Abstract
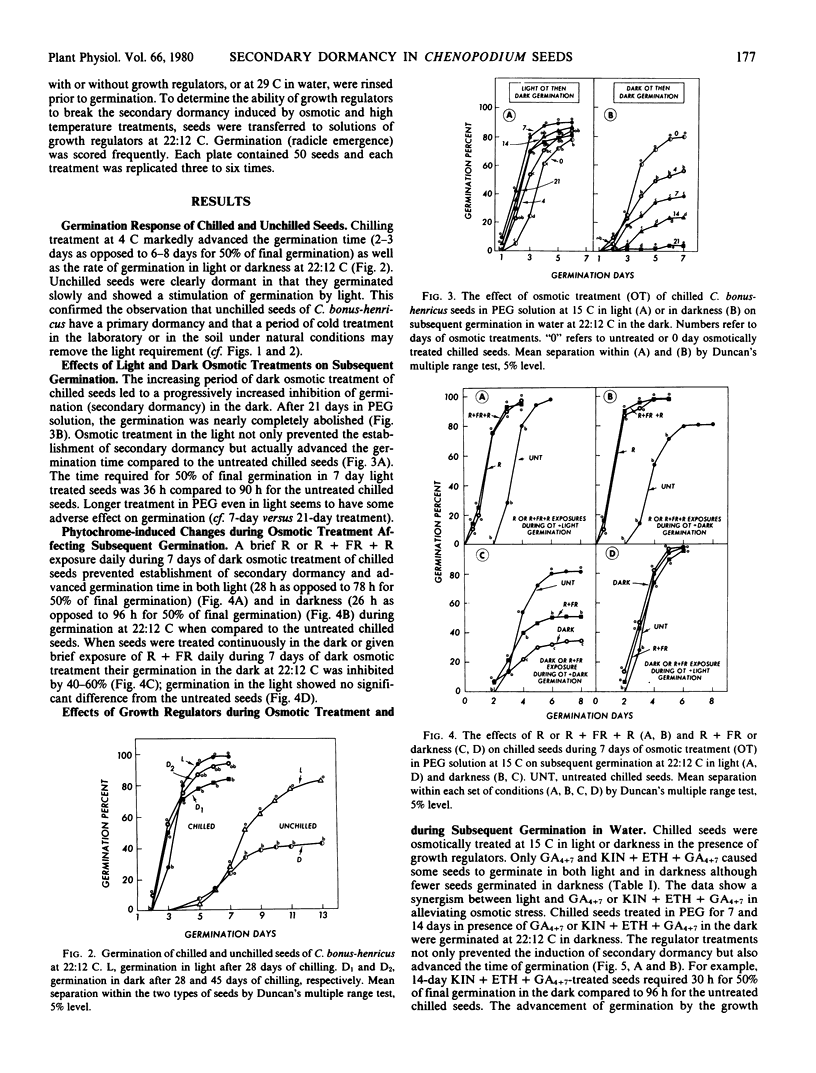
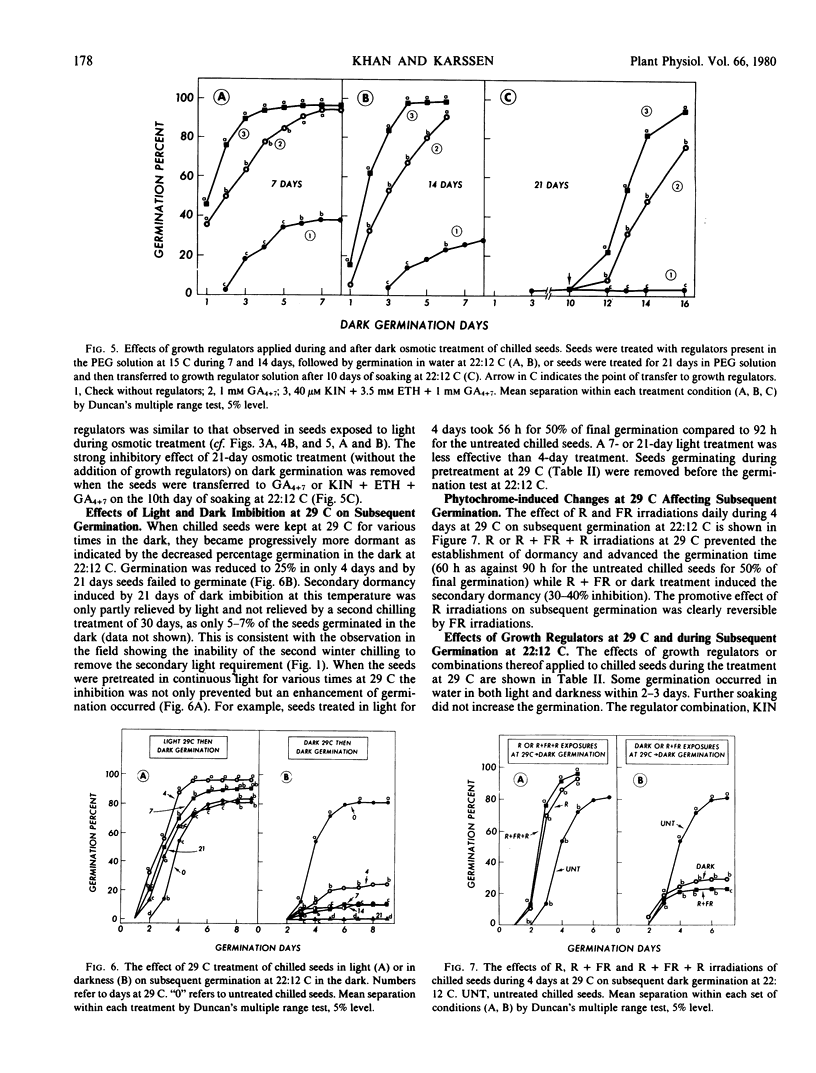
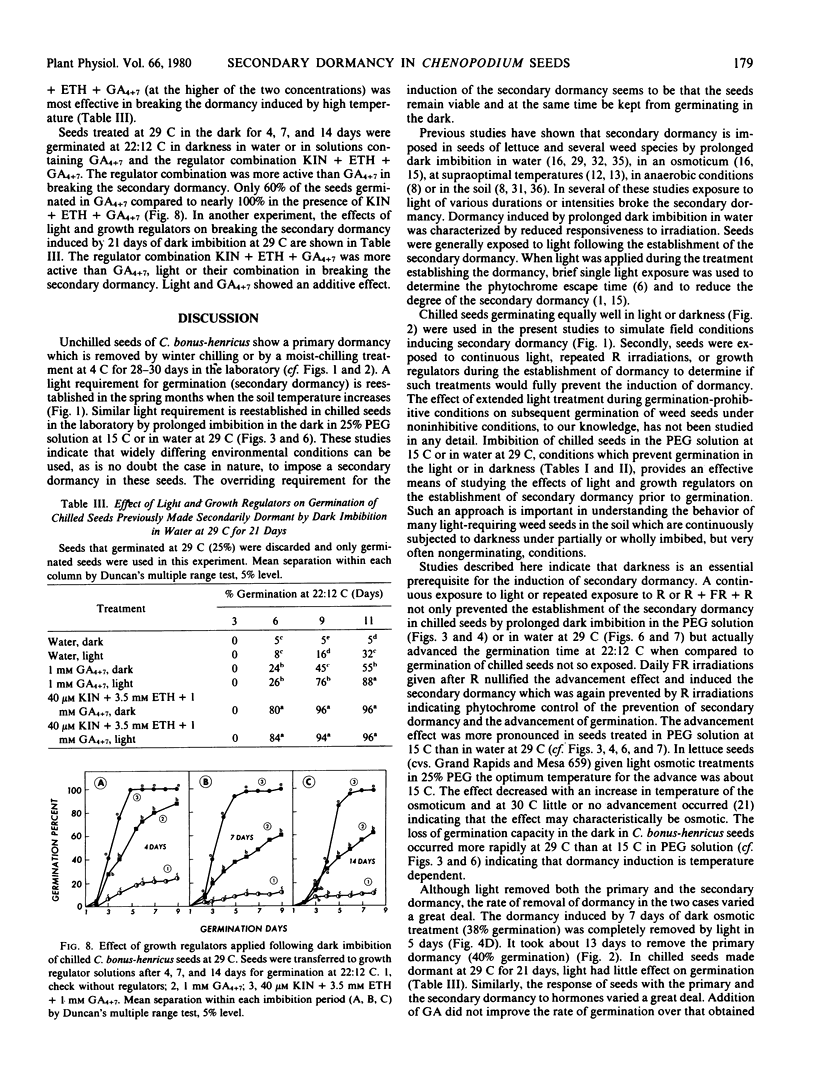
Factors controlling the establishment and removal of secondary dormancy in Chenopodium bonus-henricus L. seeds were investigated. Unchilled seeds required light for germination. A moist-chilling treatment at 4 C for 28 to 30 days removed this primary dormancy. Chilled seeds now germinated in the dark. When chilled seeds were held in the dark in −8.6 bars polyethylene glycol 6000 solution at 15 C or in water at 29 C a secondary dormancy was induced which increased progressively with time as determined by subsequent germination. These seeds now failed to germinate under the condition (darkness) which previously allowed their germination. Continuous light or daily brief red light irradiations during prolonged imbibition in polyethylene glycol solution at 15 C or in water at 29 C prevented the establishment of the secondary dormancy and caused an advancement of subsequent germination. Far red irradiations immediately following red irradiation reestablished the secondary dormancy indicating phytochrome participation in “pregerminative” processes. The growth regulator combination, kinetin + ethephon + gibberellin A4+A7 (GA4+7), and to a relatively lesser extent GA4+7, was effective in preventing the establishment of the secondary dormancy and in advancing the germination or emergence time. Following the establishment of the secondary dormancy by osmotic or high temperature treatments the regulator combination was relatively more active than light or GA4+7 in removing the