Abstract
Few studies have investigated the impact of liver cirrhosis on dendritic cell function. The purpose of this study was to compare the activation and antigen-presentation capacity of monocyte-derived dendritic cells (MoDC) from cirrhotic patients (CIR) relative to healthy donors (HD). MoDC from CIR and HD were matured, phenotyped, irradiated and pulsed with 15mer peptides for two hepatocellular carcinoma-related antigens, alphafetoprotein and glypican-3, then co-cultured with autologous T-cells. Expanded T-cells were evaluated by interferon-gamma ELISPOT and intracellular staining. 15 CIR and 7 HD were studied. While CD14+ monocytes from CIR displayed enhanced M2 polarization, under MoDC-polarizing conditions, we identified no significant difference between HD and CIR in maturation-induced upregulation of co-stimulation markers. Furthermore, no significant differences were observed between CIR and HD in subsequent expansion of tumor antigen-specific IFNγ+ T-cells.
Conclusion
MoDCs isolated from cirrhotic individuals retain similar capacity for in vitro activation, maturation and antigen-presentation as those from healthy donors.
Keywords: Dendritic cell, Cirrhosis, Hepatitis C, Monocyte, M1 macrophage, M2 macrophage
1. Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy and third leading cause of cancer death worldwide [1] and is the leading cause of death among cirrhotic patients [2]. Despite growing incidence, therapeutic options remain limited and novel approaches to treat or prevent HCC are urgently required. HCC expresses several potential tumor-associated antigen targets for immune-based therapy or prevention, including alpha-fetoprotein (AFP), MAGE-1, NY-ESO-1 and glypican-3 (GPC3) among others [3,4]. Antigen-loaded and unloaded DC have been investigated therapeutically with excellent tolerance but mixed clinical impact in Phase I–II trials in humans [3–15]. However, there are some data that suggest that DC function in hepatocellular carcinoma may be impaired [16]. In previous work, our laboratory showed that tumor antigen-specific CD8+ T-cells generated using 15mer peptide stimulation in cirrhotic patients with HCC were dysfunctional [17]. Similar findings have subsequently been reproduced by other groups [18]. Thus, by the time HCC is diagnosed it may be too late to effectively harness DC to expand tumor-reactive T-cells for optimal therapeutic benefit.
In our previous work, we identified that CD8+ T-cells from cirrhotic patients who had not yet developed HCC appeared to harbor a small population of multifunctional tumor antigen-specific CD8+ T-cells [17]. We therefore hypothesized that in the presence of optimized antigen-presenting cells that we would be able to expand multifunctional tumor antigen-specific T-cells with potential tumor preventing capacity in cirrhotic individuals. To address this hypothesis, we first aimed to evaluate the capacity of MoDC from cirrhotic donors at risk for future HCC to be generated and matured in vitro relative to those from healthy subjects. Secondly we aimed to compare the ability of MoDC derived from cirrhotic donors to prime autologous recall responses to highly immunogenic as well as tumor-related antigens with those generated from MoDC from healthy individuals. We found that MoDC from cirrhotic patients retained identical capacity for activation using a 48 h DC maturation protocol and induced a similar expansion of recall antigen- as well as tumor antigen-specific T-cell responses in vitro. These data suggest that antigen-specific cell-based vaccinations in pre-tumor cirrhotic patients could have the potential to expand functional tumor-reactive precursors in vivo that might prevent or delay progression to hepatocellular carcinoma.
2. Materials and methods
2.1. Patients
Subjects and controls were recruited from the Gastroenterology Clinic at the Philadelphia Veterans Affairs Medical Center following informed consent on an institutional review board-approved protocol. Viral status was determined using clinically-obtained hepatitis C (HCV) antibody, HCV viral load, HBsAg, and HBV DNA testing using standard definitions of chronicity. Alcohol, hemochromatosis, and non-alcoholic fatty liver disease/non-alcoholic steatohepatitis diagnoses were obtained from clinical records. Cirrhotic patients were routinely screened by sonography every 6 months to exclude interim development of HCC; data from any cirrhotic subject who developed HCC within 12 months of enrollment were excluded.
2.2. Peptides
15mer overlapping peptide pools spanning AFP and glypican-3 protein sequences were synthesized (Genscript USA Inc, Piscataway NJ). CMV, EBV, and influenza (CEF) 9–10mer control peptides (Cellular Technology Ltd., Cleveland, OH) were used as positive controls for effector T-cell responses.
2.3. Cell isolation and preparation
100–150 ml of peripheral blood was obtained, from which 100–200 million peripheral blood mononuclear cells (PBMC) were isolated using Ficoll-Histopaque (Sigma, St. Louis MO) density gradient centrifugation. T cells were purified from 30 to 40 × 106 PBMC by negative selection using the MACS Pan T-cell Isolation kit (Miltenyi Biotec, Bergisch Gladbach, Germany). Purity of CD3+ T cells was >95% as determined by flow cytometry. T-cells were plated in 24 well plates in RPMI1640 with L-glutamine (Invitrogen) with 10% human AB serum (Sigma Inc., St. Louis, MO), 1.5% HEPES (Invitrogen) and 1% penicillin/streptomycin (Invitrogen).
2.4. Antibodies and flow cytometry
All data were acquired on FACSCanto (BD) and analyzed using FlowJo (Tree Star Inc., Ashland OR) using cutoffs based on isotype antibody staining. All antibodies were purchased from Becton Dickinson (BD: Becton Dickinson, Franklin Lakes, NJ) unless specifically indicated.
2.5. Monocyte-derived dendritic cell (MoDC) maturation
CD14+ monocytes were purified from 40 × 106 PBMC using a human CD14+ cell isolation kit (Miltenyi Biotec), activated and matured using a 48 h protocol to generate MoDC as previously described [19–21]. Briefly, CD14+ monocytes were cultured in 24-well plates in X-vivo15 medium supplemented with 800 IU/ml GM-CSF (BioLegend) and 1000 IU/ml IL-4 (BioLegend) for 24h. The cells were matured for another 24 h in X-vivo15 medium supplemented a maturation cocktail (TNFα 10 ng/ml (Cell Signaling Technology), IL-1β 10 ng/ml (Cell Signaling Technology), IL-6 10 ng/ml (Cell Signaling Technology), and PGE2 1 μg/ml (Sigma), GM-CSF 1600 IU/ml and IL-4 1000 IU/ml). MoDCs (CD11chi) before and after maturation procedure were assessed using CD40 FITC, CD70 FITC, CD83 PE, CD137L PE, HLA-DR APC, OX40L APC, CD86 V450 and Live/Dead Aqua [22–27].
2.6. Ex vivo assessment of monocyte nitric oxide production and macrophage polarization
PBMCs were incubated with LPS 10 μg/mL (Escherichia coli lipopolysaccharide, Sigma) for 60 min with or without 10 min pre-incubation with the nitric oxide synthase inhibitor L-NAME 10 mM (Nω-nitro-L-arginine methyl ester, Sigma) at 37 °C, 5% CO2. Cellular levels of nitric oxide (NO) were assessed an NO probe DAF-2DA 2 μM (4,5-diaminofluorescein diacetate, Calbiochem) by flow cytometry. Briefly, cells were incubated with DAF-2DA at 37 °C for 20 min and were subsequently surface stained for CD14 (PerCP) and HLA-DR (APC) on ice. For macrophage polarization, purified CD14+ monocytes were plated in RPMI 1640 with 10% human AB serum containing with 100 ng/mL M-CSF (macrophage colony-stimulating factor, Biolegend) for 7 days for M0 differentiation. After 7 days, M1 and M2 polarization was induced by either 100 ng/mL LPS (Sigma) plus 20 ng/mL IFNγ (PeproTech), or rhIL-4 20 ng/mL (BioLegend) for 2 additional days. Cultured macrophages were stained for CD86, HLA-DR, and CD206 for analysis by flow cytometry.
