Abstract
Background
CD22 is a B-lineage differentiation antigen that has emerged as a leading therapeutic target in acute lymphoblastic leukemia (ALL).
Procedure
Properties of CD22 expression relevant to therapeutic targeting were characterized in primary samples obtained from children and young adults with relapsed and chemotherapy refractory B-precursor (pre-B) ALL.
Results
CD22 expression was demonstrated in all subjects (n=163) with detection on at least 90% of blasts in 155 cases. Median antigen site density of surface CD22 was 3,470 sites/cell (range 349 – 19,653, n=160). Blasts from patients with known 11q23 (MLL) rearrangement had lower site density (median 1,590 sites/cell, range 349-3,624, n=20 versus 3,853 sites/cell, range 451-19,653, n=140; p=<0.0001) and 6 of 21 cases had sub-populations of blasts lacking CD22 expression (22% – 82% CD22+). CD22 expression was maintained in serial studies of 73 subjects, including those treated with anti-CD22 targeted therapy. The levels of soluble CD22 in blood and marrow by ELISA were low and not expected to influence the pharmacokinetics of anti-CD22 directed agents.
Conclusions
These characteristics make CD22 an excellent potential therapeutic target in patients with relapsed and chemotherapy-refractory ALL, although cases with MLL rearrangement require close study to exclude the presence of a CD22-negative blast population.
Keywords: CD22, monoclonal antibody, acute lymphoblastic leukemia, relapse
Introduction
Despite great progress in the development of curative therapies, ALL remains a leading cause of pediatric cancer-related mortality, the prognosis is guarded for those with relapsed or refractory disease and current treatment regimens are associated with acute toxicities and long-term sequelae. [1-3] New therapies are needed to overcome chemotherapy resistance and reduce non-specific treatment-associated side effects. CD22 is a 140 kDa B-lineage differentiation antigen that has emerged as a leading therapeutic target in B cell malignancies, including childhood B-precursor acute lymphoblastic leukemia (pre-B ALL). [4-7] Multiple anti-CD22 monoclonal antibody-based therapies are under development including epratuzumab [8], inotuzumab ozogamicin, [9] moxetumomab pasudotox [10] and anti-CD22 chimeric antigen receptor transduced T cells [11].
A commonly employed threshold for eligibility on anti-CD22 directed therapeutic trials is the presence of CD22 expression on at least 20-30% of the malignant cells. [12,13] The reported expression of CD22 on pre-B ALL typically exceeds this, with reported CD22 expression levels in adult ALL ranging from 50-100% [9,12,14,15] and in children approaching 90%. [8,16] Importantly, therapeutic efficacy would clearly be impacted by the distribution of expression across the malignant cells within an individual case. Thus, the presence of a subset of CD22-negative blasts could allow for the emergence of expression-null malignant cells. The rate of “positivity” alone, however, is not the only factor of CD22 expression that may be relevant to clinical efficacy. The degree of expression, or site density/antigen binding capacity (ABC) which utilizes mean fluorescence intensity (MFI) as a measure of expression, might influence treatment response by affecting the number of therapeutic molecules that can bind the surface of the malignant blast. Furthermore, surface expression of CD22 must be maintained over time in order to assure ongoing efficacy during CD22 targeted therapy. Shedding of the CD22 antigen from the cell surface is a potential mechanism of drug resistance. Soluble shed antigen in circulation could serve as a decoy by binding monoclonal antibody-based therapeutics, adversely impacting pharmacokinetics. [17] Furthermore, sheddase activity could result in high concentrations of shed antigen in tissue sites such as solid tumors or bone marrow, further diminishing therapeutic activity. [18] Relapsed / chemotherapy-refractory ALL is the primary population for pediatric testing of anti-CD22 monoclonal antibody-based therapies. This study was designed to more fully characterize the properties of CD22 expression that might influence the efficacy of anti-CD22 directed therapies in that setting.
Methods
Patient samples
Between January 2003 and May 2014, a total of 337 samples from 163 subjects were prospectively obtained from pediatric patients with relapsed or chemotherapy-refractory pre-B ALL. All samples were obtained under protocols approved by the National Cancer Institute (NCI) Institutional Review Board. Upper age limit was set at 25 years based on eligibility for NCI protocols for which these patients were being evaluated.
