Abstract
The chemical compositions of the capsular and extracellular polysaccharides of two strains of Rhizobium japonicum (311b 138 and 110) have been determined and correlated as a function of culture age with the ability of the bacteria from which they were obtained to bind soybean seed lectin.
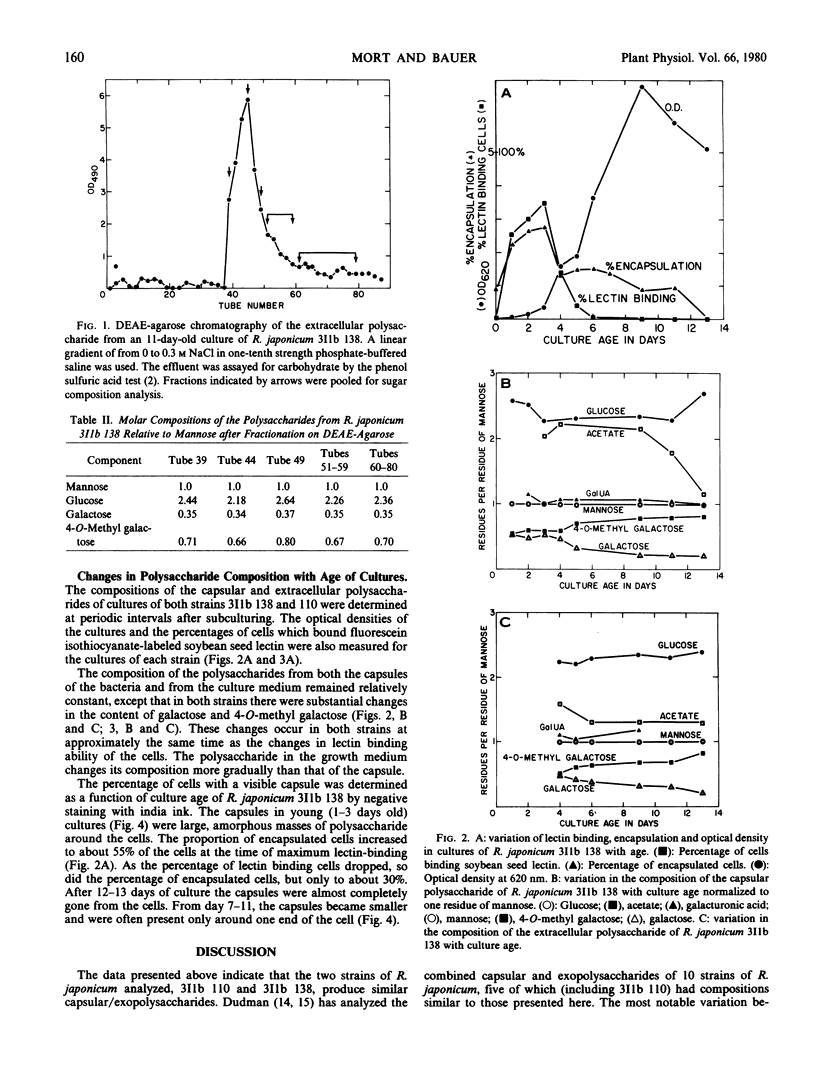
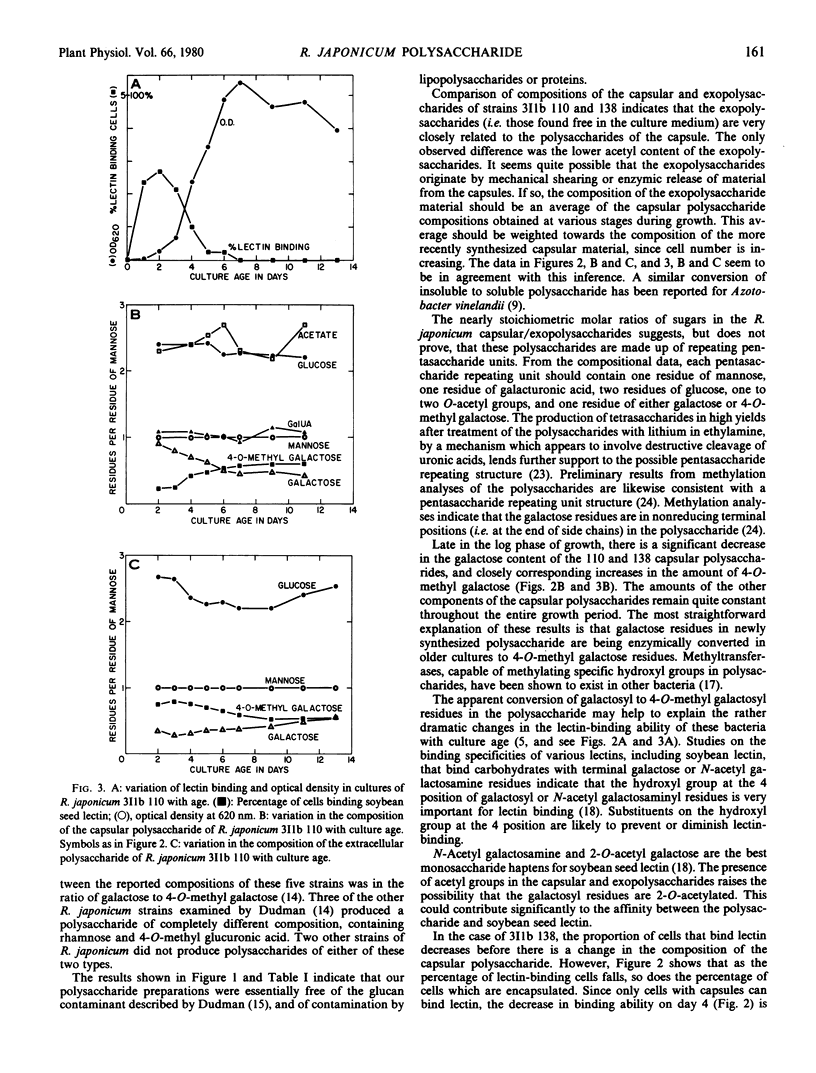
Each of the polysaccharides contains approximately constant amounts of mannosyl, glucosyl, and galacturonosyl residues in a molar ratio of 1:2:1. In addition they contain variable amounts of galactosyl and 4-O-methyl galactosyl residues. The total of galactose plus 4-O-methyl galactose, however, is constant and equivalent to the amount of mannose, indicating that the 4-O-methyl galactose residues arise by methylation of galactose residues in the polysaccharides. In both strains the proportion of galactose to methyl galactose is considerably greater in the polysaccharide from bacteria which do bind lectin than in the polysaccharide from bacteria which do not bind lectin.
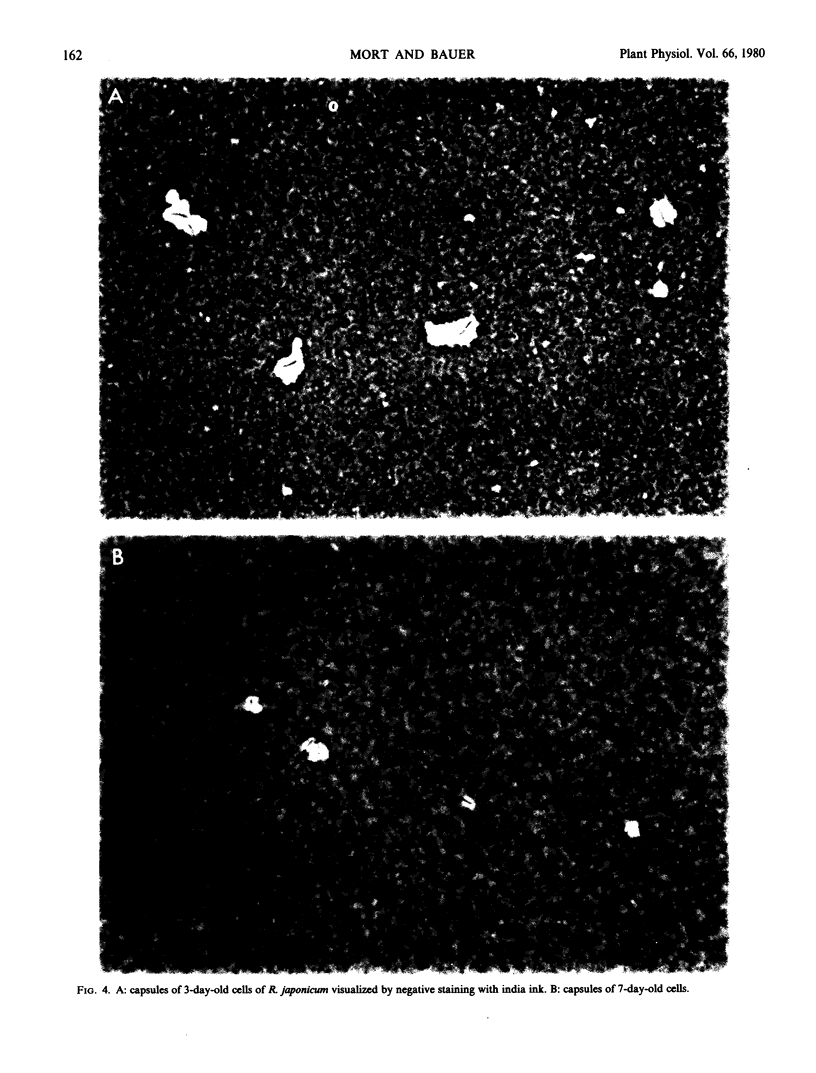
In addition to the changes in polysaccharide composition, there is a reduction of about 50% in the percentage of cells which are encapsulated as the cultures mature from early to late log phase. Since only capsulated cells bind lectin, the combination of the change in capsular composition and loss of encapsulation is probably sufficient to account for the loss of lectin binding capacity during growth of cultures of Rhizobium japonicum 311b 138 and 110.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bal A. K., Shantharam S., Ratnam S. Ultrastructure of Rhizobium japonicum in relation to its attachment to root hairs. J Bacteriol. 1978 Mar;133(3):1393–1400. doi: 10.1128/jb.133.3.1393-1400.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhatti T., Chambers R. E., Clamp J. R. The gas chromatographic properties of biologically important N-acetylglucosamine derivatives, monosaccharides, disaccharides, trisaccharides, tetrasaccharides and pentasaccharides. Biochim Biophys Acta. 1970 Nov 24;222(2):339–347. doi: 10.1016/0304-4165(70)90122-4. [DOI] [PubMed] [Google Scholar]
