Abstract
Objective
Normal childhood development is defined by age-dependent improvement across cognitive abilities, including language, memory, psychomotor speed and executive function. Epilepsy is often associated with a global disruption in cognitive development, however, it is still largely unknown how epilepsy affects the overall organization of overlapping cognitive domains. The aim of the study was to evaluate how childhood epilepsy affects the developmental interrelationships between cognitive domains.
Methods
We performed a comprehensive assessment of neuropsychological function in 127 children with new onset epilepsy and 80 typically developing children matched for age, gender, and socio-demographic status. A cross-correlation matrix between the performances across multiple cognitive tests was used to assess the interrelationship between cognitive modalities for each group (patients and controls). A weighted network composed by the cognitive domains as nodes, and pair-wise domain correlation as links, was assessed using graph theory analyses, with focus on global network structure, network hubs and community structure.
Results
Normally developing children exhibited a cognitive network with well-defined modules, with verbal intelligence, reading and spelling skills occupying a central position in the developing network. Conversely, children with epilepsy demonstrated a less well-organized network with less clear separation between modules, and relative isolation of measures of attention and executive function.
Conclusion
Our findings demonstrate that childhood-onset epilepsy, even within its early course, is associated with an extensive disruption of cognitive neurodevelopmental organization. The approach used in this study may be useful to assess the effectiveness of future interventions aimed at mitigating the cognitive consequences of epilepsy.
Keywords: cognitive development, cognitive network, early-onset epilepsy, graph theory, neuropsychological assessment
1. Introduction
Normal cognitive development is characterized by the - age dependent harmonious improvement across multiple cognitive abilities. There is now considerable agreement that childhood onset epilepsy disrupts this systematic developmental process with adverse impact on executive function (Bell, Lin, Seidenberg, & Hermann, 2011; D’Agati, Cerminara, Casarelli, Pitzianti, & Curatolo, 2012; Seidenberg et al., 1986), academic skills (Berg et al., 2005; Seidenberg et al., 1986), linguistic abilities (Caplan et al., 2008; Caplan et al., 2009) and emotional/behavioral quality of life (Berg et al., 2008; C. Camfield, Breau, & Camfield, 2003; P. Camfield & Camfield, 2003). However, cognitive domains do not operate in isolation and it remains undefined how epilepsy influences the interactions among different neuropsychological domains.
Recent cognitive training studies support the notion that the interrelationship among domains is crucial for academic success in school-aged children. First, fluid intelligence and problem solving skills can be improved when children engage in working memory exercises (Jaeggi, Buschkuehl, Jonides, & Shah, 2011). Second, training in executive function enhances other domains including language and mathematical skills (Goldin et al., 2014). Therefore, the understanding of the inter-relationship among cognitive domains can provide crucial insight on how childhood-onset epilepsy interferes with the global structure of cognitive development. It may also aid in the identification of strategies for future targeted intervention.
In this study, we employed graph theory to investigate the impact of epilepsy on the global cognitive landscape. Graph theory is a mathematical method that has recently been applied to examine brain network structure in epilepsy, revealing global disruption in brain architecture (Bonilha et al., 2013; Bonilha et al., 2012; Vaessen et al., 2014; Vaessen et al., 2012). Large –scale structural morphometrical brain changes have been correlated with specific cognitive deficits in epilepsy (Alexander, Concha, Snyder, Beaulieu, & Gross, 2014; Bonilha, Tabesh, et al., 2014). However, to date, there has not been a comprehensive examination of neuropsychological measures, as a cognitive network per se, using graph theory. To our knowledge, this study is the first to use graph theory measures to investigate the cognitive network in children with epilepsy.
We investigated the global cognitive network in a large cohort of children with new-onset epilepsy and healthy controls by examining the inter-correlations of 23 neuropsychological measures. The neuropsychological tests were selected to provide a broad profile of cognitive function, indexing abilities in six discrete cognitive domains: intelligence, academic performance, language, memory, executive function, and psychomotor speed.
We hypothesized that epilepsy may disrupt age-related cognitive development, which is manifested in altered interactions among different cognitive domains. We therefore expected to find a different arrangement of the cognitive network in children with epilepsy compared with healthy controls.
2. Methods
2.1. Subjects
We assessed data from 127 children with epilepsy (mean age: 12.31 years; SD=3.17; girls=67, boys=60)) and 80 healthy controls (mean age: 12.69 years; SD=3.17; girls=39, boys=60). There were no significant differences between the epilepsy and control families in terms of parent intelligence quotient (IQ; Wechsler Abbreviated Scale of Intelligence, controls: 109.7 ± 12.22, patients: 110.82 ± 15.41, p = 0.80), or parental employment status (full-time, part-time, unemployed; mother’s, chi-square = 4.72, p = 0.19; father’s, chi-square = 2.68, p = 0.44), indicating similar socioeconomic status between the groups. The educational background was also similar between patients and controls (patient mean grade= 6.2, control mean grade= 6.4, p=0.69. About 53% of patients with epilepsy and 20% of controls received educational services in the past. All subjects included in this study were enrolled in regular schools.
