Abstract
To determine the effects of extracellular matrix and neighboring cells on the differentiation of human embryonic stem cells (hESC) into progenitors of retinal cells and/or retinal pigment epithelium (RPE). HESC were cultured on mouse PA6 stromal cells for approximately 2 weeks to obtain neural progenitors. To induce photoreceptor marker expression, the neural progenitors were cultured on a confluent monolayer of ARPE19 or on laminin-coated dishes. To induce RPE markers, the neural progenitors were seeded onto human Bruch’s membrane or Matrigel. Cells were examined morphologically and stained with different RPE or neural progenitor markers. Microarray techniques were used to compare the gene expression profiles of hESC cultured on mouse fibroblasts or neural progenitors on PA6 cells to the transcriptome of the adult neural retina and RPE. HESC cultured on PA6 cells expressed neural progenitor markers β-tubulin III, PAX6, neural filament, GFAP and vimentin. Culturing these neural progenitors on confluent ARPE19 monolayer induced expression of the photoreceptor progenitor cell marker CRX; culturing neural progenitors on laminin substrates induced a neuronal phenotype with neurite formation. Neural progenitors expressed the RPE marker ZO-1 after culturing on Matrigel-coated dishes and the RPE marker Bestrophin after culturing on human Bruch’s membrane explants. Hierarchical clustering analysis of samples suggested that when cultured on PA6 stromal cells hESC exhibited genetic characteristics towards differentiating into neural retina. Microarray analysis showed that after culturing on PA6 cells, stem cells expressed 117 new genes; among these there were 22 genes present in neural retina or RPE cells. The functions of these genes were highly related to cell proliferation, nervous system development and cell adhesion. HESC can be induced to differentiate into neural progenitors after culturing on PA6 cells. These neural progenitors can express RPE markers when cultured on Bruch’s membrane or Matrigel, or photoreceptor markers when cultured on confluent ARPE19 or laminin. Additional studies are required to assess the function of hESC induced to express retinal or RPE markers prior to successful intraocular transplantation into animal models of retinal degeneration.
Keywords: stem cells, retinal progenitors, extracellular matrix, Bruch’s membrane, retinal transplantation
1. Introduction
Human embryonic stem cell transplantation is a promising therapeutic approach for the replacement of degenerated retinal cells in patients with age-related macular degeneration, retinitis pigmentosa, Stargardt’s disease, and other retinal degenerations. Prior to subretinal transplantation of hESC, it is important to induce stem cells to differentiate along RPE or retinal neural progenitor cell lines (Lamba et al., 2006; Ng et al., 2007). The extracellular environment can influence the function and fate of mature cells and differentiation of stem cells along different cell lineages. During embryonic development, cell cell or cell extracellular matrix contact plays a critical role in regulating stem cell differentiation, neural development, axon outgrowth and synapse formation (Bissell and Barcellos-Hoff, 1987; Comoglio et al., 2003; Meredith et al., 1996). For example, PA6 cells, derived from mouse calvarium, can induce mouse or monkey embryonic stem cells to differentiate into dopaminergic neurons (Haruta et al., 2004; Kawasaki et al., 2000, 2002; Morizane et al., 2006, 2002; Parmar and Li, 2007; Sasai, 2005); the ability of calvarium-derived cells to induce embryonic stem cells along a dopaminergic neural cell line is consistent with the notion that during development of the neural retina a developing cellular layer can induce differentiation of the adjacent cellular layer (Cepko, 1999). Embryonic stem cells from many organisms have the capacity to generate a wide variety of cell types in vitro depending on their environment (Cepko, 1999). Understanding precisely how such cells may be driven towards a specific lineage represents a major challenge prior to the wide scale use of hESC to treat human disease (Motohashi et al., 2006).
In human eyes the cells of the optic vesicle become either neural retinal progenitors expressing transcription factor Chx10, or RPE progenitors expressing transcription factor MITF (Horsford et al., 2005) that commits cells to a melanocytic lineage (Planque et al., 2004). The cell cell and cell extracellular matrix inductive phenomena that occur during vertebrate eye development are not completely understood, but the RPE plays a decisive role in the genesis of the vertebrate retina and the basement membrane plays an important role in RPE differentiation and polarization (Layer et al., 1998). The presence of normal RPE is required for normal development of the eye in vivo and to maintain the correct morphogenesis of the neural retina. After the neural retina starts to differentiate, the RPE is still necessary to maintain the organization of the retinal lamina (Raymond and Jackson, 1995). Cells exhibiting melanocyte and RPE characteristics can be induced from undifferentiated hESC grown on monolayers of specific stromal cell lines or by using a combination of Wnt3a, Endothelin-3 and stem cell factor (Motohashi et al., 2006).
The purpose of the present study was to determine the ability of extracellular matrix and cells ordinarily in contact with the neural retina or RPE to induce hESC to differentiate along neural or RPE progenitor lines. Specifically, we examined the effects of contact between hESC precultured on mouse PA6 cells and either a spontaneously immortalized human RPE line (ARPE19) or Bruch’s membrane, an acellular surface that separates the RPE from the choriocapillaris in the human eye, on induction of hESC differentiation and gene expression.
2. Materials and methods
2.1. Culturing of hESC and ARPE19 cells
The National Institutes of Health-registered BG01V hESC and ARPE19 were obtained from ATCC (American Type Culture Collection, Manassas, VA) as frozen stocks. Approximately 1 × 106 BG01V cells were plated into each of two 9.5-cm2 wells of a six-well culture plate (Corning Life Sciences, Acton, MA) containing a feeder layer of mitomycin C-treated CF-1 mouse embryonic fibroblasts (ATCC). Cells were cultured at 37 °C, 5% CO2 in an incubator in Dulbecco’s modified Eagle’s medium (DMEM)/F12 (ATCC) supplemented with 15% embryonic stem cell-qualified fetal bovine serum (FBS, ATCC), 5% knockout serum replacement (KSR, Invitrogen-Gibco, Carlsbad, CA), l-alanyl-l-glutamine (2.0 mM), MEM non-essential amino acids (1×), β-mercaptoethanol (0.1 mM), penicillin (100 IU/ml)/streptomycin (100 µg/ml) (ATCC), and 4 ng/ml basic fibroblast growth factor (bFGF, Invitrogen-Gibco). Medium was changed daily after the first 48 h of culture. Colony formation was visible within 3–4 days. Cells were passaged every 4–5 days using collagenase IV (200 units/ml, Invitrogen-Gibco). ARPE19 cells were cultured with standard cell culture protocol provided with ATCC.
2.2. SDIA-mediated differentiation of hESC
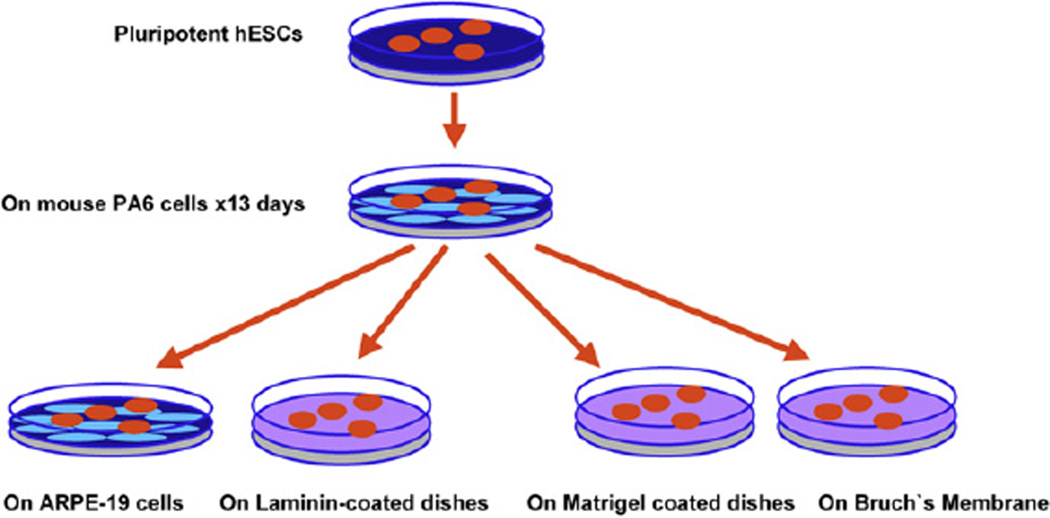
Previous studies have demonstrated that culturing embryonic stem cells on PA6 cells induces differentiation into dopaminergic neurons (Kawasaki et al., 2000, 2002). For our differentiation experiments, PA6 cells were plated at confluence prior to seeding hESC onto the confluent monolayer. HESC were dissociated after 50–60 min of treatment with type IV collagenase, and then seeded onto PA6 cells at a density of 2 × 103 cells per cm2 in embryonic stem cell differentiation medium. We used the KSR-containing Glasgow minimum essential medium (G-MEM medium, Invitrogen-Gibco) (Kawasaki et al., 2000) supplemented with 10% KSR, 2 mM glutamine, 1 mM pyruvate, 0.1 mM non-essential amino acids, 0.1 mM 2-mercaptoethanol, 100 units/ml penicillin, and 100 µg/ml streptomycin. From day 6 forward half the medium was changed every third day. Thirteen days after plating, colonies were separated from the feeder layer using type IV collagenase (200 units/ml) for about 2 h to obtain neural progenitors; these neural progenitors were then seeded onto different matrices or cells. To determine whether these neural progenitors have the capacity for the multilineage differentiation characteristic of retinal progenitors, we exposed the neural progenitors to elements of the subretinal environment such as human Bruch’s membrane, components of the extracellular matrix, or cultured them on confluent RPE monolayers. To induce photoreceptors, the neural progenitors were cultured onto ARPE19 or on laminin-coated 8-well chamber slides (BD Biosciences, San Jose CA). To induce RPE, the neural progenitors were cultured onto Matrigel (BD Biosciences) or human Bruch’s membrane (Fig. 1). The laminin source is from Engelbreth– Holm–Swarm (EHS) murine sarcoma basement membrane. Matrigel is a solubilized basement membrane preparation extracted from EHS murine sarcoma. Its major component is laminin, followed by collagen IV, heparan sulfate proteoglycans, and entactin. Matrigel contains TGF-β fibroblast growth factor, tissue plasminogen activator and other growth factors.