2.7. Antigen-specific T-cell expansion
At 48 h MoDCs were harvested, count, and phenotyped. MoDc were irradiated to 30 Gy prior to peptide loading. For peptide loading, 0.4 × 106 irradiated MoDCs per well in a 24 well plate were incubated in X-vivo15 media in the presence of AFP or GPC3 or CEF peptide pool at 1 μg/ml per peptide for 2–4 h. Pulsed MoDC were then centrifuged, washed, and co-cultured with 2.0 × 106 autologous CD3+ T-cells at T:DC ratio of 5:1 in RPMI 1640 with 10% human AB serum supplemented with IL-15 (10 ng/ml) and IL-12 (25 ng/ml). IL-2 (50 U/ml), IL-15 (10 ng/ml) and IL-21 (25 ng/ml) were added every 2–3 days. T-cells were harvested and replated for restimulation with autologous irradiated, pep-tide-pulsed MoDC (day 13) and PBMC 1.0 × 106/well (day 20). On day 27, in vitro expanded T-cells were restimulated for 6 h with media (negative control), AFP, GPC3 or CEF peptide pools at 1 μg/ml per peptide, or PMA/ionomycin (positive control) in the presence of anti-CD107a PE and monensin, fixed and permeabilized using BD Cytoperm/Cytofix (BD), then stained intracellularly for IFNγ PE-Cy7 and TNFα APC (BD). Dead cells (Live/Dead Aqua+) were excluded from analysis. Background positive responses from unrestimulated conditions were subtracted from stimulated conditions.
2.8. IFNγ Elispot
Antigen-specific T-cell IFNγ responses were examined after in vitro expansion in cytokine Elispot assay as previously described [17]. 5 × 104 antigen-expanded T-cells/well were restimulated with each peptide pool (1 μg/ml) in triplicates with positive (PHA) and negative (media) controls × 24 h in IFNγ Elispot plates. 96-well Elispot plates were pre-coated with anti-IFNγ (5 μg/ml, Thermo Scientific) and detected by biotinylated anti-IFNγ (0.5 μg/ml, Thermo Scientific). Spot-forming units were counted using IFL044 Elispot reader (AID, Strassberg Germany) excluding assays with high background (>10SFU per well) or no response to PHA. Conditions with average greater than 500SFU/106 cells were considered positive.
2.9. Statistical analysis
Median values for clinical and immunologic parameters were compared using Wilcoxon signed-rank test, the nonparametric Kruskal-Wallis, or Wilcoxon Rank Sum test. All Statistical Analysis were performed using JMP 10 (SAS Institute Inc, Cary NC). P-values of <0.05 were considered significant.
3. Results
3.1. Patient characteristics
The study cohort comprised of 22 subjects, 15 with liver cirrhosis and 7 healthy donors. Six cirrhotics patients were infected with hepatitis C, one with hepatitis B, and the remainder had non-viral causes: alcoholic cirrhosis (n = 3), Non-alcoholic steatohepatitis (n = 2), simultaneous metabolic and alcoholic cirrhosis (NASH/EtOH) (n = 1), and hemochromatosis (n = 2). Cirrhotic patients were slightly older than healthy donors (median age 62 vs 53, p = 0.02) but were overall well compensated with normal serum albumin, serum bilirubin and INR values. As expected due to portal hypertension, median platelet counts were lower in the cirrhotic group (147 vs 196 × 103/μl, p = 0.004).
3.2. Baseline and post-expansion differences between CD14+ monocytes from cirrhotic patients relative to those of healthy donors
The sequence of procedures involved in the generation of MoDC and the use of MoDC for antigen-specific T-cell expansion are shown in Supplemental Fig. 1. We isolated CD14+ monocytes from PBMC using positive selection and observed a significantly higher yield of CD14+ monocytes in cirrhotic patients than healthy donors (7.3 ± 2.8 vs 3.6 ± 0.9 from 40 × 106 PBMC, p = 0.005, Fig. 1A) which was independent of the viral or non-viral etiology of cirrhosis (Fig. 1B). This increased isolation yield resulted from an unexpected higher frequency of CD14+ monocytes in the peripheral blood of cirrhotic patients, again independent of viral or non-viral etiology, that we confirmed in an independent sampling of subjects (Supplemental Fig. 2). This higher yield of CD14+ monocytes translated at 48 h to a significantly higher yield of MoDCs (3.2 ± 1.5 vs 1.9 ± 0.6, p = 0.029) in cirrhotic patients relative to healthy donors, particularly among cirrhosis of viral etiology (Fig. 1C and D). However, after normalizing for initial CD14+ cell yield, there was no significant difference between the cirrhotics and healthy donors with regard to the yield of MoDC from CD14+ precursors which averaged 52% (data not shown).
Fig. 1.
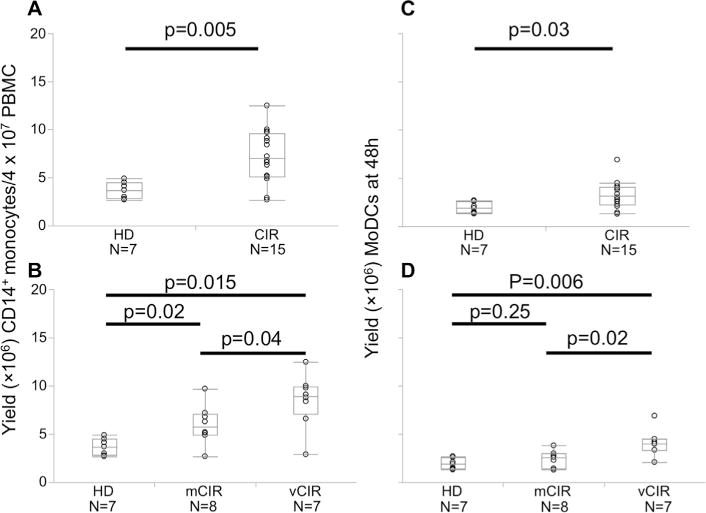
Yield of CD14+ monocytes and MoDCs in cirrhotic and healthy donors. (A) CD14+ monocytes were isolated from 40 × 106 PBMC, and the yield was enumerated comparing cirrhotic and healthy donors (B) and subdivided by etiology of cirrhosis. (C) Yield of MoDCs harvested after 48 h was similarly compared between healthy donors and cirrhotic patients (D) stratified by cirrhosis etiology. p-Values calculated using Wilcoxon-rank sum test.
3.3. Ex vivo monocyte function and macrophage polarization
To assess differences in ex vivo CD14+ monocyte function between cirrhotic and healthy donors, PBMC were incubated with lipopolysaccharide (LPS) and with or without the nitric oxide synthase inhibitor L-NAME to assess ex vivo nitric oxide (NO) production. After gating on CD14+ monocytes (Fig. 2A), we found no difference in spontaneous NO production (data not shown) or in LPS-induced NO production by CD14+ monocytes from cirrhotic or healthy donors (Fig. 2B and C; %DAF-2T+ 2.6 ± 1.2 in HD vs 1.8 ± 1.0 in CIR, p = 0.94). There was also no difference in ex vivo or LPS-induced expression in HLA-DR (Fig. 2D). No difference was present in background%DAF-2T+ in media stimulated CD14+ monocytes between healthy donors and cirrhotic patients (data not shown). By contrast, marked differences were identified in the capacity of monocytes from cirrhotic individuals to be differentiated into M1 or M2 macrophages under appropriate conditions. Under M1 conditions, cirrhotic monocyte-derived macrophages showed greater expression of CD86 (%CD86+ 1.2 ± 0.3 vs 8.8 ± 1.9, p = 0.005) and HLA-DR (%HLA-DR 6.1 ± 0.7 vs 27.1 ± 4.5, p = 0.008) than healthy donor cells (Fig. 2E and F). Under M2 conditions, cirrhotic macrophages showed enhanced M2 differentiation (%CD206+ 4.0 ± 1.0 vs 15.6 ± 3.6, p = 0.008) (Fig. 2E and F). Thus, despite similar ex vivo activation, CD14+ monocytes from cirrhotic patients exhibit enhanced polarization to either M1 or M2 phenotypes under appropriate conditions (see Table 1).
