Abstract
IMPORTANCE
New funduscopic findings in patients with enhanced S-cone syndrome (ESCS) may help clinicians in diagnosing this rare autosomal recessive retinal dystrophy.
OBJECTIVE
To expand the clinical spectrum of ESCS due to mutations in the NR2E3 gene.
DESIGN
Retrospective, noncomparative case series of 31 patients examined between 1983 and 2012.
SETTING
Academic and private ophthalmology practices specialized in retinal dystrophies.
PARTICIPANTS
A cohort of patients diagnosed with ESCS and harboring known NR2E3 mutations.
INTERVENTION
Patients had ophthalmic examinations including visual function testing that led to the original diagnosis.
MAIN OUTCOMES AND MEASURES
New fundus features captured with imaging modalities.
RESULTS
New clinical observations in ESCS include (1) torpedo-like, deep atrophic lesions with a small hyperpigmented rim, variably sized and predominantly located along the arcades; (2) circumferential fibrotic scars in the posterior pole with a spared center and large fibrotic scars around the optic nerve head; and (3) yellow dots in areas of relatively normal-appearing retina.
CONCLUSIONS AND RELEVANCE
Enhanced S-cone syndrome has more pleiotropy than previously appreciated. While the nummular type of pigmentation at the level of the retinal pigment epithelium and cystoid or schisis-like maculopathy with typical functional findings remain classic hallmarks of the disease, changes such as circumferential fibrosis of the macula or peripapillary area and “torpedo-like” lesions along the vascular arcades may also direct the clinical diagnosis and focus on screening the NR2E3 gene for a molecular diagnosis.
Enhanced S-cone syndrome (ESCS) is a rare, autosomal recessive inherited retinal dystrophy first described more than 2 decades ago.1-3 Because of the variable clinical presentation, it is not unusual that patients with ESCS are misdiagnosed as having atypical retinitis pigmentosa, congenital stationary night blindness, or X-linked retinoschisis.
The adult human retina typically consists of approximately 120 million rods and 6 million cones containing 3 cone subtypes: short-wavelength (S), medium-wavelength (M), and long-wavelength (L) cones. S-cones are the minority (about 10%)4 subset of cones in healthy human retinas whereas in ESCS, S - cones are the majori ty cone subtype.1-3,5,6 Electroretinography (ERG) and psychophysical testing play a key role in diagnosing patients with ESCS. Characteristic ERG findings are the presence of similar wave-forms in the maximum dark-adapted response and singleflash light-adapted waveform. An associated ERG feature is disproportionately reduced 30-Hz cone flicker to the single-flash cone amplitude.7-9 The basis of these waveforms has been explored.10 All of these findings occur in the absence of rod function.1-3,7,8 S-cone stimuli can show supernormal responses, although the relative increase in S-cone function compared with L- and M-cone function is diagnostic and independent of degree of disease severity. Psychophysical testing demonstrating the abnormal ratio of S-cone to L- and M-cone function was especially helpful in proving that ESCS and the more severe expression Goldmann-Favre syndrome were part of the same disease spectrum.3
Postmortem donor retinas have been studied in rare reports in which the disease was identified as ESCS and these confirmed and extended the noninvasive observations. In the donor eye from a 77-year-old woman, there were no rods detectable, a 2-fold increase in cone density with an abnormally high number of S-cones and abnormally low number of L- and M-cones, and coexpression of S-cone opsin in some L- and M-cones.5 In another study, there were no detectable rods, and cones also coexpressed different opsins.6
Enhanced S-cone syndrome is mainly caused by mutations in the NR2E3 gene that encodes the nuclear receptor class 2, subfamily E, member 3 protein (NR2E3, OMIM 604485). NR2E3 is uniquely expressed in the outer nuclear layer of the retina and there is evidence that it suppresses cone differentiation during embryogenesis. Multipotent retinal progenitor cells present in fetal eyes are directed to become rods or cones. Therefore, loss of NR2E3 results in retinas with a decreased number of rod photoreceptors but an increase in cones, predominantly expressing the S-cone opsin resulting in ESCS.11,12
A recent clinical study of ESCS reported an association with helicoid subretinal fibrosis, thereby adding another phenotypic expression to ESCS.13 The purpose of the current study was to review a cohort of established ESCS cases with mutations in NR2E3 to further determine the clinical spectrum of ESCS. New clinical findings may facilitate earlier diagnosis in patients presenting with unusual phenotypes and may increase the understanding of disease mechanisms due to NR2E3 gene causation.
