Abstract
Mutations in Pde6b lead to high levels of signaling molecules cyclic guanosine monophosphate (cGMP) and Ca2+, which ultimately result in photoreceptor cell death in certain forms of retinitis pigmentosa (RP). The level of cGMP, which is controlled by opposing activities of guanylate cyclase (GUCY) and photoreceptor phosphodiesterase-6 (PDE6), regulates the opening of cyclic nucleotide-gated ion channels [CNG] and thereby controls Ca2+ influx into the outer segments. Using a lentiviral gene therapy approach, we have previously shown that degeneration can be temporarily slowed either by introducing wild-type PDE6β or knocking down expression of GUCY2E and CNGA1 in photoreceptors of Pde6bH620Q, a mouse model for RP. Rescue was transient with either approach. Therefore, we tested a novel combination therapy using bipartite lentiviral vectors designed to both introduce wild-type PDE6β expression and knockdown GUCY2E or CNGA1. Immunoblot analysis shows simultaneous increases in PDE6β and decreases in GUCY2E or CNGA1 in retinas transduced by the vectors, indicating successful transduction. In Pde6bH620Q mutants, we observe rescue of photoreceptor function and an increase in photoreceptor rows as compared with untreated controls. However, no evidence of prolonged rescue beyond the limit of the previously tested single therapy was observed.
Keywords: gene therapy, Pde6bH620Q, mouse model, PDE6, GUCY2E, CNGA1, bipartite, retinitis pigmentosa, lentiviral vector, shRNA
Introduction
About 36,000 cases worldwide of simplex and familiar retinitis pigmentosa (RP) are caused by loss of function mutations in rod photoreceptor phosphodiesterase-6 (PDE6).1–4 Although significant advances in our understanding of RP have been made, the exact interplay between defective PDE6 and the onset of RP pathogenesis remains poorly understood.
Mouse models of human RP have been extensively used to study the mechanisms of onset and progression of the disease.5–8 Several Pde6b mutant alleles have been identified and studied, including Pde6brd1, Pde6brd10 and Pde6bH620Q.5,6,8 Loss of PDE6 enzyme activity has been shown to result in increased levels of cyclic guanosine monophosphate (cGMP) and Ca2+ in Pde6brd1 and Pde6bH620Q mice.5,9–4 Excessive influx of Ca2+ is likely the critical event leading to photoreceptor cell death.5,14–16
Under normal physiological conditions, cGMP levels are regulated by opposing activities of PDE6, which hydrolyzes cGMP into GMP, and guanylate cyclase (GUCY), which produces cGMP from guanosine-5′-triphosphate (GTP). During phototransduction, cGMP controls the excitation state of the photoreceptor through regulation of cGMP-gated (cyclic nucleotide-gated ion channel [CNG]) channels. In the dark, cGMP maintains CNG channels in an open configuration, allowing the influx of Ca2+ to the cytoplasm. In the light, PDE6 activation results in the rapid hydrolysis of cGMP and resultant closure of CNG channels. When PDE6 is absent or reduced in mutant photoreceptors, elevated concentrations of cGMP keep CNG channels open, allowing entrance of excess Ca2+, leading to cell death.8
Transduction of mutant photoreceptors with gene therapy vectors have been used to delay photoreceptor death.17 We have previously shown that expression of Pde6b cDNA in Pde6bH620Q mutant retinas transduced with Opsin::Pde6b lentivirus results in improved photoreceptor physiology and increased photoreceptor numbers.5 We also took advantage of the known relationships between PDE6, GUCY and CNG to test if reduced expression of GUCY2E or CNGA1 could suppress the degeneration phenotype. Knockdown of Gucy2e or Cnga1 was associated with improved survival and function in Pde6bH620Q mutant photoreceptors transduced with shRNA lentiviral vectors.18
Although our attempts have demonstrated these monopartite vectors can rescue the mutant phenotype, the effect was incomplete and temporary. Here we hypothesized that a combined therapy approach would have an additive or synergistic effect on photoreceptor function and survival. For that purpose, we generated two kinds of bipartite expression vectors to express Pde6b cDNA and Gucy2e or Cnga1 shRNAs. After subretinal injection of these lentiviral vectors in Pde6bH620Q mice, we analyzed the mutant photoreceptors by electroretinogram (ERG) recording and histology. Our results showed limited functional and morphological rescue, although no synergy was observed.
