Abstract
Aims
Elderberry (Sambucus spp.) is one of the oldest medicinal plants noted for its cardiovascular, anti-inflammatory, and immune-stimulatory properties. In this study, we investigated the anti-inflammatory and anti-oxidant effects of the American elderberry (Sambucus nigra subsp. canadensis) pomace as well as some of the anthocyanins (cyanidin chloride and cyanidin 3-O-glucoside) and flavonols (quercetin and rutin) in bv-2 mouse microglial cells.
Main methods
The bv-2 cells were pretreated with elderberry pomace (extracted with ethanol or ethyl acetate) or its anthocyanins and flavonols and stimulated by either lipopolysaccharide (LPS) or interferon-γ (IFNγ). Reactive oxygen species (ROS) and nitric oxide (NO) production (indicating oxidative stress and inflammatory response) were measured using the ROS detection reagent DCF-DA and the Griess reaction, respectively.
Key findings
Analysis of total monomeric anthocyanin (as cyanidin 3-O-glucoside equivalents) indicated five-fold higher amount in the freeze-dried ethanol extract as compared to that of the oven-dried extract; anthocyanin was not detected in the ethyl acetate extracts. Elderberry ethanol extracts (freeze-dried or oven-dried) showed higher anti-oxidant activities and better ability to inhibit LPS or IFNγ-induced NO production as compared with the ethyl acetate extracts. The phenolic compounds strongly inhibited LPS or IFNγ-induced ROS production, but except for quercetin, they were relatively poor in inhibiting NO production.
Significance
These results demonstrated difference in anti-oxidative and anti-inflammatory effects of elderberry extracts depending on solvents used. Results further identified quercetin as the most active component in suppressing oxidative stress and inflammatory responses on microglial cells.
Keywords: elderberry, lipopolysaccharide, quercetin, microglia, nitric oxide, reactive oxygen species
Introduction
There is increasing interest to investigate the health effects of dietary fruits and berries (Seeram et al., 2012), botanicals that have been implicated for improved cardiovascular, anti-inflammatory and immune enhancing functions (Beattie et al., 2005; Nile and Park, 2014). Elderberries (Sambucus spp.) are widely grown in Europe, Asia, North-Africa and North-America; the plant has been called “the medicine chest of country people”. Although all parts of the plant have been used in folk medicine for centuries, the berries and the flowers are most commonly described in scientific literature (Sambucus nigra, 2005). The berries contain a wide variety of anthocyanins, flavonoids and other polyphenols (Wu et al., 2004; Lee and Finn, 2007). The major flavonoids identified are quercetin and rutin and the primary anthocyanins are cyanidin-3-O-sambubioside and cyanidin 3-O-glucoside (Chandra et al., 2001; Wu et al., 2002; Sambucus nigra, 2005). In addition, the presence of iridoid glycosides, sesquiterpenes and phytosterols has also been reported (Thole et al., 2006).
Among several berry species, elderberry contains the highest amount of total flavonols (Mikulic-Petkovsek et al., 2012). In addition, hydrophilic antioxidant capacity for elderberry is among the highest that was measured in fresh fruits (Wu et al., 2004). Different extracts have been shown to possess anti-inflammatory, antiviral, anti-diabetic, anti-carcinogenic and immune-stimulatory activities (Sambucus nigra, 2005; Vlachojannis et al., 2010). Cyanidin-3-O-glucoside has 3.5 times higher antioxidant activities as compared with vitamin E (Wang et al., 1997). This compound has been shown to protect against oxidative damage (Youdim et al., 2000), and suppress the production of nitric oxide (NO) induced by lipopolysaccharide (LPS) in macrophage cells (Hu et al., 2003; Wang et al., 2008). The anthocyanins, such as cyanidin and protocatechuic acid are also effective in decreasing NO production (Min et al., 2010). In LPS stimulated RAW 264.7 macrophages, quercetin and its glycoside, rutin, inhibited inducible nitric oxide synthase (iNOS) expression (Chen et al., 2001; Kazlowska et al., 2010). Although macrophages share many properties similar to microglial cells, studies to examine elderberry extracts on oxidative and inflammatory responses of microglial cells have not been extensive.
Inflammatory processes play a major role in the progression of a number of neurodegenerative diseases and activation of microglial cells, the resident macrophages in the central nervous system, is an important initial step of the inflammatory response (Skaper et al., 2012). Upon exposure to proinflammtory cytokines and/or lipopolysaccharides (LPS), specific signal transduction pathways are activated which regulate the induction of iNOS and other oxidative and inflammatory mediators (see Glass et al., 2010, Spencer et al., 2012, for reviews). Therefore, suppression of microglial activation might lead to an earlier restoration of homeostasis and resolution of neuroinflammation. While LPS is known to directly activate TLR4 receptors and the NF-kB pathway, IFNγ, a cytokine derived from peripheral immune cells, is coupled to the JAK-STAT pathway. Some pro-inflammatory genes, such as that for iNOS, comprise of promoters that require activation of transcription factors involving NF-kB and the JAK-STAT pathways (Pawate et al., 2004). However, our earlier studies showed that LPS or IFNγ can each stimulate iNOS in bv-2 microglial cells, and this was attributed to presence of cross-talk mechanisms through activation of ERK1/2 (Shen et al., 2005; Sheng, et al., 2011; Chuang et al., 2013). Despite that LPS and IFNg can individually induce ROS and NO in microglial cells, it is not clear whether botanicals may exert differences in these pathways. In a recent study, there is evidence that the anti-inflammatory effects of certain botanical-derived compounds are stronger for IFNγ-stimulated inflammation than for LPS (Foresti et al., 2013).
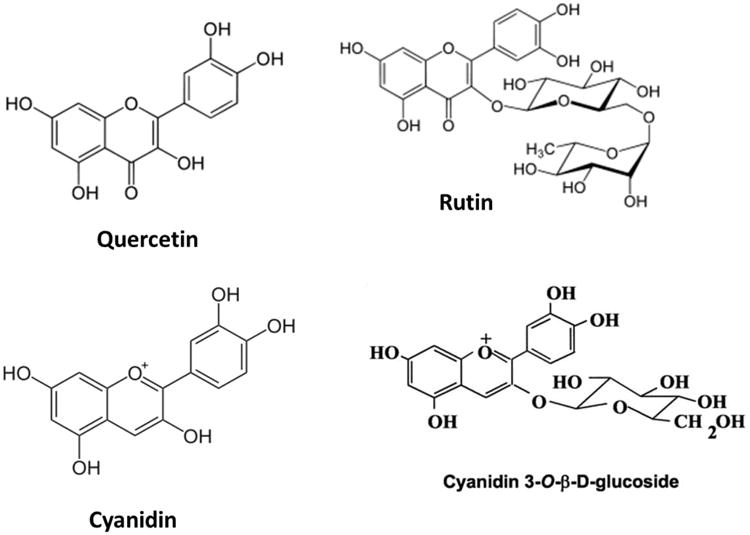
In this study, freeze-dried or oven-dried elderberry pomace was extracted by ethanol or ethyl acetate and effects of the extracts on oxidative and inflammatory responses upon stimulation of murine bv-2 microglial cells with LPS or IFNγ were determined. In addition, studies were extended to examine effects of phenolic components, such as cyanidin chloride, cyanidin 3-O-glucoside, quercetin or rutin (Fig. 1).
