Abstract
Objective
Evaluate expected tumor control and normal tissue toxicity for prostate volumetric modulated arc therapy (VMAT) with and without radiation boost to an intraprostatic dominant lesion (IDL) defined by 18F-fluorocholine PET/CT.
Methods
Thirty patients with localized prostate cancer underwent 18F-fluorocholine PET/CT before treatment. Two VMAT plans, plan79Gy and plan100-105Gy, were compared for each patient. The whole-prostate planning target volume (PTVprostate) was prescribed 79 Gy in both plans, however plan100-105Gy added simultaneous boost doses of 100 Gy and 105 Gy prescribed to IDLs defined by 60% and 70% of maximum prostatic uptake on 18F-fluorocholine PET (IDLsuv60% and IDLsuv70%, respectively, with IDLsuv70% nested inside IDLsuv60% to potentially enhance tumor specificity of the maximum point dose). Plan evaluations included histopathologic correspondence, isodose distributions, dose-volume histograms, tumor control probability (TCP), and normal tissue complication probability (NTCP).
Results
Planning objectives and dose constraints proved feasible in 30/30 cases. Prostate sextant histopathology was available from 28 cases, confirming that IDLsuv60% adequately covered all tumor-bearing prostate sextants in 27 cases and provided partial coverage in one case. Plan100-105Gy had significantly higher TCP than Plan79Gy across all prostate regions for α/β ratios ranging from 1.5 Gy to 10Gy (p < 0.001 each case). There were no significant differences in bladder and femoral head NTCP between plans, and slightly lower rectal NTCP (endpoint: grade 2+ late toxicity or rectal bleeding) for plan100-105Gy.
Conclusion
VMAT can potentially increase the likelihood of tumor control in primary prostate cancer while observing normal tissue tolerances through simultaneous delivery of a steep radiation boost to an 18F-fluorocholine PET-defined IDL.
Keywords: fluorocholine, PET/CT, prostate cancer, volumetric modulated arc therapy, boost
Introduction
Radiation dose escalation for prostate cancer external beam radiotherapy (EBRT) can lower the risk of biochemical relapse[1,2]. This radiation dose-response relationship for tumor control is evident across patients with low-, intermediate-, and high-risk prostate tumors[1,3]. Improvements in conformal radiation delivery have made it easier to intensify radiation doses to the planning target volume (PTV) without exceeding normal tissue tolerances. Unfortunately, while toxicity from intensity-modulated radiotherapy (IMRT) appears manageable at whole-prostate doses up to 86 Gy[4], recurrent disease at or near the original tumor volume can still occur at this dose magnitude [5,6]. Thus, more aggressive dose escalation may be necessary to advance local tumor control.
The selective delivery of “boost” radiation doses to tumor regions in the prostate is a promising strategy to limit tissue toxicity while pursuing radiation dose escalation [7,8]. Pathology studies have shown that prostate cancer is often a multifocal disease, and that large prostate tumors are frequently accompanied by small well-differentiated secondary tumors (< 0.5 cm diameter and Gleason score < 5). However, it is the largest tumor that almost always shows the most aggressive histologic characteristics and is thus driving prognosis [9,10]. Eradication of this so-called intraprostatic dominant lesion (IDL) could therefore have the greatest clinical impact in treating organ-confined prostate cancer.
Molecular imaging techniques such as positron emission tomography (PET) or magnetic resonance spectroscopy (MRS) are increasingly being used to detect prostate tumors [8]. Upregulated choline metabolism is common in prostate cancer and several molecular imaging modalities exploit this feature [11-13]. Such modalities include PET using carbon-11 or fluorine-18 labeled radiopharmaceuticals bearing the choline moiety [14,15], with recent studies supporting its use to define IDLs for radiotherapy planning [16,17]. In a prospective pilot trial, IMRT dose escalation to IDLs defined by fluorine-18 fluoromethylcholine (18F-choline) PET was achieved without impacting quality of life after treatment [18]. This demonstration of clinical feasibility has opened the way to exploring further dose escalation guided by 18F-choline PET.
RapidArc, a form of volumetric modulated arc therapy (VMAT), can produce steeper dose fall-off gradients than IMRT [19], and could therefore enable more intense and selective boost radiation treatment while observing normal tissue tolerances. For example, improved rectal sparing has been associated with VMAT delivering 95 Gy to an IDL defined by MRS as compared to IMRT [20]. We hypothesize that a VMAT plan to deliver a minimum dose of 79 Gy to the whole prostate can accommodate selective boosts up to 105 Gy to an IDL defined by 18F-choline PET/CT without further jeopardizing adjacent organs at risk. We preliminarily tested this hypothesis by estimating the effects of radiation boosts up to 105 Gy based on the calculated tumor control probability (TCP) and normal tissue complication probability (NTCP) of the VMAT plan.
Methods and Materials
Patients
Thirty patients with localized prostate cancer diagnosed by transrectal ultrasound guided biopsy gave written informed consent under an institutional review board approved research protocol. All patients were candidates for radical prostatectomy or radiation therapy based on National Comprehensive Cancer Network (NCCN) risk criteria [21]. Patients with non-skin malignancies diagnosed within the last 5 years, suspected extraprostatic lesions, and those who had previously received androgen deprivation therapy, prostate surgery, or radiotherapy to the pelvis were excluded.
Clinical risk status was based on age at diagnosis, pretreatment PSA, prostate biopsy results, Gleason score, and tumor staging (Table 1). The study excluded patients with very low risk or very high risk disease based on NCCN criteria. Patients with Gleason 5 and 6 tumors had expected longevities exceeding 10 years and no comorbidities precluding primary treatment of prostate cancer. Radiopharmaceutical synthesis and the PET/CT imaging protocol are detailed in the Supplemental Materials.
Table 1.
