Abstract
The authors describe fundus autofluorescence (AF) and spectral-domain optical coherence tomography (SD-OCT) findings in three patients with enhanced S-cone syndrome and their correlation around the hyperautofluorescent ring border. Patients had AF imaging in combination with SD-OCT line-scans through the fovea, at the posterior pole, and at a temporal locus centered on the ring border. All eyes demonstrated a macular ring of high-intensity AF. The inner segment ellipsoid band showed thinning and disorganization toward the ring border, where it was lost.
INTRODUCTION
Enhanced S-cone syndrome (ESCS) is a rare, slowly progressive autosomal recessive rod-cone degeneration (Online Mendelian Inheritance in Man [OMIM] identifier: 268100; http://www.ncbi.nlm.nih.gov/omim) related to mutations in the orphan nuclear receptor transcription factor NR2E3 (OMIM: 604485). Various fundus appearances can occur in ESCS, the most typical being nummular pigmentation along the arcades and macular disturbance, often associated with intraretinal cysts.1 Fundus autofluorescence (AF) imaging can display decreased or absent AF outside of the arcades, a ring of increased AF in the transitional zone between the macula region and the hypoautofluorescent periphery, hyperautofluorescent spots within the arcades, and a spoke-like increased AF centered on the fovea in the presence of foveoschisis.2,3
Although previous studies have used optical coherence tomography (OCT) and AF to describe ESCS phenotypes, to our knowledge none have investigated SD-OCT features corresponding to the hyperautofluorescent macular ring. We therefore report a case series of three patients with ESCS, describe their AF and SD-OCT findings, and correlate AF and SD-OCT changes around the ring border.
CASE REPORT
The diagnosis of ESCS was made by an experienced retinal specialist (SHT) and was supported by full-field electroretinography (ff-ERG).4 High-resolution SD-OCT line-scans and 30° AF images were simultaneously acquired with the Spectralis HRA-OCT (Heidelberg Engineering, Heidelberg, Germany). SD-OCT line-scans were obtained through the fovea, at the posterior pole, and at a temporal locus centered on the ring border. Wide-field 55° AF images were acquired separately. Molecular genetic testing was obtained when possible with previously described techniques.5 Institutional review board approval was obtained for this study.
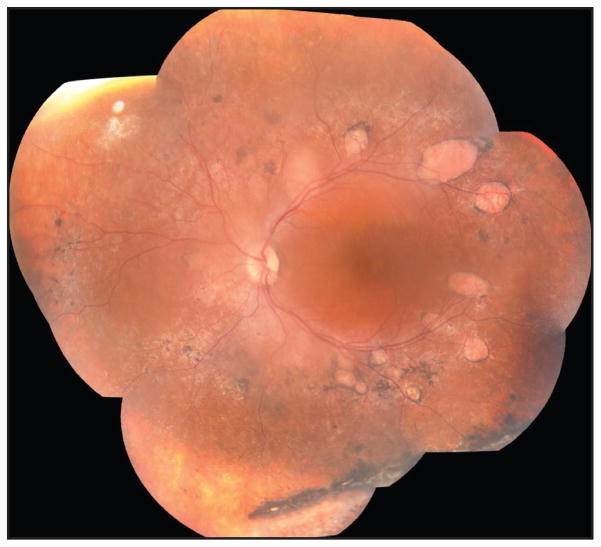
Patient 1 was a 33-year-old man with progressively worsening vision. Fundus findings showed nummular pigmentation with yellow-white dots in the midperiphery along the arcades (Figure 1). AF displayed a hyperautofluorescent macular ring demarcating the central macular area of relatively spared autofluorescence from the hypoautofluorescent periphery (Figure 2). SD-OCT demonstrated loss of the inner segment ellipsoid (ISe) band corresponding to the ring border, with thinning and disorganization of the ISe in the central macula. He carried a mutation in NR2E3 (R311Q, Q350R).
Figure 1.
Patient 1, a 33-year-old man. Color montage of the left eye showing nummular pigmentation interspersed with yellow-white dots around the arcades.