dormancy. Prolonged dark treatment at 29 C seemed to induce changes that were partially independent of light or GA4+7 control. The data presented here indicate that changes during germination preventing dark treatment determine whether the seed will germinate, show an advancement effect, or will become secondarily dormant. These changes appear to be modulated by light and hormones.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Braun J. W., Khan A. A. Endogenous abscisic Acid levels in germinating and nongerminating lettuce seed. Plant Physiol. 1975 Dec;56(6):731–733. doi: 10.1104/pp.56.6.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burdett A. N. Antagonistic effects of high and low temperature pretreatments on the germination and pregermination ethylene synthesis of lettuce seeds. Plant Physiol. 1972 Aug;50(2):201–204. doi: 10.1104/pp.50.2.201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holm R. E. Volatile metabolites controlling germination in buried weed seeds. Plant Physiol. 1972 Aug;50(2):293–297. doi: 10.1104/pp.50.2.293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsiao A. I., Vidaver W. Dark Reversion of Phytochrome in Lettuce Seeds Stored in a Water-saturated Atmosphere. Plant Physiol. 1973 Mar;51(3):459–463. doi: 10.1104/pp.51.3.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikuma H., Thimann K. V. Analysis of Germination Processes of Lettuce Seed by Means of Temperature and Anaerobiosis. Plant Physiol. 1964 Sep;39(5):756–767. doi: 10.1104/pp.39.5.756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kahn A. An Analysis of "Dark-osmotic Inhibition" of Germination of Lettuce Seeds. Plant Physiol. 