Clinical characteristics (n = 30)
| Age (years) | |
| Median (range) | 69 (52 – 87) |
| Pre-Treatment PSA (ng/mL) | |
| Median (range) | 5.9 (0.9 – 41.0) |
| Gleason Sum | |
| 5 | 1 ( 3.3) |
| 6 | 8 (26.7) |
| 7 | 12 (40.0) |
| 8 | 6 (20.0) |
| 9 | 3 (10.0) |
| 10 | 0 ( 0.0) |
| NCCN Risk Group | |
| Low (T1c, PSA <10 ng/mL, Gleason <=6, <50% core positive) | 9 (30.0) |
| Intermediate (T2b-2c, or PSA 10-20 ng/mL, or Gleason 7) | 12 (40.0) |
| High (T3a, or PSA > 20 ng/mL, or Gleason 8-10) | 9 (30.0) |
| Anatomic Stage/Prognostic Group (AJCC) | |
| I | 9 (30.0) |
| IIA | 11 (36.7) |
| IIB | 10 (33.3) |
| Prostate Volumes (cm3) | |
| Median (range) | 35.6 (15.8 – 62.8) |
| Intraprostatic Dominant Lesions (IDL) | |
| Total Number Defined | 31 |
| IDLSUV60% Volume (cm3) Median (range) |
2.7 (0.3 – 10.8) |
| IDLSUV70% Volume (cm3) Median (range) |
0.7 (0.04 – 7.10) |
Values are number (percentage) unless otherwise noted.
Delineation of Target Volumes and Organs-at-risk
PET/CT images were transferred to an image analysis workstation (MIM Maestro 6.2.7, MIM Software, Inc., Cleveland, OH). Prostate, seminal vesicles, rectum, bladder, penile bulb, and femoral heads were contoured based on guidelines from Radiation Therapy Oncology Group (RTOG) protocol 0126 [22]. IDL segmentation was performed using PET quantitation software (PMOD 3.5, PMOD Technologies, Ltd, Zurich, Switzerland) to define one or several volumes of interest (VOI) within the prostate and seminal vesicle containing those voxels with SUV exceeding 60% of prostate SUVmax (IDLsuv60%). This threshold was based on previously reported intraprostatic tumor-to-normal ratios for 18F-choline [15,23] and carbon-11 choline [24]. A smaller IDLsuv70% was drawn within IDLsuv60% as the VOI containing voxels with SUV exceeding 70% of prostate SUVmax to confine the maximum point dose to a smaller contoured region with potentially higher tumor specificity [24].
Definitions of gross tumor volume (GTV), clinical target volume (CTV), and planning target volume (PTV) were based on RTOG protocol 0126 [22]. Briefly, the GTVprostate is the prostate alone, while the CTVprostate+SV is the GTVprostate plus the proximal 1 cm of bilateral seminal vesicles (SVs). PTVprostate and the PTVprostate+SV were created by adding 6 mm lateral, superior and inferior margins and 3 mm anterior and posterior margins on the GTVprostate and CTVprostate+SV, respectively. Similarly, 3 mm and 6 mm radial and 6 mm craniocaudal expansions were used for generating PTVs60 and PTVs70 from IDLsuv60% and IDLsuv70%, respectively. PTVs60 and PTVs70 structures were also excluded from rectum and bladder structures by 6mm. If necessary, minor adjustments were made to fit all IDL vertices within the PTV.
VMAT Planning
Planning details are in the Supplemental Materials. Both Plan79Gy and Plan100-105Gy were scheduled for 39 fractions (one fraction per day, five work days per week).
TCP and NTCP Modeling
TCP was calculated via the LQ-Poisson Marsden Model using DVH data for IDLsuv60%, IDLsuv70%, and non-IDL prostate (i.e., GTVprostate - IDL suv60%) [25-27]. NCTPs for the rectum, bladder, and femoral heads were calculated using the Lyman-Kutcher-Burman formula with Nirmierko’s equivalent uniform dose (EUD) [28-32]. Modeling parameters and procedure are in the Supplemental Materials.
Histopathologic Correlation
Histopathologic data from prostate biopsy or post-surgical step-section analysis was collected to determine dominant tumor site on a prostate sextant basis [15]. Since small (< 0.5 cc) and well-differentiated (Gleason < 5) tumors are not clinically significant [10], secondary tumors meeting these criteria were not considered part of the IDL.
Statistical methods
The Wilcoxon signed-rank test was used to compare the plan parameters, TCPs and NTCPs, respectively (SAS 10.0/JMP 11, SAS Institute, Cary, NC). Median values and ranges were reported. A P-value <0.05 was taken as statistically significant.
Results
From 30 patients, one was found to have two separate foci of increased prostatic 18F-choline uptake from which two IDLsuv60%s and two IDLsuv70%s were drawn. Thus, a total of 31 IDLsuv60% and 31 IDLsuv70% volumes were analyzed. Clinical characteristics and treatment volumes are shown in Table 1. Planning results and volumes are shown in Table 2.
Table 2.