Fig. 1.
Schematic of retinal stem cell differentiation. Pluripotential hESC were grown in tissue culture dishes, harvested and seeded onto confluent monolayers of mouse PA6 stromal cells for 13 days to induce differentiation into neural progenitors. These differentiated stem cells were then harvested and cultured onto ARPE19 cell lines or laminin-coated dishes to induce photoreceptor markers; or onto human Bruch’s membrane or Matrigel to induce expression of RPE markers.
2.3. Harvesting of human Bruch’s membrane explants
Human Bruch’s membrane explants were prepared from the eyes of younger donors (aged from 20 to 40 years old) from the National Disease Research Interchange (NDRI, Philadelphia, PA) obtained within 48 h of death as described previously (Tezel et al., 2004). Briefly, the posterior pole of each eyecup was inspected visually with direct and retro illumination under a dissecting microscope, the eyecups were put in CO2-free medium (Invitrogen-Gibco), and a scleral incision was made 3 mm posterior to the limbus and extended circumferentially for 360°. Four radial incisions were then made and the sclera was peeled away. A circumferential incision was made into the subretinal space 1 mm posterior to the ora serrata. The choroid–Bruch’s membrane–RPE complex was then peeled toward the optic disc and removed after its attachment to the optic nerve was trimmed. Native RPE cells were removed by bathing the explant with 0.02 N ammonium hydroxide in a 50-mm polystyrene Petri dish (Falcon; BD Biosciences) for 20 min at room temperature, followed by washing three times in phosphate-buffered saline (PBS). The Bruch’s membrane explant was then floated in carbon dioxide-free medium over a 12–18-µm thick hydrophobic polycarbonate-polyvinylpyrrolidone membrane with 0.4-µm pores (Millipore, Bedford, MA) with the basal lamina facing the membrane. The curled edges were flattened from the choroidal side with fine forceps without touching Bruch’s membrane. Four percent agarose (Sigma-Aldrich, Saint Louis, MO) was poured onto the Bruch’s membrane–choroid complex from the choroidal side, and the tissue was kept at 4 °C for 2–3 min to solidify the agarose. The hydrophobic membrane was peeled off to expose the RPE basal lamina. The Bruch’s membrane explants were put in a 35 mm culture dish and attached with solidified agarose. The Bruch’s membrane explants were gently rinsed with PBS three times for 5 min, gamma sterilized (20,000 rad), and then stored at 4 °C.
2.4. Immunohistochemistry
Cells were fixed with 4% paraformaldehyde at 4 °C for 15 min, then permeabilized by incubation for 5 min in 0.1% Triton X-100 in PBS and incubated with 0.1% bovine serum albumin (BSA), 1% normal goat serum in PBS for 15 min. Slides were incubated with various primary monoclonal or polyclonal antibodies for 2–3 h at room temperature or overnight at 4 °C. Cell cultures were washed and incubated for 1 h at 37 °C in the dark with rabbit anti-mouse or goat anti-rabbit IgG antibodies conjugated to either Alexa TM 594 (red fluorescence) or Alexa TM 488 (green fluorescence) (Invitrogen-Molecular Probes). Nuclei were stained with diamidinophenyl indole (DAPI) by incubating the slides in the dye solution for 2 min. The slides were then washed four times in PBS. Immunologically stained cell cultures were visualized by an Olympus fluorescent microscope attached to an Olympus digital camera (Table 1).
Table 1.
List of antibodies used to stain different target cells or tissues, including the companies and dilution
| Antibody | Company | Dilution | Target cells or tissues |
|---|---|---|---|
| OCT 3/4 | BD Transduction Laboratories, San Jose, CA | 1/250 | Undifferentiated hESC |
| SSEA-4 | Millipore-Chemicon, Temecula, CA | 1/100 | Undifferentiated hESC |
| TRA-1-60 | Millipore-Chemicon, Temecula, CA | 1/100 | Undifferentiated hESC |
| TRA-1-81 | Millipore-Chemicon, Temecula, CA | 1/100 | Undifferentiated hESC |
| β-Tubulin III | Millipore-Chemicon, Temecula, CA | 1/100 | Neural progenitors |
| GFAP | Millipore-Chemicon, Temecula, CA | 1/1000 | Astrocyte |
| NF200 | Millipore-Chemicon, Temecula, CA | 1/1000 | Neuron |
| PAX6 | Covance Research Products, Berkley, CA | 1/500 | Neural progenitors |
| Vimentin | Sigma, Saint Louis, MO | 1/500 | Neural progenitors |
| CRX | Abnova Corporation, Taipei, Taiwan | 1/100 | Photoreceptor progenitor |
| ZO-1 | Invitrogen-Zymed Laboratories, South San Francisco, CA | 1/100 | RPE (tight junction) |
| Bestrophin | Novus Biologicals, Littleton, CO | 1/1000 | RPE |
| CRALBP | Abcam, Cambridge, UK | 1/200 | RPE |
Abbreviations: SSEA-4, stage-specific embryonic antigen-4; TRA-1-60, keratin sulfate- related antidgens-1-60; TRA-1-81, keratin sulfate-related antidgens-1-81; GFAP, glial fibrillary acidic protein; NF200, neurofilament 200 kDa; PAX6, paired box protein 6; CRX, cone-rod homeobox protein; CRALBP, cellular retinaldehyde-binding protein; PRE65, retinal pigment epithelium specific protein 65 kDa.
2.5. Microarray analysis
To further monitor the effects of the extracellular environment on the differentiation of stem cells and progenitor cells, we used microarray techniques to compare the gene expression profiles of the partially differentiated cells to one another and to the transcriptome of human retina and RPE. Human neural retina was prepared from the retinas of eyes of younger donors (National Disease Research Interchange (NDRI, Philadelphia, PA) aged from 20 to 40 years without evidence of eye disease; death to enucleation time was less than 10 h. The procedure was similar to harvesting Bruch’s membrane as described above except that the retina was retrieved by separating from human Bruch’s membrane (Tezel et al., 2004). Human embryonic stem cells, neural progenitors (on PA6) and ARPE19 cells were also harvested to perform the microarray analysis. Total RNA was isolated using a Qiagen RNeasy Mini Kit (Qiagen Co., Valencia, CA) as described previously (Cai et al., 2006). The gene expression profile was determined using the Affymetrix Human 133UA gene chip (Affymetrix Inc., Santa Clara, CA). Each chip contained 65,471 human gene probes and had 1090 neuron-related gene probes, and gene probes related to basic cell functions such as cell proliferation and differentiation. The advantages of using such an array included the large number of genes that were measured, and the absence of selection bias arising from the use of pathway-specific arrays containing only proliferation or apoptosis genes, for examples.
