Abstract
Cord blood transplantation (CBT) extends allograft access but is associated with a significant risk for cytomegalovirus (CMV) infection. We analyzed CMV infection in 157 CBT recipients transplanted for hematological malignancies. As compared with antigenemia testing, routine PCR monitoring was associated with increased and earlier CMV infection detection [1-year incidence if seropositive 67% (median onset 41 days) versus 100% at an earlier 33 day median (p < 0.001)] and decreased gastro-intestinal disease. One-year CMV-related transplant-related mortality was 11% in CMV+ patients with 7/9 deaths associated with initial infection. Disease-free survival was lower in seropositive compared with seronegative patients (1-yr: 55% versus 73%, p = 0.02). However, in multivariate analysis adjusting for age, treatment failure risk in CMV+ patients was not significant (HR 1.52, p = 0.11). CMV infection is a major challenge in seropositive CBT recipients. While PCR surveillance permits early detection of viremia, new prophylaxis and therapeutic strategies are needed.
Keywords: allogeneic transplantation, cord blood, cytomegalovirus, transplant-related mortality
Introduction
While cord blood (CB) transplantation (CBT) may be curative in patients with high-risk hematologic malignancies, a challenge in CBT recipients is opportunistic viral infections due to the lack of transfer of mature T-lymphocytes within the graft. CB T-cells are naïve, expand slowly in response to antigens, demonstrate a higher threshold for cytokine stimulation, and possess a lower effective cytotoxicity relative to adult-donor T-cells1. Omission of anti-thymocyte globulin (ATG) from the conditioning may assist in immune reconstitution after CBT2, and progressive T-cell recovery has been demonstrated from approximately four months after double-unit CBT3, 4. Nonetheless, patients are highly vulnerable early after transplantation, and the development of effective T-cell immunity can be delayed by graft-versus-host disease (GVHD).
CMV reactivation rates in CMV seropositive CBT recipients are as high as 100%5-7; CMV disease rates vary from 6-27%6-8. Moreover, while Beck et al did not detect differences in disease-free survival (DFS) according to CMV seropositivity in CBT recipients7, CMV seropositivity could potentially increase transplant-related mortality (TRM) risk9. Herein, we investigate the incidence, nature, and mortality of CMV infection in 157 recipients of double-unit CBT, and evaluate the effect of changing to PCR-based CMV post-transplant surveillance.
Materials and Methods
Patient and Graft Characteristics
This retrospective study was performed in patients transplanted at Memorial Sloan Kettering Cancer Center (MSKCC) between 10/2005 and 5/2012. The analysis was approved by the MSKCC Institutional Review/Privacy Board. All CBT recipients during this period received double-unit grafts. Eligible patients for this analysis included all consecutive adult and pediatric double-unit CBT recipients transplanted for hematologic malignancies. Double-unit CB grafts were selected on the basis of donor-recipient 4-6/6 HLA-A,-B antigen, -DRB1 allele match, cryopreserved total nucleated cell (TNC) dose ≥ 1.5 (increased to ≥ 2.0 × 107/kilogram (kg)/ unit in 2011), and the CB bank10.
Conditioning Regimens and GVHD Prophylaxis
Pre-transplant conditioning varied according to patient's age, diagnosis, remission status, prior therapy, and co-morbidities, and consisted of myeloablative and non-myeloablative regimens11-13 (Table 1). Granulocyte-colony-stimulating factor was given to all patients post-transplant, immunosuppression was a calcineurin inhibitor (predominantly cyclosporine-A) and mycophenolate mofetil starting day −3, and no patient received ATG.
Table 1.
Patient demographics and characteristics of the dominant unit in engraftment* (n = 157).
| Characteristics | CMV+ (n = 85) | CMV- (n = 72) | All patients (n = 157) |
|---|---|---|---|
| Median age (range), years | 47 (1-69) | 33 (0.9-61) | 39 (0.9-69) |
| Median weight (range), kg | 67 (10-111) | 69 (8-125) | 67 (8-125) |
| Diagnosis, n (%) | |||
| Acute myeloid leukemia | 33 (39%) | 20 (28%) | 53 (34%) |
| Acute lymphoblastic leukemia | 17 (20%) | 18 (25%) | 35 (22%) |
| Other acute leukemia, MDS or CML | 5 (6%) | 8 (11%) | 13 (8%) |
| Lymphoma or CLL | 30 (35%) | 26 (36%) | 56 (36%) |
| Preparative regimen*, n (%) | |||
| Myeloablative | 62 (73%) | 59 (82%) | 121 (77%) |
| Non-myeloablative | 23 (27%) | 13 (18%) | 36 (23%) |
| HLA-match** | |||
| 6/6 | - | 6 | 6 |
| 5/6 | 46 | 36 | 82 |
| 4/6 | 39 | 30 | 69 |
| Median (range) 10 allele | 6/10 (2-9/10) | 6/10 (2-9/10) | 6/10 (2-9/10) |
| Median (range) inf. cell dose** | |||
| Infused TNC × 107/kg | 2.2 (1.2-12.7) | 2.3 (1.3-11.3) | 2.2 (1.2-12.7) |
| Infused CD34+ cells × 105/kg | 0.9 (0.2-6.9) | 1.0 (0.1-4.7) | 1.0 (0.1-6.9) |
CMV indicates cytomegalovirus; + or - indicates recipient CMV serostatus positive or negative respectively; kg, kilogram; n, number; MDS, myelodysplasia; CML, chronic myelogenous leukemia; CLL, chronic lymphocytic leukemia; HLA, human leukocyte antigen; inf., infused; TNC, total nucleated cell.
