hileman SM, Nestor CC, Whited B, et al. Evidence that dopamine acts via kisspeptin to hold GnRH pulse frequency in check in anestrous ewes. Endocrinology. 2012;153(12):5918–27. doi: 10.1210/en.2012-1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Smith JT, Coolen LM, Kriegsfeld LJ, Sari IP, Jaafarzadehshirazi MR, Maltby M, et al. Variation in kisspeptin and RFamide-related peptide (RFRP) expression and terminal connections to gonadotropin-releasing hormone neurons in the brain: a novel medium for seasonal breeding in the sheep. Endocrinology. 2008;149(11):5770–82. doi: 10.1210/en.2008-0581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Goodman RL, Lehman MN, Smith JT, Coolen LM, de Oliveira CV, Jafarzadehshirazi MR, et al. Kisspeptin neurons in the arcuate nucleus of the ewe express both dynorphin A and neurokinin B. Endocrinology. 2007;148(12):5752–60. doi: 10.1210/en.2007-0961. [DOI] [PubMed] [Google Scholar]
- 39.Lehman MN, Coolen LM, Goodman RL. Minireview: kisspeptin/neurokinin B/dynorphin (KNDy) cells of the arcuate nucleus: a central node in the control of gonadotropin-releasing hormone secretion. Endocrinology. 2010;151(8):3479–89. doi: 10.1210/en.2010-0022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Meyer SL, Goodman RL. Separate neural systems mediate the steroid-dependent and steroid-independent suppression of tonic luteinizing hormone secretion in the anestrous ewe. Biology of reproduction. 1986;35(3):562–71. doi: 10.1095/biolreprod35.3.562. [DOI] [PubMed] [Google Scholar]
- 41.Sakamoto K, Murata K, Wakabayashi Y, Yayou K, Ohkura S, Takeuchi Y, et al. Central administration of neurokinin B activates kisspeptin/NKB neurons in the arcuate nucleus and stimulates luteinizing hormone secretion in ewes during the non-breeding season. The Journal of reproduction and development. 2012;58(6):700–6. doi: 10.1262/jrd.2011-038. [DOI] [PubMed] [Google Scholar]
- 42.Xiong JJ, Karsch FJ, Lehman MN. Evidence for seasonal plasticity in the gonadotropin-releasing hormone (GnRH) system of the ewe: changes in synaptic inputs onto GnRH neurons. Endocrinology. 1997;138(3):1240–50. doi: 10.1210/endo.138.3.5000. [DOI] [PubMed] [Google Scholar]
- 43.Mong JA, McCarthy MM. Steroid-induced developmental plasticity in hypothalamic astrocytes: implications for synaptic patterning. Journal of neurobiology. 1999;40(4):602–19. doi: 10.1002/(sici)1097-4695(19990915)40:4<602::aid-neu14>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 44.Turek FW. The interaction of the photoperiod and testosterone in regulating serum gonadotropin levels in castrated male hamsters. Endocrinology. 1977;101(4):1210–5. doi: 10.1210/endo-101-4-1210. [DOI] [PubMed] [Google Scholar]
- 45.Turek FW, Elliott JA, Alvis JD, Menaker M. The interaction of castration and photoperiod in the regulation of hypophyseal and serum gonadotropin levels in male golden hamsters. Endocrinology. 1975;96(4):854–60. doi: 10.1210/endo-96-4-854. [DOI] [PubMed] [Google Scholar]
- 46.Simpson SM, Follett BK, Ellis DH. Modulation by photoperiod of gonadotrophin secretion in intact and castrated Djungarian hamsters. Journal of reproduction and fertility. 1982;66(1):243–50. doi: 10.1530/jrf.0.0660243. [DOI] [PubMed] [Google Scholar]