Fig. 2.
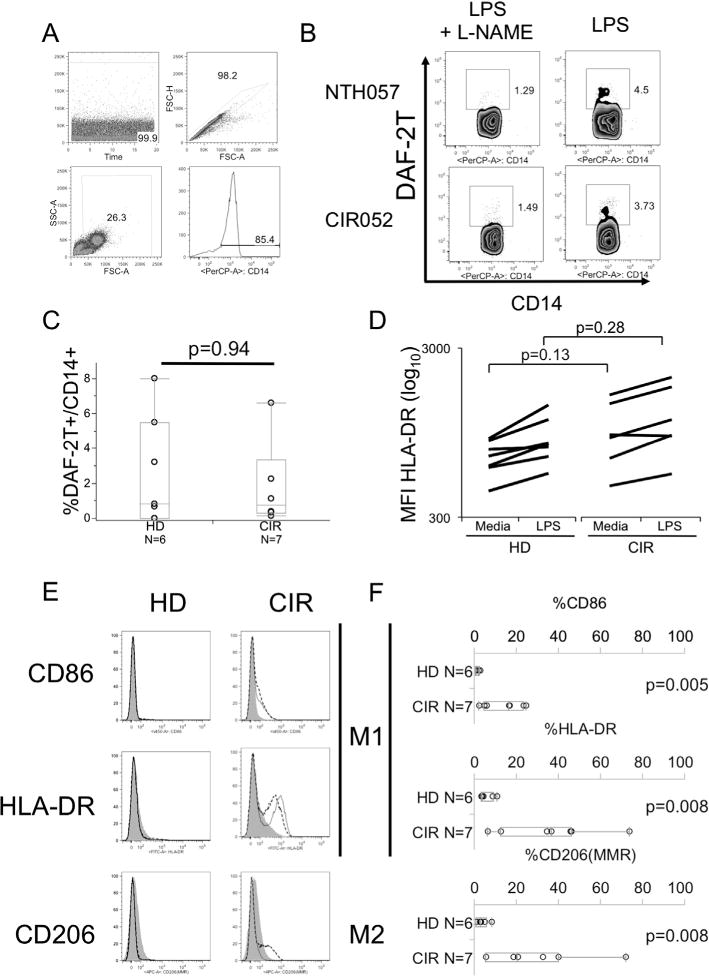
Ex vivo monocyte function in cirrhotic and healthy donors. PBMCs were incubated with LPS with or without the nitric oxide synthase inhibitor L-NAME. (A) The gating strategy used to identify monocytes (B). Representative FACS plots of HD (NTH057) and CIR (CIR052) subjects showing NO production as measured by DAF-2T after stimulation with LPS with or without L-NAME. (C) Distribution of frequency of DAF-2T+ monocytes comparing HD and CIR (D) Distribution of geometric MFI of HLA-DR expression with and without LPS-stimulation in monocytes from CIR and HD. (E) Expression of CD86 and HLA-DR under M1 and M2 polarizing conditions. Purified monocytes were incubated for 7 days with M-CSF (M0 macrophages, shaded). Expression after M1 polarization for 48 h by incubation with LPS and IFNγ (solid line) and after M2 polarization with IL-4 (dotted line) shown. FACS histogram shows the representative results (gray shaded: M0, normal black line: M1, dashed line: M2). (F) Summary of differences of M1 phenotypic markers (HLA-DR, CD86) under M1 polarizing conditions and M2 phenotype (CD206) under M2 polarizing conditions in HD and CIR donor monocyte-derived macrophages. All p-values by Wilcoxon rank-sum test.
Table 1.
Baseline characteristics.
| (A)
| ||||||||
|---|---|---|---|---|---|---|---|---|
| ID | Ethnicity | Gender | ALT | Albumin | Tbili | INR | Platelet | Etiology |
| CIR004 | H | M | 34 | 4 | 0.6 | 1.2 | 169 | HCV |
| CIR026 | B | M | 63 | 3.4 | 0.7 | 1 | 147 | HCV |
| CIR029 | W | M | 77 | 4.3 | 0.9 | 1.1 | 151 | HCV |
| CIR031 | H | M | 28 | 3.8 | 0.6 | 1.1 | 151 | HCV |
| CIR034 | W | M | 73 | 3.8 | 1 | 1.1 | 53 | HCV |
| CIR037 | B | M | 51 | 3.5 | 0.6 | 1 | 122 | HCV |
| CIR019 | B | M | 23 | 3.7 | 0.5 | 1.2 | 160 | HBV |
| CIR018 | W | M | 19 | 4.4 | 1.1 | 1.1 | 109 | EtOH |
| CIR041 | W | M | 27 | 3.9 | 0.6 | 1 | 206 | EtOH |
| CIR042 | W | M | 28 | 4.3 | 0.7 | 1.1 | 87 | EtOH |
| CIR036 | W | M | 36 | 4.5 | 0.9 | 1 | 198 | HFE |
| CIR038 | W | M | 15 | 3.7 | 0.6 | 1.1 | 146 | HFE |
| CIR035 | W | M | 98 | 4.5 | 0.7 | 1 | 175 | NASH |
| CIR043 | W | M | 31 | 3.7 | 1.4 | 1.2 | 52 | NASH |
| CIR030 | W | M | 52 | 4.4 | 0.7 | 1 | 145 | NASH/EtOH |
| (B)
| |||
|---|---|---|---|
| Healthy donor | Cirrhotic patients | p value | |
| Number of patients | |||
| Number of patients | |||
| Median age (years, IQR) | 53 (38–56) | 62 (56–65) | 0.02 |
| Ethnicity (W/B/H) | 6/1/0 | 10/3/2 | n.s |
| Gender (M/F) | 5/2 | 15/0 | n.s |
| Median alanine aminotransferase (IU/L) (IQR) | 20 (17–31) | 34 (27–63) | 0.04 |
| Median albumin (g/dl) (IQR) | 4.2 (4.1–4.3) | 3.9 (3.7–4.4) | n.s |
| Median total bilirubin (mg/dl) (IQR) | 0.7 (0.5–1.2) | 0.7 (0.6–0.9) | n.s |
| Median INR (IQR) | 1.1 (1.0–1.2) | 1.1 (1.0–1.1) | n.s |
| Median platelet count (×103/μl) (IQR) | 196 (176–271) | 147 (109–169) | 0.004 |
| Etiology (viral/metabolic) | – | 7/8 | |
n.s: not significant.
3.4. Baseline and dendritic-cell polarization-induced differences of CD14+ monocytes between cirrhotic and healthy donors
Prior to generation of monocyte-derived dendritic cells (MoDC), we assessed the baseline expression of several costimulation markers in isolated CD14+ monocytes. The gating strategy for phenotyping monocytes and MoDC is shown in Fig. 3A. As shown in Fig. 3B. there were trends for lower expression of OX40L, CD137L and CD86 and statistically significant reduced expression of CD83 on CD14+ monocytes from cirrhotic patients, but no difference was present for expression of CD40. On the other hand, there were trends towards higher expression of HLA-DR and CD70 in cirrhotic patients. There was significantly higher expression of CD70 and HLA-DR and significantly lower expression of CD83 and CD137L in monocytes from patients with cirrhosis of viral etiology compared with healthy donors (Supplemental Fig. 3). Among all groups, each of these activation/costimulation markers was significantly upregulated by dendritic-cell polarizing conditions (Fig. 3C and D) but there were no statistically significant differences in the magnitude of upregulation of each of these markers between healthy donors and cirrhotic subjects regardless of cirrhosis etiology (Table 2).