Myeloablative regimens included high dose 1320-1375 cGy total body irradiation based regimens (n = 50), high dose chemotherapy-based (n = 17, predominantly Clo/Mel/Thio), or reduced intensity but functionally myeloablative regimens (n = 56 including 47 Cy/Flu/Thio/TBI400, 8 Mel/Flu, 1 other). Non-myeloablative conditioning was Cy/Flu/TBI200 (n = 34).
Unit dominating in hematopoiesis based on blood and/or bone marrow chimerism analyses irrespective of success of clinical neutrophil engraftment.
Anti-viral Prophylaxis, CMV Surveillance and Therapy
Pre-transplant CMV serostatus was determined on all patients. All patients received standard dose intravenous acyclovir (250 mg/m2 every 8 hours) for herpes simplex and varicella zoster virus prophylaxis adjusted for renal function but no CMV-specific prophylaxis. Monitoring for CMV reactivation was done twice weekly while inpatient in CMV seropositive patients from approximately day 14 after CBT, at least once weekly in CMV seronegative patients, and at least weekly from discharge until day 100 in all patients, and thereafter as clinically indicated.
Between 10/2005 and 5/2010, surveillance was performed by pp65 antigenemia assay +/− sporadic send-out plasma quantitative polymerase chain reaction (qPCR, lower limit of quantitation 100 copies/ml) at the treating physician's discretion. Since June 2010, patients were monitored by routine MSKCC whole blood qPCR (LightCycler® CMV Quantitative Kit, Roche Diagnostics, Indianapolis, IN, quantitation lower limit 500 copies/ml). The level of viremia prompting pre-emptive therapy during the antigenemia era was usually > 1 cell/slide and in the PCR era was ≥ 1000 copies/ml. Preemptive treatment included foscarnet, ganciclovir, or valganciclovir depending on blood counts and renal function. Duration of CMV active therapy and continuation of valganciclovir as secondary prophylaxis were at the treating physician's discretion.
Study Definitions
Viremia was defined as positive antigenemia (≥ 1 cell/slide or ≥ 1 cell on one slide on ≥ 2 occasions), or detection of CMV DNA in whole blood qPCR. CMV infection was viremia and/or end-organ disease. By definition, one infection had a maximum duration of 60 days. Therefore, persistent or recurrent infection after 60 days was defined as a second infection14. CMV disease was defined by standardized criteria15, 16. Thus, CMV pneumonia was clinical pulmonary disease combined with the detection of CMV in bronchoalveolar lavage fluid or lung tissue by culture and/or histopathologic testing, and PCR positive broncho-alveolar lavage was not sufficient for the diagnosis of pneumonia16. Gastrointestinal disease was defined as clinical gastrointestinal symptoms with demonstration of CMV infection (by culture and/or histopathologic testing) in biopsies.
Statistical Methods
Descriptive statistics were used to compare patient and graft characteristics according to CMV sero-status. A Wilcoxon rank-sum statistic compared patient age according to sero-status. The competing events in cumulative incidence estimates of CMV infection was relapse or death, and for CMV-related TRM was relapse or death unrelated to CMV. DFS was calculated by Kaplan-Meier methodology. Cumulative incidence and survival were compared across CMV serostatus using Gray's test and the logrank test, respectively. Cox regression was used to assess the association between serostatus and DFS while adjusting for recipient age. Analyses were performed in R statistical software version 2.13.2 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Characteristics of Patients and Grafts
Patient and dominant unit characteristics are summarized in Table 1. Of 157 patients (median age 39 years), 85 (54%) were CMV seropositive. CMV seronegative patients (n = 72) were younger (p < 0.001). The CMV-positive and seronegative groups were otherwise similar.