A T7-(dT)24 oligomer, superscript reverse transcriptase II and DNA polymerase I (Gibco BRL) were used for first-strand and second-strand cDNA synthesis using total RNA as templates. Double-stranded cDNA was cleaned with Phase Lock Gels–phenol/chloroform extraction and ethanol precipitation. Biotin-labeled antisense cRNA was produced by an in vitro transcription reaction (ENZO BioArray High Yield RNA Transcript Labeling Kit; Affymetrix Inc.) and incubated with fragmentation buffer (Tris–acetate, KOAc and MgOAcat; 94 °C for 35 min). Target hybridization, washing, staining, and scanning probe arrays were done following an Affymetrix GeneChip Expression Analysis Manual. Data was analyzed with Affymetrix Micro array Suite 5.0, and GeneSifter web-based microarray analysis system (VizX Labs, Seattle, WA).
3. Results
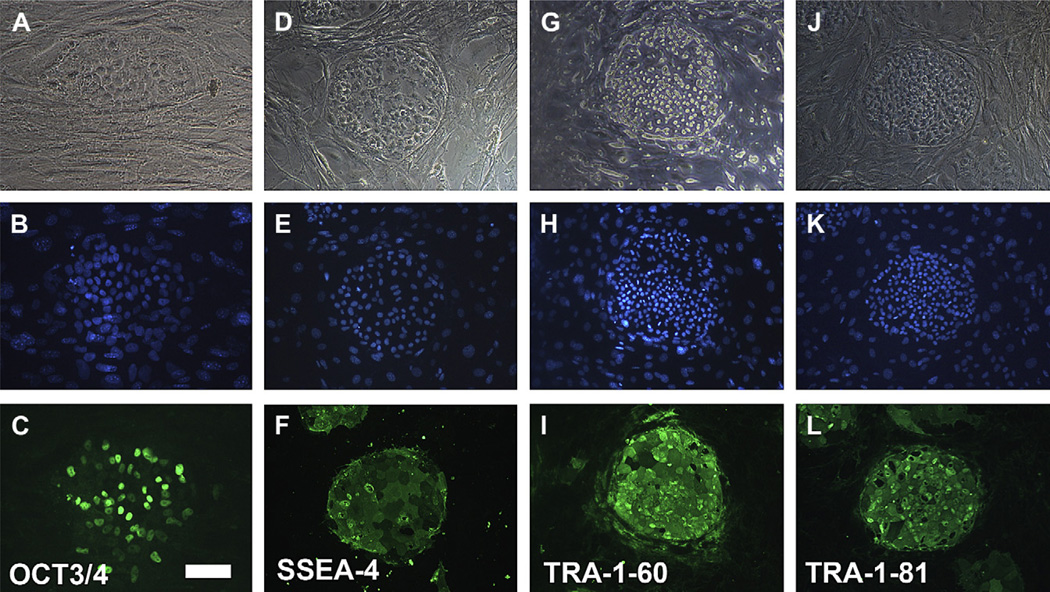
Immunocytochemistry was used to assess the expression of stem cell markers in the human embryonic stem cell line BG01V grown on mouse embryonic fibroblasts; these cells were positive for the pluripotent markers as OCT3/4, SSEA-4, TRA-1-60 and TRA-1-81 (Fig. 2). These hESC did not express neural progenitor markers β-tubulin III, MAP-2, astrocyte marker GFAP, neural filament 200 (NF200), vimentin, retinal progenitor marker Pax-6, or the RPE cell markers RPE65, CRALBP and Bestrophin as expected (Table 2).
Fig. 2.
Immunostaining of undifferentiated human embryonic stem cell line BG01V grown on mouse embryonic fibroblasts. Top row, phase-contrast micrographs; middle row, nuclear staining with DAPI; bottom row, immune fluorescence staining was positive for OCT 3/4 (C), SSEA-4 (F), TRA-1-60 (I) and TRA-1-81 (L). Bar = 100 µm.
Table 2.
Summary of marker expression studies
| Undifferentiated hESC markers | Neuroprogenitor markers | RPE markers | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| OCT 3/4 | SSEA-4 | TRA-1-60 | TRA-1-81 | β-Tubulin | GFAP | NF-200 | PAX6 | Vimentin | CRX | Bestrophin | CRALBP | RPE65 | ZO-1 | |
| hESC on feeder | + | + | + | + | − | − | − | − | − | − | − | − | ||
| hESC on PA6 | − | + | + | + | + | + | + | − | − | − | − | |||
| On ARPE 19 | + | + | + | + | ||||||||||
| On laminin | + | + | + | |||||||||||
| On Bruch’s | + | +/− | − | |||||||||||
| On Matrigel | − | − | + | |||||||||||
HESC on the feeder layer expressed undifferentiated hESC markers OCT3/4, SSEA-4, TRA-1-60 and TRA-1-81. HESC on PA6 cells expressed SSEA-4 but no longer expressed OCT 3/4; these cells then expressed neural progenitor markers β-tubulin, GFAP, NF-200, PAX6 and vimentin, but did not express CRX or the RPE markers Bestrophin, CRALBP or RPE65. After culture on ARPE19, neural progenitors continued to express neural progenitor markers β-tubulin, NF200 and vimentin and expressed the photoreceptor homeobox protein CRX. On laminin-coated dishes, neural progenitors continued to expressed β-tubulin, NF200 and PAX6 markers. On Bruch’s membrane, neural progenitors expressed RPE markers Bestrophin and sometimes expressed CRALBP but did not express RPE 65. On Matrigel-coated dishes neural progenitors expressed tight junction marker ZO-1 but did not express CRALBP or RPE65.
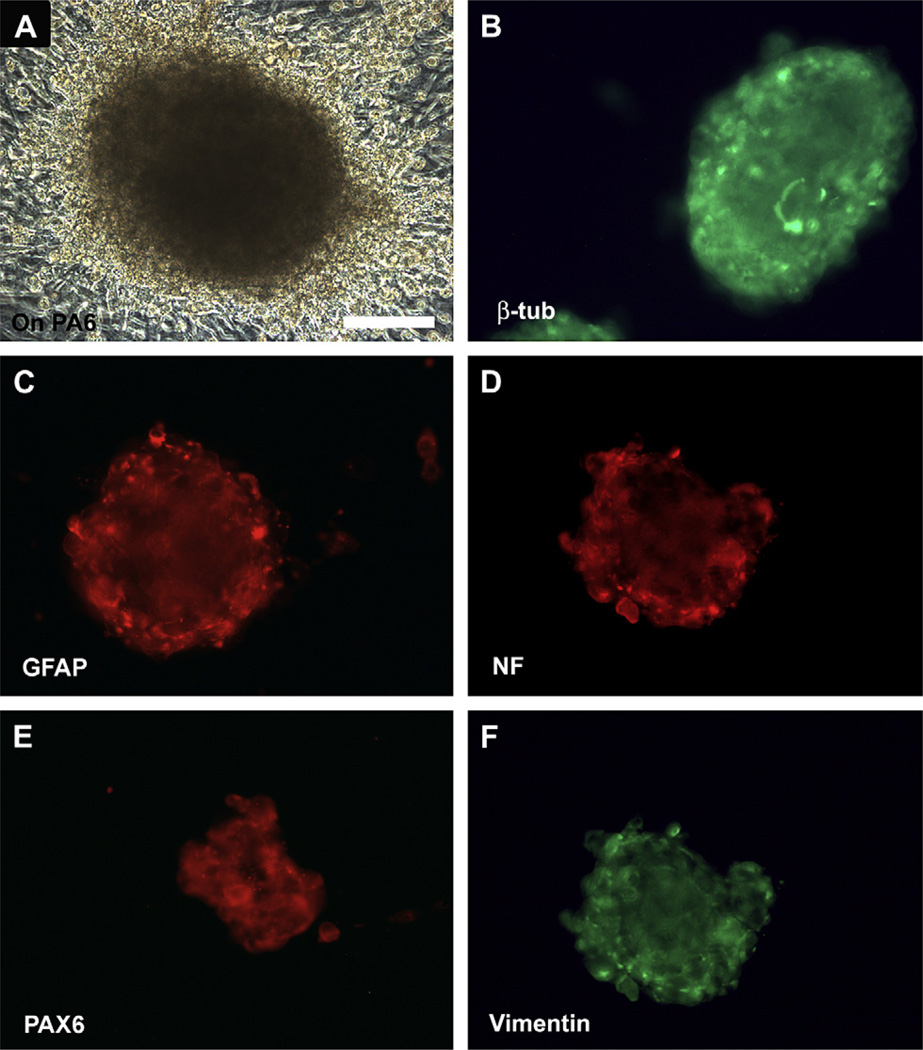
After culturing onto mouse PA6 cells, hESC became multilayered and formed pigmented spheres (Fig. 3A). These colonies contained a large population of cells that stained for β-tubulin III (>88% of the cells, n = 100 colonies), astrocyte marker GFAP, neural filament NF200 (>90%, n = 100 colonies), retinal progenitor marker Pax-6 (>88%, n = 100 colonies) and vimentin after culturing on PA6 cells for 13 days (Fig. 3B–F). These spheres were negative for the RPE cell markers as RPE65, CRALBP or Bestrophin (Table 2).