Fig. 3.
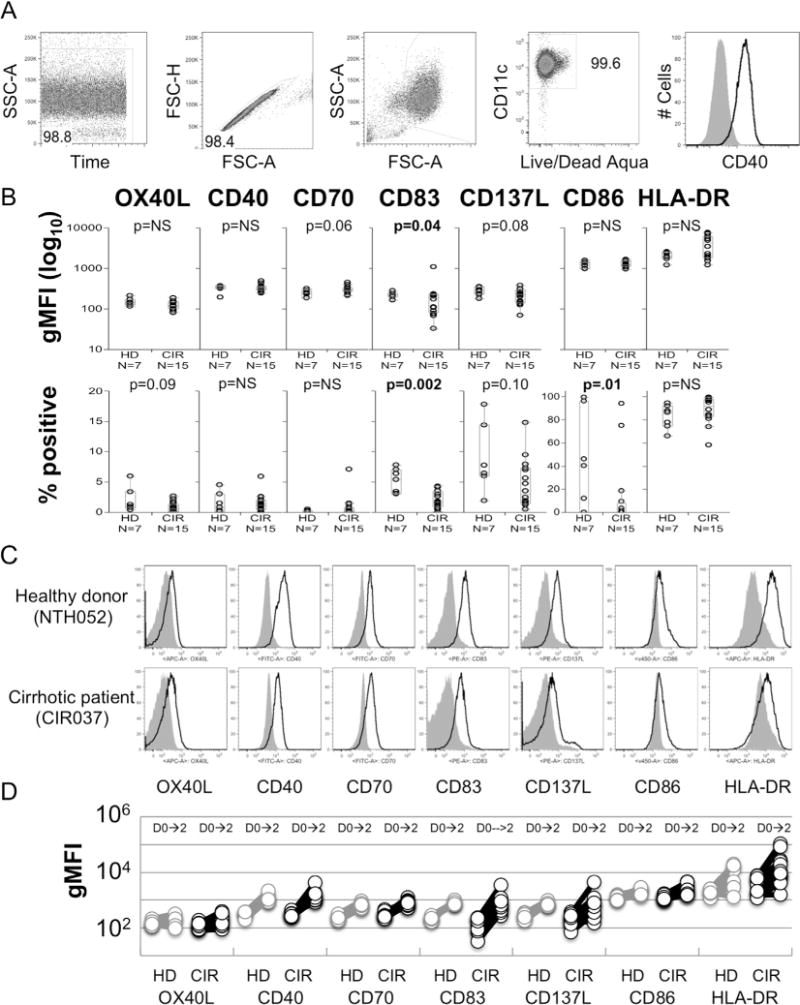
Baseline and post-maturation phenotyping of monocytes from cirrhotic and healthy donors. (A) Gating strategy used to phenotype MoDCs. (B) Comparison of the geometric MFI and frequency of positive expression of OX40L, CD40, CD70, CD83, CD137L, CD86 and HLA-DR on CD14+ monocytes prior to maturation in cirrhotic patients (CIR) and healthy donors (HD). p-Values determined by Wilcoxon Rank-Sum test. (C) Representative histograms showing expression of OX40L, CD40, CD70, CD83, CD137L, CD86 and HLA-DR from cirrhotic subject and healthy donor pre-stimulation (grey shaded) and post-maturation (black line). (D) Pre- and post-maturation changes of geometric MFI of OX40L, CD40, CD70, CD83, CD137L, CD86 and HLA-DR costimulation markers on monocytes and MoDC. No significant differences were identified between CIR and HD.
Table 2.
Experssion of costimulation markers on MoDC in cirrhotic and healthy donors.
| Immature CD14+ (Day 0)
|
MoDC (Day 2)
|
p overall (day 0 vs day 2) | p CIR vs HD | |||
|---|---|---|---|---|---|---|
| CIR gMFI ± SD | HD gMFI ± SD | CIR gMFI ± SD | HD gMFI ± SD | |||
| OX40L | 122.7 ± 8.4 | 150.1 ± 14.3 | 194.5 ± 25.1 | 201.5 ± 34.1 | 0.002 | n.s. |
| CD40 | 332.6 ± 18.2 | 328.1 ± 27.9 | 1423.4 ± 237.1 | 1260.1 ± 197.8 | <0.0001 | n.s. |
| CD70 | 308.0 ± 17.7 | 245.0 ± 22.3 | 799.2 ± 56.8 | 679.3 ± 65.8 | <0.0001 | n.s. |
| CD83 | 211.9 ± 75.9 | 224.1 ± 16.3 | 820.0 ± 221.0 | 819.1 ± 56.8 | 0.0007 | n.s. |
| CD86 | 1353.4 ± 60.2 | 1272.9 ± 102.8 | 2691.9 ± 290.6 | 2258.7 ± 245.9 | <0.0001 | n.s. |
| CD137L | 215.7 ± 25.5 | 274.7 ± 28.7 | 809.6 ± 296.4 | 742.9 ± 54.1 | 0.007 | n.s. |
| HLA-DR | 4671.8 ± 625.2 | 2032.1 ± 218.5 | 23642.1 ± 9005.3 | 11153.4 ± 3651.1 | 0.013 | n.s. |
3.5. MoDCfrom cirrhotic subjects and healthy donors similarly expand virus-specific multifunctional CD8+ T-cells
To evaluate whether or not MoDC derived from cirrhotic subjects exhibited any difference in antigen-specific T-cell expansion to epitopes derived from acute self-limited, and therefore likely non-tolerized, antigens, we measured the frequency of degranulating (CD107a+), IFNγ+ and TNFα+ T-cells after 27 day in vitro co-culture of autologous T-cells and MoDC pulsed with CMV, EBV, and Influenza optimal 9–11mer epitopes (CEF) with restimulation with MoDC at day 13 and PBMC at day 20. Results from a representative cirrhotic subject and healthy donor are presented in Fig. 4A. Upon restimulation, there was no significant difference in the generation of multifunctional (at least 2/3 responses among degranulation (CD107a+), secretion of IFNγ or TNFα with an average of 8.6 ± 2.5% multifunctional CEF-specific CD8+ T-cells in cirrhotic subjects compared with 7.0 ± 3.7% in healthy donors (p = 1.0) (Fig. 4B). Similarly, we identified no difference in the capacity of MoDC from cirrhotic subject to generate □□□□□□ IFNγ by Elispot (Fig. 4C). These data suggest that there is no impairment of antigen-presentation of highly immunogenic epitopes by MoDC derived from cirrhotic subjects relative to healthy donors.
Fig. 4.