Inclusion criteria for the patient group were: 1) diagnosis of epilepsy within the past 12 months, 2) no other neurological disorders, 3) normal neurological examinations, and 4) normal clinical imaging results. A board- certified pediatric neurologist (blinded to neuropsychological and interview data) confirmed that all patients had idiopathic epilepsies and provided independent confirmation of syndrome diagnosis. All patients were diagnosed according to the criteria defined by the International League Against Epilepsy (ILAE) (Blume et al., 2001; Engel, 2006). We excluded children with intellectual disability (IQ < 70), autism, and/or other neurological disorders. Specifics regarding the participant selection process have been described in detail in a previous publication (Hermann et al., 2006). In general, we tried to stay true to the concept of “epilepsy only” as defined broadly in the literature: normal neurological exams, average intelligence, and attendance at regular schools.
Healthy controls were age- and gender-comparable first-degree cousins of the epilepsy participants. All controls had no history of seizures, early initial precipitating injuries (e.g., febrile convulsions), other developmental or neurologic disease, or episodes of loss of consciousness. Research approval was obtained from the Institutional Review Board at the University of Wisconsin Medical School. Written informed consent was obtained for all subjects from legal guardians. Parents underwent a clinical interview and completed questionnaires to characterize gestation, delivery, neurodevelopment, and seizure history. All pertinent medical records were obtained after signed release of information was obtained from the parent. Parents were also interviewed through a structured questionnaire about their child’s school progress and any specific educational services provided to address academic problems (AP). These services included the traditional individualized educational plan (IEP) as well as early childhood interventions (e.g., speech, physical or occupational therapy), mandatory summer school, grade retention, special tutoring services, and other related services. This interview was conducted blinded to cognitive and behavioral results.
2.2. Neuropsychological Assessment
A neuropsychological test battery involving 23 different cognitive tests was administered to all subjects, all of which are listed in Table 2. This battery assessed multiple domains including intelligence, academic skills, language, memory, executive function and cognitive/psychomotor speed. The measures listed in Table 2 came from the following tests: Wechsler Abbreviated Scale of Intelligence (WASI) (Wechsler, 2003), Peabody Picture Vocabulary Test (PPVT) (Dunn & Hottel, 1961), Expressive Vocabulary Test (EVT) (Williams, 1997), Wide Range Achievement Test-IV (WRAT-IV, 2006), Wechsler Intelligence Scale for Children (WISC-IV) (Wechsler, 2003), Continuous Performance Task (CPT), (Conners, 1992), Delis-Kaplan Executive Function System (D-KEFS) (Delis, 2001), and Boston Naming Test (BNT) (Kaplan, 1983)).
Table 2.
Neuropsychological test battery
Neuropsychological test battery employed in this study. The ordering of tests demonstrated here is the same as from the adjacency matrices in Figures 1 and Supplementary
| Abbreviation | Test Name | Cognitive ability | |
|---|---|---|---|
| 1 | IQVOCS | WASI Vocabulary | Verbal intelligence |
| 2 | IQBDS | WASI Block Design | Nonverbal Intelligence |
| 3 | IQSIMS | WASI Similarities | Verbal intelligence |
| 4 | IQMRS | WASI Matrix Reasoning | Nonverbal intelligence |
| 5 | PPVTSTN | Peabody Picture Vocabulary Test | Language (word recognition) |
| 6 | EVTSTN | Expressive Vocabulary Test | Language (word naming) |
| 7 | READSTN | WRAT-IV Reading | Word recognition |
| 8 | SPELSTN | WRAT-IV Spelling | Spelling |
| 9 | ARITSTN | WRAT-IV Arithmetic | Arithmetic calculation |
| 10 | IQDSYMS | WISC-IV Digit symbol | Speed |
| 11 | WLLSS | Children’s Memory Scale-III | Verbal memory |
| 12 | WLDSS | Children’s Memory Scale-III | Verbal memory |
| 13 | CPOMT | Continuous Performance Test-II | Executive (attention) |
| 14 | CPCOMMT | Continuous Performance Test-II | Executive function |
| 15 | CPRTBLKT | Continuous Performance Test-II | Executive function (attention) |
| 16 | LETFLUS | D-KEFS Letter Fluency | Language (lexical fluency) |
| 17 | CATFLUS | D-KEFS Category Fluency | Language (semantic fluency) |
| 18 | CATSWS | D-KEFS | Executive function (category switching) |
| 19 | COLSS | D-KEFS Color-Word | Speed |
| 20 | WORDSS | D-KEFS Color-Word | Speed |
| 21 | INHSS | D-KEFS Inhibition | Executive function (response inhibition) |
| 22 | CORSORS | D-KEFS Card Sorting Test | Executive function (problem solving) |
| 23 | BNTTOT | Boston Naming Test | Language (naming) |
2.3. Network Analysis
In order to define the inter-relationship between cognitive domains, we assessed a network of cognitive functions defined as the correlation matrix between performances on the neuropsychological tests (separately for each group, i.e., patients and controls). Specifically, a correlation coefficient was calculated between each possible pair of tests, resulting in 253 possible test combinations. A correlation table was constructed based on these associations, representing an undirected (i.e., symmetrical along the diagonal) weighted adjacency matrix for each group (patients and controls).