Fig. 3.
Expression of neural progenitor markers after culturing human embryonic stem cells on mouse PA6. (A) Human embryonic stem cells became multilayered and formed pigmented spheres after culturing on mouse PA6 cells for 13 days. Immunofluorescence staining of the spheres demonstrated the presence of several neural progenitor markers including β-tubulin III (>88%) (B), GFAP (C), neural filament NF200 (>90%) (D), PAX6 (>88%) (E) and vimentin (F). Bar = 100 µm.
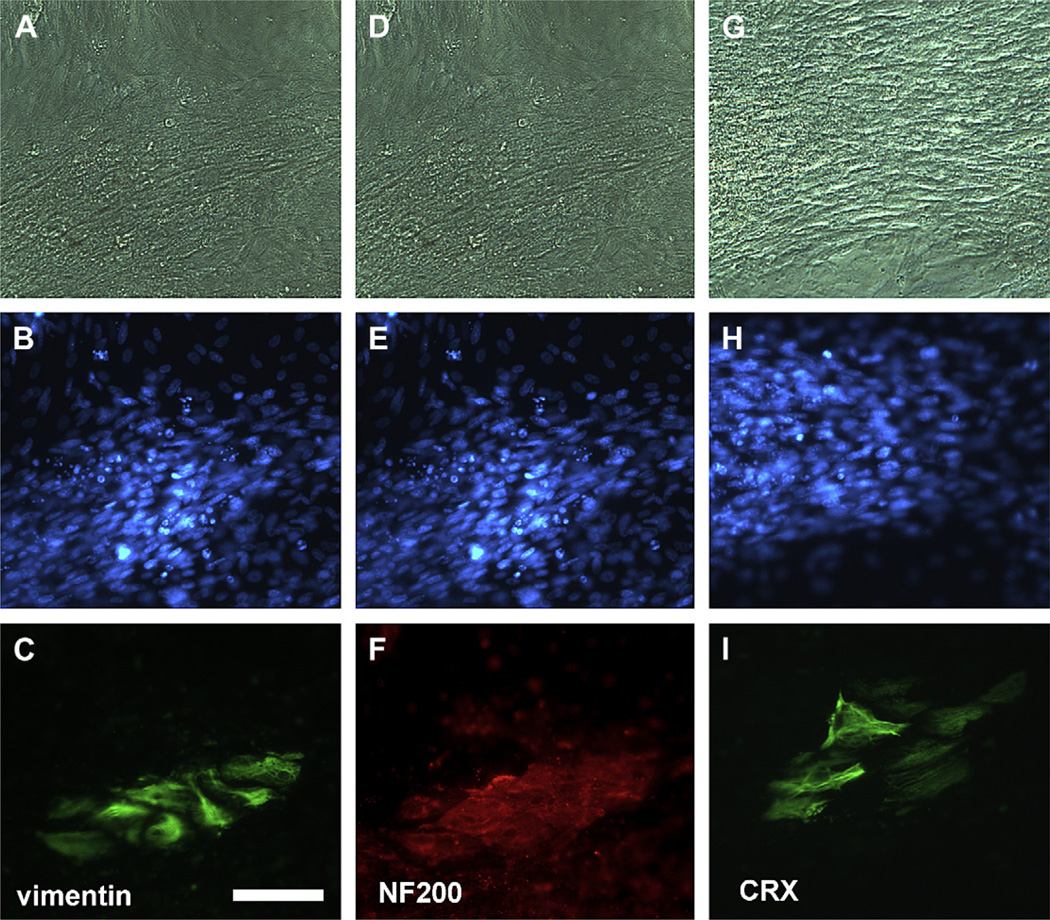
PA6-induced neural progenitors cultured on ARPE19 for 10 days continued to express neural markers vimentin, which stains Müller cells, and NF200, which stains retinal ganglion cells (Fig. 4). In addition, neural progenitors seeded onto ARPE19 also expressed photoreceptor specific homeobox protein CRX (Fig. 4G–I), which is essential during early photoreceptor development. Markers of mature retinal cells, including rhodopsin for rod photoreceptor, opsin blue for cone photoreceptor and PKC-α for rod bipolar cells were immunonegative in the culture (data not shown).
Fig. 4.
Generation of retinal precursors from neural progenitors after culturing on ARPE19 cells for 10 days. Top row, phase-contrast micrographs; middle row, nuclei in both ARPE19 and progenitors stained with DAPI. Progenitors expressed neural progenitor marker vimentin (C) and neural filament 200 (F) and photoreceptor-specific protein CRX (I) which is essential during early photoreceptor development. Bar = 50 µm.
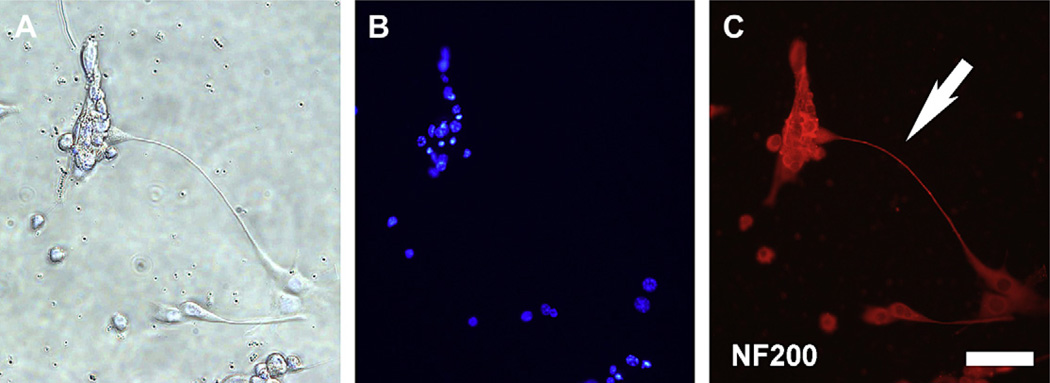
A neuronal phenotype was observed in neural progenitors cultured onto laminin-coated dish for 2 days, with staining of the neurites for neural filament 200 (Fig. 5); this is an important neuronal phenotype for establishment of polarity and the formation of synaptic connection.
Fig. 5.
Neuronal phenotype formation after culturing neural progenitors on laminin-coated dishes for 2 days. (A) Phase contrast. (B) DAPI nuclear stain. (C) Neurite outgrowth seen with staining for neural filament 200 (NF200) (arrow). Bar = 50 µm.
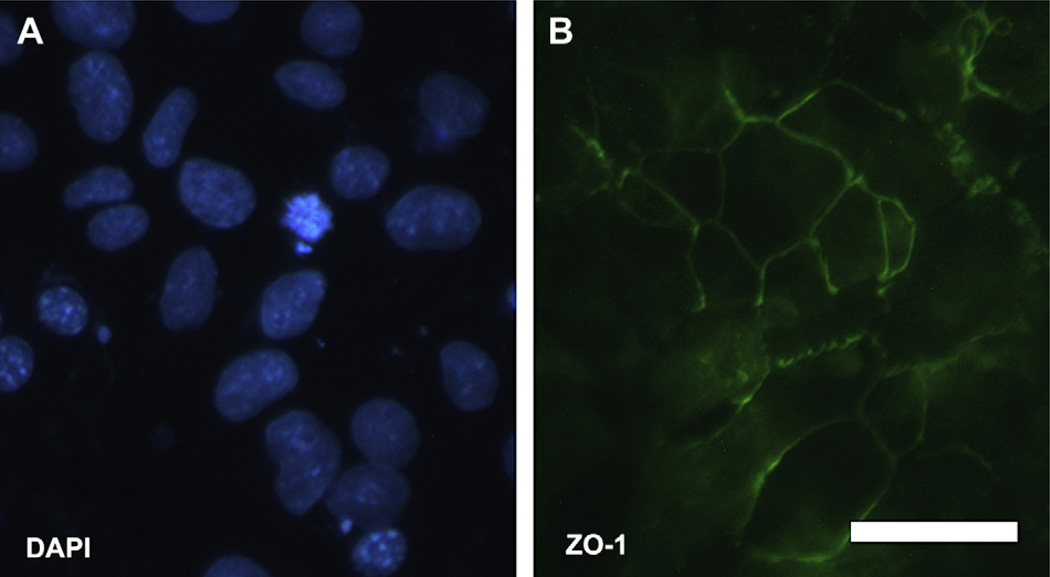
Neural progenitors attached and expanded quickly on Matrigel-coated dishes and reached confluence by day 21. These cells expressed RPE tight junction marker ZO-1 (Fig. 6) but were immunonegative for the retinal pigment epithelium markers RPE65 or CRALBP (Table 2).