Materials and methods
Animals
Mice were used in accordance with the Statement for the Use of Animals in Ophthalmic and Vision Research of the Association for Research in Vision and Ophthalmology, as well as the Policy for the Use of Animals in Neuroscience Research of the Society for Neuroscience. Pde6bH620Q mice used in this experiment were bred from a colony of mice that has been previously reported.5,19 All Pde6bH620Q mice analyzed in this study are homozygotes and in the C3H background.
Construction of lentiviral vectors
The viruses used for our experiments were self-inactivating vectors consisting of a 5′ long terminal repeat (LTR), a packaging signal ψ, a tRNA primer binding site, a reverse response element and a 3′LTR. This viral vector also contained a central polypurine tract/DNA flap (cPPT) and a Woodchuck hepatitis virus post-transcriptional regulatory element.
Three vectors were assembled: H1::Gucy2e, H1::Cnga1 and Opsin::Gucy2e (Figure 1). The vectors were designed to simultaneously express PDE6β and knockdown GUCY2E or CNGA1. To deliver rod-specific expression of PDE6β, all vectors contained the mouse rhodopsin 2.0 kb promoter cloned upstream of full-length Pde6b cDNA. Both H1::Gucy and H1::Cnga1 vectors drive shRNA expression using the human H1 RNA polymerase III promoter (as per manufacturer’s instructions; Biogenova Corporation, Frederick, MD, USA).
Figure 1.
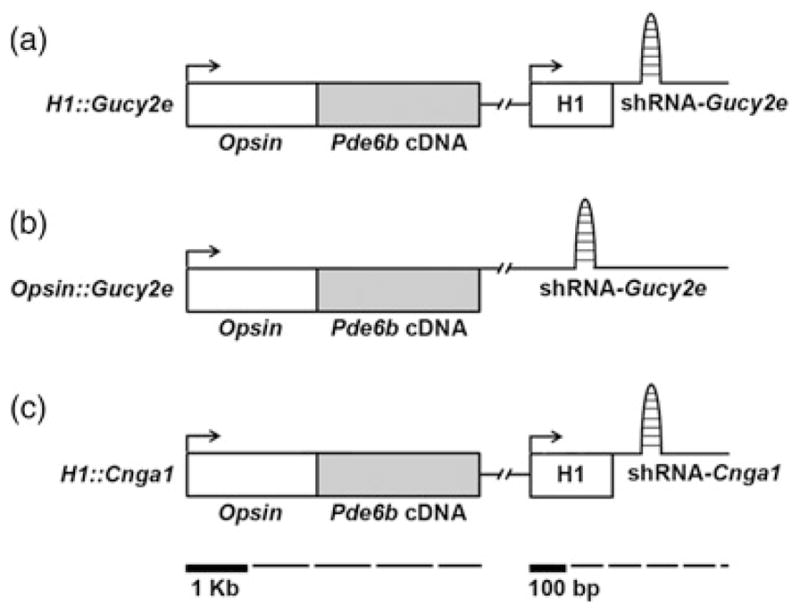
Schematic representation of the lentiviral vectors. The H1::Gucy2e (a) construct consists of a 2 kb mouse Opsin promoter, a Pde6b open reading frame (Pde6b cDNA) and the H1 promoter, which drives expression of the shRNA-Gucy2e. The Opsin::Gucy2e (b) consists of a 2 kb Opsin promoter, a Pde6b open reading frame and a shRNA-Gucy2e. The H1::Cnga1 lentiviral vector (c) was constructed using 2 kb of the mouse Opsin promoter, which directs expression of the Pde6b cDNA fragment, and the H1 promoter, which drives expression of the shRNA-Cnga1
The Opsin::Gucy2e vector was designed to direct Gucy2e shRNA expression in rods, as GUCY2E and GUCY2F are required for efficient transport of PDE6 and other proteins to outer segments.20 Opsin::Gucy2e contained Gucy2e shRNA sequence cloned within the T-cell receptor-β Cβ2.1 intron 42, which lies within the same transcription unit of the Pde6b cDNA. A unique MunI site within the intron was used for insertion of a shRNA oligonucleotide. This configuration is expected to drive expression of both the Pde6b cDNA and the pre-Gucy2e shRNA within photoreceptors, similar to the expression of pre-miR34 (as per manufacturer’s instructions; System Biosciences, Mountain View, CA, USA).21,22
Subretinal transduction of lentivirus
Subretinal injections were described before.5,18 In short, Pde6bH620Q or C57BL/6J mice at postnatal day (P) 5 were anesthetized in ice and placed under a surgical microscope. The eyelid was opened and 0.8 μL of virus particles (2 × 107 TU/mL) were injected at the 6 o’clock position 1.5 mm from the limbus. The injection produced a bubble in the subretinal space. Mice received a subretinal injection of lentivirus in the right eye, while the left eye served as a control.