Summary of the DVH analysis for the PTVs and Organs at Risk
| Parameter | Volume (cm3) Median (Range) |
Plan79Gy Median (Range) |
Plan100-105Gy Median (Range) |
p value |
|---|---|---|---|---|
| PTVprostate+SV | 101.4 (55.4-156.2) | |||
| D95% [cGy] | 6154.5 (6069.3 – 7268.3) | 6143.4 (6055.7 – 7288.5) | 0.026 | |
| Dmin [cGy] | 5676.8 (5123.4 – 5912.2) | 5672.6 (5163.9 – 5890.1) | 0.600 | |
| PTVprostate | 75.2 (42.3–121.3) | |||
| D95% [cGy] | 7938.0 (7888.1 – 7961.3) | 7967.0 (7907.0 – 8078.5) | <0.001 | |
| Dmin [cGy] | 7522.1 (7074.8 – 7665.6) | 7477.6 (6799.8 – 7746.4) | 0.178 | |
| PTVs60 | 12.9 (1.9 – 44.8) | |||
| Dmax [cGy] | 8250.6 (8148.8 – 9311.4) | 10964.6 (10751.1 – 11461.3) | <0.001 | |
| Dmean [cGy] | 8046.7 (7982.8 – 8115.3) | 10584.4 (10246.3 – 10702.1) | <0.001 | |
| D95% [cGy] | 7979.0 (7676.3 – 8029.2) | 10236.6 (10034.4 – 10483.3) | <0.001 | |
| Dmin [cGy] | 7852.0 (7553.5 – 8244.7) | 9758.3 (8646.0 – 9940.8) | <0.001 | |
| PTVs70 | 6.1 (1.5 – 25.9) | |||
| Dmax [cGy] | 8225.9 (8148.8 – 8436.8) | 10938.6 (10751.1 – 11191.4) | <0.001 | |
| Dmax [%] | 104.2 (103.1 – 106.8) | 104.2 (102.4 – 106.6) | 0.955 | |
| Dmean [cGy] | 8046.0 (7984.6 – 8112.2) | 10681.9 (10585.4 – 10805.2) | <0.001 | |
| D95% [cGy] | 7984.3 (7907.8 – 8041.2) | 10531.3 (10497.0 – 10635.6) | <0.001 | |
| Dmax,0.03cc [cGy] | 8166.1 (8094.5 – 8306.7) | 10858.9 (10685.1 – 11009.7) | <0.001 | |
| Dmin [cGy] | 7888.7 (7707.9 – 7986.9) | 10288.5 (9738.6 – 10480.4) | <0.001 | |
| PTVprostate – PTVs60 (i.e., non-IDL prostate volume) |
60.3 (20.6 – 111.8) | |||
| Dmax [cGy] | 8312.3 (8161.9 – 8606.8) | 10833.6 (10663.4 – 11078.2) | <0.001 | |
| Dmean [cGy] | 8036.5 (7971.8 – 8100.1) | 8992.4 (8449.0 – 9242.5) | <0.001 | |
| D95% [cGy] | 7930.1 (7883.3 – 7952.7) | 7947.3 (7900.1 – 8016.9) | <0.001 | |
| Rectum | 73.2 (37.4 – 164.6) | |||
| Dmax [cGy] | 8129.1 (6203.6 – 8279.0) | 8356.1 (6445.0 – 8896.4) | <0.001 | |
| Dmax,0.03cc [cGy] | 8043.2 (5953.7 – 8213.0) | 8138.0 (6091.0 – 8493.8) | <0.001 | |
| Dmean [cGy] | 3623.1 (2463.5 – 4707.2) | 3562.0 (2455.8 – 4660.4) | 0.003 | |
| D50% [cGy] | 3852.5 (1045.1 – 3141.4) | 3786.9 (1069.0 – 4775.4) | 0.005 | |
| V75Gy [%] | 2.2 (0.0 – 8.8) | 1.6 (0.0 – 9.0) | <0.001 | |
| V70Gy [%] | 4.2 (0.0 – 12.5) | 3.4 (0.0 – 12.7) | <0.001 | |
| V65Gy [%] | 6.9 (0.0 – 17.2) | 6.3 (0.0 – 17.6) | <0.001 | |
| V60Gy [%] | 11.2 (0.0–25.6) | 10.9 (0.1 – 25.0) | <0.001 | |
| V50Gy [%] | 24.5 (5.6 – 48.5) | 24.2 (5.1 – 45.8) | <0.001 | |
| Bladder | 72.2 (21.0 – 272.6) | |||
| Dmax [cGy] | 8267.8 (8158.7 – 8465.8) | 8317.1 (8123.6 – 8665.0) | <0.001 | |
| Dmax,0.03cc [cGy] | 8168.2 (8072.3 – 8363.8) | 8181.8 (8050.0 – 8454.8) | 0.180 | |
| Dmean [cGy] | 3391.4 (805.4 – 5077.4) | 3427.1 (835.3 – 5117.6) | 0.016 | |
| D50% [cGy] | 2898.6 (285.3 – 5286.6) | 2882.2 (293.7 – 5298.9) | 0.034 | |
| V80Gy [%] | 2.3 (0.4 – 6.5) | 1.6 (0.5 – 5.3) | 0.002 | |
| V75Gy [%] | 7.0 (1.2 – 19.7) | 7.0 (1.1 – 20.3) | 0.718 | |
| V70Gy [%] | 9.9 (1.6 – 26.7) | 9.2 (1.5 – 27.6) | 0.592 | |
| V65Gy [%] | 11.9 (2.0 – 33.0) | 12.1 (1.8 – 34.1) | 0.556 | |
| Left femoral head | 56.7 (35.4 – 83.2) | |||
| Dmax [cGy] | 2967.6 (486.9 – 3731.4) | 3185.8 (546.0 – 3971.0) | <0.001 | |
| Dmean [cGy] | 1602.3 (193.4 – 2357.2) | 1737.4 (206.4 – 2350.1) | <0.001 | |
| D50% [cGy] | 1585.1 (173.7 – 2398.8) | 1739.1 (185.1 – 2316.1) | <0.001 | |
| Right femoral head | 58.7 (25.9 – 103.6) | |||
| Dmax [cGy] | 2697.0 (1523.5 – 3885.6) | 3005.6 (1442.4 – 4000.6) | 0.007 | |
| Dmean [cGy] | 1518.0 (276.5 – 2185.4) | 1656.8 (298.3 – 2537.9) | 0.003 | |
| D50% [cGy] | 1550.3 (207.4 – 2275.1) | 1671.7 (220.3 – 2551.4) | 0.003 | |
| Penile bulb | 1.6 (0.9 – 2.7) | |||
| Dmax [cGy] | 6156.5 (696.6 – 8593.1) | 5919.3 (728.2 – 8794.5) | 0.011 | |
| Dmean [cGy] | 2008.4 (421.1 – 4636.5) | 1887.7 (460.9 – 4914.9) | 1.000 | |
| D50% [cGy] | 1470.1 (398.8 – 4406.9) | 1408.8 (437.3 – 4846.4) | 0.034 |
Abbreviations: Dx%: dose received by at least x% of the volume; VxGy: volume receiving at least x Gy; Dmean: the mean dose received within the designated volume; Dmax,0.03cc: the maximum point dose (size: 0.03 cc) received within the designated volume; Dmin: the minimum dose received within the designated volume; Dmax: the maximum dose received within the designated volume.