Manifestations of CMV Infection
CMV infection was only observed in CMV seropositive patients. With a 37 month (range 5-83) median follow-up in seropositive survivors, 66/85 seropositive patients had CMV infection during the study period for an one-year cumulative incidence of 76% (95%CI: 65-84). Of these, 51/66 (77%) patients developed CMV viremia only, 2 developed viremia with CMV pneumonia, 4 developed viremia with pulmonary compromise during initial infection, 8 developed CMV gastro-intestinal (GI) disease, and one had culture-positive CMV upper respiratory tract infection (URI) without viremia. Thus, 10/157 (6%) CBT recipients overall, or 10/85 (12%) of CMV seropositive patients, developed proven CMV pneumonia or GI disease.
Comparison of Surveillance Methods: Antigenemia versus PCR
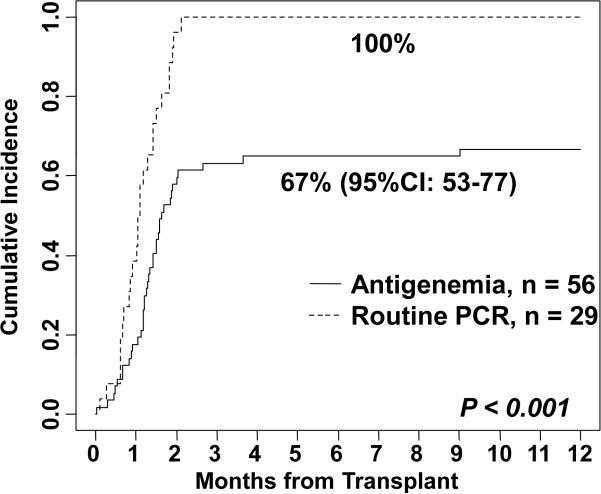
There was a significant difference in CMV detection according to monitoring method (Figure 1). Of the 56 antigenemia (+/− sporadic PCR) seropositive patients, 37 reactivated (median onset 41 days, range −1 - 274) for a 1-year cumulative incidence of 67% (95%CI: 53-77). CMV infection was first detected by antigenemia in 34 of these patients (median onset 41 days, range −1 - 274) with a median of 1 cell/slide at onset (range 1-14) and a peak viremia of 2 cells/slide (range 1 - >100) at a 51 day median (range −1 - 274) after CBT. CMV infection in the 3 remaining patients was diagnosed by sporadic PCR in one (onset 20 days), one presented with GI disease without detectable antigenemia, and one had a positive upper respiratory culture without viremia. Overall, of antigenemia patients, 1 patient developed CMV pneumonia (onset 58 days, Table 2, patient 1), 1 had pulmonary decompensation in the setting of viremia (onset 39 days, Table 2, patient 3), and 5 had GI disease [median onset 54 days (range 49-67), Table 3, patients 1-5] with initial reactivation.
Figure 1.
The cumulative incidence of CMV infection in the first year after CBT in CMV seropositive recipients according to the method of monitoring.
Table 2.
CMV-related Pneumonia or Pulmonary Complications (n = 6).
| Patients | Day of First Viremia | Day of First CMV Therapy / Agent | Day of Onset of Resp Distress | Level of Viremia at Disease Onset / Peak | Method of Diagnosis | Day of Cortico-Steroid Therapy | Day of Death / Primary COD |
|---|---|---|---|---|---|---|---|
| Proven Pneumonia | |||||||
| 1) 64 yrs, NHL, NMA | +11 | +56 / Ganciclovir | Oxygen +58, Intubation +60 | 61 cells/sl (+60)/61 cells/sl (+60) | Clinical (confirmed at autopsy) | N/A | +69 /CMV infection |
| 2) 46 yrs, NHL, MA-RIC | +32 | +42 / Foscarnet | Oxygen +53, Intubation +68 | 1580 copies (+57)/ 3720 copies (+50) | +56: BAL culture positive | +65 | +102 / CMV infection |
| Pulmonary Decompensation in the Setting Of CMV Viremia | |||||||
| 3) 10 yrs, ALL, MA-High | +20 | +22 / Foscarnet | Oxygen +39, Intubation +41 | 3000 copies (+36)/ 6500 copies (+25) | Clinical (FOB non-diagnostic) | N/A | +56 / GF |
| 4) 7 yrs, ALL, MA-High | +3 | +5 / Foscarnet | Oxygen +8, Intubation +10, Extubation +18 | 5251 copies (+10)/ 8464 copies (+28) | Clinical (no FOB) | +10 | +356 / Relapse |
| 5) 62 yrs, MDS, MA-RIC | +19 | +20 / Foscarnet | Oxygen +29, Intubation +43, Extubation +48 | 2200 copies (+29)/ 2420 copies (+33) | +44: Biopsy showed organizing pneumonitis | +43 | +228 / Relapse |
| 6) 55 yrs, AML, MA-RIC | +20 | +32 / Foscarnet | Oxygen +31, Intubation +32, Extubation +38 | 2610 copies (+31)/ 2610 copies (+31) | +32: BAL PCR positive | +34 | +51 / MOF |
CMV indicates cytomegalovirus; COD, cause of death; NHL, non-Hodgkin lymphoma; ALL, acute lymphoblastic lymphoma; MDS, myelodysplasia; AML, acute myelogenous leukemia; MA-High, high-dose myeloablative conditioning; MA-RIC, reduced intensity but functionally myeloablative conditioning; NMA, non-myeloablative conditioning; cells/sl, cells per slide; BAL, bronchioalveolar lavage; FOB, fiber-optic bronchoscopy; GF, graft failure; MOF, multi-organ failure.