Histochemical analyses
Mice were sacrificed and hematoxylin–eosin (H–E) retinal sections were obtained as described before.23,24 Shortly thereafter, animals were sacrificed and eyes were enucleated and fixed in 0.5× Karnovsky’s fixative (2% paraformaldehyde, 1.25% glutaraldehyde, 0.2 mol/L phosphate-buffered saline). After that they were embedded in paraffin and sectioned every 4 μm. Sectioning proceeded along the long axis of the segment so that each section contained both upper and lower retina as well as the posterior pole. H–E staining was then conducted. The number and morphology of photoreceptors in injected eyes were compared with control. Quantification of photoreceptor nuclei was conducted on several sections containing the optic nerve.
Immunoblot analysis
C57BL/6J mice were euthanized at eight weeks of age and retinas were homogenized in 10% sodium dodecyl sulfate (SDS) by sonication. To minimize individual differences between subretinal surgeries, retinal extracts were pooled from each virus, and each assay was carried out in duplicate. After denaturation at 100°C for five minutes, total protein was measured by the DC Protein Assay method (Bio-Rad Laboratories, Hercules, CA, USA). Proteins were then separated by SDS polyacrylamide gel electrophoresis. Samples were transferred to nitrocellulose membranes, which were preincubated in blocking solution (3% bovine serum albumin, 150 mmol/L NaCl, 100 mmol/L Tris, 0.5% Tween-20). Membranes were incubated with antibodies against PDE6β (1:2000; Thermo Scientific, Waltham, MA, USA), GUCY2E (1:2000; kindly provided by Alexander Dizhoor, Pennsylvania College of Optometry, Elkins Park, PA, USA), and CNGA1 (1:12; kindly provided by Robert Molday, The University of British Columbia, Vancouver, BC, Canada). GNAT1 is a rod-specific protein, which served as a control for photoreceptor protein content (Gαt1, 1:2000; Santa Cruz Biotechnology, Santa Cruz, CA, USA). Membranes were washed and then incubated with either goat antirabbit conjugated horseradish peroxidase secondary antibodies (1:10,000; Santa Cruz Biotechnology) or goat antimouse IgG-horseradish peroxidase secondary antibodies (1:10,000; Santa Cruz Biotechnology). Antibody complexes were visualized by chemiluminescence detection (Immobilon Western, Millipore Corporation, Billerica, MA, USA) using Kodak BioMax film (Kodak, Rochester, NY, USA). Densitometry analyses were performed using the AlphaImager imaging system (Alpha Innotech, Cell Biosciences, Santa Clara, CA, USA).
Photoreceptor functional analysis
ERGs were performed on mice at P60 and P90, as previously described.25–27 ERG b-wave enhancement of maximal response was measured. The enhancement is defined as the difference in maximum ERG responses between transduced and control fellow eyes, measured in microvolts (μV).
Results
The bipartite lentiviruses used in this study are designed to express both PDE6β and shRNAs to knockdown either GUCY2E or CNGA1 in Pde6bH620Q photoreceptors. To measure the ability of the vectors to alter retinal gene expression, we performed immunoblot analysis on lysates prepared from C57BL6 retinas injected with H1::Gucy2e, Opsin::Gucy2e or H1::Cnga1 viral particles (Figure 2). Retinas transduced with H1::Gucy2e, Opsin::Gucy2e and H1::Cnga1 showed detectably higher PDE6β levels than contralateral uninjected retinas (Figures 2a–c). Vectors H1::Gucy2e and H1::Cnga1 also produced reductions in GUCY2E and CNGA1 expression, respectively (Figures 2a and c). Similarly, transduction of the Opsin::Gucy2e vector resulted in reduced expression of GUCY2E (Figure 2b). Expression of GNAT1, a rod-specific protein measured as a control, did not vary between controls and virus-transduced eyes (Figure 2). Numerical values were obtained after measuring densitometry of different proteins and each transduced retina was compared with its control (Figure 2d).
Figure 2.