1960 Jan;35(1):1–7. doi: 10.1104/pp.35.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keys R. D., Smith O. E., Kumamoto J., Lyon J. L. Effect of Gibberellic Acid, Kinetin, and Ethylene plus Carbon Dioxide on the Thermodormancy of Lettuce Seed (Lactuca sativa L. cv. Mesa 659). Plant Physiol. 1975 Dec;56(6):826–829. doi: 10.1104/pp.56.6.826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loercher L. Persistence of red light induction in lettuce seeds of varying hydration. Plant Physiol. 1974 Mar;53(3):503–506. doi: 10.1104/pp.53.3.503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michel B. E., Kaufmann M. R. The osmotic potential of polyethylene glycol 6000. Plant Physiol. 1973 May;51(5):914–916. doi: 10.1104/pp.51.5.914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Speer H. L., Hsiao A. I., Vidaver W. Effects of Germination-promoting Substances Given in Conjunction with Red Light on the Phytochrome-mediated Germination of Dormant Lettuce Seeds (Lactuca sativa L.). Plant Physiol. 1974 Dec;54(6):852–854. doi: 10.1104/pp.54.6.852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylorson R. B., Hendricks S. B. Phytochrome Transformation and Action in Seeds of Rumex crispus L. during Secondary Dormancy. Plant Physiol. 1973 Nov;52(5):475–479. doi: 10.1104/pp.52.5.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidaver W., Hsiao A. I. Phytochrome transformation in lettuce seed irradiated at various temperatures. Plant Physiol. 1972 Aug;50(2):249–251. doi: 10.1104/pp.50.2.249. [DOI] [PMC free article] [PubMed] [Google Scholar]