Methods
All patients were evaluated by the coauthors S.G.J., S.H.T., I.B., M.J.vS., and L.A.Y.. The procedures were approved by the ethics committees of involved sites and adhered to the tenets set out in the Declaration of Helsinki.
On identification of mutations in NR2E3 in patient 1, who showed several unusual and previously undescribed findings in ESCS, we obtained clinical data from 30 other patients with established ESCS, all but 1 having proven NR2E3 mutations.14-16 The patients had been examined between 1983 and 2012. All patients underwent a standard ophthalmic examination, and most had ERGs. Color fundus photographs were obtained by camera systems available at the time of examination and were mainly of the central retina; there were no montages or attempts to cover wide retinal areas. Fundus auto-fluorescence was obtained with a Topcon TRC 50 IX DX fundus camera or Heidelberg Spectralis (Heidelberg Engineering Inc). Optical coherence tomography was obtained using the Spectralis or Stratus (Zeiss Meditec Inc) in a subset of patients.
Results
Cohort of Patients With ESCS and NR2E3 Mutations
Thirty-one patients with ESCS (30 with proven NR2E3 mutations)14-16 were studied retrospectively. Patients ranged in age from 4 to 72 years (18 female and 13 male) and had different ethnic and geographic origins. They all had symptoms of night blindness from an early age. Best-corrected visual acuity (Snellen) ranged from 20/20 to 20/200. Many patients showed the well-known fundus features of ESCS such as nummular pigment clumping at the level of the retinal pigment epithelium (RPE) along the vascular arcades and in the midperiphery, cystoid disturbances in the macula (retinoschisis), and chorioretinal atrophic changes. The patients who underwent ERG testing showed no detectable waveform to a dim flash of light intended to stimulate dark-adapted rods. A bright white flash, dark adapted, elicited a waveform that could be similar in shape to a flash of the same intensity on a white back-ground. Flicker ERGs tended to be smaller in amplitude than the light-adapted single-flash responses. When S-cone ERGs were performed, responses were abnormally increased in amplitude.1,3,7,9,10,12
New Funduscopic Observations
The new fundus features are described next and representative images are shown in Figures 1, 2, 3, 4, 5, and 6. The patients are numbered in order of appearance in the Figures, and the corresponding molecular genetic data as well as the patients’ age at time of examination are listed in the legends.
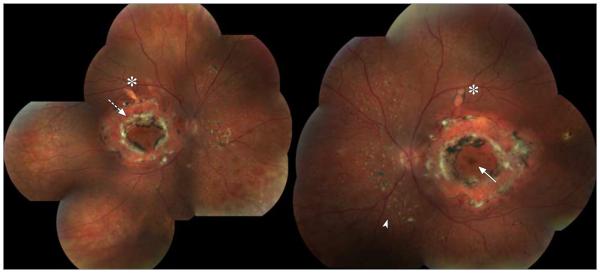
Figure 1. Color Composite of the Right and Left Eyes of Patient 1.
Twenty–five-year-old woman homozygous for the R311Q mutation. Visual acuity is 20/200 OD and 20/25 OS. There are large circumferential fibrotic lesions in the posterior poles of both eyes (interrupted arrow). The fovea is present (continuous arrow). Along the superior arcades, torpedo-like lesions are noted (asterisk). Small yellow dots interspersed with nummular pigmentations are present in both eyes, mainly nasal inferior to the disc (arrowhead).
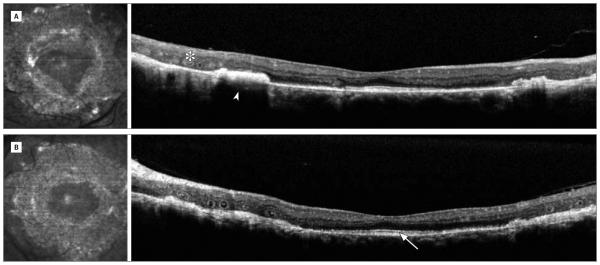
Figure 2. High-Resolution Optical Coherence Tomography Scans Horizontally Across Both Eyes of Patient 1.