Fig. 1. Chemical structures of flavonols (quercetin, rutin) and anthocyanins (cyanidin, cyanidin 3-O-β-glucoside) present in elderberry.
Materials and methods
Materials
Quercetin, rutin (Sigma-Aldrich, St. Louis, MO), cyanidin 3-O-glucoside and cyanidin chloride (Indofine Chemical Comp., Hillsborough, NJ) were dissolved in DMSO as a stock solution. Dulbecco's modified Eagle's medium (DMEM), penicillin, streptomycin, 0.05% (w/v) trypsin/EDTA, and phosphate-buffered saline (PBS) were obtained from GIBCO (Gaithersburg, MD). Lipopolysaccharide (LPS) (rough strains) from Escherichia coli F583 (Rd mutant) was obtained from Sigma-Aldrich (St. Louis, MO). Murine interferon-γ (IFNγ) was purchased from R & D Systems (Minneapolis, MN). Fetal bovine serum was from Atlanta Biologicals (Lawrenceville, GA).
Elderberry materials and preparation of extracts
Elderberry (Sambucus nigra subsp. canadensis) extracts were derived from ripe berries harvested from 4-year-old plants grown in a commercial orchard near Hartsburg, Missouri. Berries were de-stemmed by a shaker, rinsed twice with lightly bleached water, and frozen in plastic food-grade buckets (9 kg) in a -20 °C freezer after the bleached water was completely drained. Thawed berries were heated to 82 °C for 5 minutes in a 230-liter steam-jacketed kettle and pressed immediately using a cider mill to separate the juice (60% w/w) and pomace (40% w/w). Elderberry pomace (e.g., seeds and skins) was then refrozen and stored at -20 °C. Samples of elderberry pomace were either freeze-dried or oven-dried (105 °C for 18-24 hours) and subsequently extracted with 10 volumes of 95% ethanol or ethyl acetate (room temperature, overnight). Upon evaporation of the solvent under nitrogen, the dried residue was weighed, and stored at -20° C. The extracts were re-dissolved in cell culture grade DMSO before adding to the cells.
Determination of total monomeric anthocyanin content
Total monomeric anthocyanin content was measured using a pH differential method (Giusti and Wrolstad, 2001). Absorbance values were measured in triplicate at wavelengths of 520 nm and 700 nm using a PerkinElmer Enspire 2300 multimode plate reader. Cyanidin 3-O-glucoside standards were prepared and treated in the same manner as the samples. Total monomeric anthocyanin values are reported as cyanidin 3-O-glucoside equivalents (μg/mL).
Cell culture and treatments
The immortalized mouse microglial cells (bv-2) were prepared as previously described (Shen et al., 2005). Cells were cultured in 75 cm2 flasks with DMEM supplemented with 5% FBS containing 100 units/mL penicillin and 100 μg/mL streptomycin, and maintained in 5% CO2 incubator at 37 °C. Cells, 3.8 × 104 cells/well, were subcultured in 96-well plates for experiments. Cells were serum starved for 4 h prior to adding LPS (100 ng/mL) or IFNγ (10 ng/mL). Botanical compounds and extracts were added 1 h before cytokines. Cell morphology was observed by using a phase contrast Nikon DIAPHOT 300 microscope attached with a CCD cool camera linked to MagnaFire 2.1C software for image processing. Representative bright field pictures were obtained using a 20 × objective.
Assessment of cell viability
The MTT (3-(4, 5-Dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide, Sigma-Aldrich, St. Louis, MO) assay was used to quantify cell viability and cytotoxicity. Cells were treated with the specified concentrations of botanical compounds and extracts for 17 hours with or without LPS/IFNγ (added one hour after botanicals). Medium was removed and 0.1 mL of MTT reagent (0.5 mg/mL) in serum free DMEM was added into each well (96-well plates). Cells were incubated for 4 h at 37 °C, and after dissolving the formazan dye with DMSO, absorption was read at 540 nm using a Synergy-4 plate reader (BioTek Instruments, Inc., Fisher Scientific, St. Louis, MO). These assays demonstrated no significant changes in cell viabilities in any experiments (data not shown).
Measurement of reactive oxygen species production
Reactive oxygen species (ROS) production was measured using the ROS detection reagent CM-H2DCFDA (DCF, Invitrogen, Inc., OR) as described previously (Chuang et al., 2013). Briefly, bv-2 cells were seeded in a 96-well plate for 24 h. Cells were then starved for 4 h in the serum free DMEM prior to 1 h pretreatment with botanicals followed by addition of LPS/INFγ for 11 h. DCF (10 μM final concentration) was added to each well for 1 h. The fluorescence intensity of DCF was measured using the Synergy-4 plate reader with an excitation wavelength of 490 nm and an emission wavelength of 520 nm.
Nitric oxide determination in culture medium
NO released from cells was converted to nitrite in the culture medium, which was determined using the Griess reagent (Sheng et al., 2011). Cells were cultured in DMEM without phenol red. Sixteen hours after LPS/IFNγ treatments, aliquots (50 μL) of culture medium were transferred to 96-well plates and incubated with 50 μL of reagent A [1% (w/v) sulfanilamide (Sigma-Aldrich, St. Louis, MO) in 5% phosphoric acid] for 10 minutes at room temperature in the dark. Incubation with 50 μL of reagent B (0.1%, w/v, N-1-napthylethylenediamine dihydrochloride, Sigma-Aldrich, St. Louis, MO) for 10 minutes at room temperature in the dark was followed by measurement of the absorbance at 543 nm using the Synergy-4 plate reader. Sodium nitrite (0-100 μM), diluted in culture media, was used to prepare the nitrite standard curve.
Statistical analysis
Data are shown as mean ± SEM from at least three independent experiments. Results were analyzed by either one-way or two-way ANOVA followed by Bonferroni post-tests (V4.00; GraphPad Prism Software Inc., San Diego, CA). Differences were considered significant at p<0.05 for all analyses.
Results
Total monomeric anthocyanin content of the elderberry extracts
In this experiment, elderberry pomace was extracted with ethanol or ethyl acetate and an equal amount subjected to freeze-dried or oven-dried protocols. The ethanol extract from the freeze-dried pomace contained the highest amounts of total monomeric anthocyanin (103.9 ± 11 μg/mL, cyanidin-3-glucoside equivalents), and was about five times higher compared to the oven-dried extraction (20.9 ± 14 μg/mL, cyanidin-3-glucoside equivalents). The ethyl acetate extracts (both freeze-dried and oven-dried) yielded samples with undetectable amounts of total monomeric anthocyanin.