Table 3.
CMV GI Disease (n = 8)
| Patient Details | Method of Monitoring / Viremia Prior to Disease? | Day of Disease Onset / Site | Level of Viremia at Disease Onset / Peak | GVHD Prior to CMV Disease? / Max. Gr. aGVHD at Day 180 | Survival / COD |
|---|---|---|---|---|---|
| 4A) CMV Disease with Initial Infection | |||||
| 1) 48 yrs, AML, MA-High | Antigenemia / No (viremia after disease diagnosis) | +67 / Stomach | None/ 1 cells/sl (+81) | No / Grade II | Alive > 4 years |
| 2) 29 yrs, HL, NMA | Antigenemia / Yes (+36) | +54 / Colon | 1 cells/sl (+54)/ 10 cells/sl (+50) | Yes Grade II | Alive > 4 years |
| 3) 52 yrs, NHL, NMA | Antigenemia / Yes (+15) | +54 / Small Intestine (Jejunum) | 1 cells/sl (+54)/ 4 cells/sl (+26) | Yes / Grade III | Died +70 / GVHD |
| 4) 64 yrs, MDS, MA-RIC | Antigenemia / No (never viremic) | +58 / Gastric | None/ None | No / Grade 0 | Alive > 3 years |
| 5) 29 yrs, NHL, NMA | Antigenemia / No (simultaneous onset of viremia and disease) | +49 / Small Intestine (Ileum) | 1 cells/sl (+49)/ 4314 copies (+114) | No / Grade IV | Died +184 / GVHD |
| 4B) Late CMV Disease in Setting of GVHD | |||||
| 6) 23 yrs, AML, MA-High | Antigenemia / Yes (+16) | +162 / Small Intestine (Duodenum) | 4302 copies (+162)/ 18,168 copies (+161) | Yes / Grade IV | Died +266 / GVHD |
| 7) 63 yrs, NHL, NMA | PCR / Yes (+28) | +98 / Colon | None (+98)/ 9014 copies (+14) | Yes / Grade II | Died +175 / GVHD |
| 8) 47 yrs, AML, MA-RIC | PCR / Yes (+43) | +133 / All GI* | 27020 (+131)/ 118,200 copies (+117) | Yes / Grade III | Died +133 / GVHD |
Documented at autopsy.
CMV indicates cytomegalovirus; aGVHD, acute graft-versus-host disease; COD, cause of death; AML, acute myelogenous leukemia; HL, Hodgkin lymphoma; NHL, non-Hodgkin lymphoma; MDS, myelodysplasia; MA-High, high-dose myeloablative conditioning; MA-RIC, reduced intensity but functionally myeloablative conditioning; NMA, non-myeloablative conditioning; cells/sl, cells per slide; PCR, polymerase chain reaction.
In contrast, in CMV+ recipients routinely monitored by PCR, CMV reactivation was detected in all 29 (100%) patients at an earlier 33 day (range 3-64) median (p < 0.001, Figure 1). Twenty-eight of these 29 (97%) patients reactivated by day 60. The median viral load at detection was 1134 copies/ml (range 100-7901). All patients with an initial PCR < 1000 copies/ml subsequently progressed to > 1000 copies/ml at a median of 36 days (range 3-96) and the median peak viremia of 3862 copies (range 971-146,000) occurred at a median of 46 days (range 19-90). While one PCR patient developed pneumonia (Table 2, patient 2) and 3 had pulmonary decompensation during their first infection (median onset 30 days, range 8-53, Table 2, patients 4-6), no patient had GI disease during their initial infection. There was no difference in the time from initial detection to therapy initiation in the antigenemia (median 3 days, range 1-62) and routine PCR (median 4 days, range 0-18) groups (p = NS).