Prior to being submitted to the correlations, all tests were adjusted to reflect a higher score for better performance. After the adjacency matrices were constructed, only positive correlation coefficients were maintained to enable the visual representation of networks and graph theory metric calculations. Thus, the cognitive network was comprised of 23 nodes, each one representing each cognitive test, and weighted links between nodes representing the strength of positive correlation between each pair of nodes.
2.3.1. Visual representation of network structure
In order to appreciate the overall structure of cognitive development in each group, we reconstructed two-dimensional graphs based on the correlation adjacency matrices. The data were exported to the software Gephi (http://gephi.github.io/), where they were displayed using a Force Atlas algorithm (Jacomy, Venturini, Heymann, & Bastian, 2014) (attraction strength =10, repulsion strength =100, gravity=30). To improve visualization of the network structure, we reconstructed the graphs based on networks containing only the links above the 70% percentile of weight in each group, i.e., links below the 70% percentile were given weight=0, while the remaining links maintained their original weights. The use of a fixed density threshold based on link weight was chosen in order to preserve the overall network architecture, while preserving only the strongest and more meaningful links (Bonilha, Nesland, Rorden, & Fridriksson, 2014; van Wijk, Stam, & Daffertshofer, 2010; Zalesky et al., 2011).
The community structure of each network was calculated using the software Gephi and each node was coded in accordance with module participation. Nodes within a module are those with a higher strength of inter-relationship, with relative lower relationship with nodes outside the modules.
2.3.2. Global network parameters
Graph theory metrics were employed to evaluate global network properties. These calculations were performed using the Brain Connectivity Toolbox within the software MATLAB (Rubinov & Sporns, 2010). For each group, we assessed network small worldness by evaluating the relation between the normalized whole-network clustering coefficient and the normalized whole-network characteristic path length, as described by Brown et al (Brown et al., 2011). The normalized whole-network clustering coefficient was defined as the ratio between the clustering coefficient of the observed network and the average clustering coefficient from 100 random networks; similarly, the normalized whole-network characteristic path length was defined as the ratio between the path-length of the observed network and the average path-length from 100 random networks.
Other global network measures such as mean network clustering coefficient, mean betweenness centrality, mean degree and mean network efficiency were calculated. In order to enable a quantitative assessment of each metric per group, we assessed the distribution of node-wise correlation by calculating an adjacency matrix from cognitive data where the values were resampled with replacement (i.e., bootstrapped). This was repeated 1000 times, yielding 1000 adjacency matrices per group. For consistency, only positive entries were maintained.
Global network metrics were assessed for each bootstrapped matrix in each group, and differences in dispersion across groups were calculated employing t-tests.
2.3.3. Local network parameters
To investigate the participation of nodes within the same community structure, we calculated the network modularity using a well-defined stochastic test of modularity (Rubinov & Sporns, 2010). With this approach, module classification may vary slightly between calculations due to the variations in the probability distributions of local and global minima. This approach confers the advantage of enabling the frequency with which nodes are classified as belonging to the same module. Thus, after repeating the calculation of modularity 1000 times for each group, we calculated the frequency of co-association of nodes within a module (irrespective of the module label). For example, 2 nodes could have been placed in the same module 90% of the times in patients, but only 20% in controls. These frequencies were compared using a series of Chi-squared tests to evaluate differences in link-wise associations in patients versus controls.
For all quantitative analyses, the level of statistical significant was set of p<0.05 adjusted for multiple comparisons utilizing Bonferroni correction.
3. Results
3.1. Visual representation of network structure
The adjacency matrices representing the cross-correlations between tests are demonstrated in Figure 1.
Figure 1.