Twenty–five-year-old woman homozygous for the R311Q mutation. A, Right eye. B, Left eye. There is outer retinal tubulation (asterisk), subretinal fibrosis (arrowhead), retained inner segment of ellipsoid band subfoveally (arrow), and loss of retinal organization.
Figure 3. Composite of Fundus Autofluoresence Images of Both Eyes of Patient 1.

Twenty–five-year-old woman homozygous for the R311Q mutation. A large hypoautofluorescent ring is present (asterisk) with a hyperautofluorescent center (arrow). In the periphery, different patterns of autofluorescence are present. The yellow dots seen on the color photographs appear hyperautofluorescent (arrowhead).
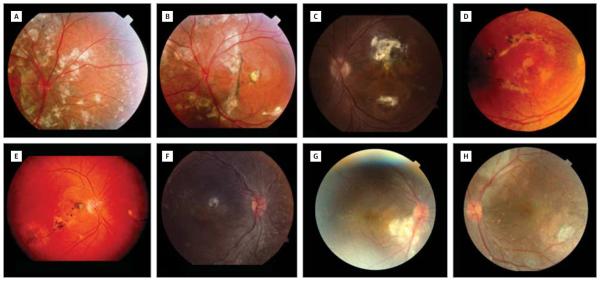
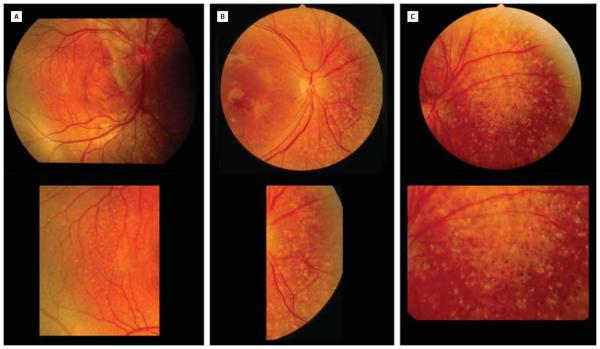
Figure 4. Varying Locations and Extents of Subretinal Fibrosis in Enhanced S-Cone Syndrome.
A, The right eye of patient 2 (15-year-old boy homozygous for the IVS1-2A>C mutation)15 shows large fibrosis surrounding the nerve head and extending superiorly. B, The left eye of patient 2 at age 15 years with a circumferential scar around the nerve head. C, Unilateral fibrotic scarring of the posterior pole in patient 3 (17-year-old boy homozygous for the R311Q mutation).15 D, Patient 4 (46-year-old woman homozygous for the R311Q mutation)15 shows bilateral scarring in the posterior pole. The right eye shows scarring in a circumferential manner around the fovea. E, Patient 5 (40-year-old woman homozygous for the R311Q mutation)15 has fibrosis in the posterior poles of both eyes. The right eye shows fibrosis extending from the peripapillary area to the temporal retina following the inferior arcade and sparing the fovea. F, Patient 6 (6-year old boy homozygous for the R311Q mutation)15 has a central scar in the right eye. The left eye shows subtle subretinal fibrosis along the inferior arcade, sparing the fovea (not shown). G, The right eye of patient 7 (4-year-old girl homozygous for the p.C67-69Gdel mutation)15 shows subretinal fibrosis inferior to the optic disc. H, The left eye of patient 7 shows subretinal fibrosis superior to the optic disc.
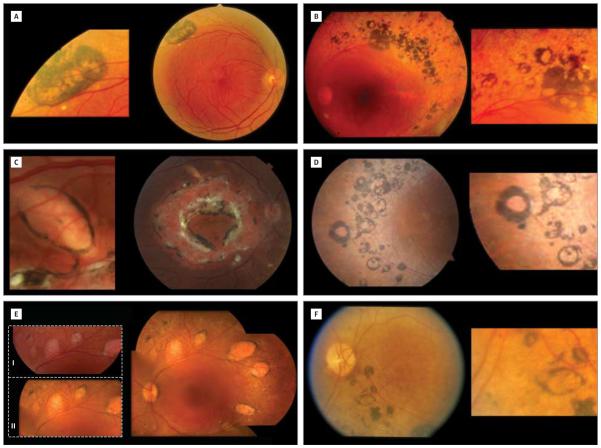
Figure 5. Torpedo-like Lesions in Enhanced S-Cone Syndrome.