Site Density
CD22 site density was measured by multiparameter flow cytometry using the BD Biosciences QuantiBRITE® system (Becton Dickinson, Franklin Lakes, NJ) at the NCI flow cytometry laboratory, which has extensive experience working with anti-CD22 antibodies over a 25 year period and have validated and optimized panels in place for routine assessment. [19] The QuantiBRITE® system was used to quantify site density by the measurement of CD22 antibody bound per cell (ABC). [20] Using anti-CD22 monoclonal antibodies conjugated 1:1 to phycoerythrin, this method is precise (median CV 1.6%, 95% confidence interval 1.2-2.3%). [19]
The majority of specimens received were shipped and not immediately processed. However, all specimens were anti-coagulated with sodium heparin that has demonstrated adequate viability in blood for up to 72 hours and in bone marrow for up to 48 hours. [21] We have previously demonstrated that prolonged sample storage is sometimes associated with a modest reduction in the CD22 site density. [19] Therefore, adequacy of specimens used for analysis of CD22 ABC was based on using only those samples with high viability, which were generally processed in less than 24 hours from shipping. Data from fresh samples were used as available.
Soluble CD22
To assess the possibility that CD22 might be shed from the surface of ALL blasts into the blood and/or concentrated in the bone marrow, we measured soluble CD22 (sCD22) in peripheral blood and bone marrow supernatants in a subset of patients based on sample availability. Using previously published methods, levels of sCD22 were measured by an enzyme-linked immunosorbent assay (ELISA). [22] To quantify sCD22, a sandwich ELISA assay was developed in which the anti-CD22 monoclonal antibody RFB4 was bound to 96-well plates, diluted serum or standards were added, and the bound sCD22 detected with a biotinylated second anti-CD22 monoclonal antibody (sHCL1) followed by standard avidin-HRP treatment and development steps.
Statistical Analysis
For those subjects with multiple samples (n=73) (range 2-10 samples/subject), the highest CD22 value per subject was used for the analysis, unless that value represented a significant outlier from other samples in the same patient, in which case the next highest value was used. A Mann-Whitney (Wilcoxon Rank sum test) was used to analyze the differences in individual samples based on site density between those with MLL-rearrangement versus those without. Wilcoxon matched-pairs signed rank test was used for paired data, when analyzing CD22 expression pre and post-CD22 targeted therapy. The samples for this latter analysis were drawn prior to and immediately after completion of therapy (at least 3 weeks following the pre-treatment sample). All p-values were two tailed and significance was set at p < 0.05. Statistical analysis was performed with Prism software (Version 6.0f, GraphPad Software, Inc.)
Results
Subjects
Samples from pediatric patients with relapsed and chemotherapy-refractory ALL were studied. A total of 337 samples from 163 subjects were evaluated, and diagnosis was confirmed. Those with flow cytometric evidence of T-cell or mature B-cell (i.e., Burkitt-type) ALL were excluded from the analysis. The median age of subjects at the time of the specimen collection was 12.5 years (range, 6 months to 25 years). (Table 1)
Table I. Characteristics of CD22 Expression on Patient Samples.
| n | |
|---|---|
| Individual cases* | 163 |
| Median age of subjects (range), years | 12.5 (0.6-25) |
| Median site density (range), sites/blastˆ | 3,470 (349-19,653) |
| Median % CD22 expression (range)# | 100% (22%-100%) |
| # Cases with less than 90% CD22 expression | 7 |
337 samples analyzed from 163 individual subjects. 73 had serial evaluations, many with multiple samples, inclusive of 47 subjects who received treatment with anti-CD22 immunotoxin therapy.
160 of 163 samples had site density evaluable for analysis.
162 of 163 subjects had CD22% available for analysis.