Whole-prostate step-section histopathology was available in 6 patients following radical prostatectomy. In each case, the dominant tumor localized to prostate sextants within the PET-defined IDL. In one case, a prostate sextant encompassed by the PET-defined IDL did not have tumor identified on step-section analysis. Biopsy results were available from the other 24 patients. In 2 patients, not all sextants were biopsied but the results were positive only on the PET-defined IDL side. This left 22 cases for sextant-level analysis. In 21/22 cases, the IDLsuv60% contour included all biopsy-positive prostate sextants. IDLsuv60% also encompassed biopsy-negative sextants in 11 cases, suggesting either overestimated tumor extent by PET or biopsy sampling error. In one case, a biopsy-positive sextant was not included in the IDLsuv60% contour. Lowering the contouring threshold to 50% of SUVmax allowed all biopsy-positive sextants to be included in this case.
All planning objectives and dose constraints were met (Table 2). Figure 1 compares isodose distributions for Plan79Gy and Plan100-105Gy along with PET. Figure 2 shows the corresponding cumulative DVH results. Both plans maintained less than 9% of rectal volumes receiving more than 75 Gy and less than 6.5% of bladder volumes receiving more than 80Gy. Femoral head and penile bulb were similarly spared in both plans.
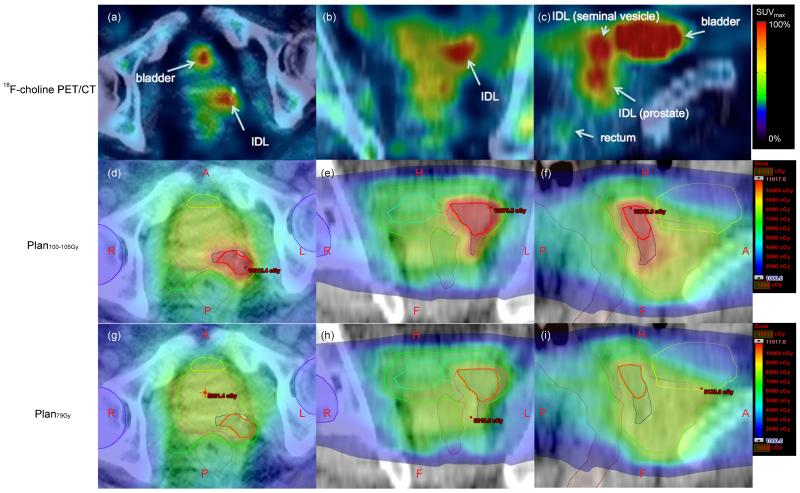
Figure 1.
Corresponding PET/CT and dose distribution images. Axial (a), coronal (b), and sagittal (c) PET/CT images show increased activity corresponding to a biopsy-confirmed IDL (b, arrow) involving the left basal prostate. Left seminal vesicle invasion is also evident (c, short arrow). Note the IDL appears distinct despite 18F-choline being present in the urinary bladder. Axial (d, g), coronal (e, h) and sagittal (f, i) projections for Plan100-105Gy (middle row) and Plan79Gy (bottom row) show contours for PTVs70 (red), PTVs60 (blue), PTVprostate (magenta), PTVprostate+SV (green), bladder (yellow), rectum (brown), penile blub (cyan), and femoral head (purple). Plan100-105Gy allocates highest dose in PTVS70, with dose dropping slightly inside PTVS60, and declining further in “non-boost” regions (i.e., PTVprostate and PTVprostate+SV). Color scale ranged from 1000 – 11017 cGy. The maximum point dose (0.03cc) in Plan100-105Gy was 11017 cGy, confined within PTVs70. In contrast, the maximum point dose (0.03cc) in Plan79Gy was 8261.4 cGy, located outside the IDL. Anchoring the “hot spot” in Plan100-105Gy close to the prostate SUVmax could have a radiobiological advantage by allocating the highest radiation dose to the tumor epicenter. A: anterior; P: posterior; L: left; R: right; H: head; F: feet.
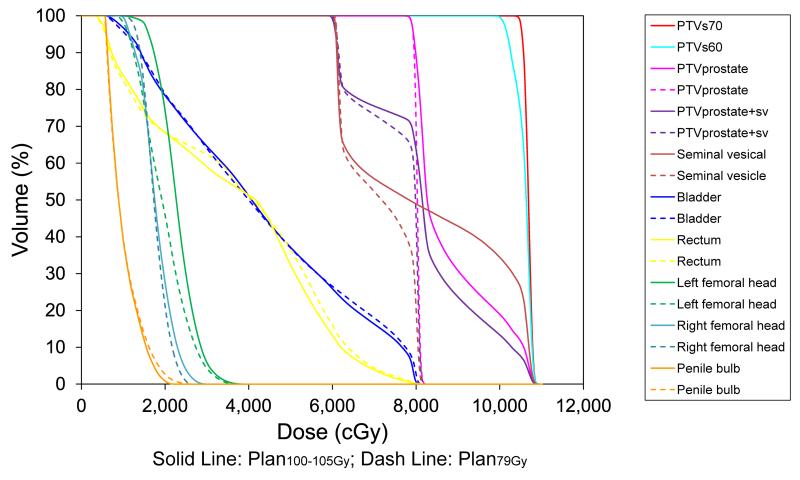
Figure 2.
Dose volume histogram corresponding to Figure 1 demonstrates significant differences in PTV dose coverage and comparable doses to surrounding organs-at-risk between Plan100-105Gy and Plan79Gy.