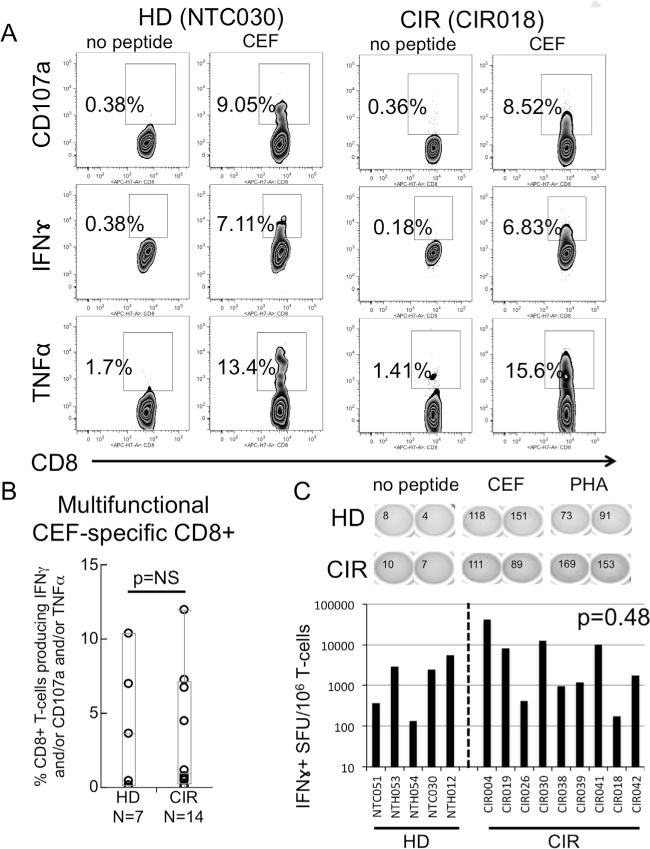
Cytokine and degranulation profile of CEF peptide in vitro-expanded T-cells. (A) Representative intracellular cytokine density plots showing CEF-specific CD8+ TNFα+, IFNγ+ or CD107a+ T-cells for CIR and HD patients after in vitro restimulation using 9–10mer pooled CEF peptides. (B) Summary of frequency of multifunctional CD8+ T-cells (any 2 or 3 positive of TNFα+ and/or IFNγ+ and/or CD107a) among HD and CIR patients (C). CEF-specific IFNγ ELISPOT results of CEF-expanded T-cells from HD and CIR. p-Value by Wilcoxon Rank-Sum test.
3.6. MoDCfrom cirrhotic subjects and healthy donors similarly, weakly expand tumor antigen-specific multifunctional T-cells
To evaluate whether or not MoDC derived from cirrhotic subjects exhibited any difference in antigen-specific T-cell expansion to tumor-related, likely tolerized, epitopes, we measured the frequency of degranulating (CD107a+), IFNγ+ and TNFα+ T-cells after in vitro co-culture of autologous T-cells and MoDC after pulsing with 15mer peptides derived from two liver cancer-related antigens, AFP and GPC3. As shown in Fig. 5A and B, by intracellular cytokine staining the detection of AFP- and GPC3-specific CD4+ and CD8+ T-cell responses were low frequency in both HD and CIR. Single positive cytokine responses >0.25% were present in 4/7 HD and 3/14 CIR for AFP-specific CD4+ T-cells, 3/7 HD and 10/14 CIR for AFP-specific CD8+ T-cells, 4/7 HD and 3/14 CIR for GPC3-specific CD4+ T-cells, and 5/7 HD and 6/14 CIR for GPC3-specific CD8+ T-cells. There were no significant differences in the presence of multiple-function T-cells responses against either antigen in CIR and HD: AFP-specific CD4+ (0.22 ± 0.12 vs 0.05 ± 0.02, p = 0.22); AFP-specific CD8+ (0.49 ± 0.27 vs 0.10 ± 0.06, p = 0.18); GPC3-specific CD4+ (0.40 ± 0.30 vs 0.04 ± 0.01, p = 0.12); GPC3-specific CD8+ (0.87 ± 0.80 vs 0.10 ± 0.05, p = 0.51). As shown in Fig. 5C, significant IFNγ responses (arbitrarily >500 SFU/106 cells) were low frequency and not significantly more frequent among healthy donor and cirrhotic subjects. These data indicate that expanded T-cells with multifunctional cytokine/degranulation profiles targeting highly tolerized tumor antigens can be modestly expanded utilizing MoDC cocultured with T-cells from both cirrhotic subjects and healthy donors.
Fig. 5.
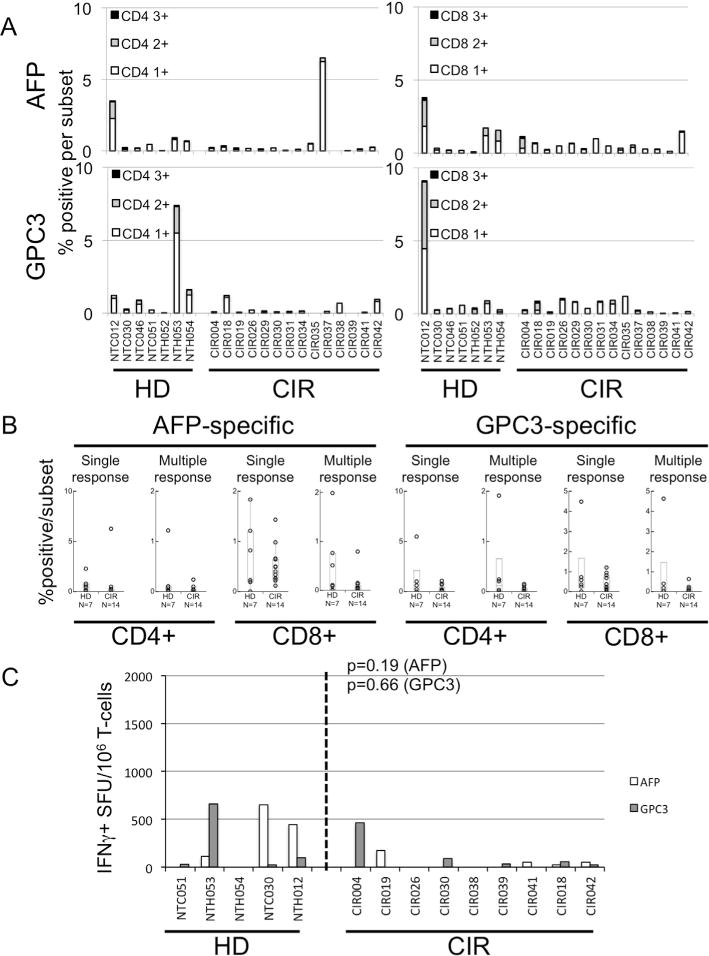
Cytokine and degranulation profile of AFP- or GPC3-peptide in vitro-expanded T-cells. (A) Stacked column chart showing the frequency of cytokine-producting/degranulating CD4+ or CD8+ T-cells after expansion and restimulation with either AFP or GPC3 15mer peptide pools. Black columns represent frequency of T-cells producing TNFα+ and IFNγ+ and staining for CD107a+. Grey columns represent any 2 responses, and white column any single responses. (B) Percentage of MoDC-primed CD4+ or CD8+ T-cells with positive (>0.25%) responses, either single response (producing TNFα+ or IFNγ+ or staining for CD107a+) or multiple response (2 or 3 of above), against AFP or GPC3. (C) Results of AFP- or GPC3-specific IFNγ ELISPOT showing IFNγ SFU per million cells after restimulation of MoDC-primed T-cells. p-Value by Wilcoxon Rank-Sum test.
4. Discussion
Hepatocellular carcinoma (HCC) is the most common cause of death among patients with HCV cirrhosis [2]. Dendritic cell-based therapeutics have been proposed to boost tumor-related antigen-specific T-cell responses in HCC [15,28,29]. Since HCC most commonly arises in the context of cirrhosis, the functional capacity of MoDC from cirrhotic subjects would likely impact upon the success of such approaches. However little is known about the impact of cirrhosis on MoDC generation, maturation and T-cell stimulation. In this study, we demonstrate that MoDCs derived from cirrhotic individuals are phenotypically and functionally indistinguishable from those derived from healthy donors despite subtle baseline phenotypic differences among CD14+ CD11chigh monocytes.