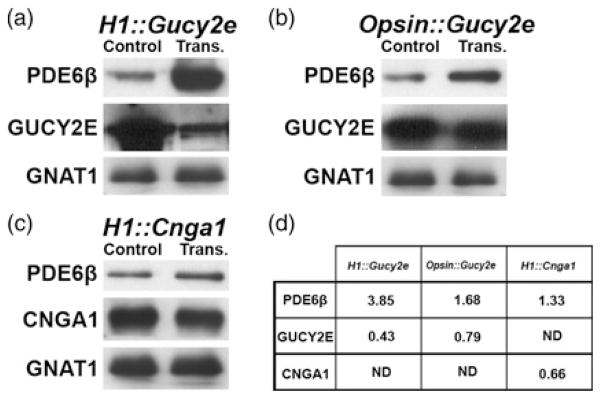
Immunoblotting analysis of retinal lysates from C57BL/6J mice transduced with bipartite vectors. PDE6β was increased after transduction with either H1::Gucy2e, Opsin::Gucy2e or H1::Cnga1 (a–c). GUCY2E is depressed in retinas transduced with H1::Gucy2e (a) and Opsin::Gucy2e (b) compared with controls. CNGA1 expression is reduced in retinas transduced with H1::Cnga1 (c) compared with controls. GNAT1 was used as control for photoreceptor protein content and its expression was not reduced by any of the three viruses. Intensities were determined by densitometry to calculate an integral density value for each band. The values were normalized to total protein and GNAT1 expression as a percentage of GNAT1 signal in 20 μg of control lysate (d). Samples from each experimental group were pooled in order to minimize variation resulting from differences in subretinal surgeries; immunoblot analyses were carried out in duplicate (average values shown). ND, not determined; trans., transduced
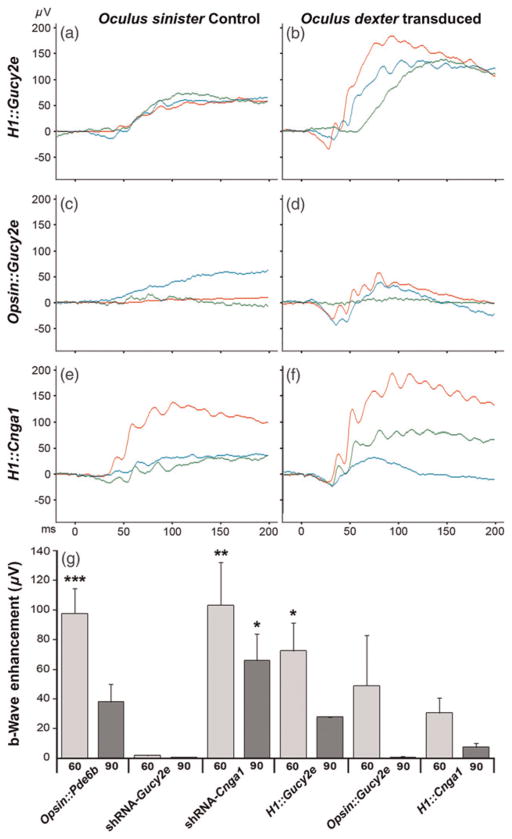
To measure the ability of the three vectors to retard Pde6bH620Q degeneration, we analyzed the consequences of vector transduction on photoreceptor function and morphology. ERG analysis was performed on injected and control eyes in Pde6bH620Q mice at P60 and P90 (Figure 3). In general, maximal ERG responses were higher for transduced than control eyes for all three vectors (Figures 3a–f). In particular, photoreceptor-generated a-waves and inner retina-mediated b-waves were observed at P60 in eyes injected with the bipartite lentiviruses (Figures 3b, d and f) but not in control untreated eyes (Figures 3a, c and e).
Figure 3.