CMV Pneumonia or Pulmonary Decompensation in the Setting of Viremia
The details of CMV pneumonia or pulmonary decompensation in the setting of viremia are summarized in Table 2. All pulmonary complications occurred in the first 2 months after CBT as part of the first infection. Two patients with advanced lymphoid malignancies had lethal CMV pneumonia. In each, treatment was initiated 45 days and 10 days after initial CMV detection, respectively, due to marked cytopenia and renal impairment complicating therapy. Four additional patients had pulmonary failure in the setting of CMV viremia without fulfilling criteria for CMV pneumonia (although one had a PCR+ broncho-alveolar lavage, Table 2, patient 6). In these patients diffuse alveolar hemorrhage and other documented infections were excluded and there was no relationship with regimen intensity. All 4 patients were intubated; 3 treated with corticosteroids for a potential inflammatory component of their decompensation were subsequently extubated. However, all subsequently died of transplant-related causes (n = 2) or relapse (n = 2).
CMV-Related GI Disease
The details of CMV-related GI disease (n = 8) are summarized in Table 3. Five antigenemia patients had early GI disease during their first CMV infection (median onset 54 days, range 49-67) (Table 3A). GI disease was the first manifestation of CMV infection in 3 (with one never having viremia) whereas 2 patients with GVHD developed GI disease despite initially receiving pre-emptive therapy for viremia. Two of these 5 patients with GI disease with their initial CMV infection died from GVHD. No patient monitored by routine PCR developed GI disease with initial infection. However, late CMV GI disease developed in 3 GVHD patients (Table 3B). All had prior viremia, and all died.
Recurrent Infection
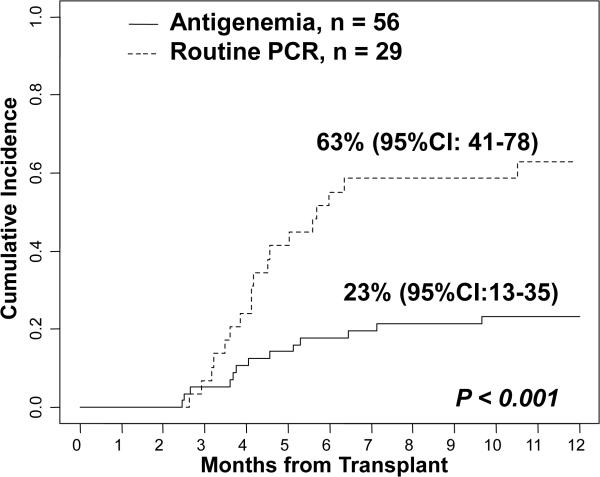
The cumulative incidence of a second CMV infection by one-year post-transplant in patients monitored by antigenemia was 23% (95%CI: 13-35). This compared to 63% (95%CI: 41-78) in those monitored by PCR (p < 0.001, Figure 2).
Figure 2.
The cumulative incidence of a second CMV infection in CMV seropositive patients monitored by antigenemia (n = 56) versus routine PCR (n = 29).
Transplant Outcomes and Mortality By CMV Serostatus
CMV infection was the primary cause of death in 2 patients with pneumonia. In 2 with viremia and pulmonary compromise, CMV likely contributed to death (primary cause one graft failure and one multi-organ failure, Table 2, patients 3 and 6). In 5 patients with CMV GI disease who died, the primary cause of death was GVHD but active CMV likely contributed (Table 3). Thus, with a median 39 month (range 5-83) follow-up of survivors, the 1-year cumulative incidence of CMV-related TRM in CMV seropositive patients was 11% (95%CI: 5-18). Seven of 9 deaths occurred with the initial infection. No end-organ disease or CMV-related death occurred in the absence of active acute GVHD beyond day 100 post-CBT, and all CMV deaths occurred within 9 months post-CBT.
As only CMV seropositive CBT recipients reactivated CMV, and the majority monitored by antigenemia and all monitored by PCR reactivated CMV, transplant outcomes were compared in all CMV seropositive versus all CMV seronegative recipients (Table 4). The speed and success of neutrophil recovery was similar in the two groups. While the incidence of day 100 grade II-IV acute GVHD was not different, there was a trend that GVHD lethality was higher in CMV seropositive patients independent of age. Of adults ≥ 30 years, for example, 29/63 seropositive patients had grade 2-4 aGVHD and 8/29 (28%) died of GVHD. By comparison, of 38 seronegative patients, 25 had grade 2-4 aGVHD and only 2/25 (8%) died of GVHD (p = 0.09).
Table 4.