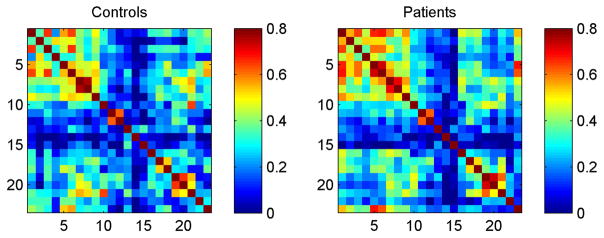
Adjacency matrices demonstrating the cross-correlations between scores in neuropsychological tests for controls and patients. Neuropsychological tests are numbered in accordance with Table 2
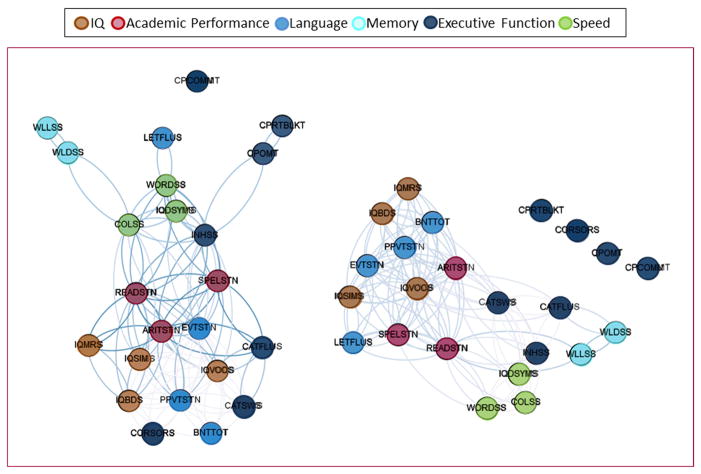
The two-dimensional graphs illustrating the structure of the cognitive network for each group is demonstrated in Figure 2.
Figure 2.
This two-dimensional graph representation illustrates the cognitive networks representing the spatial relationship between cognitive tests. The spatial distribution of nodes was calculated using a force-atlas graph algorithm, where nodes that demonstrated stronger connections are located closer in space, whilst nodes with fewer connections tend to drift away. Nodes with a similar color belong to the same module, whereas each module is composed of nodes with the highest association (i.e., connectivity strength) between in-module nodes, and the lowest association with nodes outside the module.
From a qualitative perspective, there were remarkable differences in network arrangement between groups. While controls demonstrated an organized separation between functional modules, patients exhibited a poorly organized pattern, with significant overlap between multiple modalities.
In controls, there was a clear separation between modules composed mostly of executive function tests, verbal and arithmetic performance, memory and attention. Non-verbal intelligence served as the interface between verbal performance and executive function modules, while speed and response inhibition represented the interface between executive function and verbal skills. Interesting, reading and writing abilities were central in the cognitive structure of normally developing children.
Among children with epilepsy, a poor separation between modules was observed, with multiple domains being represented in the same module, indicating a higher degree of co-dependence between variable functions. Measures of attention and executive function were relatively peripheral to the network, possibly suggesting an immature pattern of development.
Children with new onset epilepsy exhibited a significantly higher rate of academic problems (AP) compared to healthy controls. AP was defined in the broad sense of requiring ancillary educational services to address school based struggles [e.g., IEP, school based tutoring, summer school]). 59% of children with epilepsy exhibited AP, while 20% of controls exhibited AP. We did not observe a significant qualitative interaction between AP and epilepsy on the conformation of the cognitive networks, indicating that the diagnosis of epilepsy, whether concurrent or not with AP, was the most important determinant of the arrangement of the cognitive network conformation.
3.2. Global network metrics
We observed a mildly reduced normalized measure of small-worldness in the cognitive network of patients, compared with controls (1.003 versus 1.004).
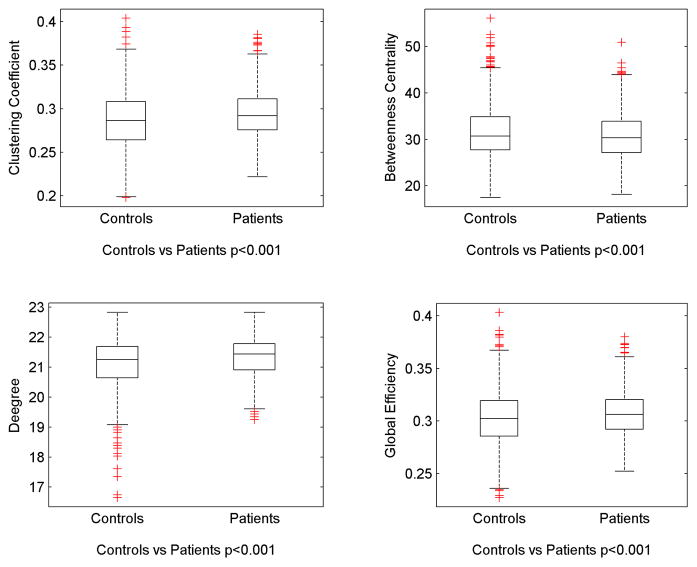
Overall, global network metrics were different in patients compared with controls. These are demonstrated in Figure 3. Children with epilepsy demonstrated an increase in average clustering coefficient (controls=0.28± 0.04, patients=0.29± 0.03), degree (controls=20.6± 1.0, patients=21.4± 0.6) and efficiency (controls=0.30± 0.03, patients=0.31± 0.02), while average betweenness centrality was reduced in patients (controls=32.6± 5.9, patients=31.2± 5.6). These results indicate that children with epilepsy exhibited a network less organized into a well-segregated structure.