CD22 Expression
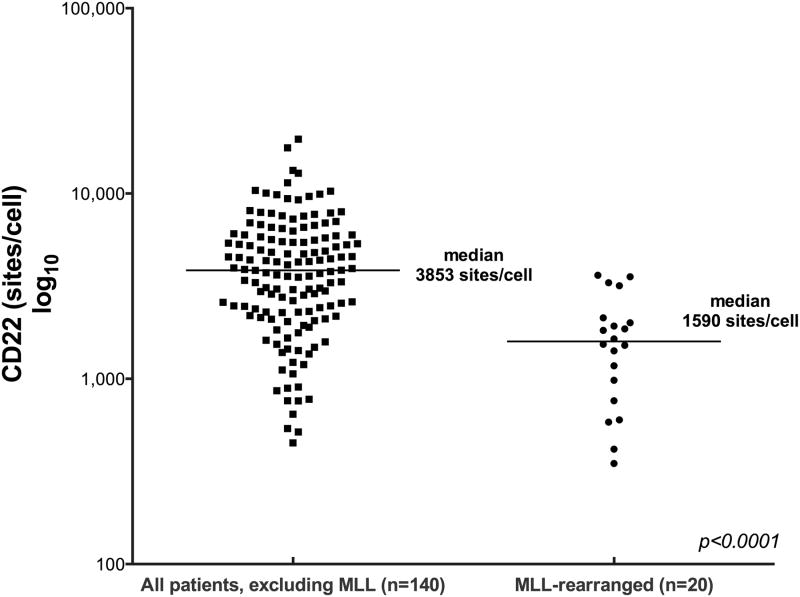
All samples demonstrated CD22 expression. (Table 1, Figure 1) CD22 was detected on at least 90% of blasts in 155 cases. Six of 21 cases with known 11q23 (MLL) rearrangement had sub-populations of blasts that lacked CD22 expression (22%, 29%, 31% 73%, 75% and 82% CD22 positive blasts). Determination of the antibody binding capacity per cell revealed that the median CD22 site density for all cases was 3,470 sites/cell (range, 349 to 19,653). Site density was lower in cases of known MLL rearrangement (median 1,590 sites/cell, range 349-3,624, n=20) in comparison to those without MLL rearrangement (median 3,853 sites/cell, range 451-19,653, n=140); p=<0.0001). (Figure 2)
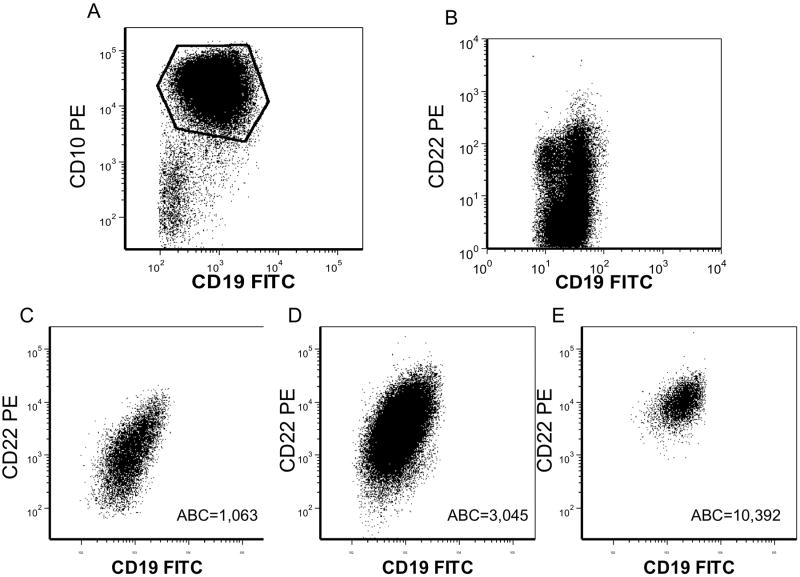
Figure 1. CD22 Expression in Representative Samples.
Quantification of CD22 expression (antigen binding capacity [ABC] value) by flow cytometry using QuantiBRITE method (BD Biosciences) A. Gating strategy for analysis of ALL blasts. CD10+ CD19+ cells analyzed. B. ALL with partial CD22 positivity (MLL-rearranged). C. ALL with CD22 ABC = 1,063. D. ALL with CD22 ABC = 3,045. E. ALL with CD22 ABC = 10,392.
Figure 2. CD22 Antigen Site Density on Patient Lymphoblasts.
Quantification of CD22 expression on patient blasts was conducted using the QuantiBRITE method (BD Biosciences). Median density for subjects with known MLL rearrangement was 1,590 sites/blasts (range, 349-3,624). Median site density for those without MLL rearrangement was 3,470 sites/blasts (range, 451-19,653).