Effects of elderberry extracts on ROS production
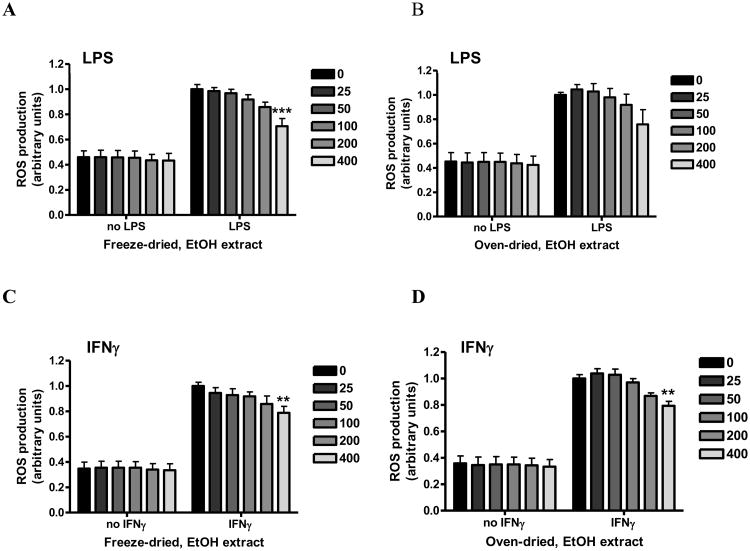
In bv-2 microglial cells, none of the elderberry extracts had an influence on basal ROS production. When cells were treated with freeze-dried and oven-dried elderberry ethanol extracts prior to LPS or IFNγ exposure, there was a dose-dependent attenuation in ROS production but significant decrease was not attained until 400 μg/mL (Fig. 2). Under similar treatment conditions, none of the ethyl acetate extracts showed any effects (data not shown).
Fig. 2. Effects of elderberry extracts on ROS production induced by LPS or IFNγ in microglial cells.
Elderberry extracts (0 to 400 μg/mL), (A and C, freeze-dried, B and D, oven-dried), were applied to cells 1 h prior to exposure to LPS (A, B) (100 ng/mL) or IFNγ (C, D) (10 ng/mL) for 11 h. ROS production was measured using CM-H2DCFDA as described in the text. Results are expressed as the mean ± SEM (n = 4) and analyzed by two-way ANOVA with Bonferroni post-tests. **p < 0.01 and ***p<0.001 as compared to the respective LPS or IFNγ-stimulated group.
Effects of elderberry extracts on NO production
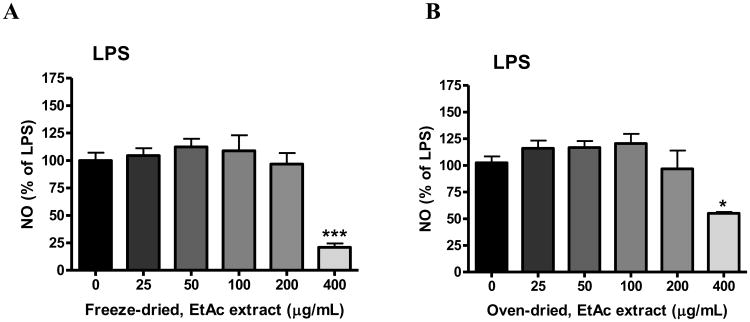
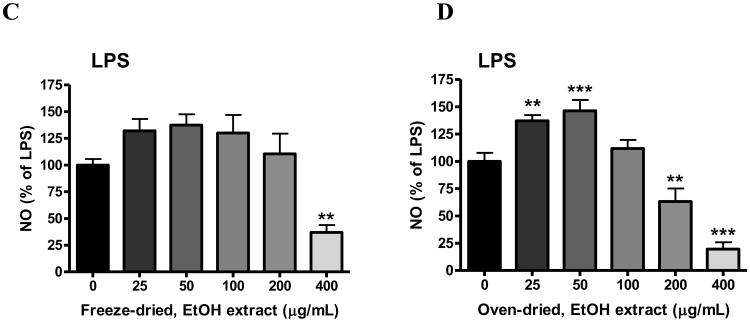
In bv-2 microglial cells, none of the elderberry extracts alone increased NO production; thus data were expressed as % of the values obtained for LPS or IFNγ. In this study, both freeze-dried and oven-dried ethyl acetate extracts significantly attenuated LPS-induced NO production at 400 μg/mL (p< 0.001, Fig. 3A; p<0.05, Fig. 3B). Both freeze-dried and oven-dried ethanol extracts also significantly inhibited LPS-induced NO production (p<0.01, Fig. 3C and 3D). Interestingly, besides inhibition at 200-400 μg/mL, ethanol extracts appear to show a biphasic effect. In particular, the oven-dried extract significantly increased LPS-induced NO production at 25 and 50 μg/mL (p<0.01 and p<0.001, Fig. 3D). Studies with IFNγ-induced NO production indicated similar results (data not shown).
Fig. 3. Effects of elderberry extracts on NO production induced by LPS in microglia.
Cells were treated with elderberry extracts (0 to 400 μg/mL) for 1 h followed by stimulation with LPS (100 ng/mL) for 16 h. Culture media were collected for determination of NO using the Griess reaction protocol as described in the text. Results are expressed as the mean ± SEM (n = 3-5) and significant difference from the LPS-stimulated group was determined by one-way ANOVA followed by Bonferroni post-tests, *p < 0.05, **p< 0.01, ***p<0.001. (A) Freeze-dried, ethyl acetate extract; (B) Oven-dried, ethyl acetate extract; (C) Freeze-dried, ethanol extract; (D) Oven-dried, ethanol extract.
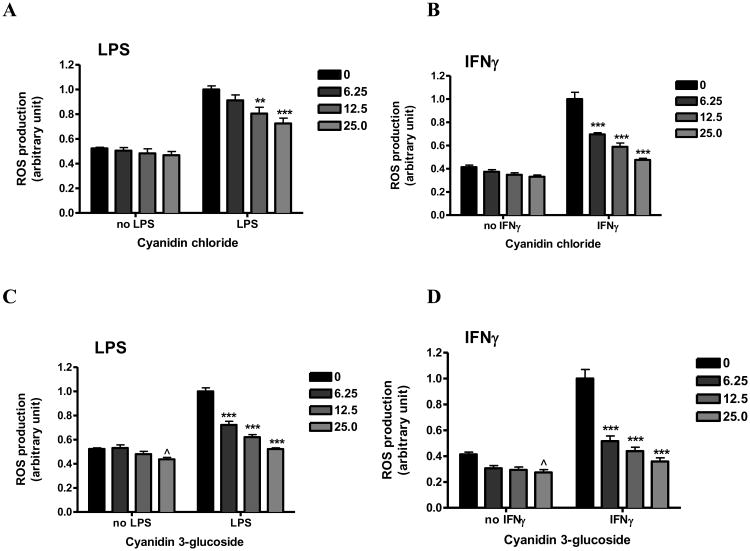
Effects of anthocyanins on ROS production
In a preliminary study, cyanidin and cyanidin 3-glucoside were shown to offer anti-oxidative effects on ROS production stimulated with LPS or IFNγ in bv-2 cells in a dose dependent manner ranging to 25 μM. Cyanidin 3-glucoside slightly reduced basal ROS production (25 μM, p<0.05, Fig. 4C and D) but cyanidin chloride had no effect (p>0.05, Fig. 4A and B). Both compounds attenuated LPS or IFNγ-induced ROS production in a concentration-dependent manner (Fig. 4). Interestingly, both cyanidin chloride and cyanidin 3-O-glucoside were more effective in inhibition of ROS due to stimulation with IFNγ as compared to that with LPS. Furthermore, in cells stimulated with either LPS or IFNγ, cyanidin 3-O-glucoside appeared to be more effective in inhibiting ROS production as compared with cyanidin chloride (Fig 4). Under these conditions, cyanidin 3-O-glucoside was more potent in inhibiting ROS production due to stimulation of IFNγ, showing a significant decrease of more than 80% at 6.25 μM (Fig. 4D).