Figure 3.
Box-whisker plots demonstrating global network measures. Average clustering coefficient, degree and efficiency were increased in patients, while average betweenness centrality was reduced in patients.
3.3. Local network metrics
We observed a significant rearrangement of nodal participation in community structure across groups. Statistical results comparing nodal modularity co-occurrence matrices are demonstrated in Supplementary Figure 1. As expected from the visual demonstration of network structures shown in Figure 1, several tests associations were different in patients compared with controls. A comprehensive list of significant changes in associations is presented in Supplementary Table 1. Overall, it is possible to note a pattern of changes indicating that patients exhibited a higher association between non-verbal intelligence with other tests, while a relative decrement of associations between verbal skills and other modalities.
4. Discussion
In this study, we employed graph theory to chart the structure of the cognitive network across multiple neuropsychological domains in children with new-onset epilepsy compared to normally developing children. To our knowledge, this study is the first to use graph theory measures to investigate the cognitive network in children with epilepsy. There were two main findings. First, children with epilepsy, compared to healthy controls, demonstrated a less well-organized network with poorly separated modules. Moreover, measures of attention and executive function were isolated from other modules. Second, the less demarcated modularity arrangement was also reflected by global network measures. Specifically, the cognitive networks of children with epilepsy were organized into a less well-segregated architecture with higher cluster coefficient, greater degree connectivity, and shorter path length.
Whereas numerous studies have documented the impact of epilepsy on cognition, our study stands out from the traditional paediatric cognitive literature. Instead of focusing on general intelligence or multiple domain specific deficits, which, when simultaneously examined, can be confounded by multiple comparisons (i.e. type 1) errors (for a review see (Lin, Mula, & Hermann, 2012)), we characterized the cognitive network organization in its entirety using graph theory (Fair, Bathula, Nikolas, & Nigg, 2012). Using community detection methods, we demarcated the internal network organization and found important distinctions between children with epilepsy and healthy controls. Compared to controls, children with epilepsy demonstrated less distinct separation among the modules and greater dependency among cognitive domains. Whereas higher-level skills such as reading, arithmetic, and spelling were central to the modular organization of healthy controls, these academic skills were intermixed among other cognitive abilities in children with epilepsy. Importantly, speed and executive function were not well integrated and placed conspicuously peripheral in the network of children with epilepsy. The lack of interaction of processing speed and attention into the other cognitive domains in the epilepsy group might support the consistent findings in the literature of impaired attention and processing speed in these children (Austin et al., 2001; Austin et al., 2010; Helmstaedter, 2012; Semrud-Clikeman & Wical, 1999).
Academic skills are uniquely complex and require integration of other cognitive abilities including general intelligence, speed, and executive function (Rohde, 2007). Specific learning disorders, such as those affecting reading, writing or mathematical skills, are more frequently impaired in people with epilepsy than in the general population, even in children with normal intelligence (Sillanpaa, 1992). This poor relationship between executive function and academic skills exhibited in children with epilepsy provides potentially important insights into the underlying mechanism of poor academic performance. Indeed, attention deficit hyperactive disorder (ADHD) is significantly overrepresented in children at the time of diagnosis of epilepsy compared to controls.
Further, patients compared to controls exhibited a higher association between non-verbal intelligence with other tests, while a relative decrement of associations between verbal skills and other modalities. The association with nonverbal tests, albeit only few of these were included in the study, might suggest a compensatory mechanism for the poor verbal related functioning.
In summary, children with epilepsy exhibited a pattern of suboptimal network arrangement with greater inter-relationship among most of the cognitive domains but fragmented from executive function.
Global network measures also highlighted the greater inter-reliance among cognitive domains in children with epilepsy. Overall, the network architecture in epilepsy exhibited reduced small-worldness, suggesting less optimal balance between local connectivity and global integration. Further, it showed greater average degree of connectivity among the cognitive domains and increased network segregation as indicated by augmented cluster coefficient. The altered cognitive landscape in epilepsy may represent a compensatory process, the underlying abnormality, or a combination of the two effects.
5. Limitations and considerations
In spite of its success, a few conceptual limitations of this study should be considered. Although our sample was not balanced between children with epilepsy and controls, our sample size in general was quite high. We also want to note that our sample did not differ significantly in age, education or parental education and employment status. We did not present specific information about those subjects experiencing academic problems (AP) as defined in this investigation, a factor that one might suspect to drive findings in the epilepsy group, being more common in the epilepsy than control group (52% vs 20%). We did inquire into the impact of this concept on the obtained findings and there was not a significant qualitative interaction between AP and epilepsy on the conformation of the cognitive networks, indicating that the diagnosis of epilepsy, whether concurrent or not with AP, was the most important determinant of the arrangement of the cognitive network.