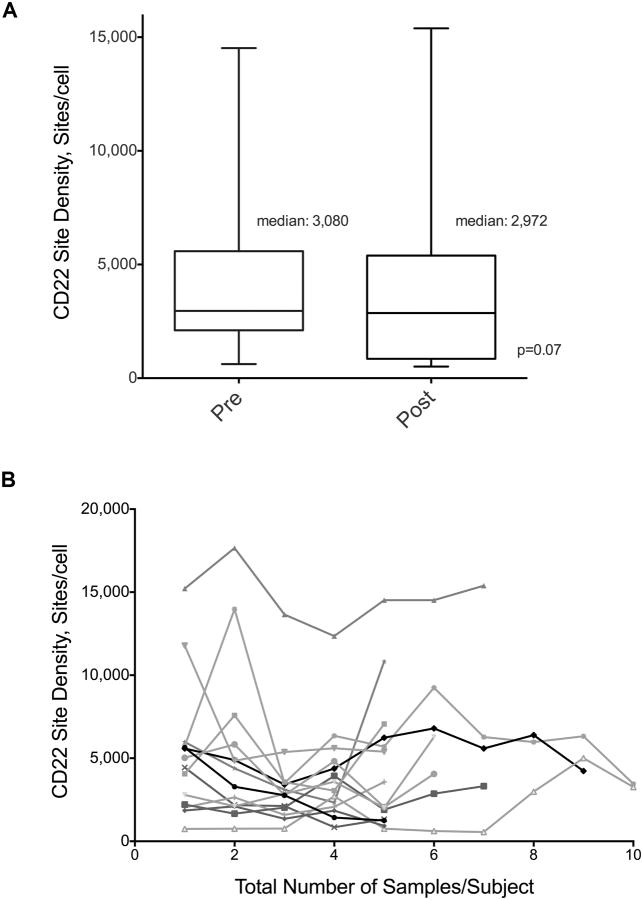
73 subjects had serial measurements of CD22. Amongst 44 non-MLL rearranged subjects with paired samples before and after anti-CD22 targeted therapy, no loss of expression was seen in any subject. (Figure 3A) Median CD22 site density pre-therapy was 3,080 (range, 620-14,519 sites/cell) and post-therapy was 2,972 (range, 549-15,392 sites/cell) (p=0.07). Additionally, stability of CD22 expression was seen over a prolonged period (up to 2 years) in patients with multiple samples who had received anti-CD22 targeted therapy (Figure 3B). Three patients with MLL-rearranged ALL treated with an anti-CD22 immunotoxin demonstrated emergence of a subpopulation of CD22 dim/negative blasts that were detectable on the pre-treatment sample and predominated after treatment (Median CD22 sites per cell pre/post therapy for the two patients with available site density data: 1,643/605; 1,824/ 511). (Figure 4)
Figure 3. CD22 Expression Before and After Anti-CD22 Targeted Therapy.
A. Quantification of CD22 expression on blasts from 44 non-MLL rearranged patients before and after treatment with anti-CD22 targeted therapy demonstrates no loss of CD22 expression following CD22 targeted therapy (p=0.07). Median CD22 site density pre-therapy was 3,080 (range, 620-14,519 sites/cell) and post-therapy was 2,972 (range, 549-15,392 sites/cell). B. Quantification of serial CD22 expression from 14 subjects who received anti-CD22 targeted therapy (≥ 5 samples/patient over a 2-year period). Maximum number of screens/patient=10
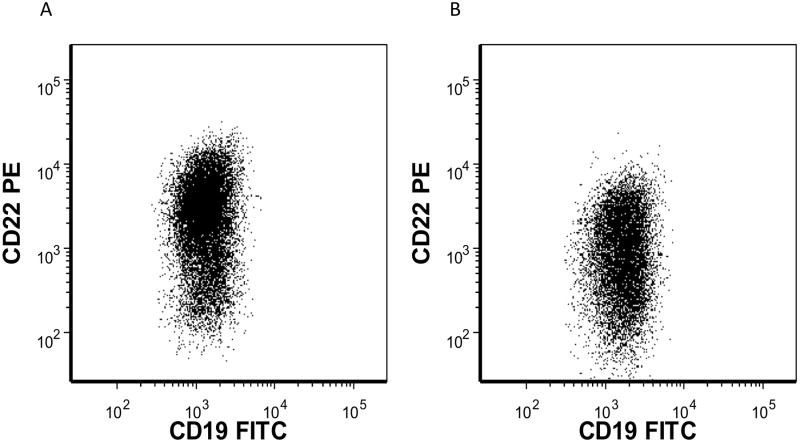
Figure 4. Emergence of a CD22 Dim Population in a Patient with MLL-Rearranged ALL Treated with Anti-CD22 Targeted Therapy.
A 1 year old with MLL-rearranged ALL was treated with an anti-CD22 immunotoxin. A subpopulation of CD22 dim blasts was detectable on the pre-treatment sample (median site density 1,643) (A). An initial dramatic reduction in circulating lymphoblasts was followed by emergence of a CD22 dim blast population (median site density 605) (B) despite ongoing therapy.