- 47.Maywood ES, Bittman EL, Hastings MH. Lesions of the melatonin- and androgen-responsive tissue of the dorsomedial nucleus of the hypothalamus block the gonadal response of male Syrian hamsters to programmed infusions of melatonin. Biology of reproduction. 1996;54(2):470–7. doi: 10.1095/biolreprod54.2.470. [DOI] [PubMed] [Google Scholar]
- 48.Maywood ES, Hastings MH. Lesions of the iodomelatonin-binding sites of the mediobasal hypothalamus spare the lactotropic, but block the gonadotropic response of male Syrian hamsters to short photoperiod and to melatonin. Endocrinology. 1995;136(1):144–53. doi: 10.1210/endo.136.1.7828525. [DOI] [PubMed] [Google Scholar]
- 49.Badura LL, Goldman BD. Central sites mediating reproductive responses to melatonin in juvenile male Siberian hamsters. Brain research. 1992;598(1–2):98–106. doi: 10.1016/0006-8993(92)90172-6. [DOI] [PubMed] [Google Scholar]
- 50.Bartness TJ, Goldman BD, Bittman EL. SCN lesions block responses to systemic melatonin infusions in Siberian hamsters. The American journal of physiology. 1991;260(1 Pt 2):R102–12. doi: 10.1152/ajpregu.1991.260.1.R102. [DOI] [PubMed] [Google Scholar]
- 51.Song CK, Bartness TJ. The effects of anterior hypothalamic lesions on short-day responses in Siberian hamsters given timed melatonin infusions. Journal of biological rhythms. 1996;11(1):14–26. doi: 10.1177/074873049601100102. [DOI] [PubMed] [Google Scholar]
- 52.Dardente H, Hazlerigg DG, Ebling FJ. Thyroid hormone and seasonal rhythmicity. Frontiers in endocrinology. 2014;5:19. doi: 10.3389/fendo.2014.00019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yasuo S, Yoshimura T, Ebihara S, Korf HW. Photoperiodic control of TSH-beta expression in the mammalian pars tuberalis has different impacts on the induction and suppression of the hypothalamo-hypopysial gonadal axis. Journal of neuroendocrinology. 2010;22(1):43–50. doi: 10.1111/j.1365-2826.2009.01936.x. [DOI] [PubMed] [Google Scholar]
- 54.Klosen P, Sebert ME, Rasri K, Laran-Chich MP, Simonneaux V. TSH restores a summer phenotype in photoinhibited mammals via the RF-amides RFRP3 and kisspeptin. FASEB journal : official publication of the Federation of American Societies for Experimental Biology. 2013;27(7):2677–86. doi: 10.1096/fj.13-229559. [DOI] [PubMed] [Google Scholar]
- 55.Goodman RL, Karsch FJ. A critique of the evidence on the importance of steroid feedback to seasonal changes in gonadotrophin secretion. Journal of reproduction and fertility Supplement. 1981;30:1–13. [PubMed] [Google Scholar]
- 56.Revel FG, Saboureau M, Pevet P, Mikkelsen JD, Simonneaux V. Melatonin regulates type 2 deiodinase gene expression in the Syrian hamster. Endocrinology. 2006;147(10):4680–7. doi: 10.1210/en.2006-0606. [DOI] [PubMed] [Google Scholar]
- 57.Yasuo S, Yoshimura T, Ebihara S, Korf HW. Temporal dynamics of type 2 deiodinase expression after melatonin injections in Syrian hamsters. Endocrinology. 2007;148(9):4385–92. doi: 10.1210/en.2007-0497. [DOI] [PubMed] [Google Scholar]
- 58.Watanabe T, Yamamura T, Watanabe M, Yasuo S, Nakao N, Dawson A, et al. Hypothalamic expression of thyroid hormone-activating and -inactivating enzyme genes in relation to photorefractoriness in birds and mammals. American journal of physiology Regulatory, integrative and comparative physiology. 2007;292(1):R568–72. doi: 10.1152/ajpregu.00521.2006. [DOI] [PubMed] [Google Scholar]