Fig. 6.
Induction of ZO-1 immune positive cells from neural progenitors after culturing on Matrigel-coated dishes for 21 days. (A) Nuclei in cells stained with DAPI. (B) Cells cultured on Matrigel expressed ZO-1 protein, which is a tight junction marker of epithelium cells. Bar = 20 µm.
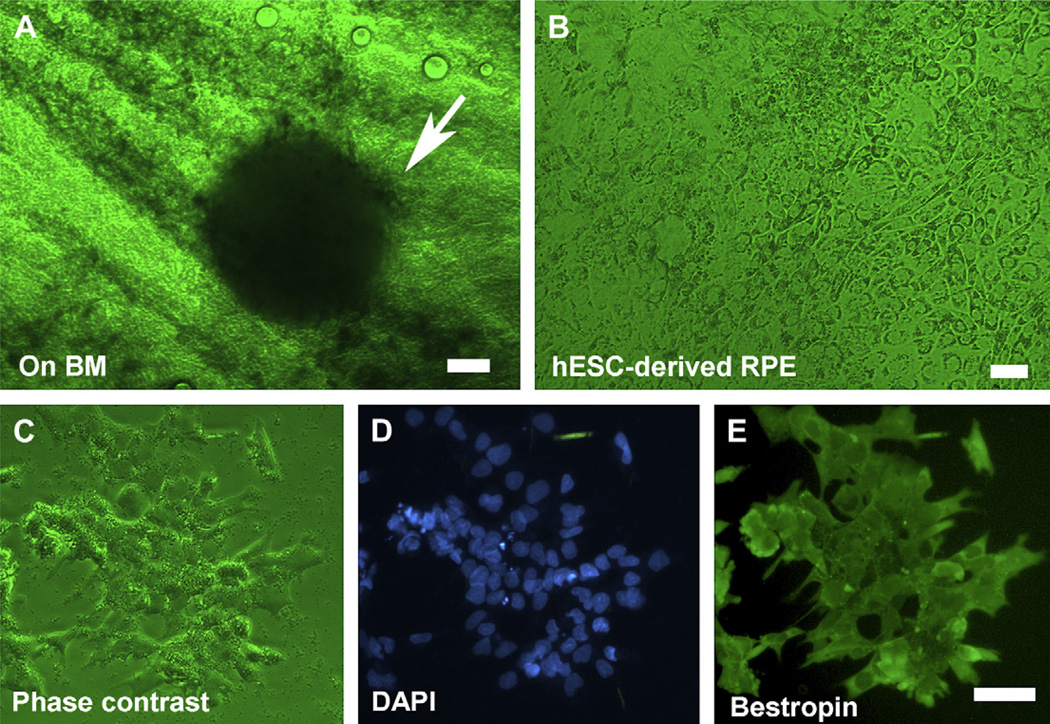
Clusters of pigmented hESC were observed 4 days after seeding onto human Bruch’s membrane explants and showed pigment epithelium-like cells after 15 days (Fig. 7A,B). These cells were immunopositive for Bestrophin protein, which is a RPE cell marker (Fig. 7C–E), but negative for RPE65 (Table 2).
Fig. 7.
Induction of RPE markers in human embryonic stem cells cultured on human Bruch’s membrane. (A) A cluster of pigmented human embryonic stem cells 4 days after growing on human Bruch’s membrane explants (arrow). (B) Phase-contrast micrograph of flattened pigmented epithelium-like cells on human Bruch’s membrane. (C)–(E) hESC-derived RPE under phase contrast, DAPI and Bestrophin protein staining respectively. Bar = 50 µm in (A), (C)–(E); bar = 20 µm in (B).
DNA microarray analysis data showed that when human embryonic stem cells were cultured on PA6 cells the resulting progenitor cells expressed 117 new genes, including 22 genes expressed in either human retina and/or RPE cells (Table 3). The functions of these genes were diverse but were related to cell proliferation, nervous system development and cell adhesion. One of those genes, vitronectin, is a ubiquitous extracellular protein involved in cell adhesion.
Table 3.
Neural retinal and/or RPE genes expressed within human embryo stem cells induced by cultured on PA6 cells
| Probe set ID | Gene title | Gene symbol | GO biological process description | Present in |
|---|---|---|---|---|
| 204534_at | Vitronectin | VTN | Cell adhesion; cell-matrix adhesion | Retina |
| 206825_at | Oxytocin receptor | OXTR | GPCRDB Class A Rhodopsin-like; phospholipase C activating | Both |
| 209420_s_at | Sphingomyelin phosphodiesterase 1 | SMPD1 | signal transduction; nervous system development | Both |
| 211896_s_at | Decorin | DCN | Organ morphogenesis; integral to membrane | Retina |
| 212494_at | Tensin-like C1 domain containing phosphatase (tensin 2) | TENC1 | Intracellular singing cascade; Zinc ion binding; diacylglycerol binding | Retina |
| 212956_at | TBC1 domain family, member 9 (with GRAM domain) | TBC1D9 | Regulation of Rab GTPase activity | Both |
| 213540_at | Hydroxysteroid (17-beta) dehydrogenase 8 | HSD17B8 | Steroid biosynthetic process; lipid biosynthetic process | Both |
| 217522_at | Potassium channel, subfamily V, member 2 | KCNV2 | Ion transport; visual perception; response to stimulae | Retina |
| 218501_at | Rho guanine nucleotide exchange factor (GEF) 3 | ARHGEF3 | Rho protein signal transduction | Both |
| 227178_at | CUG triplet repeat, RNA binding protein 2 | CUGBP2 | RNA processing; neuromuscular junction development | Both |
| 228268_at | Flavin containing monooxygenase 2 (non-functional) | FMO2 | Oxygen and reactive oxygen species metabolic process | Retina |
| 230242_at | Neurofascin homolog (chicken) | NFASC | Cell adhesion; proteinaceous extracellular matrix | Both |
| 230475_at | Similar to RIKEN cDNA 6030419C18 gene | LOC388135 | Unknown | Retina |
| 233333_X_at | Advillin | AVIL | Cytoskeleton organization and biogenesis; nervous system development | Both |
| 243504_at | Transcribed locus | – | Unknown | Retina |
| 1555968_a_at | CDNA FLJ30424, similar to ZINC FINGER PROTEIN 195 | – | Unknown | Both |
| 1556281_at | Full length insert cDNA clone YI54A07 | YI54A07 | Unknown | Both |
| 1556464_a_at | Hypothetical protein LOC257407 | LOC257407 | Unknown | Retina |
| 1558401_at | CDNA FLJ37332 fis, clone BRAMY2019710 | – | Unknown | Retina |
| 1560792_at | CDNA FLJ37191 fis, clone BRALZ2003453 | FLJ37191 | Unknown | RPE |
| 1569078_at | Ring finger protein 213 | RNF213 | Nucleotide, protein AND zinc ion binding: nucleoside-triphosphatase activity | Retina |
| 1570155_at | Homo sapiens, clone IMAGE:4427279, mRNA | – | Unknown | RPE |
Culturing hESC on PA6 cells induced the expression of 22 new genes that were also expressed in neural retina and RPE. The functions of these 22 genes were diverse but were related to cell proliferation, nervous system development and cell adhesion.