A, Patient 8 (54-year-old man homozygous for the R311Q mutation)15 shows a large hyperpigmented lesion with multiple atrophic areas along the superior arcade in the right eye. B, Patient 9 (41-year-old man with the R311Q and IVS1-2A>C mutations)15 has multiple hyperpigmented spots. Some of these lesions show atrophic centers. C, A large and more typical oval torpedo-like lesion just inferior of the superior arcade of the right eye of patient 1 (25-year-old woman homozygous for the R311Q mutation).15 D, Multiple hyperpigmented round lesions variable in size, most of which have an atrophic center, in the right eye of patient 10 (55-year-old woman homozygous for the R311Q mutation).15 E, The left eye of patient 11 (35-year-old man with the R311Q and Q350R mutations)14 shows multiple oval torpedo-like lesions along the arcades. The 2 enlarged images are from photographs taken in 1999 (I) and 2010 (II) and show no obvious change in appearance. F, One larger oval torpedo-like lesion in an area of hyperpigmentation inferior to the disc and arcade in the left eye of patient 12 (72-year-old woman).
Figure 6. Yellow Dots in Enhanced S-Cone Syndrome.
A, The right eye of patient 2 (15-year-old boy homozygous for the IVS1-2A>C mutation)15 shows multiple small subretinal yellow dots only present in an area of normal-appearing retina. The dots are located temporal to the fovea. There is fibrosis along the disc. B, The right eye of patient 4 (46-year-old woman homozygous for the R311Q mutation)15 shows different-sized yellow dots nasal to the optic nerve. The yellow dots are interspersed with hyperpigmented dots. There is fibrosis in the posterior pole. C, The left eye of patient 4 (46-year-old woman homozygous for the R311Q mutation)15 shows a large area of confluent-appearing white dots nasal and superior to the nerve head. The nummular type of pigmentation is also present.
Circumferential Subretinal Fibrosis
A 25-year-old woman (patient 1) with a history of night blindness since early childhood presented with bilateral, large circumferential fibrosis in the macula, with hyperpigmentation and sparing of the fovea (Figure 1). Based on high-resolution optical coherence tomography images of the central area, the fibroses appeared to be located subretinally (Figure 2). There was loss of normal outer retinal architecture across most of the scanned region except at the fovea, which had a normal-appearing outer nuclear layer and a retained inner segment ellipsoid band of the photoreceptors.17 An epiretinal membrane, some disorganization of the retinal layers, and outer retinal tubulations (circular pattern of degenerate photoreceptors)18 were also present. Fundus autofluoresence images of both eyes showed a large hypoautofluorescent ring with a hyperautofluorescent center. The hypoautofluorescent ring corresponded to the area of subretinal fibrosis seen on color images. The midperiphery and far periphery showed areas of normal autofluorescence and areas of hypoautofluorescence. Within the normal and hypoautofluorescent areas, there were multiple small hyperautofluorescent and hypoautofluorescent dots (Figure 3).
Subretinal fibrosis was subsequently noted in 6 other patients (23%) (Figure 4). These fibrotic lesions were typically found in the posterior pole. Two patients showed peripapillary subretinal fibrosis. The subretinal fibrosis in patients presented herein showed different patterns. Most patients had bilateral and symmetrical subretinal fibrosis. Subretinal fibrosis was found as early as 4 years of age (patients 2 and 7). Unilateral subretinal fibrosis was seen only in 1 patient (patient 3).
Torpedo-like Lesions
Unusual torpedo-like lesions along the superior arcades were observed in patient 1. She had 1 large choroidal-RPE-retinal atrophic lesion along the superior arcades in the right and left eyes. These were located in relatively healthy-appearing retina and showed a hyperpigmented rim. They were oval and showed hypoautofluorescence on fundus autofluoresence. The presence of torpedo-like lesions was subsequently observed in 9 additional patients (32%) (5 of whom are shown in Figure 5). These lesions were variable in size. The exact location varied but all torpedolike lesions were observed in a ring along the superior and/or inferior arcade. Typically, they consisted of central depigmentation or chorioretinal atrophy with a hyperpigmented border.