A significant body of literature has associated chronic hepatitis C infection, a frequent cause of cirrhosis, with abnormalities of DC function including reduced dextran uptake [30], defective maturation [30,31], and impaired allostimulation primarily in mixed lymphocyte reactions [30,32–34]. However, other studies have demonstrated no significant differences in the phenotype of, maturation of, or naïve T-cell priming by MoDC [35–37] in chronic HCV. Fewer studies have specifically interrogated the role of chronic hepatitis B infection on DC generation [38,39]. Alcohol consumption, another common etiology of cirrhosis, has been associated with reduced allo- and recall-antigen specific T-cell priming by MoDC [34,40] primarily after short-term alcohol exposure. In this context, we found no difference in MoDC activation between HCV cirrhosis, non-HCV cirrhosis and healthy individuals, and no downstream impact on recall antigen-specific and tumor antigen-specific T-cell stimulation. These data suggest that neither chronic viral infection nor cirrhosis significantly reduces the likelihood of effective MoDC generation for therapeutic targeting of HCC.
There is still no consensus on the optimal procedure for preparation of MoDCs with clinical good manufacturing practice (cGMP) grade reagents. In this study, we adopted a 48-h DC maturation procedure as previously described [19,20,41,42]. It is possible that discrepancies between cirrhotic and normal MoDC could have become detected with a longer MoDC generation schema. Specifically related to cirrhosis, Kakuzu et al. demonstrated that depletion of branched chain amino acids (BCAAs) such as valine alters the expression of CD83 on MoDC, downregulates mTOR/S6 K signaling, and reduces MoDC allostimulatory capacity [43]. The XVivo15 medium we used to generate MoDC is fully supplemented with BCAAs, and thus might in part explain the lack of differences we observed between cirrhotic and healthy subjects. Attention to the essential amino acid component to growth media ex vivo, or supplementation of BCAAs to patients, could be critical in the context of cirrhosis to optimize antigen presentation.
Nearly one third of patients with HCV-related cirrhosis will ultimately develop hepatocellular carcinoma [2]. While virus-directed vaccines in chronic hepatitis B and human papillomavirus have had profound effects on cancer incidence, a licensed preventive vaccine for non-viral cancer has yet to be developed largely because there are few disease states with a large, well-defined population that have an annual cancer risk similar to that seen in cirrhosis. Our previous work showed that tumor antigen-specific CD8+ T-cells in patients with established HCC are dysfunctional [5] suggesting that immune stimulation in the present of macroscopic tumor is unlikely to have meaningful benefit. By contrast, using cells from pre-tumor cirrhotic patients, we were able to expand potentially tumor-reactive multifunctional T-cells in a significant minority of subjects using 15mer peptide antigens. Further optimization of the approach with techniques such as codon-optimized DNA [44], co-transfection with cytokine -encoding DNA [45–47], or co-transfection with PAMPs [48–50] could increase the frequency of tumor-reactive T-cells generatable with cell-based vaccinations. We postulate that translation of an optimized MoDC approach using HCC-related antigens in pre-tumor cirrhotic patients has the potential to prevent or delay progression to hepatocellular carcinoma in this high risk population.
Several novel observations were made regarding monocyte function in cirrhosis that merit note. First, we identified a higher frequency of circulating monocytes in patients with cirrhosis relative to other cell subsets. While this could reflect relative monocytosis, it could also reflect relative preservation of this population in the setting of decreased frequency of circulating T- or B-cells. Second, circulating CD14+ monocytes of cirrhotic individuals of viral etiology exhibited evidence of increased activation even relative to other cirrhotic patients that suggests a potential additional role for chronic viral infection in altering monocyte function. Macrophage activation in cirrhosis in vivo has previously been associated with enhanced gut permeability [51] by increased NO production. However, we did not observe enhanced ex vivo NO production in our study. Third, under macrophage polarizing conditions, cirrhotic monocytes demonstrated enhanced M1 and M2 polarization relative to healthy donors, possibly due to the basal activation of these cells, similar to an observation of enhanced M1 and M2 macrophage polarization in a rat model of cirrhosis [52]. The clinical implications and etiopathogenesis of these observations merits further study.
5. Conclusion
We conclude that using adequate conditions for Mo-DC maturation and T-cell priming, Mo-DCs from cirrhotic patients retain similar capacity for activation, maturation and antigen-presentation as those from healthy donors. Moreover, these findings support the investigation of the use of autologous DC-based vaccination in cirrhotic patients to reduce liver cancer risk among patients with cirrhosis.
Supplementary Material
Abbreviations
- CIR
cirrhotic group
- HCC
hepatocellular carcinoma
- HCV
hepatitis C virus
- HD
healthy donor
- PBMC
peripheral blood mononuclear cells
- MoDC
monocyte-derived dendritic cell
- AFP
alpha-fetoprotein
- GPC3
glypican-3
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cellimm.2015.02.008.
Footnotes
Research Support: This work was supported by the NIH R01 CA166111 (DEK) and the Pennsylvania Commonwealth Universal Research Endowment. The content of this article does not reflect the views of the VA or of the US Government.
References
- 1.Parkin DM. Global cancer statistics in the year 2000. Lancet Oncol. 2001;2:533–543. doi: 10.1016/S1470-2045(01)00486-7. [DOI] [PubMed] [Google Scholar]
- 2.Sangiovanni A, Prati GM, Fasani P, Ronchi G, Romeo R, Manini M, Del Ninno E, Morabito A, Colombo M. The natural history of compensated cirrhosis due to hepatitis C virus: a 17-year cohort study of 214 patients. Hepatology. 2006;43:1303–1310. doi: 10.1002/hep.21176. [DOI] [PubMed] [Google Scholar]
- 3.Butterfield LH, Ribas A, Dissette VB, Lee Y, Yang JQ, De la Rocha P, Duran SD, Hernandez J, Seja E, Potter DM, McBride WH, Finn R, Glaspy JA, Economou JS. A phase I/II trial testing immunization of hepatocellular carcinoma patients with dendritic cells pulsed with four alpha-fetoprotein peptides. Clin Cancer Res. 2006;12:2817–2825. doi: 10.1158/1078-0432.CCR-05-2856. [DOI] [PubMed] [Google Scholar]