Fig. 4. Effects of anthocyanins on ROS production induced by LPS or IFNγ in microglia.
Cyanidin chloride (A, B) or cyanidin 3-O-glucoside (C, D) (0-25 μM) were applied to cells 1 h prior to either LPS (A, C) (100 ng/mL) or IFNγ (B, D) (10 ng/mL) for 11 h. ROS production was measured using CM-H2DCFDA as described in the text. Results are expressed as the mean ± SEM (n = 3) and analyzed by two-way ANOVA with Bonferroni post-tests. ˆp<0.05 as compared to the unstimulated control; **p < 0.01, ***p<0.001 as compared to the respective LPS/IFNγ-stimulated group.
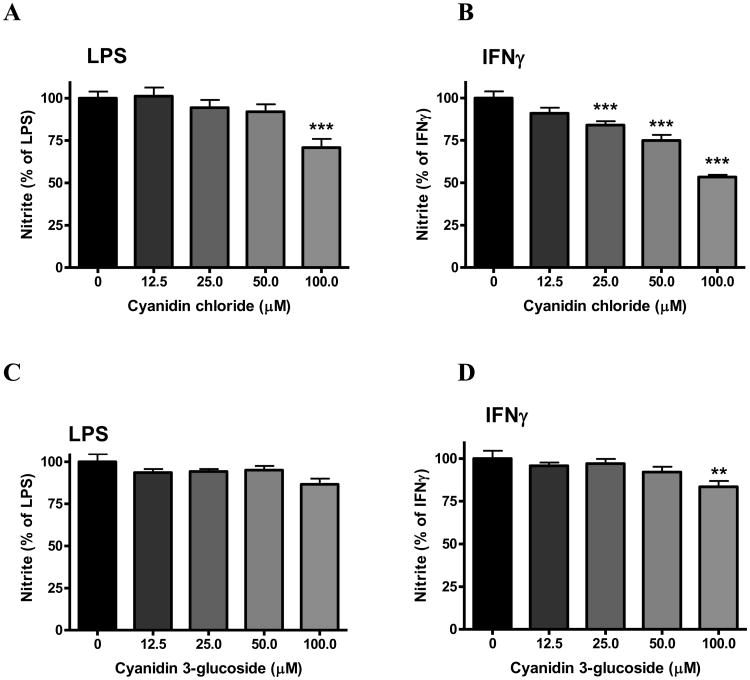
Effects of anthocyanins on NO production
In bv-2 microglial cells, neither cyanidin chloride nor cyanidin 3-O-glucoside alone increased NO production, and so data were expressed as % of the values obtained for LPS or IFNγ. Pretreatment of the cells with cyanidin chloride attenuated both LPS- (100 μM, p<0.001, Fig. 5A) and IFNγ-induced NO production (25-100 μM, p<0.001, Fig. 5B). However, there were minimal effects for cyanidin 3-O-glucoside to reduce NO production with only a small but significant decrease observed at 100 μM (p<0.01, Fig. 5D).
Fig. 5. Effects of anthocyanins on NO production induced by LPS or IFNγ in microglia.
Cells were treated with cyanidin chloride (A, B) or cyanidin 3-O-glucoside (C, D) (0 to 100 μM) for 1 h followed by stimulation with either LPS (A, C) (100 ng/mL) or IFNγ (B, D) (10 ng/mL) for 16 h. Culture media were collected for determination of NO using the Griess reaction protocol as described in the text. Results are expressed as the mean ± SEM (n = 4-6) and significant difference from the LPS/IFNγ-stimulated group was determined by one-way ANOVA followed by Bonferroni post-tests, **p<0.01, ***p < 0.001.
Effects of flavonols on ROS production
Quercetin (12.5 μM, p<0.05, Fig. 6A and B) but not rutin (Fig. 6C and D) was able to suppress basal ROS production. However, similar to the anthocyanins, both quercetin and rutin attenuated LPS- or IFNγ-induced ROS production in a concentration-dependent manner (Fig. 6).
Fig. 6. Effects of flavonols on ROS production induced by LPS or IFNγ in microglia.
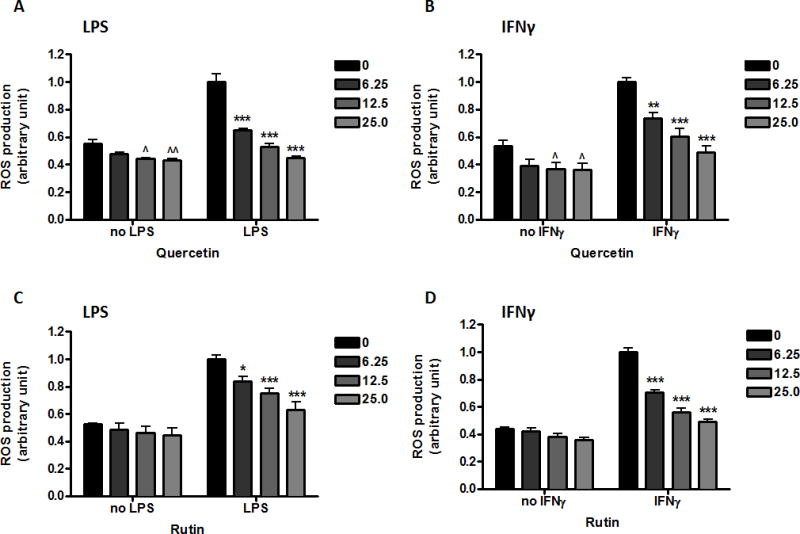
Quercetin (A, B) or rutin (C, D) (0-25 μM) were applied to cells 1 h prior to either LPS (A, C) (100 ng/mL) or IFNγ (B, D) (10 ng/mL) for 11 h. ROS production was measured using CM-H2DCFDA as described in the text. Results are expressed as the mean ± SEM (n = 3) and analyzed by two-way ANOVA with Bonferroni post-tests. ˆp<0.05, ˆˆp<0.01 as compared to the unstimulated control; *p<0.05, **p < 0.01, ***p<0.001 as compared to the respective LPS/IFNγ-stimulated group.