Finally, most existing neuropsychological tests require verbal and language skills, even if the test does target these domains. Our findings were predominantly related to the differential influence of language domains between groups. Therefore, they should be interpreted in the context of the neuropsychological tests that were utilized.
6. Conclusion
The current approach provides new insights regarding the impact of epilepsy on cognition and the response of the cognitive systems to epilepsy. Characterization of network perturbations might serve as the basis for the assessment of specific effects associated with epilepsy phenotypes, or to evaluate future therapies designed to mitigate the neuropsychological effects of epilepsy. Whereas childhood onset epilepsy influences diverse cognitive abilities, current therapy has focused on domain specific cognitive rehabilitation such as memory and attention. Overall, these treatment approaches have produced mixed results and why only some children improve is unclear. Understanding the inter-relationship between cognitive domains will improving our understanding of how performances in one domain may influence abilities in other domains. Such insights derived from this study will allow the development of rationale cognitive rehabilitation in individual with epilepsy and hopefully intervene at early in the course of epilepsy.
Supplementary Material
The matrix on the left side of the image represents the Chi-square obtained by calculating whether each pair of nodes was more likely to belong to the same module in patients versus controls. The matrix on the right side of the image demonstrates the p-values associated with these Chi-square values. Note that the direction of the difference (patients> controls or vice-versa) is not demonstrated here, but are summarized in the Supplementary Table 1.
Nodes that were more frequently associated within the same module in controls as opposed to patients, and vice-versa, are listed in this table.
Table 1. Group characteristics.
Characteristics of controls and epilepsy participants by subsyndrome (mean and SD); FSIQ, full-scale intelligence quotient; BECT=benign epilepsy of childhood with centrotemporal spikes; BOE=benign occipital epilepsy; TLE=temporal lobe epilepsy; CAE=childhood absence epilepsy; JAE=juvenile absence epilepsy; JME=juvenile myoclonic epilepsy; PGE=primary generalized epilepsy.
| Variable | Healthy Controls (n=80) | Patients (n=127) |
|---|---|---|
| Age (y, SD) | 12.69 (3.17) | 12.31 (3.17) |
| Sex (number female) | 39 | 60 |
| FSIQ (total, SD) | 107.65 (12.01) | 101.29(13.61) |
| Age of seizure onset (y, SD) | 11.44 (3.25) | |
| Duration (months, SD) | 8.65 (3.53) | |
| Specific Syndrome | BECT=31 BOE=2 TLE=12 Focal=11 CAE=10 JAE=6 JME=32 PGE/IGE NOS=12 Frontal=9 n.s.=2 |
Acknowledgments
The work was supported by grants from the NIH National Institute of Neurological Disorders and Stroke (K23 NS060993, J.J.L.; 3RO1-44351, B.P.H.). The project was also supported by the Clinical and Translational Science Award program, through the NIH National Center for Advancing Translational Sciences (grant number UL1TR000427), and the University of Wisconsin. We thank Raj Sheth MD, Monica Koehn MD, and Jason Dozier MD for study participation and subject recruitment. Also greatly appreciated are Dace Almane, Melissa Hanson, Kate Young, and Bjorn Hanson for overall study coordination, participant recruitment, cognitive assessment, and data management. Also appreciated are the contributions of Drs. Hsu, Stafstrom, Edelman, Jones, Jackson and Seidenberg.
Footnotes
Disclosure: The authors report no financial or nonfinancial conflicts of interest associated with this study.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alexander RP, Concha L, Snyder TJ, Beaulieu C, Gross DW. Correlations between Limbic White Matter and Cognitive Function in Temporal-Lobe Epilepsy, Preliminary Findings. Front Aging Neurosci. 2014;6:142. doi: 10.3389/fnagi.2014.00142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Austin JK, Harezlak J, Dunn DW, Huster GA, Rose DF, Ambrosius WT. Behavior problems in children before first recognized seizures. Pediatrics. 2001;107(1):115–122. doi: 10.1542/peds.107.1.115. [DOI] [PubMed] [Google Scholar]
- Austin JK, Perkins SM, Johnson CS, Fastenau PS, Byars AW, deGrauw TJ, Dunn DW. Self-esteem and symptoms of depression in children with seizures: relationships with neuropsychological functioning and family variables over time. Epilepsia. 2010;51(10):2074–2083. doi: 10.1111/j.1528-1167.2010.02575.x. [DOI] [PubMed] [Google Scholar]