- 4.Tada F, Abe M, Hirooka M, Ikeda Y, Hiasa Y, Lee Y, Jung NC, Lee WB, Lee HS, Bae YS, Onji M. Phase I/II study of immunotherapy using tumor antigen-pulsed dendritic cells in patients with hepatocellular carcinoma. Int J Oncol. 2012;41:1601–1609. doi: 10.3892/ijo.2012.1626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Iwashita Y, Tahara K, Goto S, Sasaki A, Kai S, Seike M, Chen CL, Kawano K, Kitano S. A phase I study of autologous dendritic cell-based immunotherapy for patients with unresectable primary liver cancer. Cancer Immunol Immunother. 2003;52:155–161. doi: 10.1007/s00262-002-0360-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikeda H, Chamoto K, Tsuji T, Suzuki Y, Wakita D, Takeshima T, Nishimura T. The critical role of type-1 innate and acquired immunity in tumor immunotherapy. Cancer Sci. 2004;95:697–703. doi: 10.1111/j.1349-7006.2004.tb03248.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mellman I, Coukos G, Dranoff G. Cancer immunotherapy comes of age. Nature. 2011;480:480–489. doi: 10.1038/nature10673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shang XY, Chen HS, Zhang HG, Pang XW, Qiao H, Peng JR, Qin LL, Fei R, Mei MH, Leng XS, Gnjatic S, Ritter G, Simpson AJ, Old LJ, Chen WF. The spontaneous CD8+ T-cell response to HLA-A2-restricted NY-ESO-1b peptide in hepatocellular carcinoma patients. Clin Cancer Res. 2004;10:6946–6955. doi: 10.1158/1078-0432.CCR-04-0502. [DOI] [PubMed] [Google Scholar]
- 9.Butterfield LH, Ribas A, Potter DM, Economou JS. Spontaneous and vaccine induced AFP-specific T cell phenotypes in subjects with AFP-positive hepatocellular cancer. Cancer Immunol Immunother. 2007;56:1931–1943. doi: 10.1007/s00262-007-0337-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cui J, Wang N, Zhao H, Jin H, Wang G, Niu C, Terunuma H, He H, Li W. Combination of radiofrequency ablation and sequential cellular immunotherapy improves progression-free survival for patients with hepatocellular carcinoma. Int J Cancer. 2014;134:342–351. doi: 10.1002/ijc.28372. [DOI] [PubMed] [Google Scholar]
- 11.Nobuoka D, Yoshikawa T, Sawada Y, Fujiwara T, Nakatsura T. Peptide vaccines for hepatocellular carcinoma. Hum vaccines immunother. 2013;9:210–212. doi: 10.4161/hv.22473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhong JH, Ma L, Wu LC, Zhao W, Yuan WP, Wu FX, Zhang ZM, Huang S, You XM, Li LQ. Adoptive immunotherapy for postoperative hepatocellular carcinoma: a systematic review. Int J Clin Pract. 2012;66:21–27. doi: 10.1111/j.1742-1241.2011.02814.x. [DOI] [PubMed] [Google Scholar]
- 13.Qiu Y, Xu MB, Yun MM, Wang YZ, Zhang RM, Meng XK, Ou-Yang XH, Yun S. Hepatocellular carcinoma-specific immunotherapy with synthesized alpha1,3-galactosyl epitope-pulsed dendritic cells and cytokine-induced killer cells. World J Gastroenterol. 2011;17:5260–5266. doi: 10.3748/wjg.v17.i48.5260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nakamoto Y, Mizukoshi E, Kitahara M, Arihara F, Sakai Y, Kakinoki K, Fujita Y, Marukawa Y, Arai K, Yamashita T, Mukaida N, Matsushima K, Matsui O, Kaneko S. Prolonged recurrence-free survival following OK432-stimulated dendritic cell transfer into hepatocellular carcinoma during transarterial embolization. Clin Exp Immunol. 2011;163:165–177. doi: 10.1111/j.1365-2249.2010.04246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Palmer DH, Midgley RS, Mirza N, Torr EE, Ahmed F, Steele JC, Steven NM, Kerr DJ, Young LS, Adams DH. A phase II study of adoptive immunotherapy using dendritic cells pulsed with tumor lysate in patients with hepatocellular carcinoma. Hepatology. 2009;49:124–132. doi: 10.1002/hep.22626. [DOI] [PubMed] [Google Scholar]
- 16.Ninomiya T, Akbar SM, Masumoto T, Horiike N, Onji M. Dendritic cells with immature phenotype and defective function in the peripheral blood from patients with hepatocellular carcinoma. J Hepatol. 1999;31:323–331. doi: 10.1016/s0168-8278(99)80231-1. [DOI] [PubMed] [Google Scholar]
- 17.Xu Y, Li H, Gao RL, Adeyemo O, Itkin M, Kaplan DE. Expansion of interferon-gamma-producing multifunctional CD4+ T-cells and dysfunctional CD8+ T-cells by glypican-3 peptide library in hepatocellular carcinoma patients. Clin Immunol. 2011 doi: 10.1016/j.clim.2011.02.014. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt N, Neumann-Haefelin C, Thimme R. Cellular immune responses to hepatocellular carcinoma: lessons for immunotherapy. Dig Dis. 2012;30:483–491. doi: 10.1159/000341697. [DOI] [PubMed] [Google Scholar]
- 19.Kvistborg P, Boegh M, Pedersen AW, Claesson MH, Zocca MB. Fast generation of dendritic cells. Cell Immunol. 2009;260:56–62. doi: 10.1016/j.cellimm.2009.09.003. [DOI] [PubMed] [Google Scholar]
- 20.Dauer M, Obermaier B, Herten J, Haerle C, Pohl K, Rothenfusser S, Schnurr M, Endres S, Eigler A. Mature dendritic cells derived from human monocytes within 48 hours: a novel strategy for dendritic cell differentiation from blood precursors. J Immunol. 2003;170:4069–4076. doi: 10.4049/jimmunol.170.8.4069. [DOI] [PubMed] [Google Scholar]
- 21.Dauer M, Schad K, Herten J, Junkmann J, Bauer C, Kiefl R, Endres S, Eigler A. FastDC derived from human monocytes within 48 h effectively prime tumor antigen-specific cytotoxic T cells. J Immunol Methods. 2005;302:145–155. doi: 10.1016/j.jim.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 22.Bak SP, Barnkob MS, Bai A, Higham EM, Wittrup KD, Chen J. Differential requirement for CD70 and CD80/CD86 in dendritic cell-mediated activation of tumor-tolerized CD8 T cells. J Immunol. 2012;189:1708–1716. doi: 10.4049/jimmunol.1201271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jenkins SJ, Perona-Wright G, Worsley AG, Ishii N, MacDonald AS. Dendritic cell expression of OX40 ligand acts as a costimulatory, not polarizing, signal for optimal Th2 priming and memory induction in vivo. J Immunol. 2007;179:3515–3523. doi: 10.4049/jimmunol.179.6.3515. [DOI] [PubMed] [Google Scholar]
- 24.Ju S, Ju S, Ge Y, Qiu H, Lu B, Qiu Y, Fu J, Liu G, Wang Q, Hu Y, Shu Y, Zhang X. A novel approach to induce human DCs from monocytes by triggering 4-1BBL reverse signaling. Int Immunol. 2009;21:1135–1144. doi: 10.1093/intimm/dxp077. [DOI] [PubMed] [Google Scholar]
- 25.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 26.Sun M, Fink PJ. A new class of reverse signaling costimulators belongs to the TNF family. J Immunol. 2007;179:4307–4312. doi: 10.4049/jimmunol.179.7.4307. [DOI] [PubMed] [Google Scholar]
- 27.Ma DY, Clark EA. The role of CD40 and CD154/CD40L in dendritic cells. Semin Immunol. 2009;21:265–272. doi: 10.1016/j.smim.2009.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sun K, Wang L, Zhang Y. Dendritic cell as therapeutic vaccines against tumors and its role in therapy for hepatocellular carcinoma. Cell Mol Immunol. 2006;3:197–203. [PubMed] [Google Scholar]
- 29.Bray SM, Vujanovic L, Butterfield LH. Dendritic cell-based vaccines positively impact natural killer and regulatory T cells in hepatocellular carcinoma patients. Clin Dev Immunol. 2011;2011:249281. doi: 10.1155/2011/249281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Auffermann-Gretzinger S, Keeffe EB, Levy S. Impaired dendritic cell maturation in patients with chronic, but not resolved, hepatitis C virus infection. Blood. 2001;97:3171–3176. doi: 10.1182/blood.v97.10.3171. [DOI] [PubMed] [Google Scholar]