Soluble CD22 Levels
The median levels of sCD22 measured in the serum and bone marrow supernatants were 4.2 ng/ml (range 0-39.6 ng/mL) (n=30) and 0.28 ng/mL (range 0-32.9 ng/mL) (n=26) respectively. These were low in comparison to pretreatment serum levels observed in adults with hairy cell leukemia (2.1-163 ng/mL; median, 18 ng/mL). [22]
Discussion
CD22 is a differentiation antigen expressed on the surface of B-lineage cells from the early progenitor stage of development (pro-B cell IgD+) until prior to terminal differentiation into plasma cells, which are CD22 negative. [5,6] Multiple new therapies that target CD22 are currently under investigation and have shown promise in early phase trials. [8-10] The aims of this study were to more completely characterize properties of CD22 expression relevant to clinical activity of such agents in the treatment of ALL. Multiply relapsed and chemotherapy-refractory pre-B ALL represents a high-risk population with a major unmet therapeutic need. Targeting CD22 has great potential in this patient group as CD22 expression was observed in 100% of individuals studied. Furthermore, CD22 was expressed on at least 90% of blasts in 155 of 162 cases. The surface density of CD22 on lymphoblasts ranged from 349 to 19,653 sites per cell, which is above the theoretical threshold required for intracytoplasmic action of conjugates that require internalization for activity. [23,24] To date, the level of CD22 expression has not been reported to correlate with response to CD22 targeted therapy. [9,10] Some of the observed variability in CD22 expression on this study was related to the underlying cytogenetic subtype, as patients with MLL rearrangement had lower average CD22 site density. These findings are consistent with studies in adults with ALL where MLL-rearranged samples had reduced CD22 expression in comparison with other cytogenetic abnormalities. [13,14] MLL-rearranged leukemia in infants is commonly associated with an undifferentiated phenotype [25] and the level of CD22 expression has been shown to correlate with the stage of B-cell maturation. [13] Notably, in this series 6 of 21 cases with MLL-rearranged ALL had CD22-negative blast populations ranging from 22% - 82%. Consequently, targeting CD22 in infant ALL with MLL rearrangement may not be optimal for single agent clinical efficacy.
Maintenance of expression of the targeted antigen on malignant cells is critical to clinical efficacy. For example, loss of CD20 expression following treatment with the anti-CD20 monoclonal antibody rituximab [26] and CD19 negative relapse after anti-CD19 chimeric antigen receptor T cell therapy [27] has emerged as an important resistance mechanism. In this series, CD22 expression was maintained in patients treated with anti-CD22 immunotoxins and the level of expression did not correlate with response to CD22 targeted therapy.
High sCD22 levels could potentially bind anti-CD22 directed therapy and reduce therapeutic effect. [17,28] To assess the possibility that CD22 might be shed from the surface of ALL blasts and concentrated in the bone marrow, we measured soluble CD22 (sCD22) in peripheral blood and bone marrow supernatants. The levels of sCD22 were low in both blood and marrow (median 4.2 ng/ml and 0.28 ng/mL respectively. All samples were <40 ng/ml, which is well below the maximum plasma concentrations of anti-CD22 agents achieved in adults and children with leukemia (200-1,500 ng/ml). [10,29] Thus, antigen shedding does not appear to be an obstacle to therapeutic targeting of CD22.
There are a number of potential limitations of this study. As a referral center, there is a selection bias for patients pursuing early phase clinical trials, some of which have specifically targeted CD22. Thus, subjects who were previously known to be CD22 positive at other institutions might have skewed the study population. However, samples from subjects previously reported to be “CD22-negative” or “dim” were among those studied and in all cases these were found to be CD22 positive using our sensitive and validated techniques. [19] CD22 is not routinely studied by commercial and some hospital based flow cytometry laboratories and there may be limited experience in evaluating expression of CD22 with potential for incomplete validation of performance to accurately assess the dim CD22 positivity observed in ALL. [30] Thus, caution is advised before declaring a case of non-T cell ALL in children as CD22 negative unless methods, such as those used in this study, which incorporate use of antibodies conjugated to the PE fluorochrome, are employed. As noted, the study population represents subjects with relapsed or refractory ALL. Most prior reports have studied newly diagnosed cases. It is possible that CD22 expression differs from diagnosis to relapse. Notably, however, Raponi et al analyzed 36-paired samples from adults with ALL at diagnosis and relapse and found no major changes in CD22 expression. [13] Finally, the majority of specimens were shipped overnight. However, we have previously demonstrated that this has limited impact on CD22 expression. [19]
Conclusions
The properties of CD22 expression in relapsed and chemotherapy-refractory ALL make this an attractive therapeutic target. Multiple characteristics are likely to be important in reference to clinical efficacy including the expression in all cases of pre-B ALL and on most, if not all, blasts within individual cases. Furthermore, to date there has been no apparent antigenic loss of CD22 expression despite targeting this antigen with monoclonal antibody-based therapies. The concentrations of shed soluble CD22 detected in circulation and bone marrow are low and not expected to impact the pharmacology of anti-CD22 directed agents. These results suggest that CD22 is an excellent target for monoclonal antibody-based therapies in childhood ALL. The subpopulations of CD22 negative blasts that were detected in some cases of MLL-rearranged ALL indicate that CD22 may not be the ideal target for single-agent therapy; in such cases detailed study of CD22 expression is warranted.