- Bell B, Lin JJ, Seidenberg M, Hermann B. The neurobiology of cognitive disorders in temporal lobe epilepsy. Nat Rev Neurol. 2011;7(3):154–164. doi: 10.1038/nrneurol.2011.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berg AT, Langfitt JT, Testa FM, Levy SR, DiMario F, Westerveld M, Kulas J. Global cognitive function in children with epilepsy: a community-based study. Epilepsia. 2008;49(4):608–614. doi: 10.1111/j.1528-1167.2007.01461.x. [DOI] [PubMed] [Google Scholar]
- Berg AT, Smith SN, Frobish D, Levy SR, Testa FM, Beckerman B, Shinnar S. Special education needs of children with newly diagnosed epilepsy. Dev Med Child Neurol. 2005;47(11):749–753. doi: 10.1017/S001216220500157X. [DOI] [PubMed] [Google Scholar]
- Blume WT, Luders HO, Mizrahi E, Tassinari C, van Emde Boas W, Engel J., Jr Glossary of descriptive terminology for ictal semiology: report of the ILAE task force on classification and terminology. Epilepsia. 2001;42(9):1212–1218. doi: 10.1046/j.1528-1157.2001.22001.x. [DOI] [PubMed] [Google Scholar]
- Bonilha L, Helpern JA, Sainju R, Nesland T, Edwards JC, Glazier SS, Tabesh A. Presurgical connectome and postsurgical seizure control in temporal lobe epilepsy. Neurology. 2013;81(19):1704–1710. doi: 10.1212/01.wnl.0000435306.95271.5f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Nesland T, Martz GU, Joseph JE, Spampinato MV, Edwards JC, Tabesh A. Medial temporal lobe epilepsy is associated with neuronal fibre loss and paradoxical increase in structural connectivity of limbic structures. J Neurol Neurosurg Psychiatry. 2012;83(9):903–909. doi: 10.1136/jnnp-2012-302476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Nesland T, Rorden C, Fridriksson J. Asymmetry of the structural brain connectome in healthy older adults. Front Psychiatry. 2014;4:186. doi: 10.3389/fpsyt.2013.00186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonilha L, Tabesh A, Dabbs K, Hsu DA, Stafstrom CE, Hermann BP, Lin JJ. Neurodevelopmental alterations of large-scale structural networks in children with new-onset epilepsy. Hum Brain Mapp. 2014 doi: 10.1002/hbm.22428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JA, Terashima KH, Burggren AC, Ercoli LM, Miller KJ, Small GW, Bookheimer SY. Brain network local interconnectivity loss in aging APOE-4 allele carriers. Proc Natl Acad Sci U S A. 2011;108(51):20760–20765. doi: 10.1073/pnas.1109038108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camfield C, Breau L, Camfield P. Assessing the impact of pediatric epilepsy and concomitant behavioral, cognitive, and physical/neurologic disability: Impact of Childhood Neurologic Disability Scale. Dev Med Child Neurol. 2003;45(3):152–159. doi: 10.1017/s0012162203000306. [DOI] [PubMed] [Google Scholar]
- Camfield P, Camfield C. Childhood epilepsy: what is the evidence for what we think and what we do? J Child Neurol. 2003;18(4):272–287. doi: 10.1177/08830738030180041401. [DOI] [PubMed] [Google Scholar]
- Caplan R, Siddarth P, Stahl L, Lanphier E, Vona P, Gurbani S, Shields WD. Childhood absence epilepsy: behavioral, cognitive, and linguistic comorbidities. Epilepsia. 2008;49(11):1838–1846. doi: 10.1111/j.1528-1167.2008.01680.x. [DOI] [PubMed] [Google Scholar]
- Caplan R, Siddarth P, Vona P, Stahl L, Bailey C, Gurbani S, Donald Shields W. Language in pediatric epilepsy. Epilepsia. 2009;50(11):2397–2407. doi: 10.1111/j.1528-1167.2009.02199.x. [DOI] [PubMed] [Google Scholar]
- Conners CK. Conners’ Continuous Performance Test user’s manual. Toronto, Canada: Multi-Health Systems; 1992. [Google Scholar]
- D’Agati E, Cerminara C, Casarelli L, Pitzianti M, Curatolo P. Attention and executive functions profile in childhood absence epilepsy. Brain Dev. 2012;34(10):812–817. doi: 10.1016/j.braindev.2012.03.001. [DOI] [PubMed] [Google Scholar]
- Delis DC, Kaplan E, Kramer JH. Delis-Kaplan Executive Function System (D-KEFS) examiner’s manual. San Antonio, TX: The Psychological Corporation; 2001. pp. 1–218. [Google Scholar]
- Dunn LM, Hottel JV. Peabody picture vocabulary test performance of trainable mentally retarded children. Am J Ment Defic. 1961;65:448–452. [PubMed] [Google Scholar]
- Engel J., Jr Report of the ILAE classification core group. Epilepsia. 2006;47(9):1558–1568. doi: 10.1111/j.1528-1167.2006.00215.x. [DOI] [PubMed] [Google Scholar]