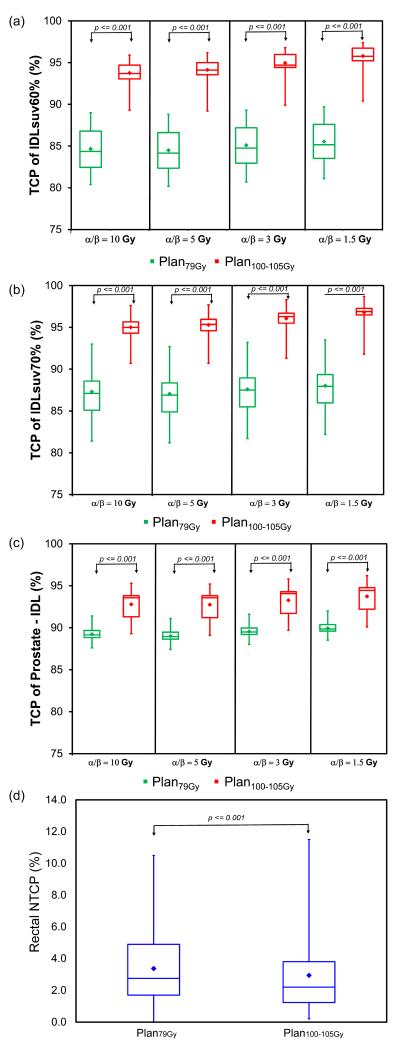
Figure 3 illustrates the TCP and NTCP results. Plan100-105Gy was associated with significantly higher TCP in IDLsuv60%, IDLsuv70% and non-IDL prostate regions compared to Plan79Gy at all four α/β ratios examined (p < 0.001 in each case). The lower α/β ratio, the greater the benefit of radiation boost to the IDL, with gains in median TCP IDLSUV60% of 9.4% (from 84.4% to 93.7%) for an α/β ratio of 10 Gy compared to 9.9% (from 84.2% to 94.1%) for an α/β ratio of 5 Gy, and 10.0% (from 84.8% to 94.7%) for an α/β ratio of 3 Gy, and 10.6% (from 85.2% to 95.8%) for an α/β ratio of 1.5 Gy. The absolute difference in rectal NTCP for grade 2+ late toxicity or rectal bleeding between Plan100-105Gy and Plan79Gy was small but statistically significant in paired testing (2.2% vs. 2.8%, p < 0.001) (Figure 3). There were no significant differences in bladder and femoral head NTCPs between two plans.
Figure 3.
Box-whisker plots comparing Plan79Gy and Plan100-105Gy for (a) IDLsuv60% TCP, (b) IDLsuv70% TCP, (c) non-IDL TCP, and (d) rectal NTCP (endpoint: grade 2+ late toxicity or rectal bleeding). Boxes represent interquartile range between 25th and 75th percentiles. Diamond and horizontal lines represent mean and median, respectively. Vertical lines represent range from maximum and minimum values.
Discussion
This study examined the technical feasibility of simultaneous integrated boost for primary prostate cancer using VMAT to deliver up to 105 Gy to an IDL defined by 18F-choline PET/CT. A similar study by Ost et al. compared IMRT with VMAT for prostate cancer treatment incorporating radiation boosts to an IDL defined by MRS [20]. In that study, both IMRT and VMAT delivered a median of 93 Gy to the IDL, however VMAT was associated with better sparing of the rectum. VMAT has also been associated with improved rectal sparing in treatment plans not involving an IDL boost [19]. These gains may be due to greater control over dose distribution and fall-off with VMAT. Other clinical advantages of VMAT are improved patient comfort and delivery time.
Choline PET has been used previously to define IDLs for planning IMRT boost. Pinkawa et al. conducted several investigations using 18F-choline PET/CT leading to a prospective pilot trial of PET-guided IMRT boost treatment [17,18,33]. They combined a 76 Gy whole-prostate dose with a PET-directed boost to 80 Gy, finding comparable EUD to the rectum and bladder between boost and non-boost treatment [17]. Longitudinal follow-up revealed no significant differences in urinary and bowel quality of life after boost treatment [18]. Chang et al. similarly applied 11C-choline PET to generate boost IMRT contours that were prescribed 90 Gy [16]. They reported slightly lower rectal NTCPs and higher TCPs for boost treatment relative to whole-prostate IMRT at doses of 72 Gy or 78 Gy. A high-dose salvage radiation therapy trial to deliver up to 80 Gy to 18F-choline PET-positive areas of the prostate also reported a low rate of toxicity with 1.7% of grade 2 gastrointestinal late toxicity and 0% of grade 2 genitourinary late toxicity [34]. The current study follows these previous technical and clinical accomplishments with results supporting even more aggressive dose escalation in the context of VMAT.
Dose escalation is tempered by increased risks of normal tissue toxicity and long-term morbidity. This study based dose constraints on relatively conservative values from QUANTEC [35] and RTOG protocol 0126 [22]. Adherence to these constraints should result in ≤15% late grade 2+ rectal complications [35]. Chang et al. reported that 11C-choline PET guided boost IMRT for localized prostate cancer could achieve a 4.6% of late grade 2+ rectal NTCP value for the non-boost plan (78Gy), and a 3.7% of rectal NTCP value for the boost plan (90 Gy prescribed to IDLs) [16]. In a ten-year outcomes study evaluating high-dose prostate IMRT prescribing 81 Gy to the PTV, the 10-year likelihood of grade 2 and 3 late genitourinary toxicity was 11% and 5%, respectively, and grade 2 and 3 late gastrointestinal toxicity was 2% and 1%, respectively [36]. The current study using a 79 Gy PTV dose with simultaneous 100-105 Gy boost in IDLs achieved similar NTCP results.
Our radiobiological results associate higher TCP with VMAT boost while keeping NTCP within ranges reported for high-dose IMRT for primary and salvage radiation therapy [34,36]. Similar to the study by Chang et al. [16], rectal NTCP was slightly lower for the boost plan. While probably not clinically significant, this statistical difference (Fig 3d) may seem curious at first glance. However, consistent with adequate confinement of boost dose fall off during the process of VMAT planning, EUDs to the rectum were higher in Plan79Gy than in Plan100-105Gy (5766.8 cGy median rectal EUD in Plan79Gy (Range 3761.1 – 6435.2 cGy) vs. 5677.2 cGy median rectal EUD in Plan100-105Gy (range 3829.5 – 6488.6 cGy), paired test p < 0.001). Since NTCP calculations are based on EUD measures, Plan100-105Gy resulted in lower rectal NTCPs as compared to Plan79Gy.