In patient 11, the torpedo-like lesions appeared to be non-progressive given that they had not changed over a 10-year follow-up period starting at age 24 years (Figure 5). The patients with torpedo-like lesions in this study were 24 to 55 years of age at the time of documentation of the lesions.
Yellow Dots in the Periphery
Patient 1 had yellow dots in peripheral areas that also showed small nummular hyperpigmentation. The dots were mainly seen nasal to the optic disc and below the inferior arcade. The location, size, and distribution of these yellow dots were variable among patients (Figure 6). The dots were not perfectly round and were also not of a uniform size within retinas of individual patients studied. Hyperpigmented nummular dots were present in areas of yellow dots in most patients except for patients 2 and 7. The dots in patients 2 and 7 were mainly found temporal to the fovea, whereas in the other patients, they were primarily located nasal to the disc. The yellow dots were noted in areas of relatively normal-appearing retina. All patients in whom subretinal fibrosis was observed also had yellow dots.
Discussion
Our review of funduscopic abnormalities in a cohort of patients with ESCS and NR2E3 mutations allowed us to identify additional, previously underappreciated features of this autosomal recessive retinal degeneration and thereby expand the clinical spectrum. A novel observation in ESCS is the presence of torpedo-like lesions. These lesions are variable in size, oval, and show a central area of RPE depigmentation or chorioretinal atrophy with a hyperpigmented margin. Most of these lesions were located along the arcades. The hyperpigmented rim in torpedo-like lesions was not surrounded by normal RPE and the lesions were located in retinal areas with many other retinal and RPE abnormalities (Figure 5). The torpedo-like lesions differed from the torpedo lesions described in the literature. Torpedo lesions tend to be solitary and relatively large with a sharp narrowed end that points to the foveola and are located temporal to or under the fovea. Surrounding the hyperpigmented rim is normal RPE. The torpedo lesions are congenital and have been thought to be due to abnormal choroidal or ciliary vasculature development or a fetal RPE defect.19,20
Subretinal fibrosis as seen in our patient 1 was previously observed in 2 patients with ESCS and NR2E3 mutations.13 The basis for the distribution of these lesions, in the region of the arcades in some patients and in the peripapillary or submacular areas in others, remains unexplained. A recent study showed subretinal hemorrhage and subretinal fibrosis in a 14-monthold boy with ESCS.21 Subretinal hemorrhages early in the course of the disease have been documented and may be the cause of the subretinal fibrosis.22,23 It remains unclear why subretinal hemorrhages occur in ESCS. A submacular vascular abnormality was suggested to cause the fibrosis and macular hemorrhage in the 14-month-old child.21
The retinal region with many of the observed abnormalities in ESCS is the perimacular elliptical ring near the vascular arcades that also extends into the nasal retina. The NR2E3 mutations in ESCS lead to transcriptional misregulation and abnormal photoreceptor development with an increase in the number of S-cone–type photoreceptors at the expense of rods that fail to differentiate.24,25 In normal retinas, the perimacular elliptical ring is a rod-rich zone.4 In ESCS, the perimacular region is thought to be filled with S-cone photoreceptors instead of rods. Studies of retinal organization in patients with ESCS have shown disturbed structure in this region; optical coherence tomography indicated thick and bulging retina in the ring with abnormal laminar architecture.24 It has been speculated that larger cone cells in place of rods could cause abnormal packing of photoreceptors, secondary impairment in phagocytosis by the RPE, and resulting degeneration.26 This mismatch between photoreceptor cell type and underlying RPE would be expected to be exaggerated in the usually dense rod ring filled with S-cones.24 Patchy loss of RPE cells or their pigment or increased density of melanin granules in remaining RPE cells could be the funduscopically visible result of a chronically abnormal interface of photoreceptors and RPE. Histopathological analysis of an ungenotyped eye donor with clumped pigmentary retinopathy showed such findings.27,28 There were patches of more densely pigmented RPE near areas of less pigment, accounting for the clumping appearance. Such images in donor eyes of patients with ESCS from NR2E3 mutations have also been published (Milam et al Figure 2E5 and Bonilha et al Figure 1C6). The basis of the torpedo-like configuration of some of these RPE disturbances is unknown. There is the possibility that patches of retina with more abnormal photoreceptor and RPE approximation, possibly caused by retinal folds or pseudorosettes, as seen in Nr2e3rd7 mouse models and in ESCS histopathological analysis,5,6,25,26,29-31 could lead to round or oval regions of RPE cell loss.