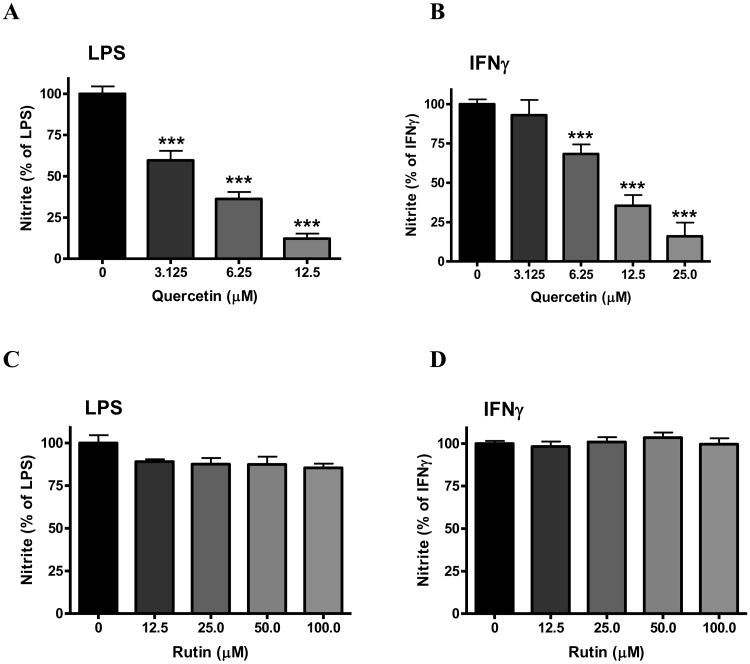
Effects of flavonols on NO production
In bv-2 microglial cells, neither quercetin nor rutin alone increased NO production, so data were expressed as % of the values obtained for LPS or IFNγ. Pretreatment of the cells with quercetin prior to exposure to LPS or IFNγ resulted in a concentration-dependent reduction in NO release (Fig. 7A and B). Quercetin was more effective against LPS, and 12.5 μM quercetin attenuated LPS-induced NO production by 90% (p<0.001, Fig. 7A). Pretreatment of the cells with rutin (0-100 μM) did not change NO production induced by either LPS (Fig. 7C) or IFNγ (Fig. 7D).
Fig. 7. Effects of flavonols on NO production induced by LPS or IFNγ in microglia.
Cells were treated with quercetin (A, B) (0 to 25 μM) or rutin (C, D) (0-100 μM) for 1 h followed by stimulation with either LPS (A, C) (100 ng/mL) or IFNγ (B, D) (10 ng/mL) for 16 h. Culture media were collected for determination of NO using the Griess reaction protocol as described in the text. Results are expressed as the mean ± SEM (n = 3-9) and significant difference from the LPS/IFNγ-stimulated group was determined by one-way ANOVA followed by Bonferroni post-tests, ***p < 0.001.
Discussion
Regulated production of ROS and NO is an important part of redox signaling and they serve as mediators of physiological responses. Under normal physiological conditions, ROS and NO are generated in a stimulus-dependent way in different compartments of the cell, and they participate in signaling cascades and modulate important cell functions. However, under inflammatory conditions, microglial cells generate a significant amount of ROS and NO by the induction and activation of NADPH oxidase and iNOS. In line with our previous studies, LPS or IFNγ could independently ROS and NO production in microglial cells (Shen et al., 2005). Our studies further provided evidence for a cross-talk mechanism between LPS stimulated NF-κB and IFNγ-stimulated JAK/STAT pathways through activation of the ERK1/2 (Chuang et al., 2013). U0126, a specific inhibitor for MEK1/2, the kinases leading to phosphorylation of ERK1/2, readily abrogated IFNγ induced NO in these cells. Besides induction of ROS and NO, microglia activation is also accompanied by secretion of pro-inflammatory cytokines and chemokines, including TNFα, IL-1β and IL-6 (Kang et al., 2013; Lim, et al., 2012). Many botanical compounds offer neuroprotective effects by suppressing microglial activation. In this study, we observed differences in effects of botanicals on LPS-induced ROS and NO. Although elderberry extracts inhibited LPS- or IFNγ-induced ROS and NO, there is no biphasic effect with the dose response for ROS. Furthermore, while most polyphenols tested were effective in suppressing LPS-induced ROS production, polyphenols such as rutin and cyanidin 3-glucoside were not effective in suppressing LPS-induced NO.
Results here demonstrated the anti-oxidative effects of elderberry ethanol extracts (both freeze-dried and oven-dried) but not ethyl acetate extracts. On the other hand, both ethanol and ethyl acetate extracts could inhibit inflammatory (NO) responses on microglial cells, albeit at high concentrations. These results are in line with previous studies using other berries such as blueberry, blackberry, raspberry, cranberry, strawberry and acai (Spencer et al., 2012; Nile and Park, 2014). However, this is the first study to show that some elderberry extracts showed a biphasic effect, with increases in NO production at lower concentrations and inhibition at higher concentrations. Similar biphasic effect has been reported on cytokine-production in dendritic cells (Frøkiær et al., 2012), and pro- and anti-inflammatory activities of elderberry extracts have also been observed (Vlachojannis et al., 2010). This type of immunomodulation in our study as well as in other studies may be caused by a specific constituent in the extracts or by synergistic effects of several ingredients. Obviously, more studies are needed to identify these bioactive compounds.
Anthocyanins are abundant in berries and are reported to have beneficial effects to human health, especially for the cardiovascular functions, anti-carcinogenic, anti-viral and anti-inflammatory effects (Prior and Wu, 2006; Zafra-Stone et al., 2007). Berry anthocyanins have been shown to prevent age-associated increase in oxidative stress and improve neuronal and cognitive functions in animal models (Joseph et al., 2009; Poulose et al., 2012). Cyanidin 3-O-glucoside was shown to protect against cerebral ischemic damage, ethanol-induced neurodegeneration, and attenuates amyloid-beta-induced memory impairments (Ke et al., 2011; Min et al., 2011; Qin et al., 2013). Similarly, both quercetin and rutin showed neuroprotective effects in vivo (Pu et al., 2007; Koda et al., 2009; Yao et al., 2012; Karuppagounder et al., 2013). The in vivo effects suggest that berry flavonoids or their metabolites are bioavailable to the brain. Elderberry contains especially high amounts of anthocyanins and flavonols (Wu et al., 2004; Lee and Finn, 2007; Mikulic-Petkovsek et al., 2012), and there is evidence for anthocyanins to cross the blood-brain barrier. Study by Andres-Lacueva et al. (2005) detected anthocyanins in rat brains given a diet supplemented with a blueberry extract for 10 weeks. Similarly, anthocyanins and their metabolites have been found in the brains of blueberry-fed pigs (Kalt et al., 2008; Milbury and Kalt, 2010). A study with endothelial cells also demonstrated incorporation of anthocyanins into the cell plasma membrane and cytosol (Youdim et al., 2000). Considering the bioavailability of flavonols, several studies have provided evidence for the accumulation of quercetin and its metabolites in the brain. Quercetin-3-O-glucuronide, one of the major metabolites, has also been identified in rat and human brain tissues (Ishisaka et al., 2011, 2014; Ho et al., 2013).