In this study, TCP in the IDL and non-IDL regions were evaluated based on expected tumoral variation in clonogen density and other radiobiologic parameters. Several studies show dose escalation up to 95 Gy with EBRT to be feasible for most patients with a resulting absolute increase in TCP of 2–15% based on the assumption of higher radioresistance in IDLs [16-18,24,33,37,38]. These results are consistent with the current study findings of higher TCPs associated with Plan100-105Gy. It may be advantageous in terms of local tumor control to pursue both a high whole-prostate dose and a high boost differential, since a high boost dose at the expense of a lower whole prostate dose (< 70–75 Gy) has been associated with relatively high failure rates [39-42]. In contrast to the > 20 Gy differential boost dose of the current study, the average differential dose (PTVIDL–PTVprostate) reported in a systematic review was 8 Gy (biologically effective dose 2 Gy per fraction, α/β = 3 Gy, range 3–35 Gy) and the most common rectal dose constraints used were V70Gy < 15–30% with Dmax of 76–80 Gy [8]. While some studies used relatively low (60 Gy) whole prostate dose combined with IDL boost delivered by HDR or IMRT, their favorable control rates (78% and 98% 5-year biochemical disease free survival rate, respectively) may have stemmed from having large boost volumes that encompassed most of the gland [41,43]. Thus, both the IDL and non-boost regions of the PTV should be considered in boost treatment plan evaluations.
In addition to applying VMAT to pursue dose intensification without further compromising normal tissues, this study explored the use of a second focal boost region (IDLsuv70%) within a larger boost region (IDLsuv60%) to confine the maximum point dose centrally in the tumor with histopathology confirming the tumor-affected prostate sextants or lobes in all cases. Chang et al. also verified the appropriateness of using a 60% SUV threshold to define IDLs on 11C-choline PET, through correspondence with coregistered prostate specimens [16]. Although limited to a prostate sextant basis, our histologic correlation results bear resemblance to a study by Pinkawa et al. that reported 97% PET concordance with the biopsy positive lobe in 66 patients that underwent 18F-choline PET/CT for IMRT planning [17]. Also similar is our finding that biopsy-negative sextants were included within the IDL contour in more than one-third of cases. While increased 18F-choline uptake due to benign prostatic disease could cause this apparent radiopathologic discordance [44], this is not a forgone conclusion since false-negative biopsy could also lead to underestimated tumor extent and discordance with PET. Since NTCP estimates for boost-treatment compared favorably against non-boost treatment plans, no apparent penalty from potentially overestimating the IDL boundary was found in this study.
Multi-parametric MRI has also been used to define IDLs [8]. MRI provides superior soft tissue contrast and additionally can measure chemical composition, vasculature, perfusion, and water mobility within potential IDLs. In contrast, choline PET isolates intracellular delivery and uptake of choline [8,15,45]. IDLs defined by one modality may therefore not fully overlap with that of the other, and differences may exist in how diagnostic sensitivity and specificity is weighted by each modality. Sensitivity may be desirable for delineating IDLs for radiotherapy, and our NTCP results suggest that VMAT can be suitably paired with imaging that favors tumor sensitivity over specificity. With the advent of PET/MRI, it is possible that complementary information from both MRI and PET will lead to more optimized IDL definitions.
This study has limitations as a single institution study to determine the expected radiobiologic effects in patients eligible for radiation therapy based on NCCN guidelines. This study estimates the advantage of selective dose escalation through calculations of TCP and NTCP. As such, these results require validation in a prospective trial that will be susceptible to many of the common radiation therapy clinical uncertainties. Interventions to minimize variations in setup and organ position/motion, as well as image-guidance, were not considered in the approach of this study, and yet may help to truly realize the theoretical benefits of selective dose augmentation.
The current study included patients with low-, intermediate-, and high-risk tumors. It has been debated whether all patients with Gleason 5 or 6 tumors require radical treatment. The reason low-risk patients were included in the current study was not only because they qualified for radiotherapy based on current guidelines, but also to demonstrate feasibility for applying 18F-choline PET/CT to define IDLs in low as well as high risk patients. As imaging and treatment planning tools used for selective dose escalation can also be applied for dose sparing, it may be worthwhile to explore more focused treatment options for lower risk patients [10,46].
Conclusion
This radiobiologic analysis shows potential for VMAT to achieve a high differential boost dose (21 to 26 Gy) to a 18F-choline PET/CT defined IDL while not exceeding theoretical normal tissue tolerances beyond that of conventional high-dose (79 Gy) whole-prostate radiotherapy. Based on histopathologic correspondence, contouring derived by static 18F-choline PET threshholding can potentially lead to an overestimated IDL volume. However, no corresponding penalty with regards to NTCP was observed in this preliminary study.
Supplementary Material
Acknowledgements
This work was supported in part by Congressionally Directed Medical Research Programs Prostate Cancer Research Program grant PC04130, National Institutes of Health/National Cancer Institute grant R41CA110121, and by the Mountain West Clinical Translational Research - Infrastructure Network under grant 1U54GM104944 from the National Institute of General Medical Sciences and the UNLV Lincy Endowed Assistant Professorship. This content does not necessarily represent the views of the National Institutes of Health or Queen’s Medical Center.