We found intraretinal yellow dots in several patients (Figures 1, 2, 3, 4, 5, and 6). Although this has been described before in ESCS and other retinal dystrophies, it is of interest that all patients with subretinal scarring had intraretinal yellow dots.32 In another study of patients with NR2E3 mutations, hyperautofluorescent loci were noted and colocalized cross-sectional optical coherence tomography images showed these features to be intraretinal and associated with dysmorphology of the photoreceptor layer.26 The Nr2e3rd7 mutant mouse also has white-yellow dots visible ophthalmoscopically at certain ages; autofluorescent dots were studied in these mice and attributed to material within macrophages, which were associated with retinal pseudorosettes.31
Were there any clear genotype-phenotype relationships? The question was asked only of those patients with common NR2E3 mutations: p.R311Q and c.119-2A>C (IVS1-2A>C).15,16 In the present cohort, these 2 mutations were present in the homozygous state in 19 patients: 14 patients for R311Q and 5 patients for IVS1-2A>C . We asked whether the torpedo-like lesions and subretinal fibrosis were present in all or most of the patients with one or the other mutation. Among the 14 R311Q homozygotes, 5 of 14 (36%) showed torpedo-like lesions, 4 of 14 (29%) showed subretinal fibrosis in the posterior pole, and 1 patient showed both. None of the 5 homozygotes for IVS1-2A>C showed torpedo-like lesions, and 1 had subretinal fibrosis surrounding the optic nerves. Yellow dots mainly were seen in association with the subretinal fibrosis. The torpedo-like lesions, however, were not exclusive to the R311Q homozygotes and subretinal fibrosis was not exclusive to the 2 genotypes. Other genotypes in our cohort (but only represented anecdotally by 1 patient each) showed these fundus features. We therefore could not establish a clear genotypephenotype relationship.
In conclusion, patients with torpedo-like lesions along the vascular arcades or circumferential macular fibrosis should have ESCS considered among the differential diagnoses. While fibrosis and torpedo-like lesions may be the most striking funduscopic abnormalities in some individuals, careful clinical observation usually reveals other characteristics such as nummular pigmentary changes, interspersed with yellow dots; cystoid maculopathy; and macular or peripheral schisis. Regardless of the clinical presentation, the diagnosis of ESCS will need to be confirmed by ERG, psychophysical testing, and/or mutation analysis of the NR2E3 gene. Phenotype recognition and improved knowledge of disease causation should aid in developing treatment strategies to prevent progressive vision loss in ESCS in the future. Such insight is also valuable for understanding normal retinal and photoreceptor development.
Acknowledgments
Funding/Support: This work was supported by the Macula Foundation Inc.
Footnotes
Conflict of Interest Disclosures: None reported.