Different types of elderberry fruit extracts contain different profiles of polyphenols. In the current study, quantification of total monomeric anthocyanins showed high concentration in the ethanol extracts but not in the ethyl acetate extracts. This difference in extraction procedure reflected the ability of the extracts to inhibit ROS production (see Fig. 2). Similar findings have been reported in the literature (Dawidowicz et al., 2006; Vlachojannis et al., 2010). The elderberry extracts generated by different extraction methods also exhibited significantly different effects on their ability to inhibit LPS-induced NO production (see Fig. 3).
Cyanidin 3-glucoside is one of the major anthocyanins in elderberry, and showed higher antioxidant capacity than its aglycone form (Wang et al., 1997). In agreement with the literature, cyanidin 3-glucoside was more effective at inhibiting LPS or IFNγ-induced ROS production than cyanidin (Fig. 4). In contrast, cyanidin 3-glucoside showed weak inhibitory effects on the level of NO (Fig. 5). Similar findings have been demonstrated on primary mixed glial cells (Vafeiadou et al., 2009) and RAW264.7 macrophages (Jin et al., 2006), although others have reported stronger anti-inflammatory effects in vitro (Hu et al., 2003; Min et al., 2010). Nevertheless, several studies provided evidence for the neuroprotective and anti-inflammatory effectiveness of cyanidin 3-O-glucoside in vivo (Ke et al., 2011; Min et al., 2011; Qin et al., 2013). The possibility that cyanidin 3-O-glucoside exerts activity in vivo by converting to its aglycone form remains to be investigated.
Among the elderberry phenolic compounds used in our assays, quercetin showed the strongest effects against oxidative and inflammatory stress in microglia (see Fig. 6 and 7). Pretreatment with quercetin inhibited the formation of NO in a concentration-dependent manner, with IC50 values of 3.4 and 13.6 μM for LPS and IFNγ induction, respectively. Similar values have been obtained by other studies (Chen et al., 2005; Kao et al., 2010). Quercetin may directly affect signal transduction pathways and exert its anti-oxidative/anti-inflammatory effects by multiple mechanisms. These mechanisms may be cell type specific. In our present study, we observed a difference in anti-inflammatory effect between quercetin and its glucoside rutin (Fig. 7). It is known that quercetin metabolites can be less, or equally active than the aglycone and sometimes even an inverse activity can be found (see Beekmann et al., 2012 for further discussion). Considering the extensive metabolism of quercetin in the body (Del Rio et al., 2013), and the presence of its glucuronide metabolites at the extracellular level, future studies should include examining bioavailability of these compounds in animal models.
Conclusions
This study demonstrated that elderberry extracts and some of its phenolic constituents can exert anti-oxidative and anti-inflammatory effects on LPS or IFNγ-stimulated murine microglial bv-2 cells. The study further identified differences in response between ROS and NO and among the polyphenols, quercetin is a particularly active component to mitigate the oxidative and inflammatory responses in microglial cells.
Acknowledgments
This work is supported by NIH grants 2P01 AG08357 from NIA, 1P50 AT006273 from NCCAM, ODS and NCI.
Footnotes
Author contribution to study: GYS, AS, ZG and DBL conceived idea and designed experiments for the project; ZC, JJ, YZ, DYC provided technical help; ALT, CHL and KLF provided help on elderberry collection and processing; CMG and GER provided technical help on chemical analysis. All authors have read/edited and approved the manuscript.
Conflict of interest: The authors declare that there are no conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Andres-Lacueva C, Shukitt-Hale B, Galli RL, Jauregui O, Lamuela-Raventos RM, Joseph JA. Anthocyanins in aged blueberry-fed rats are found centrally and may enhance memory. Nutr Neurosci. 2005;8:111–120. doi: 10.1080/10284150500078117. [DOI] [PubMed] [Google Scholar]
- Beattie J, Crozier A, Duthie GG. Potential health benefits of berries. Curr Nutr Food Sci. 2005;1:71–86. [Google Scholar]
- Beekmann K, Actis-Goretta L, van Bladeren PJ, Dionisi F, Destaillats F, Rietjens IM. A state-of-the-art overview of the effect of metabolic conjugation on the biological activity of flavonoids. Food Funct. 2012;3:1008–1018. doi: 10.1039/c2fo30065f. [DOI] [PubMed] [Google Scholar]
- Chandra A, Rana J, Li Y. Separation, identification, quantification, and method validation of anthocyanins in botanical supplement raw materials by HPLC and HPLC-MS. J Agric Food Chem. 2001;49:3515–3521. doi: 10.1021/jf010389p. [DOI] [PubMed] [Google Scholar]
- Chen YC, Shen SC, Lee WR, Hou WC, Yang LL, Lee TJ. Inhibition of nitric oxide synthase inhibitors and lipopolysaccharide induced inducible NOS and cyclooxygenase-2 gene expressions by rutin, quercetin, and quercetin pentaacetate in RAW 264.7 macrophages. J Cell Biochem. 2001;82:537–548. doi: 10.1002/jcb.1184. [DOI] [PubMed] [Google Scholar]
- Chen JC, Ho FM, Pei-Dawn Lee Chao, Chen CP, Jeng KC, Hsu HB, Lee ST, Wen Tung Wu, Lin WW. Inhibition of iNOS gene expression by quercetin is mediated by the inhibition of IkappaB kinase, nuclear factor-kappa B and STAT1, and depends on heme oxygenase-1 induction in mouse BV-2 microglia. Eur J Pharmacol. 2005;521:9–20. doi: 10.1016/j.ejphar.2005.08.005. [DOI] [PubMed] [Google Scholar]
- Chuang DY, Chan MH, Zong Y, Sheng W, He Y, Jiang JH, Simonyi A, Gu Z, Fritsche KL, Cui J, Lee JC, Folk WR, Lubahn DB, Sun AY, Sun GY. Magnolia polyphenols attenuate oxidative and inflammatory responses in neurons and microglial cells. J Neuroinflammation. 2013;10:15. doi: 10.1186/1742-2094-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dawidowicz AL, Wianowska D, Baraniak B. The antioxidant properties of alcoholic extracts from Sambucus nigra L. (antioxidant properties of extracts) LWT. 2006;39:308–315. [Google Scholar]