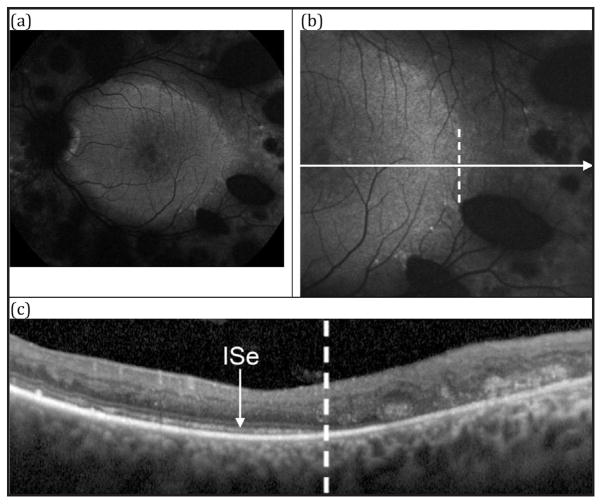
Figure 2.
Patient 1. (A) 55° fundus autofluorescence (AF) in the left eye showing a hyperautofluorescent ring demarcating the central macular area of relatively spared autofluorescence from the hypoautofluorescent periphery. (B) 30° AF centered temporally on the border of the ring. (C) SD-OCT horizontal line-scan through the fovea in the left eye extending temporally, corresponding to the cut from B. The dashed vertical line in C corresponds to the position marked in B with a dashed vertical line. Note the loss of the inner segment ellipsoid at the border of the ring.
Patient 2 was an 11-year-old boy with nyctalopia. Fundus findings included nummular pigmentation with jagged-like zones of pale, mottled atrophy in the midperiphery along the arcades (Figure 3). AF imaging revealed a hyperautofluorescent ring demarcating the central macular area of mottled hyperautofluorescence from the hypoautofluorescent periphery. SD-OCT demonstrated ISe loss at the ring border and subtle ISe disorganization in the central macula (Figure 4). His parents declined genetic testing.
Figure 3.
Patient 2, an 11-year-old boy. Color montage of the left eye showing nummular pigmentation accompanied by pale mottling around the vascular arcades.
Figure 4.