Footnotes
Conflict of interest None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kupelian PA, Ciezki J, Reddy CA, et al. Effect of increasing radiation doses on local and distant failures in patients with localized prostate cancer. International journal of radiation oncology, biology, physics. 2008;71:16–22. doi: 10.1016/j.ijrobp.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 2.Zelefsky MJ, Pei X, Chou JF, et al. Dose escalation for prostate cancer radiotherapy: Predictors of long-term biochemical tumor control and distant metastases-free survival outcomes. European urology. 2011;60:1133–1139. doi: 10.1016/j.eururo.2011.08.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Viani GA, Stefano EJ, Afonso SL. Higher-than-conventional radiation doses in localized prostate cancer treatment: A meta-analysis of randomized, controlled trials. International journal of radiation oncology, biology, physics. 2009;74:1405–1418. doi: 10.1016/j.ijrobp.2008.10.091. [DOI] [PubMed] [Google Scholar]
- 4.Spratt DE, Pei X, Yamada J, et al. Long-term survival and toxicity in patients treated with high-dose intensity modulated radiation therapy for localized prostate cancer. International journal of radiation oncology, biology, physics. 2013;85:686–692. doi: 10.1016/j.ijrobp.2012.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cellini N, Morganti AG, Mattiucci GC, et al. Analysis of intraprostatic failures in patients treated with hormonal therapy and radiotherapy: Implications for conformal therapy planning. International journal of radiation oncology, biology, physics. 2002;53:595–599. doi: 10.1016/s0360-3016(02)02795-5. [DOI] [PubMed] [Google Scholar]
- 6.Pucar D, Hricak H, Shukla-Dave A, et al. Clinically significant prostate cancer local recurrence after radiation therapy occurs at the site of primary tumor: Magnetic resonance imaging and step-section pathology evidence. International journal of radiation oncology, biology, physics. 2007;69:62–69. doi: 10.1016/j.ijrobp.2007.03.065. [DOI] [PubMed] [Google Scholar]
- 7.Nutting CM, Corbishley CM, Sanchez-Nieto B, et al. Potential improvements in the therapeutic ratio of prostate cancer irradiation: Dose escalation of pathologically identified tumour nodules using intensity modulated radiotherapy. The British journal of radiology. 2002;75:151–161. doi: 10.1259/bjr.75.890.750151. [DOI] [PubMed] [Google Scholar]
- 8.Bauman G, Haider M, Van der Heide UA, et al. Boosting imaging defined dominant prostatic tumors: A systematic review. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2013;107:274–281. doi: 10.1016/j.radonc.2013.04.027. [DOI] [PubMed] [Google Scholar]
- 9.Huang CC, Deng FM, Kong MX, et al. Re-evaluating the concept of “dominant/index tumor nodule” in multifocal prostate cancer. Virchows Archiv: an international journal of pathology. 2014;464:589–594. doi: 10.1007/s00428-014-1557-y. [DOI] [PubMed] [Google Scholar]
- 10.Mouraviev V, Villers A, Bostwick DG, et al. Understanding the pathological features of focality, grade and tumour volume of early-stage prostate cancer as a foundation for parenchyma-sparing prostate cancer therapies: Active surveillance and focal targeted therapy. BJU international. 2011;108:1074–1085. doi: 10.1111/j.1464-410X.2010.10039.x. [DOI] [PubMed] [Google Scholar]
- 11.Glunde K, Bhujwalla ZM, Ronen SM. Choline metabolism in malignant transformation. Nature reviews Cancer. 2011;11:835–848. doi: 10.1038/nrc3162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Yamaguchi T, Lee J, Uemura H, et al. Prostate cancer: A comparative study of 11c-choline pet and mr imaging combined with proton mr spectroscopy. Eur J Nucl Med Mol Imaging. 2005;32:742–748. doi: 10.1007/s00259-004-1755-y. [DOI] [PubMed] [Google Scholar]
- 13.Kwee SA, DeGrado TR, Talbot JN, et al. Cancer imaging with fluorine-18-labeled choline derivatives. Semin Nucl Med. 2007;37:420–428. doi: 10.1053/j.semnuclmed.2007.07.003. [DOI] [PubMed] [Google Scholar]
- 14.Piert M, Park H, Khan A, et al. Detection of aggressive primary prostate cancer with 11c-choline pet/ct using multimodality fusion techniques. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2009;50:1585–1593. doi: 10.2967/jnumed.109.063396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kwee SA, Thibault GP, Stack RS, et al. Use of step-section histopathology to evaluate 18f-fluorocholine pet sextant localization of prostate cancer. Molecular imaging. 2008;7:12–20. [PubMed] [Google Scholar]
- 16.Chang JH, Lim Joon D, Lee ST, et al. Intensity modulated radiation therapy dose painting for localized prostate cancer using (1)(1)c-choline positron emission tomography scans. International journal of radiation oncology, biology, physics. 2012;83:e691–696. doi: 10.1016/j.ijrobp.2012.01.087. [DOI] [PubMed] [Google Scholar]
- 17.Pinkawa M, Holy R, Piroth MD, et al. Intensity-modulated radiotherapy for prostate cancer implementing molecular imaging with 18f-choline pet-ct to define a simultaneous integrated boost. Strahlentherapie und Onkologie: Organ der Deutschen Rontgengesellschaft [et al] 2010;186:600–606. doi: 10.1007/s00066-010-2122-5. [DOI] [PubMed] [Google Scholar]
- 18.Pinkawa M, Piroth MD, Holy R, et al. Dose-escalation using intensity-modulated radiotherapy for prostate cancer - evaluation of quality of life with and without (18)f-choline pet-ct detected simultaneous integrated boost. Radiation oncology. 2012;7:14. doi: 10.1186/1748-717X-7-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Quan EM, Li X, Li Y, et al. A comprehensive comparison of imrt and vmat plan quality for prostate cancer treatment. International journal of radiation oncology, biology, physics. 2012;83:1169–1178. doi: 10.1016/j.ijrobp.2011.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ost P, Speleers B, De Meerleer G, et al. Volumetric arc therapy and intensity-modulated radiotherapy for primary prostate radiotherapy with simultaneous integrated boost to intraprostatic lesion with 6 and 18 mv: A planning comparison study. International journal of radiation oncology, biology, physics. 2011;79:920–926. doi: 10.1016/j.ijrobp.2010.04.025. [DOI] [PubMed] [Google Scholar]
- 21.Mohler J, Bahnson RR, Boston B, et al. Nccn clinical practice guidelines in oncology: Prostate cancer. Journal of the National Comprehensive Cancer Network: JNCCN. 2010;8:162–200. doi: 10.6004/jnccn.2010.0012. [DOI] [PubMed] [Google Scholar]
- 22.Michalski J, Purdy J, Bruner D, et al. A phase iii randomized study of high dose 3dcrt/imrt versus standard dose 3dcrt/imrt in patients treated for localized prostate cancer. RTOG 0126. 2014 http://www.rtog.org/ClinicalTrials/ProtocolTable/StudyDetails.aspx?study=0126 [Google Scholar]