- Del Rio D, Rodriguez-Mateos A, Spencer JP, Tognolini M, Borges G, Crozier A. Dietary (poly)phenolics in human health: structures, bioavailability, and evidence of protective effects against chronic diseases. Antioxid Redox Signal. 2013;18:1818–1892. doi: 10.1089/ars.2012.4581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foresti R, Bains SK, Pitchumony TS, de Castro Bras LE, Drago F, Dubois-Rande JC, Bucolo C, Motterlini R. Small molecule activators of Nrf2-HO-1 antioxidant axis modulate heme metabolism and inflammation in BV2 microglia cells. Pharmacol Res. 2013;76:132–148. doi: 10.1016/j.phrs.2013.07.010. [DOI] [PubMed] [Google Scholar]
- Frøkiær H, Henningsen L, Metzdorff SB, Weiss G, Roller M, Flanagan J, Fromentin E, Ibarra A. Astragalus root and elderberry fruit extracts enhance the IFN-β stimulatory effects of Lactobacillus acidophilus in murine-derived dendritic cells. PLoS One. 2012;7:e47878. doi: 10.1371/journal.pone.0047878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glass CK, Saijo K, Winner B, Marchetto MC, Gage FH. Mechanisms underlying inflammation in neurodegeneration. Cell. 2010;140:918–934. doi: 10.1016/j.cell.2010.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giusti MM, Wrolstad RE. Characterization and measurement of anthocyanins by UV-visible spectroscopy. Unit F 1.2. In: Wrolstad RE, Schwartz SJ, editors. Current Protocols in Food Analytical Chemistry. John Wiley & Sons, Inc; New York, NY: 2001. pp. F1.2.1–F1.2.13. [Google Scholar]
- Ho L, Ferruzzi MG, Janle EM, Wang J, Gong B, Chen TY, Lobo J, Cooper B, Wu QL, Talcott ST, Percival SS, Simon JE, Pasinetti GM. Identification of brain-targeted bioactive dietary quercetin-3-O-glucuronide as a novel intervention for Alzheimer's disease. FASEB J. 2013;27:769–781. doi: 10.1096/fj.12-212118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu C, Zawistowski J, Ling W, Kitts DD. Black rice (Oryza sativa L. indica) pigmented fraction suppresses both reactive oxygen species and nitric oxide in chemical and biological model systems. J Agric Food Chem. 2003;51:5271–5277. doi: 10.1021/jf034466n. [DOI] [PubMed] [Google Scholar]
- Ishisaka A, Ichikawa S, Sakakibara H, Piskula MK, Nakamura T, Kato Y, Ito M, Miyamoto K, Tsuji A, Kawai Y, Terao J. Accumulation of orally administered quercetin in brain tissue and its antioxidative effects on rats. Free Radic Biol Med. 2011;51:1329–1336. doi: 10.1016/j.freeradbiomed.2011.06.017. [DOI] [PubMed] [Google Scholar]
- Ishisaka A, Mukai R, Terao J, Shibata N, Kawai Y. Specific localization of quercetin-3-O-glucuronide in human brain. Arch Biochem Biophys. 2014;557:11–17. doi: 10.1016/j.abb.2014.05.025. [DOI] [PubMed] [Google Scholar]
- Jin XH, Ohgami K, Shiratori K, Suzuki Y, Koyama Y, Yoshida K, Ilieva I, Tanaka T, Onoe K, Ohno S. Effects of blue honeysuckle (Lonicera caerulea L.) extract on lipopolysaccharide-induced inflammation in vitro and in vivo. Exp Eye Res. 2006;82:860–867. doi: 10.1016/j.exer.2005.10.024. [DOI] [PubMed] [Google Scholar]
- Joseph JA, Shukitt-Hale B, Willis LM. Grape juice, berries, and walnuts affect brain aging and behavior. J Nutr. 2009;139:1813S–7S. doi: 10.3945/jn.109.108266. [DOI] [PubMed] [Google Scholar]
- Kalt W, Blumberg JB, McDonald JE, Vinqvist-Tymchuk MR, Fillmore SA, Graf BA, O'Leary JM, Milbury PE. Identification of anthocyanins in the liver, eye, and brain of blueberry-fed pigs. J Agric Food Chem. 2008;56:705–712. doi: 10.1021/jf071998l. [DOI] [PubMed] [Google Scholar]
- Kang CH, Jayasooriya RG, Choi YH, Moon SK, Kim WJ, Kim GY. β-lonone attenuates LPS-induced pro-inflammatory mediators such as NO, PGE2 and TNF-α in BV2 microglial cells via suppression of the NF-κB and MAPK pathway. Toxicol In Vitro. 2013;27:782–787. doi: 10.1016/j.tiv.2012.12.012. [DOI] [PubMed] [Google Scholar]
- Kao TK, Ou YC, Raung SL, Lai CY, Liao SL, Chen CJ. Inhibition of nitric oxide production by quercetin in endotoxin/cytokine-stimulated microglia. Life Sci. 2010;86:315–321. doi: 10.1016/j.lfs.2009.12.014. [DOI] [PubMed] [Google Scholar]
- Karuppagounder SS, Madathil SK, Pandey M, Haobam R, Rajamma U, Mohanakumar KP. Quercetin up-regulates mitochondrial complex-I activity to protect against programmed cell death in rotenone model of Parkinson's disease in rats. Neuroscience. 2013;236:136–148. doi: 10.1016/j.neuroscience.2013.01.032. [DOI] [PubMed] [Google Scholar]
- Kazłowska K, Hsu T, Hou CC, Yang WC, Tsai GJ. Anti-inflammatory properties of phenolic compounds and crude extract from Porphyra dentata. J Ethnopharmacol. 2010;128:123–130. doi: 10.1016/j.jep.2009.12.037. [DOI] [PubMed] [Google Scholar]
- Ke Z, Liu Y, Wang X, Fan Z, Chen G, Xu M, Bower KA, Frank JA, Ou X, Shi X, Luo J. Cyanidin-3-glucoside ameliorates ethanol neurotoxicity in the developing brain. J Neurosci Res. 2011;89:1676–1684. doi: 10.1002/jnr.22689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koda T, Kuroda Y, Imai H. Rutin supplementation in the diet has protective effects against toxicant-induced hippocampal injury by suppression of microglial activation and pro-inflammatory cytokines: protective effect of rutin against toxicant-induced hippocampal injury. Cell Mol Neurobiol. 2009;29:523–531. doi: 10.1007/s10571-008-9344-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Finn CE. Anthocyanins and other polyphenolics in American elderberry (Sambucus canadensis) and European elderberry (S. nigra) cultivars. J Sci Food Agric. 2007;87:2665–2675. doi: 10.1002/jsfa.3029. [DOI] [PubMed] [Google Scholar]
- Lim JY, Hwang BY, Hwang KW, Park SY. Methylalpinumisoflavone inhibits lipopolysaccharide-induced inflammation in microglial cells by the NF-kappaB and MAPK signaling pathway. Phytother Res. 2012;26:1948–1956. doi: 10.1002/ptr.4810. [DOI] [PubMed] [Google Scholar]
- Mikulic-Petkovsek M, Slatnar A, Stampar F, Veberic R. HPLC-MSn identification and quantification of flavonol glycosides in 28 wild and cultivated berry species. Food Chemistry. 2012;135:2138–2146. doi: 10.1016/j.foodchem.2012.06.115. [DOI] [PubMed] [Google Scholar]