- Fair DA, Bathula D, Nikolas MA, Nigg JT. Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proc Natl Acad Sci U S A. 2012;109(17):6769–6774. doi: 10.1073/pnas.1115365109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin AP, Hermida MJ, Shalom DE, Elias Costa M, Lopez-Rosenfeld M, Segretin MS, Sigman M. Far transfer to language and math of a short software-based gaming intervention. Proc Natl Acad Sci U S A. 2014;111(17):6443–6448. doi: 10.1073/pnas.1320217111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helmstaedter C, Witt JA. Clinical neuropsychology in epilepsy: theoretical and practical issues. Handb Clin Neurol. 2012;107:437–459. doi: 10.1016/B978-0-444-52898-8.00036-7. [DOI] [PubMed] [Google Scholar]
- Hermann B, Jones J, Sheth R, Dow C, Koehn M, Seidenberg M. Children with new-onset epilepsy: neuropsychological status and brain structure. Brain. 2006;129(Pt 10):2609–2619. doi: 10.1093/brain/awl196. [DOI] [PubMed] [Google Scholar]
- Jacomy M, Venturini T, Heymann S, Bastian M. ForceAtlas2, a Continuous Graph Layout Algorithm for Handy Network Visualization Designed for the Gephi Software. PLoS One. 2014;9(6):e98679. doi: 10.1371/journal.pone.0098679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaeggi SM, Buschkuehl M, Jonides J, Shah P. Short- and long-term benefits of cognitive training. Proc Natl Acad Sci U S A. 2011;108(25):10081–10086. doi: 10.1073/pnas.1103228108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan E, Godglass H, Weintraub S. Boston Naming Test. Philadelphia: Lea & Febiger; 1983. [Google Scholar]
- Lin JJ, Mula M, Hermann BP. Uncovering the neurobehavioural comorbidities of epilepsy over the lifespan. Lancet. 2012;380(9848):1180–1192. doi: 10.1016/S0140-6736(12)61455-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohde TE, Thompson L. Predicting academic achievement with cognitive ability. Intelligence. 2007;35(1):83–92. [Google Scholar]
- Rubinov M, Sporns O. Complex network measures of brain connectivity: uses and interpretations. Neuroimage. 2010;52(3):1059–1069. doi: 10.1016/j.neuroimage.2009.10.003. [DOI] [PubMed] [Google Scholar]
- Seidenberg M, Beck N, Geisser M, Giordani B, Sackellares JC, Berent S, Boll TJ. Academic achievement of children with epilepsy. Epilepsia. 1986;27(6):753–759. doi: 10.1111/j.1528-1157.1986.tb03606.x. [DOI] [PubMed] [Google Scholar]
- Semrud-Clikeman M, Wical B. Components of attention in children with complex partial seizures with and without ADHD. Epilepsia. 1999;40(2):211–215. doi: 10.1111/j.1528-1157.1999.tb02077.x. [DOI] [PubMed] [Google Scholar]
- Sillanpaa M. Epilepsy in children: prevalence, disability, and handicap. Epilepsia. 1992;33(3):444–449. doi: 10.1111/j.1528-1157.1992.tb01689.x. [DOI] [PubMed] [Google Scholar]
- Vaessen MJ, Jansen JF, Braakman HM, Hofman PA, De Louw A, Aldenkamp AP, Backes WH. Functional and structural network impairment in childhood frontal lobe epilepsy. PLoS One. 2014;9(3):e90068. doi: 10.1371/journal.pone.0090068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaessen MJ, Jansen JF, Vlooswijk MC, Hofman PA, Majoie HJ, Aldenkamp AP, Backes WH. White matter network abnormalities are associated with cognitive decline in chronic epilepsy. Cereb Cortex. 2012;22(9):2139–2147. doi: 10.1093/cercor/bhr298. [DOI] [PubMed] [Google Scholar]
- van Wijk BC, Stam CJ, Daffertshofer A. Comparing brain networks of different size and connectivity density using graph theory. PLoS One. 2010;5(10):e13701. doi: 10.1371/journal.pone.0013701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wechsler D. Wechsler intelligence scale for children. 4. San Antonio, TX: The Psychological Corporation; 2003. [Google Scholar]
- Williams K. Expressive Vocabulary Test. Circle Pines, MN: American Guidance Service; 1997. [Google Scholar]
- Zalesky A, Fornito A, Seal ML, Cocchi L, Westin CF, Bullmore ET, Pantelis C. Disrupted axonal fiber connectivity in schizophrenia. Biol Psychiatry. 2011;69(1):80–89. doi: 10.1016/j.biopsych.2010.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The matrix on the left side of the image represents the Chi-square obtained by calculating whether each pair of nodes was more likely to belong to the same module in patients versus controls. The matrix on the right side of the image demonstrates the p-values associated with these Chi-square values. Note that the direction of the difference (patients> controls or vice-versa) is not demonstrated here, but are summarized in the Supplementary Table 1.
Nodes that were more frequently associated within the same module in controls as opposed to patients, and vice-versa, are listed in this table.