Acknowledgments
We gratefully acknowledge the patients, their families and referring physicians and medical care teams for providing study samples. We would like to recognize the technical assistance of Hong Zhou regarding soluble CD22 assays. We would also like to recognize the statistical assistance provided by Seth Steinberg.
A.S.W., R.J.K., and I.P. are inventors of anti-CD22 immunotoxins with patents held by the National Institutes of Health. A.S.W., R.J.K., and I.P. have received research support from MedImmune related to the development of CD22 targeted agents. A.S.W. has received travel support and an honorarium from MedImmune.
Research support: This work was supported in part by the Intramural Research Program of the National Institutes of Health, National Cancer Institute, Center for Cancer Research and the Warren Grant Magnuson Clinical Center.
Footnotes
Conflict of Interest: All other authors indicate they have no financial disclosures or conflicts of interest to report.
References
- 1.Gloeckler Ries L, Mortality CC. Cancer Incidence and Survival among Children and Adolescents: United States SEER Program 1975-1995. In: Reis LAGSM, Gurney JG, editors. SEER Program. National Cancer Institute; Bethesda: 1999. pp. 165–170. NIH Pub No 99-4649. [Google Scholar]
- 2.Ko RH, Ji L, Barnette P, Bostrom B, Hutchinson R, Raetz E, Seibel NL, Twist CJ, Eckroth E, Sposto R, Gaynon PS, Loh ML. Outcome of patients treated for relapsed or refractory acute lymphoblastic leukemia: a Therapeutic Advances in Childhood Leukemia Consortium study. J Clin Oncol. 2010;28(4):648–654. doi: 10.1200/JCO.2009.22.2950. doi:JCO.2009.22.2950 [pii] 10.1200/JCO.2009.22.2950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oeffinger KC, Mertens AC, Sklar CA, Kawashima T, Hudson MM, Meadows AT, Friedman DL, Marina N, Hobbie W, Kadan-Lottick NS, Schwartz CL, Leisenring W, Robison LL. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355(15):1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 4.Law CL, Aruffo A, Chandran KA, Doty RT, Clark EA. Ig domains 1 and 2 of murine CD22 constitute the ligand-binding domain and bind multiple sialylated ligands expressed on B and T cells. J Immunol. 1995;155(7):3368–3376. [PubMed] [Google Scholar]
- 5.Tedder TF, Tuscano J, Sato S, Kehrl JH. CD22, a B lymphocyte-specific adhesion molecule that regulates antigen receptor signaling. Annual review of immunology. 1997;15:481–504. doi: 10.1146/annurev.immunol.15.1.481. [DOI] [PubMed] [Google Scholar]
- 6.Tedder TF, Poe JC, Haas KM. CD22: a multifunctional receptor that regulates B lymphocyte survival and signal transduction. Advances in immunology. 2005;88:1–50. doi: 10.1016/S0065-2776(05)88001-0. [DOI] [PubMed] [Google Scholar]
- 7.Clark EA. CD22, a B cell-specific receptor, mediates adhesion and signal transduction. J Immunol. 1993;150(11):4715–4718. [PubMed] [Google Scholar]
- 8.Raetz EA, Cairo MS, Borowitz MJ, Blaney SM, Krailo MD, Leil TA, Reid JM, Goldenberg DM, Wegener WA, Carroll WL, Adamson PC. Chemoimmunotherapy reinduction with epratuzumab in children with acute lymphoblastic leukemia in marrow relapse: a Children's Oncology Group Pilot Study. J Clin Oncol. 2008;26(22):3756–3762. doi: 10.1200/JCO.2007.15.3528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kantarjian H, Thomas D, Jorgensen J, Kebriaei P, Jabbour E, Rytting M, York S, Ravandi F, Garris R, Kwari M, Faderl S, Cortes J, Champlin R, O'Brien S. Results of inotuzumab ozogamicin, a CD22 monoclonal antibody, in refractory and relapsed acute lymphocytic leukemia. Cancer. 