- Milbury PE, Kalt W. Xenobiotic metabolism and berry flavonoid transport across the blood-brain barrier. J Agric Food Chem. 2010;58:3950–3956. doi: 10.1021/jf903529m. [DOI] [PubMed] [Google Scholar]
- Min J, Yu SW, Baek SH, Nair KM, Bae ON, Bhatt A, Kassab M, Nair MG, Majid A. Neuroprotective effect of cyanidin-3-O-glucoside anthocyanin in mice with focal cerebral ischemia. Neurosci Lett. 2011;500:157–1561. doi: 10.1016/j.neulet.2011.05.048. [DOI] [PubMed] [Google Scholar]
- Min SW, Ryu SN, Kim DH. Anti-inflammatory effects of black rice, cyanidin-3-O-β-D-glycoside, and its metabolites, cyanidin and protocatechuic acid. Int Immunopharmacol. 2010;10:959–966. doi: 10.1016/j.intimp.2010.05.009. [DOI] [PubMed] [Google Scholar]
- Nile SH, Park SW. Edible berries: Bioactive components and their effect on human health. Nutrition. 2014;30:134–144. doi: 10.1016/j.nut.2013.04.007. [DOI] [PubMed] [Google Scholar]
- Pawate S, Shen Q, Fan F, Bhat NR. Redox regulation of glial inflammatory response to lipopolysaccharide and interferongamma. J Neurosci Res. 2004;77:540–551. doi: 10.1002/jnr.20180. [DOI] [PubMed] [Google Scholar]
- Poulose SM, Carey AN, Shukitt-Hale B. Improving brain signaling in aging: Could berries be the answer? Expert Rev Neurother. 2012;12:887–889. doi: 10.1586/ern.12.86. [DOI] [PubMed] [Google Scholar]
- Prior RL, Wu X. Anthocyanins: structural characteristics that result in unique metabolic patterns and biological activities. Free Radic Res. 2006;40:1014–1028. doi: 10.1080/10715760600758522. [DOI] [PubMed] [Google Scholar]
- Pu F, Mishima K, Irie K, Motohashi K, Tanaka Y, Orito K, Egawa T, Kitamura Y, Egashira N, Iwasaki K, Fujiwara M. Neuroprotective effects of quercetin and rutin on spatial memory impairment in an 8-arm radial maze task and neuronal death induced by repeated cerebral ischemia in rats. J Pharmacol Sci. 2007;104:329–334. doi: 10.1254/jphs.fp0070247. [DOI] [PubMed] [Google Scholar]
- Qin L, Zhang J, Qin M. Protective effect of cyanidin 3-O-glucoside on beta-amyloid peptide-induced cognitive impairment in rats. Neurosci Lett. 2013;534:285–288. doi: 10.1016/j.neulet.2012.12.023. [DOI] [PubMed] [Google Scholar]
- Sambucus nigra (Elderberry). Monograph. Alternative Med Rev. 2005;10:51–55. [PubMed] [Google Scholar]
- Seeram NP. Emerging research supporting the positive effects of berries on human health and disease prevention. J Agric Food Chem. 2012;60:5685–86. doi: 10.1021/jf203455z. [DOI] [PubMed] [Google Scholar]
- Shen S, Yu S, Binek J, Chalimoniuk M, Zhang X, Lo SC, Hannink M, Wu J, Fritsche K, Donato R, Sun GY. Distinct signaling pathways for induction of type II NOS by IFNgamma and LPS in BV-2 microglial cells. Neurochem Int. 2005;47:298–307. doi: 10.1016/j.neuint.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Sheng W, Zong Y, Mohammad A, Ajit D, Cui J, Han D, Hamilton JL, Simonyi A, Sun AY, Gu Z, Hong JS, Weisman GA, Sun GY. Pro-inflammatory cytokines and lipopolysaccharide induce changes in cell morphology, and upregulation of ERK1/2, iNOS and sPLA2-IIA expression in astrocytes and microglia. J Neuroinflammation. 2011;8:121. doi: 10.1186/1742-2094-8-121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaper SD, Giusti P, Facci L. Microglia and mast cells: two tracks on the road to neuroinflammation. FASEB J. 2012;26:3103–3117. doi: 10.1096/fj.11-197194. [DOI] [PubMed] [Google Scholar]
- Spencer JP, Vafeiadou K, Williams RJ, Vauzour D. Neuroinflammation: modulation by flavonoids and mechanisms of action. Mol Aspects Med. 2012;33:83–97. doi: 10.1016/j.mam.2011.10.016. [DOI] [PubMed] [Google Scholar]
- Thole JM, Kraft TF, Sueiro LA, Kang YH, Gills JJ, Cuendet M, Pezzuto JM, Seigler DS, Lila MA. A comparative evaluation of the anticancer properties of European and American elderberry fruits. J Med Food. 2006;9:498–504. doi: 10.1089/jmf.2006.9.498. [DOI] [PubMed] [Google Scholar]
- Vafeiadou K, Vauzour D, Lee HY, Rodriguez-Mateos A, Williams RJ, Spencer JP. The citrus flavanone naringenin inhibits inflammatory signalling in glial cells and protects against neuroinflammatory injury. Arch Biochem Biophys. 2009;484:100–109. doi: 10.1016/j.abb.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Vlachojannis JE, Cameron M, Chrubasik S. A systematic review on the sambuci fructus effect and efficacy profiles. Phytother Res. 2010;24:1–8. doi: 10.1002/ptr.2729. [DOI] [PubMed] [Google Scholar]
- Wang H, Cao G, Prior RL. Oxygen radical absorbing capacity of anthocyanins. J Agric Food Chem. 1997;45:304–309. [Google Scholar]
- Wang Q, Xia M, Liu C, Guo H, Ye Q, Hu Y, Zhang Y, Hou M, Zhu H, Ma J, Ling W. Cyanidin-3-O-beta-glucoside inhibits iNOS and COX-2 expression by inducing liver X receptor alpha activation in THP-1 macrophages. Life Sci. 2008;83:176–84. doi: 10.1016/j.lfs.2008.05.017. [DOI] [PubMed] [Google Scholar]
- Wu X, Cao G, Prior RL. Absorption and metabolism of anthocyanins in elderly women after consumption of elderberry or blueberry. J Nutr. 2002;132:1865–1871. doi: 10.1093/jn/132.7.1865. [DOI] [PubMed] [Google Scholar]
- Wu X, Gu L, Prior RL, McKay S. Characterization of anthocyanins and proanthocyanidins in some cultivars of Ribes, Aronia, and Sambucus and their antioxidant capacity. J Agric Food Chem. 2004;52:7846–7856. doi: 10.1021/jf0486850. [DOI] [PubMed] [Google Scholar]
- Yao RQ, Qi DS, Yu HL, Liu J, Yang LH, Wu XX. Quercetin attenuates cell apoptosis in focal cerebral ischemia rat brain via activation of BDNF-TrkB-PI3K/Akt signaling pathway. Neurochem Res. 2012;37:2777–2786. doi: 10.1007/s11064-012-0871-5. [DOI] [PubMed] [Google Scholar]
- Youdim KA, Martin A, Joseph JA. Incorporation of the elderberry anthocyanins by endothelial cells increases protection against oxidative stress. Free Radic Biol Med. 2000;29:51–60. doi: 10.1016/s0891-5849(00)00329-4. [DOI] [PubMed] [Google Scholar]
- Zafra-Stone S, Yasmin T, Bagchi M, Chatterjee A, Vinson JA, Bagchi D. Berry anthocyanins as novel antioxidants in human health and disease prevention. Mol Nutr Food Res. 2007;51:675–683. doi: 10.1002/mnfr.200700002. [DOI] [PubMed] [Google Scholar]