- 31.Sarobe P, Lasarte JJ, Zabaleta A, Arribillaga L, Arina A, Melero I, Borras-Cuesta F, Prieto J. Hepatitis C virus structural proteins impair dendritic cell maturation and inhibit in vivo induction of cellular immune responses. J Virol. 2003;77:10862–10871. doi: 10.1128/JVI.77.20.10862-10871.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kanto T, Hayashi N, Takehara T, Tatsumi T, Kuzushita N, Ito A, Sasaki Y, Kasahara A, Hori M. Impaired allostimulatory capacity of peripheral blood dendritic cells recovered from hepatitis C virus-infected individuals. J Immunol. 1999;162:5584–5591. [PubMed] [Google Scholar]
- 33.Bain C, Fatmi A, Zoulim F, Zarski JP, Trepo C, Inchauspe G. Impaired allostimulatory function of dendritic cells in chronic hepatitis C infection. Gastroenterology. 2001;120:512–524. doi: 10.1053/gast.2001.21212. [DOI] [PubMed] [Google Scholar]
- 34.Dolganiuc A, Kodys K, Kopasz A, Marshall C, Mandrekar P, Szabo G. Additive inhibition of dendritic cell allostimulatory capacity by alcohol and hepatitis C is not restored by DC maturation and involves abnormal IL-10 and IL-2 induction. Alcohol Clin Exp Res. 2003;27:1023–1031. doi: 10.1097/01.ALC.0000071745.63433.32. [DOI] [PubMed] [Google Scholar]
- 35.Longman RS, Talal AH, Jacobson IM, Albert ML, Rice CM. Presence of functional dendritic cells in patients chronically infected with hepatitis C virus. Blood. 2004;103:1026–1029. doi: 10.1182/blood-2003-04-1339. [DOI] [PubMed] [Google Scholar]
- 36.Piccioli D, Tavarini S, Nuti S, Colombatto P, Brunetto M, Bonino F, Ciccorossi P, Zorat F, Pozzato G, Comar C, Abrignani S, Wack A. Comparable functions of plasmacytoid and monocyte-derived dendritic cells in chronic hepatitis C patients and healthy donors. J Hepatol. 2005;42:61–67. doi: 10.1016/j.jhep.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 37.Barnes E, Salio M, Cerundolo V, Francesco L, Pardoll D, Klenerman P, Cox A. Monocyte derived dendritic cells retain their functional capacity in patients following infection with hepatitis C virus. J Viral Hepatol. 2008;15:219–228. doi: 10.1111/j.1365-2893.2007.00934.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hatipoglu I, Ercan D, Acilan C, Basalp A, Durali D, Baykal AT. Hepatitis B virus e antigen (HBeAg) may have a negative effect on dendritic cell generation. Immunobiology. 2014 doi: 10.1016/j.imbio.2014.07.020. [DOI] [PubMed] [Google Scholar]
- 39.Agrawal A, Samrat SK, Agrawal B, Tyrrell DL, Kumar R. Co-incubation with core proteins of HBV and HCV leads to modulation of human dendritic cells. Viral Immunol. 2014 doi: 10.1089/vim.2014.0056. [DOI] [PubMed] [Google Scholar]
- 40.Szabo G, Catalano D, White B, Mandrekar P. Acute alcohol consumption inhibits accessory cell function of monocytes and dendritic cells. Alcohol Clin Exp Res. 2004;28:824–828. doi: 10.1097/01.alc.0000127104.80398.9b. [DOI] [PubMed] [Google Scholar]
- 41.Ho WY, Nguyen HN, Wolfl M, Kuball J, Greenberg PD. In vitro methods for generating CD8+ T-cell clones for immunotherapy from the naive repertoire. J Immunol Methods. 2006;310:40–52. doi: 10.1016/j.jim.2005.11.023. [DOI] [PubMed] [Google Scholar]
- 42.Jarnjak-Jankovic S, Hammerstad H, Saeboe-Larssen S, Kvalheim G, Gaudernack G. A full scale comparative study of methods for generation of functional dendritic cells for use as cancer vaccines. BMC Cancer. 2007;7:119. doi: 10.1186/1471-2407-7-119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kakazu E, Kanno N, Ueno Y, Shimosegawa T. Extracellular branched-chain amino acids, especially valine, regulate maturation and function of monocyte-derived dendritic cells. J Immunol. 2007;179:7137–7146. doi: 10.4049/jimmunol.179.10.7137. [DOI] [PubMed] [Google Scholar]
- 44.Van Gulck ER, Vanham G, Heyndrickx L, Coppens S, Vereecken K, Atkinson D, Florence E, Kint I, Berneman ZN, Van Tendeloo V. Efficient in vitro expansion of human immunodeficiency virus (HIV)-specific T-cell responses by gag mRNA-electroporated dendritic cells from treated and untreated HIV type 1-infected individuals. J Virol. 2008;82:3561–3573. doi: 10.1128/JVI.02080-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vogt A, Sievers E, Lukacs-Kornek V, Decker G, Raskopf E, Meumann N, Buning H, Sauerbruch T, Strassburg CP, Schmidt-Wolf IG, Gonzalez-Carmona MA. Improving immunotherapy of hepatocellular carcinoma (HCC) using dendritic cells (DC) engineered to express IL-12 in vivo. Liver Int. 2013 doi: 10.1111/liv.12284. [DOI] [PubMed] [Google Scholar]
- 46.Kayashima H, Toshima T, Okano S, Taketomi A, Harada N, Yamashita Y, Tomita Y, Shirabe K, Maehara Y. Intratumoral neoadjuvant immunotherapy using IL-12 and dendritic cells is an effective strategy to control recurrence of murine hepatocellular carcinoma in immunosuppressed mice. J Immunol. 2010;185:698–708. doi: 10.4049/jimmunol.0900187. [DOI] [PubMed] [Google Scholar]
- 47.Tuting T, Wilson CC, Martin DM, Kasamon YL, Rowles J, Ma DI, Slingluff CL, Jr, Wagner SN, van der Bruggen P, Baar J, Lotze MT, Storkus WJ. Autologous human monocyte-derived dendritic cells genetically modified to express melanoma antigens elicit primary cytotoxic T cell responses in vitro: enhancement by cotransfection of genes encoding the Th1-biasing cytokines IL-12 and IFN-alpha. J Immunol. 1998;160:1139–1147. [PubMed] [Google Scholar]
- 48.Szabo A, Gogolak P, Pazmandi K, Kis-Toth K, Riedl K, Wizel B, Lingnau K, Bacsi A, Rethi B, Rajnavolgyi E. The two-component adjuvant IC31(R) boosts type i interferon production of human monocyte-derived dendritic cells via ligation of endosomal TLRs. PLoS ONE. 2013;8:e55264. doi: 10.1371/journal.pone.0055264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Pen JJ, De Keersmaecker B, Maenhout SK, Van Nuffel AM, Heirman C, Corthals J, Escors D, Bonehill A, Thielemans K, Breckpot K, Aerts JL. Modulation of regulatory T cell function by monocyte-derived dendritic cells matured through electroporation with mRNA encoding CD40 ligand, constitutively active TLR4, and CD70. J Immunol. 2013;191:1976–1983. doi: 10.4049/jimmunol.1201008. [DOI] [PubMed] [Google Scholar]
- 50.Bonehill A, Tuyaerts S, Van Nuffel AM, Heirman C, Bos TJ, Fostier K, Neyns B, Thielemans K. Enhancing the T-cell stimulatory capacity of human dendritic cells by co-electroporation with CD40L, CD70 and constitutively active TLR4 encoding mRNA. Mol Ther. 2008;16:1170–1180. doi: 10.1038/mt.2008.77. [DOI] [PubMed] [Google Scholar]
- 51.Goode EC, Warburton RC, Gelson WT, Watson AJ. Activated intestinal macrophages in patients with cirrhosis release NO and IL-6 that may disrupt intestinal barrier function. Gastroenterology. 2013;145:1481–1484. doi: 10.1053/j.gastro.2013.10.031. [DOI] [PubMed] [Google Scholar]
- 52.Wijesundera KK, Izawa T, Tennakoon AH, Murakami H, Golbar HM, Katou-Ichikawa C, Tanaka M, Kuwamura M, Yamate J. M1- and M2-macrophage polarization in rat liver cirrhosis induced by thioacetamide (TAA), focusing on Iba1 and galectin-3. Exp Mol Pathol. 2014;96:382–392. doi: 10.1016/j.yexmp.2014.04.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