Univariate analysis of transplant outcomes according to CMV serostatus (n = 157).
| Outcome* | CMV+ (n = 85) | CMV- (n = 72) | P value |
|---|---|---|---|
| Day 45 Engraftment | 97% (95%CI:87-99) | 94% (95%CI:86-98) | P = 0.10 |
| Day 100 Grade II-IV aGVHD | 41% (95%CI:31-51) | 60% (95%CI:47-70) | p = 0.21 |
| 1-yr Chronic GVHD | 11% (95%CI: 5-18) | 14% (95%CI:7-23) | P = 0.54 |
| 1-yr Relapse | 16% (95%CI:9-24) | 13% (95%CI:6-21) | p = 0.48 |
| 1-yr TRM | 29% (95%CI:20-39) | 14% (95%CI:7-23) | p = 0.04 |
| 1-yr DFS | 55% (95%CI:45-67) | 73% (95%CI:64-84) | p = 0.02 |
Engraftment, aGVHD, relapse, and TRM reflect cumulative incidence estimates whereas DFS is calculated using Kaplan-Meier methodology.
The 29% (95%CI:20-39) 1-year TRM in CMV-seropositive recipients was higher than that of CMV-seronegative recipients, but there were no differences in relapse (Table 4). While DFS was lower in seropositive CBT recipients compared with seronegative patients [1-yr: 55% (95%CI: 45-67) versus 73% (95%CI: 64-84) p = 0.02), in multivariate analysis adjusting for age, the trend of higher treatment failure (death or relapse) in CMV seropositive CBT recipients did not reach significance [hazard ratio 1.52 (95%CI: 0.91-2.54) p = 0.11].
Discussion
In this study, 10/85 seropositive patients had CMV disease (2 pneumonias, 8 GI disease), 7 of whom developed disease with their initial infection. CMV caused or likely contributed to TRM in 11% of CMV seropositive CBT recipients by 1-year post-transplant. While only 2 patients developed pneumonia, both died. GI disease in the setting of GVHD had a high lethality with death in 5/7 patients. Four additional patients had pulmonary decompensation in the setting of viremia early post-transplant without fulfilling diagnostic criteria for CMV pneumonia. It is possible that in these cases donor-derived T-cells are inadequate to control the infection but are able to initiate an inflammatory response resulting in pulmonary decompensation. Overall, while lower DFS in CMV seropositive CBT patients was not significant when controlling for age, CMV infection remains a major cause of morbidity and mortality in seropositive CBT recipients. Furthermore, a higher GVHD lethality in CMV seropositive CBT recipients was suggested.
Our rate of CMV infection detection was dependent on the surveillance method with all CMV+ patients reacting when routinely monitored by a highly sensitive whole blood PCR. Implementation of PCR monitoring enabled earlier detection and initiation of pre-emptive treatment. However, it is possible this assay is overly sensitive. Additionally, although quantitative PCR methods for CMV detection have replaced antigenemia for routine monitoring, until recently there has been a poor inter-laboratory correlation of viral load due to the lack of an international standard. Assays vary by specimen (whole blood or plasma), the detection limit, the limit of quantification, the assay's linear range, and the reproducibility within the institution. The recently approved COBAS® AmpliPrep/COBAS® TaqMan® (CAP/CTM) CMV test calibrated to the first WHO CMV standard should enable comparisons across different centers and patient populations.
These limitations notwithstanding, all patients with low level PCR positivity progressed to > 1000 copies/ml supporting initiation of pre-emptive therapy at first CMV detection in CBT recipients. However, it remains to be definitively proven that such an approach improves clinical outcomes, and the toxicity of currently available anti-viral agents must be considered. It is notable, however, that since the introduction of routine PCR monitoring, no patient has developed GI disease with their initial infection. We postulate the earlier detection and treatment of viremia in PCR monitored patients has abrogated GI disease with initial CMV reactivation. Larger prospective studies using standardized PCR methodologies and clinical endpoints are needed to establish clinically relevant thresholds of CMV viremia in CBT patients and the effect of early therapy on TRM.
The high level of CMV reactivation in PCR monitored seropositive CBT recipients suggests that new strategies for CMV prophylaxis and therapy are needed. In response to this analysis, we have recently implemented ganciclovir prophylaxis during the conditioning in CMV seropositive recipients as recommended by Milano et al6, and evaluation of the efficacy of this strategy is forthcoming. While only a randomized prospective trial of pre-emptive therapy would be definitive, we now also initiate CMV specific anti-viral therapy at any level of PCR detection early post-CBT6. New agents such as maribavir17 or liposomal cidofovir (brincidofovir)18 should also be explored as prophylaxis or pre-emptive therapy. This is of particular importance in CBT recipients in whom delayed myeloid recovery and the use of nephrotoxic calcineurin-inhibitor based immunosuppression increases the toxicity of ganciclovir and foscarnet, respectively. CMV-specific cytotoxic T-cells could also be beneficial in selected patients19, 20, and more effective GVHD prevention could be advantageous provided it does not hamper CMV-specific T-cell recovery.