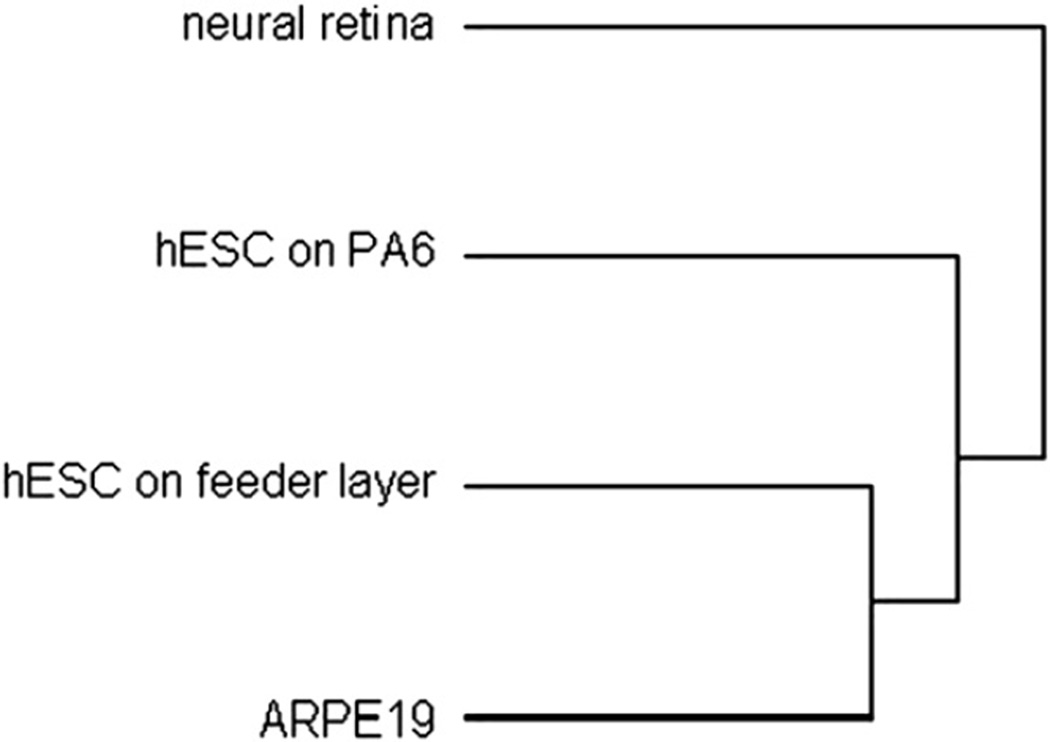
Hierarchical clustering analysis (Fig. 8) showed that RPE and hESC maintained on a feeder layer cluster together. HESC cultured on PA6 (i.e., neural progenitors) had a transcriptome that was more closely related to adult human retinal samples. This suggested that when cultured on PA6 stroma cells, hESC became progenital cells which had the genetic characteristics towards differentiating into neural retina.
Fig. 8.
Hierarchical clustering analysis of DNA microarray study. Dendrogram showed the gene expression profile of hESC maintained on a feeder layer was most similar to that of ARPE19. Culturing on PA6 shifted the transcriptome of hESC towards neural retina.
4. Discussion
Our data demonstrates that elements of the extracellular environment normally present in the subretinal space and adjacent tissues, such as RPE and Bruch’s membrane, have a significant effect on the differentiation of hESC along retinal or RPE progenitor cell lines, respectively. Human Bruch’s membrane and Matrigel, which is a solubilized basement membrane preparation extracted from a mouse sarcoma line that has been used as a substitution for basement membrane (Albini et al., 1992; Taniguchi et al., 1989; Terranova et al., 1986), can induce expression of RPE markers. In vivo the outer retina is in contact with the retinal pigment epithelium, which is a pigmented monolayer that is responsible for re-isomerization of visual pigments, maintaining the integrity of the outer blood–retinal barrier and phagocytosis of outer segments. In addition, numerous authors have shown that an intact RPE is necessary to maintain the perfusion of the underlying choriocapillaris. Surgical removal of the retinal pigment epithelial with choroidal neovascular membranes in humans (Akduman et al., 1997; Castellarin et al., 1997, 1998; Del Priore et al., 1995; Del Priore and Kaplan, 1995; Del Priore et al., 1996; Desai et al., 1995; Henkind and Gartner, 1983; Ivert et al., 2003; Korte, 1989; Korte et al., 1984, 1986; Leonard et al., 1997; Nasir et al., 1997; Pollack et al., 1996; Tezel et al., 2006; Valentino et al., 1995); pharmacological destruction of the RPE with intravitreal or intravenous sodium iodate leads to RPE damage followed by secondary choriocapillaris atrophy (Henkind and Gartner, 1983; Ivert et al., 2003; Korte, 1989; Korte et al., 1984, 1986). Our study demonstrated that human ARPE19 cells were effective at inducing differentiation of hESC into retinal progenitor cells in vitro (Fig. 4). During eye development, RPE and neural retina share the same origin, namely, the optic vesicle. The notion that extracellular matrix or cells can induce differentiation of hESC into the adjacent cell layer is appealing simple from a teleological point of view (Cepko, 1999; Jadhav et al., 2006).
To date several authors have determined the effects of other cells and/or extracellular matrix on hESC differentiation. Culturing primate embryonic stem cells on mouse skull stromal cells (PA6 cells) induces differentiation into dopaminergic neurons and RPE (Haruta et al., 2004; Kawasaki et al., 2000, 2002; Morizane et al., 2002, 2006; Parmar and Li, 2007; Sasai, 2005). Interestingly, this neuronal-inducing activity is also present on paraformaldehyde-fixed PA6 cells (Kawasaki et al., 2000), suggesting that matrix of PA6 cells is necessary and sufficient for induction. Those hESC-derived RPE cells express specific RPE cell markers, phagocytose latex beads and show visual rescue after transplantation into the Royal College of Surgeons rat, which has a known defect in phagocytosis (Haruta et al., 2004). Mouse and human ESC in serum-free medium could differentiate into RPE after culturing on bare (for mouse) or laminin-coated (for human) amniotic membrane (Ueno et al., 2006). Putative RPE could be isolated from spontaneously differentiating hESC grown on gelatin-coated matrix (Klimanskaya et al., 2004). In all of these studies only a small percentage of the stem cells expressed RPE markers, thus necessitating harvesting of RPE-like clumps prior to expansion of the population.
In human eyes in vivo, the native RPE is attached to the inner aspects of Bruch’s membrane, which contains 5 anatomic layers rich in extracellular matrix components that include collagen IV, laminin, fibronectin and vitronectin. After 3 weeks on human Bruch’s membrane, hESC became pigmented and expressed Bestrophin protein (Fig. 7). To induce retinal neurons, RPE appears a good candidate for neural inducing activity because of the anatomic relationship between RPE and photoreceptor outer segments in the vertebrate eye and the importance of the interrelationship of these cells for phagocytosis (Finnemann et al., 1997). Pigment epithelium-derived factor (PEDF), which is released by the RPE, can also support retinal neuronal growth (Jablonski et al., 2000), thus demonstrating that trophic factors, in addition to cell–cell contact, play an important role in the differentiation of hESCs. HESCs receive messages from adjacent cells via gap junctions and receptor/ligand interactions between adjacent cells (Kehat et al., 2002; Krause, 2002). Other authors have demonstrated that a cocktail containing noggin, DKK1 (a secreted antagonist of the Wnt/β-catenin signaling pathway) and IGF-1 or combination of DKK1, lefty, FCS and activin can induce the differentiation of stem cells into retinal neurons (Ikeda et al., 2005; Lamba et al., 2006; MacLaren et al., 2006). The fact that expression of certain growth factors facilitates neuronal differentiation could allow us to determine specific matrix–ligand interactions responsible for differentiation of hESC into RPE or photoreceptor progenitors.
We have shown that hESC cultured on Bruch’s membrane expressed the RPE-specific protein Bestrophin and appeared structurally similar to RPE in situ, as they were organized in a monolayer and showed pigmentation (Fig. 7). We detected the presence of the tight junction protein ZO-1 in hESC cultured on Matrigel-coated dishes, as reported previously (Haruta et al., 2004), but not RPE65. Although RPE65 is an RPE-specific protein essential to maintain normal function of photoreceptors and a key protein for regenerating 11-cis-retinal in the RPE (Fan et al., 2003), RPE65 was not specifically detected by others in hESC-derived RPE by immune staining (Haruta et al., 2004; Klimanskaya et al., 2004). Some authors have demonstrated that low levels of RPE65 mRNA could be detected by RT-PCR in putative RPE generated from hESC (Klimanskaya et al., 2004).
There was extensive overlap between the gene expression profile of neural progenitors grown on PA6 cells and neural retina. Some specific genes, such as vitronectin, were absent in undifferentiated hESC but present in both human retina and neural progenitors. Vitronectin is a secreted glycoprotein that is present in blood plasma and in the extracellular matrix of many tissues during development, including brain. Vitronectin mRNA is detected in the neural retina, including photoreceptor cells, retinal ganglion cells and in the RPE (Anderson et al., 1999; Cai and Del Priore, 2006). Functionally, vitronectin regulates cell differentiation, neuronal survival, and neurite outgrowth in the developing chicken retina (Martinez-Morales et al., 1995). Vitronectin can bind integrins on the cell surface through the RGD sequence, thus connecting cells to matrix proteins. In addition, vitronectin also has regulatory roles in complement-mediated cell lysis, fibrinolysis, thrombosis, inflammation, and phagocytosis (Miceli et al., 1997; Nandrot et al., 2007). The integrin αvβ5 vitronectin receptor is also expressed on the apical membrane of human RPE. This vitronectin receptor participates in the binding of photoreceptor rod outer segment during phagocytosis by cultured human RPE (Finnemann et al., 1997). An increase of vitronectin in neural progenitors may be important to the development of hESC into neural retinal photoreceptors, as it is important to subsequent phagocytosis of outer segments by the RPE.