- Bhuvaneswari T. V., Pueppke S. G., Bauer W. D. Role of lectins in plant-microorganism interactions: I. Binding of soybean lectin to rhizobia. Plant Physiol. 1977 Oct;60(4):486–491. doi: 10.1104/pp.60.4.486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenkrantz N., Asboe-Hansen G. New method for quantitative determination of uronic acids. Anal Biochem. 1973 Aug;54(2):484–489. doi: 10.1016/0003-2697(73)90377-1. [DOI] [PubMed] [Google Scholar]
- Calvert H. E., Lalonde M., Bhuvaneswari T. V., Bauer W. D. Role of lectins in plant--microorganism interactions. IV. Ultrastructural localization of soybean lectin binding sites of Rhizobium japonicum. Can J Microbiol. 1978 Jul;24(7):785–793. doi: 10.1139/m78-132. [DOI] [PubMed] [Google Scholar]
- Carlson R. W., Sanders R. E., Napoli C., Albersheim P. Host-Symbiont Interactions: III. Purification and Partial Characterization of Rhizobium Lipopolysaccharides. Plant Physiol. 1978 Dec;62(6):912–917. doi: 10.1104/pp.62.6.912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couperwhite I., McCallum M. F. Polysaccharide production and the possible occurrence of GDP-D-mannose dehydrogenase in Azotobacter vinelandii. Antonie Van Leeuwenhoek. 1975;41(1):25–32. doi: 10.1007/BF02565034. [DOI] [PubMed] [Google Scholar]
- DUGUID J. P. The demonstration of bacterial capsules and slime. J Pathol Bacteriol. 1951 Oct;63(4):673–685. doi: 10.1002/path.1700630413. [DOI] [PubMed] [Google Scholar]
- Grellert E., Ballou C. E. Biosynthesis of a mycobacterial lipopolysaccharide. Evidence for an acylpolysaccharide methyltransferase. J Biol Chem. 1972 May 25;247(10):3236–3241. [PubMed] [Google Scholar]