Research into the determinants of CMV-specific immune recovery such as the studies of Brown et al21 and McGoldrick et al22 is also a priority for CBT recipients. This could assist in the determination of patients’ vulnerability for end-organ disease during the first infection, and the risk of CMV infection in asymptomatic patients in whom recurrent low level viremia is detected later in their transplant course. It is notable that despite ATG omission, all seropositive PCR patients reactivated demonstrating no advantage to avoiding ATG from the standpoint of initial CMV infection. This is in contrast to the risk of EBV infection, for example, that is increased in ATG-based CBT. However, no CMV seropositive patient died of CMV-related illness beyond day 100 in the absence of significant acute GVHD in this study. This could represent indirect evidence of the development of CMV-specific immunity in CBT survivors. We have demonstrated progressive T-cell recovery from approximately four months after double-unit CBT and recently showed that TCR diversity (as measured by deep sequencing of CD4+ and CD8+ T-cells) in CBT survivors approximates that of healthy individuals by 6 months after CBT3,4. Further investigation is required to identify determinants of CB-derived CMV-specific T-cell immune reconstitution and how this could be augmented.
Acknowledgements
This work was supported in part by the Gabrielle's Angel Foundation for Cancer Research (J.N.B.), the Society of Memorial Sloan-Kettering Cancer Center (J.N.B. and S.G.), the Memorial Sloan-Kettering Cancer Center Translational and Integrative Medicine Research Program (J.N.B.), the American Society of Clinical Oncology Young Investigator Award (C.S.), and P01 CA23766 from the National Cancer Institute, National Institutes of Health.
Footnotes
Author Contributions
P.B.D. collected and analyzed the data and wrote the manuscript. M.A.P, S.M.D., M. L., A.M.G. analyzed the data. A.O., A.S., N.A.K., R.O'R., S.G., A.J., G.K., E.B.P., D.P., C.S., and G.P. wrote the manuscript. J.N.B designed the study, collected and analyzed the data and wrote the manuscript.
Conflict of Interest
The authors have no relevant conflicts of interest to declare.
References
- 1.Szabolcs P. T-lymphocyte recovery and function after cord blood transplantation. Immunol Res. 2011;49(1-3):56–69. doi: 10.1007/s12026-010-8194-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Chiesa R, Gilmour K, Qasim W, Adams S, Worth AJ, Zhan H, et al. Omission of in vivo T- cell depletion promotes rapid expansion of naive CD4+ cord blood lymphocytes and restores adaptive immunity within 2 months after unrelated cord blood transplant. Br J Haematol. 2012;156(5):656–66. doi: 10.1111/j.1365-2141.2011.08994.x. [DOI] [PubMed] [Google Scholar]
- 3.Sauter C, Abboud M, Jia X, Heller G, Gonzales AM, Lubin M, et al. Serious infection risk and immune recovery after double-unit cord blood transplantation without antithymocyte globulin. Biol Blood Marrow Transplant. 2011;17(10):1460–71. doi: 10.1016/j.bbmt.2011.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.van Heijst JW, Ceberio I, Lipuma LB, Samilo DW, Wasilewski GD, Gonzales AM, et al. Quantitative assessment of T cell repertoire recovery after hematopoietic stem cell transplantation. Nat Med. 2013;19(3):372–7. doi: 10.1038/nm.3100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomonari A, Iseki T, Ooi J, Takahashi S, Shindo M, Ishii K, et al. Cytomegalovirus infection following unrelated cord blood transplantation for adult patients: a single institute experience in Japan. Br J Haematol. 2003;121(2):304–11. doi: 10.1046/j.1365-2141.2003.04264.x. [DOI] [PubMed] [Google Scholar]
- 6.Milano F, Pergam SA, Xie H, Leisenring WM, Gutman JA, Riffkin I, et al. Intensive strategy to prevent CMV disease in seropositive umbilical cord blood transplant recipients. Blood. 2011;118(20):5689–96. doi: 10.1182/blood-2011-06-361618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beck JC, Wagner JE, DeFor TE, Brunstein CG, Schleiss MR, Young JA, et al. Impact of cytomegalovirus (CMV) reactivation after umbilical cord blood transplantation. Biol Blood Marrow Transplant. 2010;16(2):215–22. doi: 10.1016/j.bbmt.2009.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Walker CM, van Burik JA, De For TE, Weisdorf DJ. Cytomegalovirus infection after allogeneic transplantation: comparison of cord blood with peripheral blood and marrow graft sources. Biol Blood Marrow Transplant. 2007;13(9):1106–15. doi: 10.1016/j.bbmt.2007.06.006. [DOI] [PubMed] [Google Scholar]