In conclusion, this study demonstrated that neighboring cells or extracellular matrix were able to induce hESC along photoreceptor or RPE progenitor cell lines, potentially allowing for the enrichment of populations of differentiated progenitors. Further work is needed prior to the use of hESC for the treatment of retinal degenerations.
Acknowledgements
This work was supported by the Foundation Fighting Blindness, the Hickey Foundation, Robert L. Burch III Fund, the Macula Foundation, and unrestricted funds from Research to Prevent Blindness.
References
- Akduman L, Del Priore LV, Desai VN, Olk RJ, Kaplan HJ. Perfusion of the subfoveal choriocapillaris affects visual recovery after submacular surgery in presumed ocular histoplasmosis syndrome. Am. J. Ophthalmol. 1997;123:90–96. doi: 10.1016/s0002-9394(14)70997-3. [DOI] [PubMed] [Google Scholar]
- Albini A, Melchiori A, Garofalo A, Noonan DM, Basolo F, Taraboletti G, et al. Matrigel promotes retinoblastoma cell growth in vitro and in vivo. Int. J. Cancer. 1992;52:234–240. doi: 10.1002/ijc.2910520214. [DOI] [PubMed] [Google Scholar]
- Anderson DH, Hageman GS, Mullins RF, Neitz M, Neitz J, Ozaki S, et al. Vitronectin gene expression in the adult human retina. Invest. Ophthalmol. Vis. Sci. 1999;40:3305–3315. [PubMed] [Google Scholar]
- Bissell MJ, Barcellos-Hoff MH. The influence of extracellular matrix on gene expression: is structure the message? J. Cell Sci. Suppl. 1987;8:327–343. doi: 10.1242/jcs.1987.supplement_8.18. [DOI] [PubMed] [Google Scholar]
- Cai H, Del Priore LV. Bruch membrane aging alters the gene expression profile of human retinal pigment epithelium. Curr. Eye Res. 2006;31:181–189. doi: 10.1080/02713680500514628. [DOI] [PubMed] [Google Scholar]
- Cai H, Shin MC, Tezel TH, Kaplan HJ, Del Priore LV. Use of iris pigment epithelium to replace retinal pigment epithelium in age-related macular degeneration: a gene expression analysis. Arch. Ophthalmol. 2006;124:1276–1285. doi: 10.1001/archopht.124.9.1276. [DOI] [PubMed] [Google Scholar]
- Castellarin AA, Sugino IK, Nasir M, Zarbin MA. Clinicopathological correlation of an excised choroidal neovascular membrane in pseudotumour cerebri. Br. J. Ophthalmol. 1997;81:994–1000. doi: 10.1136/bjo.81.11.994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castellarin AA, Nasir M, Sugino IK, Zarbin MA. Progressive presumed choriocapillaris atrophy after surgery for age-related macular degeneration. Retina. 1998;18:143–149. doi: 10.1097/00006982-199818020-00008. [DOI] [PubMed] [Google Scholar]
- Cepko CL. The roles of intrinsic and extrinsic cues and bHLH genes in the determination of retinal cell fates. Curr. Opin. Neurobiol. 1999;9:37–46. doi: 10.1016/s0959-4388(99)80005-1. [DOI] [PubMed] [Google Scholar]
- Comoglio PM, Boccaccio C, Trusolino L. Interactions between growth factor receptors and adhesion molecules: breaking the rules. Curr. Opin. Cell Biol. 2003;15:565–571. doi: 10.1016/s0955-0674(03)00096-6. [DOI] [PubMed] [Google Scholar]
- Del Priore LV, Kaplan HJ. Pathogenesis of AMD. Ophthalmology. 1995;102:1125–1126. doi: 10.1016/s0161-6420(95)30903-7. [DOI] [PubMed] [Google Scholar]
- Del Priore LV, Hornbeck R, Kaplan HJ, Jones Z, Valentino TL, Mosinger-Ogilvie J, et al. Debridement of the pig retinal pigment epithelium in vivo. Arch. Ophthalmol. 1995;113:939–944. doi: 10.1001/archopht.1995.01100070113034. [DOI] [PubMed] [Google Scholar]
- Del Priore LV, Kaplan HJ, Hornbeck R, Jones Z, Swinn M. Retinal pigment epithelial debridement as a model for the pathogenesis and treatment of macular degeneration. Am. J. Ophthalmol. 1996;122:629–643. doi: 10.1016/s0002-9394(14)70481-7. [DOI] [PubMed] [Google Scholar]
- Desai VN, Del Priore LV, Kaplan HJ. Choriocapillaris atrophy after submacular surgery in presumed ocular histoplasmosis syndrome. Arch. Ophthalmol. 1995;113:408–409. doi: 10.1001/archopht.1995.01100040023014. [DOI] [PubMed] [Google Scholar]
- Fan J, Rohrer B, Moiseyev G, Ma JX, Crouch RK. Isorhodopsin rather than rhodopsin mediates rod function in RPE65 knock-out mice. Proc. Natl. Acad. Sci. USA. 2003;100:13662–13667. doi: 10.1073/pnas.2234461100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finnemann SC, Bonilha VL, Marmorstein AD, Rodriguez-Boulan E. Phagocytosis of rod outer segments by retinal pigment epithelial cells requires alpha(v)beta5 integrin for binding but not for internalization. Proc. Natl. Acad. Sci. USA. 1997;94:12932–12937. doi: 10.1073/pnas.94.24.12932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haruta M, Sasai Y, Kawasaki H, Amemiya K, Ooto S, Kitada M, et al. In vitro and in vivo characterization of pigment epithelial cells differentiated from primate embryonic stem cells. Invest. Ophthalmol. Vis. Sci. 2004;45:1020–1025. doi: 10.1167/iovs.03-1034. [DOI] [PubMed] [Google Scholar]
- Henkind P, Gartner S. The relationship between retinal pigment epithelium and the choriocapillaris. Trans. Ophthalmol. Soc. UK. 1983;103(Pt 4):444–447. [PubMed] [Google Scholar]
- Horsford DJ, Nguyen MT, Sellar GC, Kothary R, Arnheiter H, McInnes RR. Chx10 repression of Mitf is required for the maintenance of mammalian neuroretinal identity. Development. 2005;132:177–187. doi: 10.1242/dev.01571. [DOI] [PubMed] [Google Scholar]
- Ikeda H, Osakada F, Watanabe K, Mizuseki K, Haraguchi T, Miyoshi H, et al. Generation of Rx+/Pax6+ neural retinal precursors from embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2005;102:11331–11336. doi: 10.1073/pnas.0500010102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ivert L, Kong J, Gouras P. Changes in the choroidal circulation of rabbit following RPE removal. Graefes Arch. Clin. Exp. Ophthalmol. 2003;241:656–666. doi: 10.1007/s00417-003-0653-5. [DOI] [PubMed] [Google Scholar]
- Jablonski MM, Tombran-Tink J, Mrazek DA, Iannaccone A. Pigment epithelium-derived factor supports normal development of photoreceptor neurons and opsin expression after retinal pigment epithelium removal. J. Neurosci. 2000;20:7149–7157. doi: 10.1523/JNEUROSCI.20-19-07149.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav AP, Mason HA, Cepko CL. Notch 1 inhibits photoreceptor production in the developing mammalian retina. Development. 2006;133:913–923. doi: 10.1242/dev.02245. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Mizuseki K, Nishikawa S, Kaneko S, Kuwana Y, Nakanishi S, et al. Induction of midbrain dopaminergic neurons from ES cells by stromal cell-derived inducing activity. Neuron. 2000;28:31–40. doi: 10.1016/s0896-6273(00)00083-0. [DOI] [PubMed] [Google Scholar]
- Kawasaki H, Suemori H, Mizuseki K, Watanabe K, Urano F, Ichinose H, et al. Generation of dopaminergic neurons and pigmented epithelia from primate ES cells by stromal cell-derived inducing activity. Proc. Natl. Acad. Sci. USA. 2002;99:1580–1585. doi: 10.1073/pnas.032662199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehat I, Gepstein A, Spira A, Itskovitz-Eldor J, Gepstein L. High-resolution electrophysiological assessment of human embryonic stem cell-derived cardiomyocytes: a novel in vitro model for the study of conduction. Circ. Res. 2002;91:659–661. doi: 10.1161/01.res.0000039084.30342.9b. [DOI] [PubMed] [Google Scholar]