Patient 2. (A) 55° fundus autofluorescence (AF) in the left eye showing a hyperautofluorescent ring demarcating the central macular area of mottled hyperautofluorescence from the hypoautofluorescent periphery. (B) 30° AF centered temporally on the border of the ring. (C) SD-OCT horizontal line-scan through the fovea in the left eye extending temporally, corresponding to the cut from B. The dashed vertical line in C corresponds to the position marked in B with a dashed vertical line. Note the loss of the inner segment ellipsoid at the border of the ring.
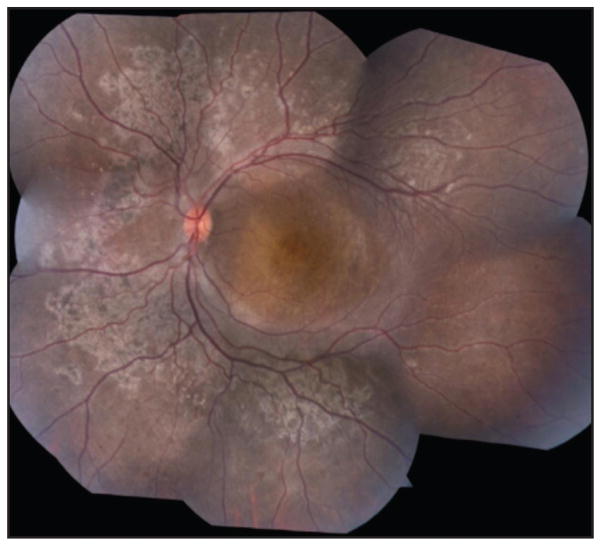
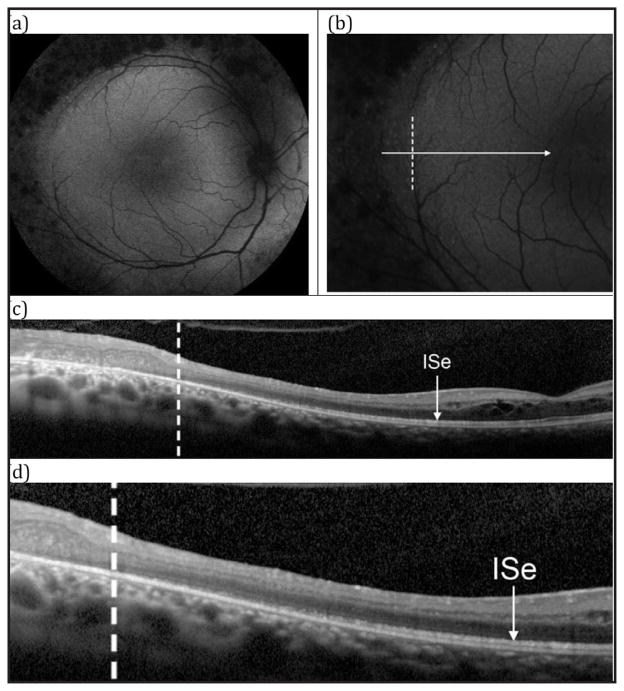
Patient 3 was a 27-year-old woman reporting blurry vision. Fundus examination showed nummular pigmentation with yellow-white dots in the midperiphery along the arcades (Figure 5). AF revealed a weakly hyperautofluorescent ring in the macula demarcating a central region of relatively spared autofluorescence and peripheral hypoautofluorescence (Figure 6). SD-OCT demonstrated a wide region of gradual ISe disorganization toward the ring border, where it was lost. However, the ISe in the central macula appeared spared. She carried a mutation in NR2E3 (IVS1-2A > Chom).
Figure 5.
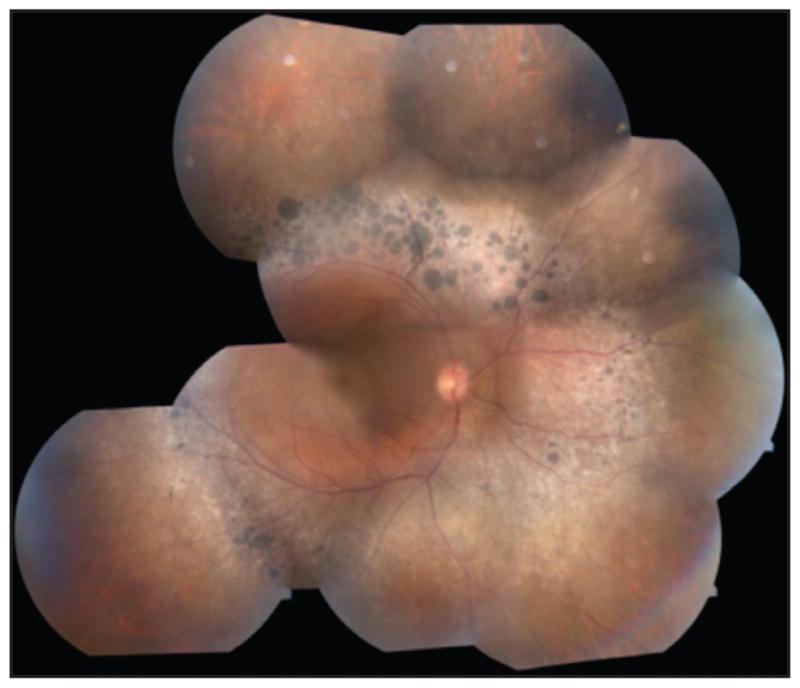
Patient 3, a 27-year-old woman. Color montage in the right eye showing nummular pigmentation accompanied by yellow-white dots around the arcades.
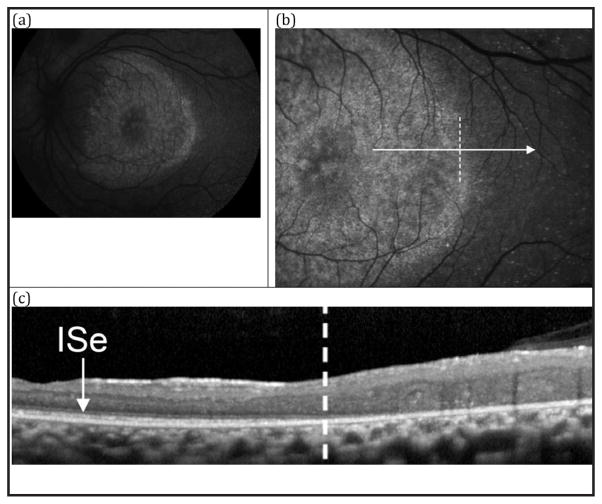
Figure 6.