- Hammarström S., Murphy L. A., Goldstein I. J., Etzler M. E. Carbohydrate binding specificity of four N-acetyl-D-galactosamine- "specific" lectins: Helix pomatia A hemagglutinin, soy bean agglutinin, lima bean lectin, and Dolichos biflorus lectin. Biochemistry. 1977 Jun 14;16(12):2750–2755. doi: 10.1021/bi00631a025. [DOI] [PubMed] [Google Scholar]
- Karkhanis Y. D., Zeltner J. Y., Jackson J. J., Carlo D. J. A new and improved microassay to determine 2-keto-3-deoxyoctonate in lipopolysaccharide of Gram-negative bacteria. Anal Biochem. 1978 Apr;85(2):595–601. doi: 10.1016/0003-2697(78)90260-9. [DOI] [PubMed] [Google Scholar]
- Kennedy L. D., Bailey R. W. Monomethyl sugars in extracellular polysaccharides from slow-growing Rhizobia. Carbohydr Res. 1976 Jul;49:451–454. doi: 10.1016/s0008-6215(00)83162-6. [DOI] [PubMed] [Google Scholar]
- Mackenzie S. L., Tenaschuk D. Rapid formation of amino acid isobutyl esters for gas chromatography. J Chromatogr. 1975 Sep 3;111(2):413–415. doi: 10.1016/s0021-9673(00)99292-6. [DOI] [PubMed] [Google Scholar]
- Pope D. G. Relationships between Hydroxyproline-containing Proteins Secreted into the Cell Wall and Medium by Suspension-cultured Acer pseudoplatanus Cells. Plant Physiol. 1977 May;59(5):894–900. doi: 10.1104/pp.59.5.894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pritchard D. G., Todd C. W. Gas chromatography of methyl glycosides as their tri-methylsilyl ethers. The methanolysis and re-N-acetylation steps. J Chromatogr. 1977 Mar 11;133(1):133–139. doi: 10.1016/s0021-9673(00)89212-2. [DOI] [PubMed] [Google Scholar]
- SLONEKER J. H., ORENTAS D. G. Pyruvic acid, a unique component of an exocellular bacterial polysaccharide. Nature. 1962 May 5;194:478–479. doi: 10.1038/194478a0. [DOI] [PubMed] [Google Scholar]
- Sutherland I. W. Structural studies on colanic acid, the common exopolysaccharide found in the enterobacteriaceae, by partial acid hydrolysis. Oligosaccharides from colanic acid. Biochem J. 1969 Dec;115(5):935–945. doi: 10.1042/bj1150935. [DOI] [PMC free article] [PubMed] [Google Scholar]