REFERENCES
- 1.Jacobson SG, Marmor MF, Kemp CM, Knighton RW. SWS (blue) cone hypersensitivity in a newly identified retinal degeneration. Invest Ophthalmol Vis Sci. 1990;31(5):827–838. [PubMed] [Google Scholar]
- 2.Marmor MF, Jacobson SG, Foerster MH, Kellner U, Weleber RG. Diagnostic clinical findings of a new syndrome with night blindness, maculopathy, and enhanced S cone sensitivity. Am J Ophthalmol. 1990;110(2):124–134. doi: 10.1016/s0002-9394(14)76980-6. [DOI] [PubMed] [Google Scholar]
- 3.Jacobson SG, Román AJ, Román MI, Gass JD, Parker JA. Relatively enhanced S cone function in the Goldmann-Favre syndrome. Am J Ophthalmol. 1991;111(4):446–453. doi: 10.1016/s0002-9394(14)72379-7. [DOI] [PubMed] [Google Scholar]
- 4.Curcio CA, Sloan KR, Kalina RE, Hendrickson AE. Human photoreceptor topography. J Comp Neurol. 1990;292(4):497–523. doi: 10.1002/cne.902920402. [DOI] [PubMed] [Google Scholar]
- 5.Milam AH, Rose L, Cideciyan AV, et al. The nuclear receptor NR2E3 plays a role in human retinal photoreceptor differentiation and degeneration. Proc Natl Acad SciUS A. 2002;99(1):473–478. doi: 10.1073/pnas.022533099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bonilha VL, Fishman GA, Rayborn ME, Hollyfield JG. Retinal pathology of a patient with Goldmann-Favre syndrome. Ophthalmic Genet. 2009;30(4):172–180. doi: 10.3109/13816810903176765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hood DC, Cideciyan AV, Roman AJ, Jacobson SG. Enhanced S cone syndrome: evidence for an abnormally large number of S cones. Vision Res. 1995;35(10):1473–1481. doi: 10.1016/0042-6989(95)98727-q. [DOI] [PubMed] [Google Scholar]
- 8.Greenstein VC, Zaidi Q, Hood DC, Spehar B, Cideciyan AV, Jacobson SG. The enhanced S cone syndrome: an analysis of receptoral and post-receptoral changes. Vision Res. 1996;36(22):3711–3722. doi: 10.1016/0042-6989(96)00073-9. [DOI] [PubMed] [Google Scholar]
- 9.Fishman GA, Jampol LM, Goldberg MF. Diagnostic features of the Favre-Goldmann syndrome. Br J Ophthalmol. 1976;60(5):345–353. doi: 10.1136/bjo.60.5.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Román AJ, Jacobson SG. S cone-driven but not S cone-type electroretinograms in the enhanced S cone syndrome. Exp Eye Res. 1991;53(5):685–690. doi: 10.1016/0014-4835(91)90230-c. [DOI] [PubMed] [Google Scholar]
- 11.Mollema N, Haider NB. Focus on molecules: nuclear hormone receptor Nr2e3: impact on retinal development and disease. Exp Eye Res. 2010;91(2):116–117. doi: 10.1016/j.exer.2010.04.013. [DOI] [PubMed] [Google Scholar]
- 12.Haider NB, Mollema N, Gaule M, et al. Nr2e3-directed transcriptional regulation of genes involved in photoreceptor development and cell-type specific phototransduction. Exp Eye Res. 2009;89(3):365–372. doi: 10.1016/j.exer.2009.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Khan AO, Aldahmesh MA, Al-Harthi E, Alkuraya FS. Helicoid subretinal fibrosis associated with a novel recessive NR2E3 mutation p.S44X. Arch Ophthalmol. 2010;128(3):344–348. doi: 10.1001/archophthalmol.2010.15. [DOI] [PubMed] [Google Scholar]
- 14.Pachydaki SI, Klaver CC, Barbazetto IA, et al. Phenotypic features of patients with NR2E3 mutations. Arch Ophthalmol. 2009;127(1):71–75. doi: 10.1001/archophthalmol.2008.534. [DOI] [PubMed] [Google Scholar]
- 15.Haider NB, Jacobson SG, Cideciyan AV, et al. Mutation of a nuclear receptor gene, NR2E3, causes enhanced S cone syndrome, a disorder of retinal cell fate. Nat Genet. 2000;24(2):127–131. doi: 10.1038/72777. [DOI] [PubMed] [Google Scholar]
- 16.Wright AF, Reddick AC, Schwartz SB, et al. Mutation analysis of NR2E3 and NRL genes in enhanced S cone syndrome. Hum Mutat. 2004;24(5):439. doi: 10.1002/humu.9285. [DOI] [PubMed] [Google Scholar]