- 9.Barker JN, Ponce DM, Devlin SM, et al. Double-Unit Cord Blood Transplantation (DCBT) for Acute Leukemia: High Disease-Free Survival in Adults and Children with Comparable Survival in European and Minority Patients. Biol Blood Marrow Transplant. 2013;19(2):S354. [Google Scholar]
- 10.Barker JN, Byam C, Scaradavou A. How I treat: the selection and acquisition of unrelated cord blood grafts. Blood. 2011;117(8):2332–9. doi: 10.1182/blood-2010-04-280966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barker JN, Abboud M, Rice RD, Hawke R, Schaible A, Heller G, et al. A “no-wash” albumin-dextran dilution strategy for cord blood unit thaw: high rate of engraftment and a low incidence of serious infusion reactions. Biol Blood Marrow Transplant. 2009;15(12):1596–602. doi: 10.1016/j.bbmt.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ponce DM, Sauter C, Devlin S, Lubin M, Gonzales AM, Kernan NA, et al. A novel reduced-intensity conditioning regimen induces a high incidence of sustained donor- derived neutrophil and platelet engraftment after double-unit cord blood transplantation. Biol Blood Marrow Transplant. 2013;19(5):799–803. doi: 10.1016/j.bbmt.2013.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ponce DM, Gonzales A, Lubin M, Castro-Malaspina H, Giralt S, Goldberg JD, et al. Graft- versus-Host Disease after Double-Unit Cord Blood Transplantation Has Unique Features and an Association with Engrafting Unit-to-Recipient HLA Match. Biol Blood Marrow Transplant. 2013;19(6):904–911. doi: 10.1016/j.bbmt.2013.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.van Burik JA, Carter SL, Freifeld AG, High KP, Godder KT, Papanicolaou GA, et al. Higher risk of cytomegalovirus and aspergillus infections in recipients of T cell-depleted unrelated bone marrow: analysis of infectious complications in patients treated with T cell depletion versus immunosuppressive therapy to prevent graft-versus-host disease. Biol Blood Marrow Transplant. 2007;13(12):1487–98. doi: 10.1016/j.bbmt.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 15.Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clin Infect Dis. 2002;34(8):1094–7. doi: 10.1086/339329. [DOI] [PubMed] [Google Scholar]
- 16.Boeckh M, Ljungman P. How I treat cytomegalovirus in hematopoietic cell transplant recipients. Blood. 2009;113(23):5711–5719. doi: 10.1182/blood-2008-10-143560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Winston DJ, Young JA, Pullarkat V, Papanicolaou GA, Vij R, Vance E, et al. Maribavir prophylaxis for prevention of cytomegalovirus infection in allogeneic stem cell transplant recipients: a multicenter, randomized, double-blind, placebo-controlled, dose-ranging study. Blood. 2008;111(11):5403–10. doi: 10.1182/blood-2007-11-121558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marty FM, Winston DJ. Rowley Sea. CMX001 to Prevent Cytomegalovirus disease in Hematopoietic-Cell Transplantation. N Engl J Med. 2013;369:1227–1236. doi: 10.1056/NEJMoa1303688. [DOI] [PubMed] [Google Scholar]
- 19.Hanley PJ, Cruz CR, Savoldo B, Leen AM, Stanojevic M, Khalil M, et al. Functionally active virus-specific T cells that target CMV, adenovirus, and EBV can be expanded from naive T-cell populations in cord blood and will target a range of viral epitopes. Blood. 2009;114(9):1958–67. doi: 10.1182/blood-2009-03-213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leen AM, Bollard CM, Mendizabal AM, Shpall EJ, Szabolcs P, Antin JH, et al. Multicenter study of banked third-party virus-specific T cells to treat severe viral infections after hematopoietic stem cell transplantation. Blood. 2013;121(26):5113–23. doi: 10.1182/blood-2013-02-486324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brown JA, Stevenson K, Kim HT, Cutler C, Ballen K, McDonough S, et al. Clearance of CMV viremia and survival after double umbilical cord blood transplantation in adults depends on reconstitution of thymopoiesis. Blood. 2010;115(20):4111–9. doi: 10.1182/blood-2009-09-244145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McGoldrick SM, Bleakley ME, Guerrero A, Turtle CJ, Yamamoto TN, Pereira SE, et al. Cytomegalovirus-specific T cells are primed early after cord blood transplant but fail to control virus in vivo. Blood. 2013;121(14):2796–803. doi: 10.1182/blood-2012-09-453720. [DOI] [PMC free article] [PubMed] [Google Scholar]