Patient 3. (A) 55° fundus autofluorescence (AF) in the left eye showing a weakly hyperautofluorescent ring demarcating the central macular area of relatively spared autofluorescence from the hypoautofluorescent periphery. Nummular pigmentation in the periphery is shown to lack fluorescence. (B) 30° AF centered temporally on the border of the ring. (C) SD-OCT horizontal line-scan through the fovea in the left eye extending temporally, corresponding to the cut from B. The dashed vertical line in C corresponds to position marked in B with a dashed vertical line. Note the small cystic changes (possibly foveal schisis) in the parafoveal region. (D) Magnified view of C, demonstrating gradual disorganization of the ISe towards the ring border, where it is lost.
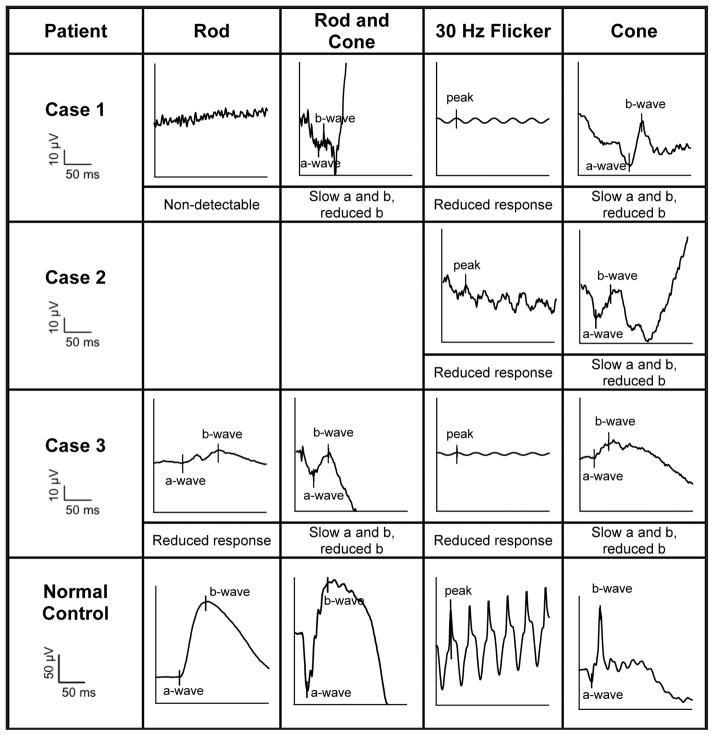
For all three patients, ff-ERG testing was obtained. Patient 2 was unable to tolerate scotopic testing but completed photopic testing. The clinical diagnosis of ESCS was supported by ff-ERG tracings (Figure 7).
Figure 7.
ISCEV-standard ERG tracings of each enhanced S-cone study patient. Rod-specific, maximal mixed rod and cone, 30-Hz flicker stimulus, and transient single-cone ERG waveforms are shown. Waveforms are from the left eye of patients 1 and 2 and the right eye of patient 3. The ERG responses to a standard single flash (cone) had similar waveforms under photopic (cone) and maximal scotopic conditions, except in waveform amplitude. The 30-Hz flicker stimulus waveforms have reduced amplitudes compared to single flash photopic (cone) a-waves. Note that patient 2 was unable to complete scotopic testing.