- 59.Barrett P, Ebling FJ, Schuhler S, Wilson D, Ross AW, Warner A, et al. Hypothalamic thyroid hormone catabolism acts as a gatekeeper for the seasonal control of body weight and reproduction. Endocrinology. 2007;148(8):3608–17. doi: 10.1210/en.2007-0316. [DOI] [PubMed] [Google Scholar]
- 60.Watanabe M, Yasuo S, Watanabe T, Yamamura T, Nakao N, Ebihara S, et al. Photoperiodic regulation of type 2 deiodinase gene in Djungarian hamster: possible homologies between avian and mammalian photoperiodic regulation of reproduction. Endocrinology. 2004;145(4):1546–9. doi: 10.1210/en.2003-1593. [DOI] [PubMed] [Google Scholar]
- 61.Robinson JE, Karsch FJ. Refractoriness to inductive day lengths terminates the breeding season of the Suffolk ewe. Biology of reproduction. 1984;31(4):656–63. doi: 10.1095/biolreprod31.4.656. [DOI] [PubMed] [Google Scholar]
- 62.Robinson JE, Wayne NL, Karsch FJ. Refractoriness to inhibitory day lengths initiates the breeding season of the Suffolk ewe. Biology of reproduction. 1985;32(5):1024–30. doi: 10.1095/biolreprod32.5.1024. [DOI] [PubMed] [Google Scholar]
- 63.Karsch FJ, Robinson JE, Woodfill CJ, Brown MB. Circannual cycles of luteinizing hormone and prolactin secretion in ewes during prolonged exposure to a fixed photoperiod: evidence for an endogenous reproductive rhythm. Biology of reproduction. 1989;41(6):1034–46. doi: 10.1095/biolreprod41.6.1034. [DOI] [PubMed] [Google Scholar]
- 64.Woodfill CJ, Robinson JE, Malpaux B, Karsch FJ. Synchronization of the circannual reproductive rhythm of the ewe by discrete photoperiodic signals. Biology of reproduction. 1991;45(1):110–21. doi: 10.1095/biolreprod45.1.110. [DOI] [PubMed] [Google Scholar]
- 65.Simonneaux V, Ancel C, Poirel VJ, Gauer F. Kisspeptins and RFRP-3 Act in Concert to Synchronize Rodent Reproduction with Seasons. Frontiers in neuroscience. 2013;7:22. doi: 10.3389/fnins.2013.00022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Revel FG, Saboureau M, Masson-Pevet M, Pevet P, Mikkelsen JD, Simonneaux V. Kisspeptin mediates the photoperiodic control of reproduction in hamsters. Current biology : CB. 2006;16(17):1730–5. doi: 10.1016/j.cub.2006.07.025. [DOI] [PubMed] [Google Scholar]
- 67.Ansel L, Bolborea M, Bentsen AH, Klosen P, Mikkelsen JD, Simonneaux V. Differential regulation of kiss1 expression by melatonin and gonadal hormones in male and female Syrian hamsters. Journal of biological rhythms. 2010;25(2):81–91. doi: 10.1177/0748730410361918. [DOI] [PubMed] [Google Scholar]
- 68.Ansel L, Bentsen AH, Ancel C, Bolborea M, Klosen P, Mikkelsen JD, et al. Peripheral kisspeptin reverses short photoperiod-induced gonadal regression in Syrian hamsters by promoting GNRH release. Reproduction. 2011;142(3):417–25. doi: 10.1530/REP-10-0313. [DOI] [PubMed] [Google Scholar]
- 69.Greives TJ, Mason AO, Scotti MA, Levine J, Ketterson ED, Kriegsfeld LJ, et al. Environmental control of kisspeptin: implications for seasonal reproduction. Endocrinology. 2007;148(3):1158–66. doi: 10.1210/en.2006-1249. [DOI] [PubMed] [Google Scholar]
- 70.Simonneaux V, Ansel L, Revel FG, Klosen P, Pevet P, Mikkelsen JD. Kisspeptin and the seasonal control of reproduction in hamsters. Peptides. 2009;30(1):146–53. doi: 10.1016/j.peptides.2008.06.006. [DOI] [PubMed] [Google Scholar]