2013;119(15):2728–2736. doi: 10.1002/cncr.28136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kreitman RJ, Tallman MS, Robak T, Coutre S, Wilson WH, Stetler-Stevenson M, Fitzgerald DJ, Lechleider R, Pastan I. Phase I trial of anti-CD22 recombinant immunotoxin moxetumomab pasudotox (CAT-8015 or HA22) in patients with hairy cell leukemia. J Clin Oncol. 2012;30(15):1822–1828. doi: 10.1200/JCO.2011.38.1756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Haso W, Lee DW, Shah NN, Stetler-Stevenson M, Yuan CM, Pastan IH, Dimitrov DS, Morgan RA, Fitzgerald DJ, Barrett DM, Wayne AS, Mackall CL, Orentas RJ. Anti-CD22-chimeric antigen receptors targeting B cell precursor acute lymphoblastic leukemia. Blood. 2012 doi: 10.1182/blood-2012-06-438002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hoelzer D, Gokbuget N. Chemoimmunotherapy in acute lymphoblastic leukemia. Blood Rev. 2012;26(1):25–32. doi: 10.1016/j.blre.2011.08.001. [DOI] [PubMed] [Google Scholar]
- 13.Raponi S, De Propris MS, Intoppa S, Milani ML, Vitale A, Elia L, Perbellini O, Pizzolo G, Foa R, Guarini A. Flow cytometric study of potential target antigens (CD19, CD20, CD22, CD33) for antibody-based immunotherapy in acute lymphoblastic leukemia: analysis of 552 cases. Leuk Lymphoma. 2011;52(6):1098–1107. doi: 10.3109/10428194.2011.559668. [DOI] [PubMed] [Google Scholar]
- 14.Piccaluga PP, Arpinati M, Candoni A, Laterza C, Paolini S, Gazzola A, Sabattini E, Visani G, Pileri SA. Surface antigens analysis reveals significant expression of candidate targets for immunotherapy in adult acute lymphoid leukemia. Leuk Lymphoma. 2011;52(2):325–327. doi: 10.3109/10428194.2010.529206. [DOI] [PubMed] [Google Scholar]
- 15.Chevallier P, Robillard N, Houille G, Ayari S, Guillaume T, Delaunay J, Harousseau JL, Avet-Loiseau H, Mohty M, Garand R. Simultaneous study of five candidate target antigens (CD20, CD22, CD33, CD52, HER2) for antibody-based immunotherapy in B-ALL: a monocentric study of 44 cases. Leukemia. 2009;23(4):806–807. doi: 10.1038/leu.2008.303. [DOI] [PubMed] [Google Scholar]
- 16.Iwamoto S, Deguchi T, Ohta H, Kiyokawa N, Tsurusawa M, Yamada T, Takase K, Fujimoto J, Hanada R, Hori H, Horibe K, Komada Y. Flow cytometric analysis of de novo acute lymphoblastic leukemia in childhood: report from the Japanese Pediatric Leukemia/Lymphoma Study Group. Int J Hematol. 2011;94(2):185–192. doi: 10.1007/s12185-011-0900-1. [DOI] [PubMed] [Google Scholar]
- 17.Singh R, Zhang Y, Pastan I, Kreitman RJ. Synergistic antitumor activity of anti-CD25 recombinant immunotoxin LMB-2 with chemotherapy. Clin Cancer Res. 2012;18(1):152–160. doi: 10.1158/1078-0432.CCR-11-1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang Y, Hansen JK, Xiang L, Kawa S, Onda M, Ho M, Hassan R, Pastan I. A flow cytometry method to quantitate internalized immunotoxins shows that taxol synergistically increases cellular immunotoxins uptake. Cancer research. 2010;70(3):1082–1089. doi: 10.1158/0008-5472.CAN-09-2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jasper GA, Arun I, Venzon D, Kreitman RJ, Wayne AS, Yuan CM, Marti GE, Stetler-Stevenson M. Variables affecting the quantitation of CD22 in neoplastic B cells. Cytometry B Clin Cytom. 2011;80(2):83–90. doi: 10.1002/cyto.b.20567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schwartz A, Marti GE, Poon R, Gratama JW, Fernandez-Repollet E. Standardizing flow cytometry: a classification system of fluorescence standards used for flow cytometry. Cytometry. 1998;33(2):106–114. [PubMed] [Google Scholar]