DISCUSSION
We report a case series of three patients with ESCS imaged with AF and high-resolution SD-OCT. In our series, we targeted the imaging on the border of the hyperautofluorescent macular ring and illustrated disruption of the ISe band toward the border of the ring, where it was lost. The observed hyperautofluorescence suggests increased deposition of lipofuscin in the RPE cells from degenerating photoreceptor outer segments and likely indicates impending photoreceptor loss in that region.6 However, the ISe was also somewhat disorganized central to the ring and at the fovea in two patients, while the third showed ISe sparing in the central macula but with a wide zone of gradual ISe disorganization central to the ring border. The ISe disturbance within the ring is in line with multifocal-ERG analysis showing subnormal macular function in ESCS.7 The decreased autofluorescence observed outside of the ring may be due to the loss of photoreceptors, as has been previously suggested.2 Alternately, the AF signal may have been attenuated by the marked retinal thickening observed outside of the ring. This thickening involved the entire extent of remaining retinal layers and was associated with a distortion of retinal architecture. Additionally, reflective material was observed in the remaining outer layers, which likely signified remnants of cellular breakdown. Similar to those found in ESCS, rings of increased AF intensity can be found in the parafoveal region in certain conditions, including retinitis pigmentosa, X-linked retinoschisis, Leber congenital amaurosis, and cone-rod dystrophy. These rings colocalize with regions of decreased visual sensitivity and have been shown to constrict in retinitis pigmentosa and expand in cone-rod dystrophy, with subsequent loss of structure and function.8–11 Therefore, hyperautofluorescent rings are likely a nonspecific manifestation of certain conditions that indicates areas of impending photoreceptor loss.
In summary, we present three cases of ESCS imaged with high-resolution AF and SD-OCT. Novel to our series was that we correlated AF and SD-OCT across the border of the ring, demonstrating that the ISe band is lost in this region.
Footnotes
The authors have no financial or proprietary interest in the materials presented herein.
References
- 1.Jacobson SG, Marmor MF, Kemp CM, et al. SWS (Blue)cone hypersensitivity in a newly identified retinal degeneration. Invest Ophthalmol Vis Sci. 1990;31:827–838. [PubMed] [Google Scholar]
- 2.Audo I, Michaelides M, Robson AG, et al. Phenotypic variation in enhanced S-cone syndrome. Invest Ophthalmol Vis Sci. 2008;49:2082–2093. doi: 10.1167/iovs.05-1629. [DOI] [PubMed] [Google Scholar]
- 3.Wang N, Fine H, Chang S, et al. Cellular origin of fundus autofluorescence in patients and mice with defective NR2E3 gene. Br J Ophthalmol. 2009;93:1234–1240. doi: 10.1136/bjo.2008.153577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marmor MF, Fulton AB, Holder GE, et al. ISCEV Standard for full-field clinical electrophysiology (2008 update) Doc Ophthalmol. 2009;118:69–77. doi: 10.1007/s10633-008-9155-4. [DOI] [PubMed] [Google Scholar]
- 5.Park SP, Hong IH, Tsang SH, et al. Disruption of the human cone photoreceptor mosaic from a defect in NR2E3 transcription factor function in young adults. Graefes Arch Clin Exp Ophthalmol. 2013;251:2299–2309. doi: 10.1007/s00417-013-2296-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sparrow JR, Yoon KD, Wu Y, et al. Interpretations of fundus autofluorescence from studies of the bisretinoids of the retina. Invest Ophthalmol Vis Sci. 2010;51:4351–4357. doi: 10.1167/iovs.10-5852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marmor MF, Tan F, Sutter EE, et al. Topography of cone electrophysiology in the enhanced S con syndrome. Invest Ophthalmol Vis Sci. 1999;40:1866–1873. [PubMed] [Google Scholar]
- 8.Lima LH, Cella W, Greenstein VC, et al. Structural analysis of hyperautofluorescent ring in patients with retinitis pigmentosa. Retina. 2009;29:1025–1031. doi: 10.1097/IAE.0b013e3181ac2418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Robson AG, Michaelides M, Luong VA, et al. Functional correlates of fundus autofluorescence abnormalities in patients with RPGR or RIMS1 mutations causing cone or cone rod dystrophy. Br J Ophthalmol. 2008;92:95–102. doi: 10.1136/bjo.2007.124008. [DOI] [PubMed] [Google Scholar]
- 10.Tsang SH, Vaclavik V, Bird AC, et al. Novel phenotypic and genotypic findings in X-linked retinoschisis. Arch Ophthalmol. 2007;125:259–267. doi: 10.1001/archopht.125.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Scholl HP, Chong NH, Robson AG, et al. Fundus autofluorescence in patients with leber congenital amaurosis. Invest Ophthalmol Vis Sci. 2004;45:2747–2752. doi: 10.1167/iovs.03-1208. [DOI] [PubMed] [Google Scholar]