- 23.Kwee SA, Wei H, Sesterhenn I, et al. Localization of primary prostate cancer with dual-phase 18f-fluorocholine pet. Journal of nuclear medicine: official publication, Society of Nuclear Medicine. 2006;47:262–269. [PubMed] [Google Scholar]
- 24.Chang JH, Joon DL, Lee ST, et al. Histopathological correlation of (11)c-choline pet scans for target volume definition in radical prostate radiotherapy. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2011;99:187–192. doi: 10.1016/j.radonc.2011.03.012. [DOI] [PubMed] [Google Scholar]
- 25.Murray LJ, Lilley J, Thompson CM, et al. Prostate stereotactic ablative radiation therapy using volumetric modulated arc therapy to dominant intraprostatic lesions. International journal of radiation oncology, biology, physics. 2014;89:406–415. doi: 10.1016/j.ijrobp.2014.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Uzan J, Nahum AE. Radiobiologically guided optimisation of the prescription dose and fractionation scheme in radiotherapy using biosuite. The British journal of radiology. 2012;85:1279–1286. doi: 10.1259/bjr/20476567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nahum A, Sanchez-Nieto B. Tumour control probability modelling: Basic principles and applications in treatment planning. Physica Medica. 2001;17:13–23. [Google Scholar]
- 28.Lyman JT. Complication probability as assessed from dose-volume histograms. Radiation research Supplement. 1985;8:S13–19. [PubMed] [Google Scholar]
- 29.Kutcher GJ, Burman C. Calculation of complication probability factors for non-uniform normal tissue irradiation: The effective volume method. International journal of radiation oncology, biology, physics. 1989;16:1623–1630. doi: 10.1016/0360-3016(89)90972-3. [DOI] [PubMed] [Google Scholar]
- 30.Niemierko A. Reporting and analyzing dose distributions: A concept of equivalent uniform dose. Medical physics. 1997;24:103–110. doi: 10.1118/1.598063. [DOI] [PubMed] [Google Scholar]
- 31.Michalski JM, Gay H, Jackson A, et al. Radiation dose-volume effects in radiation-induced rectal injury. International journal of radiation oncology, biology, physics. 2010;76:S123–129. doi: 10.1016/j.ijrobp.2009.03.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burman C, Kutcher GJ, Emami B, et al. Fitting of normal tissue tolerance data to an analytic function. International journal of radiation oncology, biology, physics. 1991;21:123–135. doi: 10.1016/0360-3016(91)90172-z. [DOI] [PubMed] [Google Scholar]
- 33.Pinkawa M, Attieh C, Piroth MD, et al. Dose-escalation using intensity-modulated radiotherapy for prostate cancer--evaluation of the dose distribution with and without 18f-choline pet-ct detected simultaneous integrated boost. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2009;93:213–219. doi: 10.1016/j.radonc.2009.07.014. [DOI] [PubMed] [Google Scholar]
- 34.D’Angelillo RM, Sciuto R, Ramella S, et al. (1)(8)f-choline positron emission tomography/computed tomography-driven high-dose salvage radiation therapy in patients with biochemical progression after radical prostatectomy: Feasibility study in 60 patients. International journal of radiation oncology, biology, physics. 2014;90:296–302. doi: 10.1016/j.ijrobp.2014.05.050. [DOI] [PubMed] [Google Scholar]
- 35.Marks LB, Yorke ED, Jackson A, et al. Use of normal tissue complication probability models in the clinic. International journal of radiation oncology, biology, physics. 2010;76:S10–19. doi: 10.1016/j.ijrobp.2009.07.1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alicikus ZA, Yamada Y, Zhang Z, et al. Ten-year outcomes of high-dose, intensity-modulated radiotherapy for localized prostate cancer. Cancer. 2011;117:1429–1437. doi: 10.1002/cncr.25467. [DOI] [PubMed] [Google Scholar]
- 37.Seppala J, Seppanen M, Arponen E, et al. Carbon-11 acetate pet/ct based dose escalated imrt in prostate cancer. Radiotherapy and oncology: journal of the European Society for Therapeutic Radiology and Oncology. 2009;93:234–240. doi: 10.1016/j.radonc.2009.08.010. [DOI] [PubMed] [Google Scholar]
- 38.Riches SF, Payne GS, Desouza NM, et al. Effect on therapeutic ratio of planning a boosted radiotherapy dose to the dominant intraprostatic tumour lesion within the prostate based on multifunctional mr parameters. The British journal of radiology. 2014;87:20130813. doi: 10.1259/bjr.20130813. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nguyen PL, Chen M-H, Zhang Y, et al. Updated results of magnetic resonance imaging guided partial prostate brachytherapy for favorable risk prostate cancer: Implications for focal therapy. The Journal of urology. 2012;188:1151–1156. doi: 10.1016/j.juro.2012.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Vainshtein J, Abu-Isa E, Olson K, et al. Randomized phase ii trial of urethral sparing intensity modulated radiation therapy in low-risk prostate cancer: Implications for focal therapy. Radiation oncology. 2012;7:82. doi: 10.1186/1748-717X-7-82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Miralbell R, Mollà M, Rouzaud M, et al. Hypofractionated boost to the dominant tumor region with intensity modulated stereotactic radiotherapy for prostate cancer: A sequential dose escalation pilot study. International Journal of Radiation Oncology*Biology*Physics. 2010;78:50–57. doi: 10.1016/j.ijrobp.2009.07.1689. [DOI] [PubMed] [Google Scholar]
- 42.Miralbell R, Buchegger F. Pet/ct imaging and the oligometastatic prostate cancer patient: An opportunity for a curative approach with high-dose radiotherapy? European journal of nuclear medicine and molecular imaging. 2014;41:1267–1269. doi: 10.1007/s00259-014-2793-8. [DOI] [PubMed] [Google Scholar]
- 43.Schick U, Popowski Y, Nouet P, et al. High-dose-rate brachytherapy boost to the dominant intra-prostatic tumor region: Hemi-irradiation of prostate cancer. Prostate. 2011;71:1309–1316. doi: 10.1002/pros.21347. [DOI] [PubMed] [Google Scholar]
- 44.Schmid DT, John H, Zweifel R, et al. Fluorocholine pet/ct in patients with prostate cancer: Initial experience. Radiology. 2005;235:623–628. doi: 10.1148/radiol.2352040494. [DOI] [PubMed] [Google Scholar]
- 45.Bauman G, Belhocine T, Kovacs M, et al. 18f-fluorocholine for prostate cancer imaging: A systematic review of the literature. Prostate Cancer Prostatic Dis. 2012;15:45–55. doi: 10.1038/pcan.2011.35. [DOI] [PubMed] [Google Scholar]
- 46.Tareen B, Godoy G, Sankin A, et al. Can contemporary transrectal prostate biopsy accurately select candidates for hemi-ablative focal therapy of prostate cancer? BJU international. 2009;104:195–199. doi: 10.1111/j.1464-410X.2009.08347.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.