- 21.Stetler-Stevenson M, Ahmad E, Barnett D, Braylan RC, DiGiuseppe JA, Marti G, Menozzi D, Oldaker TA, Orfao A, Rabellino E, Stone EC, W C. Clinical and Laboratory Standards Institute. Approved Guideline-Second Edition CLSI document H43-A2. Clinical and Laboratory Standards Institute; Wayne, Pennsylvania: 2005. Clinical Flow Cytometric Analysis of Neoplastic Hematolymphoid Cells. [Google Scholar]
- 22.Matsushita K, Margulies I, Onda M, Nagata S, Stetler-Stevenson M, Kreitman RJ. Soluble CD22 as a tumor marker for hairy cell leukemia. Blood. 2008;112(6):2272–2277. doi: 10.1182/blood-2008-01-131987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kreitman RJ, Pastan I. Accumulation of a recombinant immunotoxin in a tumor in vivo: fewer than 1000 molecules per cell are sufficient for complete responses. Cancer research. 1998;58(5):968–975. [PubMed] [Google Scholar]
- 24.Ogata M, Chaudhary VK, Pastan I, FitzGerald DJ. Processing of Pseudomonas exotoxin by a cellular protease results in the generation of a 37,000-Da toxin fragment that is translocated to the cytosol. The Journal of biological chemistry. 1990;265(33):20678–20685. [PubMed] [Google Scholar]
- 25.Jansen MW, Corral L, van der Velden VH, Panzer-Grumayer R, Schrappe M, Schrauder A, Marschalek R, Meyer C, den Boer ML, Hop WJ, Valsecchi MG, Basso G, Biondi A, Pieters R, van Dongen JJ. Immunobiological diversity in infant acute lymphoblastic leukemia is related to the occurrence and type of MLL gene rearrangement. Leukemia. 2007;21(4):633–641. doi: 10.1038/sj.leu.2404578. [DOI] [PubMed] [Google Scholar]
- 26.Kennedy GA, Tey SK, Cobcroft R, Marlton P, Cull G, Grimmett K, Thomson D, Gill D. Incidence and nature of CD20-negative relapses following rituximab therapy in aggressive B-cell non-Hodgkin's lymphoma: a retrospective review. Br J Haematol. 2002;119(2):412–416. doi: 10.1046/j.1365-2141.2002.03843.x. [DOI] [PubMed] [Google Scholar]
- 27.Grupp SA, Kalos M, Barrett D, Aplenc R, Porter DL, Rheingold SR, Teachey DT, Chew A, Hauck B, Wright JF, Milone MC, Levine BL, June CH. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. The New England journal of medicine. 2013;368(16):1509–1518. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang Y, Pastan I. High shed antigen levels within tumors: an additional barrier to immunoconjugate therapy. Clin Cancer Res. 2008;14(24):7981–7986. doi: 10.1158/1078-0432.CCR-08-0324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Wayne AS, Kreitman RJ, Findley HW, Lew G, Delbrook C, Steinberg SM, Stetler-Stevenson M, Fitzgerald DJ, Pastan I. Anti-CD22 immunotoxin RFB4(dsFv)-PE38 (BL22) for CD22-positive hematologic malignancies of childhood: preclinical studies and phase I clinical trial. Clin Cancer Res. 2010;16(6):1894–1903. doi: 10.1158/1078-0432.CCR-09-2980. doi:1078-0432.CCR-09-2980 [pii] 10.1158/1078-0432.CCR-09-2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wood BL, Arroz M, Barnett D, DiGiuseppe J, Greig B, Kussick SJ, Oldaker T, Shenkin M, Stone E, Wallace P. 2006 Bethesda International Consensus recommendations on the immunophenotypic analysis of hematolymphoid neoplasia by flow cytometry: optimal reagents and reporting for the flow cytometric diagnosis of hematopoietic neoplasia. Cytometry B Clin Cytom. 2007;72(Suppl 1):S14–22. doi: 10.1002/cyto.b.20363. [DOI] [PubMed] [Google Scholar]