- 17.Spaide RF, Curcio CA. Anatomical correlates to the bands seen in the outer retina by optical coherence tomography: literature review and model. Retina. 2011;31(8):1609–1619. doi: 10.1097/IAE.0b013e3182247535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zweifel SA, Engelbert M, Laud K, Margolis R, Spaide RF, Freund KB. Outer retinal tubulation: a novel optical coherence tomography finding. Arch Ophthalmol. 2009;127(12):1596–1602. doi: 10.1001/archophthalmol.2009.326. [DOI] [PubMed] [Google Scholar]
- 19.Shields CL, Guzman JM, Shapiro MJ, Fogel LE, Shields JA. Torpedo maculopathy at the site of the fetal “bulge”. Arch Ophthalmol. 2010;128(4):499–501. doi: 10.1001/archophthalmol.2010.29. [DOI] [PubMed] [Google Scholar]
- 20.Golchet PR, Jampol LM, Mathura JR, Jr, Daily MJ. Torpedo maculopathy. Br J Ophthalmol. 2010;94(3):302–306. doi: 10.1136/bjo.2009.162669. [DOI] [PubMed] [Google Scholar]
- 21.Cassiman C, Spileers W, De Baere E, de Ravel T, Casteels I. Peculiar fundus abnormalities and pathognomonic electrophysiological findings in a 14-month-old boy with NR2E3 mutations. Ophthalmic Genet. 2013;34(1-2):105–108. doi: 10.3109/13816810.2012.726395. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura M, Hotta Y, Piao CH, Kondo M, Terasaki H, Miyake Y. Enhanced S-cone syndrome with subfoveal neovascularization. Am J Ophthalmol. 2002;133(4):575–577. doi: 10.1016/s0002-9394(01)01428-3. [DOI] [PubMed] [Google Scholar]
- 23.Lam BL, Goldberg JL, Hartley KL, Stone EM, Liu M. Atypical mild enhanced S-cone syndrome with novel compound heterozygosity of the NR2E3 gene. Am J Ophthalmol. 2007;144(1):157–159. doi: 10.1016/j.ajo.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 24.Jacobson SG, Sumaroka A, Aleman TS, et al. Nuclear receptor NR2E3 gene mutations distort human retinal laminar architecture and cause an unusual degeneration. Hum Mol Genet. 2004;13(17):1893–1902. doi: 10.1093/hmg/ddh198. [DOI] [PubMed] [Google Scholar]
- 25.Chen J, Rattner A, Nathans J. The rod photoreceptor-specific nuclear receptor Nr2e3 represses transcription of multiple cone-specific genes. J Neurosci. 2005;25(1):118–129. doi: 10.1523/JNEUROSCI.3571-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Mustafi D, Kevany BM, Genoud C, et al. Defective photoreceptor phagocytosis in a mouse model of enhanced S-cone syndrome causes progressive retinal degeneration. FASEB J. 2011;25(9):3157–3176. doi: 10.1096/fj.11-186767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.To KW, Adamian M, Jakobiec FA, Berson EL. Clinical and histopathologic findings in clumped pigmentary retinal degeneration. Arch Ophthalmol. 1996;114(8):950–955. doi: 10.1001/archopht.1996.01100140158008. [DOI] [PubMed] [Google Scholar]
- 28.Sharon D, Sandberg MA, Caruso RC, Berson EL, Dryja TP. Shared mutations in NR2E3 in enhanced S-cone syndrome, Goldmann-Favre syndrome, and many cases of clumped pigmentary retinal degeneration. Arch Ophthalmol. 2003;121(9):1316–1323. doi: 10.1001/archopht.121.9.1316. [DOI] [PubMed] [Google Scholar]
- 29.Mears AJ, Kondo M, Swain PK, et al. Nrl is required for rod photoreceptor development. Nat Genet. 2001;29(4):447–452. doi: 10.1038/ng774. [DOI] [PubMed] [Google Scholar]
- 30.Chen J, Nathans J. Genetic ablation of cone photoreceptors eliminates retinal folds in the retinal degeneration 7 (rd7) mouse. Invest Ophthalmol Vis Sci. 2007;48(6):2799–2805. doi: 10.1167/iovs.06-0922. [DOI] [PubMed] [Google Scholar]
- 31.Wang NK, Fine HF, Chang S, et al. Cellular origin of fundus autofluorescence in patients and mice with a defective NR2E3 gene. Br J Ophthalmol. 2009;93(9):1234–1240. doi: 10.1136/bjo.2008.153577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Audo I, Michaelides M, Robson AG, et al. Phenotypic variation in enhanced S-cone syndrome. Invest Ophthalmol Vis Sci. 2008;49(5):2082–2093. doi: 10.1167/iovs.05-1629. [DOI] [PubMed] [Google Scholar]