- Klimanskaya I, Hipp J, Rezai KA, West M, Atala A, Lanza R. Derivation and comparative assessment of retinal pigment epithelium from human embryonic stem cells using transcriptomics. Cloning Stem Cells. 2004;6:217–245. doi: 10.1089/clo.2004.6.217. [DOI] [PubMed] [Google Scholar]
- Korte GE. Choriocapillaris regeneration in the rabbit. Ultrastructure of new endothelial tube formation. Invest. Ophthalmol. Vis. Sci. 1989;30:1938–1950. [PubMed] [Google Scholar]
- Korte GE, Reppucci V, Henkind P. RPE destruction causes choriocapillary atrophy. Invest. Ophthalmol. Vis. Sci. 1984;25:1135–1145. [PubMed] [Google Scholar]
- Korte GE, Gerszberg T, Pua F, Henkind P. Choriocapillaris atrophy after experimental destruction of the retinal pigment epithelium in the rat. A study in thin sections and vascular casts. Acta Anat. (Basel) 1986;127:171–175. doi: 10.1159/000146277. [DOI] [PubMed] [Google Scholar]
- Krause DS. Regulation of hematopoietic stem cell fate. Oncogene. 2002;21:3262–3269. doi: 10.1038/sj.onc.1205316. [DOI] [PubMed] [Google Scholar]
- Lamba DA, Karl MO, Ware CB, Reh TA. Efficient generation of retinal progenitor cells from human embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2006;103:12769–12774. doi: 10.1073/pnas.0601990103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Layer PG, Rothermel A, Willbold E. Inductive effects of the retinal pigmented epithelium (RPE) on histogenesis of the avian retina as revealed by retinospheroid technology. Semin. Cell Dev. Biol. 1998;9:257–262. doi: 10.1006/scdb.1998.0234. [DOI] [PubMed] [Google Scholar]
- Leonard DS, Zhang XG, Panozzo G, Sugino IK, Zarbin MA. Clinicopathologic correlation of localized retinal pigment epithelium debridement. Invest. Ophthalmol. Vis. Sci. 1997;38:1094–1109. [PubMed] [Google Scholar]
- MacLaren RE, Pearson RA, MacNeil A, Douglas RH, Salt TE, Akimoto M, et al. Retinal repair by transplantation of photoreceptor precursors. Nature. 2006;444:203–207. doi: 10.1038/nature05161. [DOI] [PubMed] [Google Scholar]
- Martinez-Morales JR, Marti E, Frade JM, Rodriguez-Tebar A. Developmentally regulated vitronectin influences cell differentiation, neuron survival and process outgrowth in the developing chicken retina. Neuroscience. 1995;68:245–253. doi: 10.1016/0306-4522(95)00089-2. [DOI] [PubMed] [Google Scholar]
- Meredith JE, Jr, Winitz S, Lewis JM, Hess S, Ren XD, Renshaw MW, et al. The regulation of growth and intracellular signaling by integrins. Endocr. Rev. 1996;17:207–220. doi: 10.1210/edrv-17-3-207. [DOI] [PubMed] [Google Scholar]
- Miceli MV, Newsome DA, Tate DJ., Jr Vitronectin is responsible for serum-stimulated uptake of rod outer segments by cultured retinal pigment epithelial cells. Invest. Ophthalmol. Vis. Sci. 1997;38:1588–1597. [PubMed] [Google Scholar]
- Morizane A, Takahashi J, Takagi Y, Sasai Y, Hashimoto N. Optimal conditions for in vivo induction of dopaminergic neurons from embryonic stem cells through stromal cell-derived inducing activity. J. Neurosci. Res. 2002;69:934–939. doi: 10.1002/jnr.10363. [DOI] [PubMed] [Google Scholar]
- Morizane A, Takahashi J, Shinoyama M, Ideguchi M, Takagi Y, Fukuda H, et al. Generation of graftable dopaminergic neuron progenitors from mouse ES cells by a combination of coculture and neurosphere methods. J. Neurosci. Res. 2006;83:1015–1027. doi: 10.1002/jnr.20799. [DOI] [PubMed] [Google Scholar]
- Motohashi T, Aoki H, Yoshimura N, Kunisada T. Induction of melanocytes from embryonic stem cells and their therapeutic potential. Pigment Cell Res. 2006;19:284–289. doi: 10.1111/j.1600-0749.2006.00317.x. [DOI] [PubMed] [Google Scholar]
- Nandrot EF, Anand M, Almeida D, Atabai K, Sheppard D, Finnemann SC. Essential role for MFG-E8 as ligand for alphavbeta5 integrin in diurnal retinal phagocytosis. Proc. Natl. Acad. Sci. USA. 2007;104:12005–12010. doi: 10.1073/pnas.0704756104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nasir MA, Sugino I, Zarbin MA. Decreased choriocapillaris perfusion following surgical excision of choroidal neovascular membranes in age-related macular degeneration. Br. J. Ophthalmol. 1997;81:481–489. doi: 10.1136/bjo.81.6.481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng TF, Lavik E, Keino H, Taylor AW, Langer RS, Young MJ. Creating an immune-privileged site using retinal progenitor cells and biodegradable polymers. Stem Cells. 2007;25:1552–1559. doi: 10.1634/stemcells.2006-0780. [DOI] [PubMed] [Google Scholar]
- Parmar M, Li M. Early specification of dopaminergic phenotype during ES cell differentiation. BMC Dev. Biol. 2007;7:86. doi: 10.1186/1471-213X-7-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Planque N, Raposo G, Leconte L, Anezo O, Martin P, Saule S. Microphthalmia transcription factor induces both retinal pigmented epithelium and neural crest melanocytes from neuroretina cells. J. Biol. Chem. 2004;279:41911–41917. doi: 10.1074/jbc.M404964200. [DOI] [PubMed] [Google Scholar]
- Pollack JS, Del Priore LV, Smith ME, Feiner MA, Kaplan HJ. Post-operative abnormalities of the choriocapillaris in exudative age-related macular degeneration. Br. J. Ophthalmol. 1996;80:314–318. doi: 10.1136/bjo.80.4.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond SM, Jackson IJ. The retinal pigmented epithelium is required for development and maintenance of the mouse neural retina. Curr. Biol. 1995;5:1286–1295. doi: 10.1016/s0960-9822(95)00255-7. [DOI] [PubMed] [Google Scholar]
- Sasai Y. Directed differentiation of neural and sensory tissues from embryonic stem cells in vitro. Ernst Schering Research Foundation Workshop. 2005:101–109. doi: 10.1007/3-540-37644-5_7. [DOI] [PubMed] [Google Scholar]
- Taniguchi S, Tatsuka M, Nakamatsu K, Inoue M, Sadano H, Okazaki H, et al. High invasiveness associated with augmentation of motility in a fos-transferred highly metastatic rat 3Y1 cell line. Cancer Res. 1989;49:6738–6744. [PubMed] [Google Scholar]
- Terranova VP, Hujanen ES, Loeb DM, Martin GR, Thornburg L, Glushko V. Use of a reconstituted basement membrane to measure cell invasiveness and select for highly invasive tumor cells. Proc. Natl. Acad. Sci. USA. 1986;83:465–469. doi: 10.1073/pnas.83.2.465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tezel TH, Del Priore LV, Kaplan HJ. Reengineering of aged Bruch’s membrane to enhance retinal pigment epithelium repopulation. Invest. Ophthalmol. Vis. Sci. 2004;45:3337–3348. doi: 10.1167/iovs.04-0193. [DOI] [PubMed] [Google Scholar]
- Tezel TH, Geng L, Kaplan HJ, Del Priore LV. Retinal pigment epithelium rescues vascular endothelium from retinoic acid-induced apoptosis. Invest. Ophthalmol. Vis. Sci. 2006;47:5075–5087. doi: 10.1167/iovs.05-1557. [DOI] [PubMed] [Google Scholar]
- Ueno M, Matsumura M, Watanabe K, Nakamura T, Osakada F, Takahashi M, et al. Neural conversion of ES cells by an inductive activity on human amniotic membrane matrix. Proc. Natl. Acad. Sci. USA. 2006;103:9554–9559. doi: 10.1073/pnas.0600104103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valentino TL, Kaplan HJ, Del Priore LV, Fang SR, Berger A, Silverman MS. Retinal pigment epithelial repopulation in monkeys after submacular surgery. Arch. Ophthalmol. 1995;113:932–938. doi: 10.1001/archopht.1995.01100070106033. [DOI] [PubMed] [Google Scholar]