- 71.Greives TJ, Humber SA, Goldstein AN, Scotti MA, Demas GE, Kriegsfeld LJ. Photoperiod and testosterone interact to drive seasonal changes in kisspeptin expression in Siberian hamsters (Phodopus sungorus) Journal of neuroendocrinology. 2008;20(12):1339–47. doi: 10.1111/j.1365-2826.2008.01790.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Greives TJ, Kriegsfeld LJ, Demas GE. Exogenous kisspeptin does not alter photoperiod-induced gonadal regression in Siberian hamsters (Phodopus sungorus) General and comparative endocrinology. 2008;156(3):552–8. doi: 10.1016/j.ygcen.2008.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Ramaswamy S, Seminara SB, Pohl CR, DiPietro MJ, Crowley WF, Jr, Plant TM. Effect of continuous intravenous administration of human metastin 45–54 on the neuroendocrine activity of the hypothalamic-pituitary-testicular axis in the adult male rhesus monkey (Macaca mulatta) Endocrinology. 2007;148(7):3364–70. doi: 10.1210/en.2007-0207. [DOI] [PubMed] [Google Scholar]
- 74.Caraty A, Smith JT, Lomet D, Ben Said S, Morrissey A, Cognie J, et al. Kisspeptin synchronizes preovulatory surges in cyclical ewes and causes ovulation in seasonally acyclic ewes. Endocrinology. 2007;148(11):5258–67. doi: 10.1210/en.2007-0554. [DOI] [PubMed] [Google Scholar]
- 75.Ebling FJ, Alexander IH, Urbanski HF, Hastings MH. Effects of N-methyl-D-aspartate (NMDA) on seasonal cycles of reproduction, body weight and pelage colour in the male Siberian hamster. Journal of neuroendocrinology. 1995;7(7):555–66. doi: 10.1111/j.1365-2826.1995.tb00792.x. [DOI] [PubMed] [Google Scholar]
- 76.Revel FG, Saboureau M, Pevet P, Simonneaux V, Mikkelsen JD. RFamide-related peptide gene is a melatonin-driven photoperiodic gene. Endocrinology. 2008;149(3):902–12. doi: 10.1210/en.2007-0848. [DOI] [PubMed] [Google Scholar]
- 77.Mason AO, Duffy S, Zhao S, Ubuka T, Bentley GE, Tsutsui K, et al. Photoperiod and reproductive condition are associated with changes in RFamide-related peptide (RFRP) expression in Syrian hamsters (Mesocricetus auratus) Journal of biological rhythms. 2010;25(3):176–85. doi: 10.1177/0748730410368821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Ubuka T, Inoue K, Fukuda Y, Mizuno T, Ukena K, Kriegsfeld LJ, et al. Identification, expression, and physiological functions of Siberian hamster gonadotropin-inhibitory hormone. Endocrinology. 2012;153(1):373–85. doi: 10.1210/en.2011-1110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Ancel C, Bentsen AH, Sebert ME, Tena-Sempere M, Mikkelsen JD, Simonneaux V. Stimulatory effect of RFRP-3 on the gonadotrophic axis in the male Syrian hamster: the exception proves the rule. Endocrinology. 2012;153(3):1352–63. doi: 10.1210/en.2011-1622. [DOI] [PubMed] [Google Scholar]
- 80.Paul MJ, Pyter LM, Freeman DA, Galang J, Prendergast BJ. Photic and nonphotic seasonal cues differentially engage hypothalamic kisspeptin and RFamide-related peptide mRNA expression in Siberian hamsters. Journal of neuroendocrinology. 2009;21(12):1007–14. doi: 10.1111/j.1365-2826.2009.01924.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Anderson GM, Hardy SL, Valent M, Billings HJ, Connors JM, Goodman RL. Evidence that thyroid hormones act in the ventromedial preoptic area and the premammillary region of the brain to allow the termination of the breeding season in the ewe. Endocrinology. 2003;144(7):2892–901. doi: 10.1210/en.2003-0322. [DOI] [PubMed] [Google Scholar]