Functional rescue due to bipartite vector transduction. Maximal dark-adapted electroretinograms (ERGs) were performed on both eyes (transduced and control) of Pde6bH620Q animals at approximately P60 and P90. In each ERG panel (a–f), the tracings represent different mice from the same treatment group. Compared with untreated controls (a, c, e), the H1::Gucy2e (b), Opsin::Gucy2e (d) and H1::Cnga1 (f) vectors improved visual function. Function was measured as both the photoreceptor-mediated a-wave and inner retina-mediated b-wave. ERG traces from three mice are shown for each virus. Enhancement of b-wave was calculated by comparing ERG values from transduced and untreated eyes. Results of the bipartite therapies described in this study (H1::Gucy2e, Opsin::Gucy2e and H1::Cnga1) were compared with previous results of the monopartite therapies (Opsin::Pde6b, shRNA-Gucy2e and shRNA-Cnga1)5,18 (g). Significant values: *P < 0.05, **P < 0.01, ***P < 0.001 (A color version of this figure is available in the online journal)
To compare the effectiveness of these newly created bipartite vectors to the previously tested monopartite vectors (Opsin::Pde6b, shRNA-Gucy2e, shRNA-Cnga1),5,18 we calculated b-wave enhancement values and compared them between all vectors (Figure 3g). We have previously shown that expression of wild-type PDE6β in Pde6bH620Q photoreceptors partially rescues the ERG phenotype (Opsin::Pde6b). Similarly, knockdown of CNGA1 by shRNA expression results in partial ERG rescue (shRNA-Cnga1). In contrast, shRNA knockdown of GUCY2E does not detectably restore Pde6bH620Q ERGs, although photoreceptor cell rescue was observed, likely due to the known effect of Gucy2e loss of function on photoreceptor physiology.20 In this study, we found that transduction of the new bipartite vectors resulted in partial Pde6bH620Q ERG rescue. H1::Gucy2e and Opsin::Gucy2e vectors produced higher b-wave enhancement than the shRNA-Gucy2e vector, suggesting that co-expression of PDE6β can mitigate the effect of Gucy2e knockdown on phototransduction. However, the apparent rescue of the b-wave was only significant (P = 0.0297) when the eye was transduced with the H1::Gucy2e vector (b-wave: 132.69 ± 26.02 μV), compared with the non-treated eye (b-wave: 61.17 ±7.53 μV). H1::Cnga1 b-wave enhancement was lower than either Opsin::Pde6b or shRNA-Cnga1, suggesting that simultaneous expression of wild-type PDE6β and knockdown of CNGA1 negatively affect Pde6bH620Q mutant photoreceptor function.
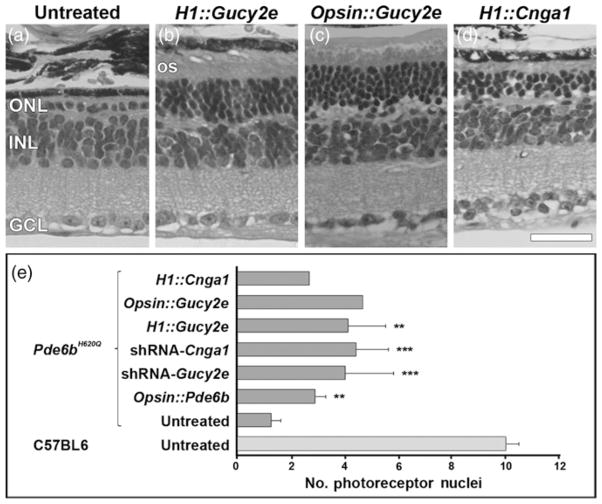
To confirm rescue of cell viability, we quantified the number of photoreceptor nuclear rows in the transduced retinas (Figure 4). In untreated control Pde6bH620Q retina, the photoreceptor layer contained one row of nuclei at week 8 (Figure 4a). Retinas transduced with bipartite vectors H1::Gucy2e, Opsin::Gucy2e and H1::Cnga1 showed greater than one row of photoreceptors in the injected portion of the retina (Figures 4b–d). In some cases the outer nuclear layer had around four rows of photoreceptor cells. Photoreceptors in transduced retinas showed outer segments that were not present in Pde6bH620Q untreated retinas at this age. The inner nuclear layer and ganglion cell layer remained intact in both treated and untreated retinas. Quantification of the number of nuclear rows for both single and bipartite vectors showed evidence of photoreceptor rescue when compared with untreated control (Figure 4e). Wild-type control mice had approximately 10 cell rows.
Figure 4.
Photoreceptor cell rescue due to bipartite vector transduction. Hematoxylin–eosin labeling in Pde6bH620Q retinal sections at P60 stained the different layers of the retina: ONL, INL, GCL (a–d). In non-treated Pde6bH620Q retinas, the ONL contained only a single row of photoreceptors (a). Treated retinas with H1::Gucy (b), Opsin::Gucy (c) and H1::Cnga1 (d) showed a higher number of photoreceptor rows within the ONL. Outer segments (OS) were also preserved in transduced but not in untreated retinas. Photoreceptor cell rescue in Pde6bH620Q using bipartite therapies described in this study (H1::Gucy2e, Opsin::Gucy2e, and H1::Cnga1) was compared with previous data obtained from the use of monopartite therapies (Opsin::Pde6b, shRNA-Gucy2e and shRNA-Cnga1) and from untreated Pde6bH620Q and healthy, untreated C57BL65,18 (e). ONL, outer nuclear layer; INL, inner nuclear layer; GCL, ganglion cell layer. Scale bar in (d) is 50 μm. Bars in (e) represent mean and SEM. Significant values: **P < 0.005, ***P < 0.001
Reduction of GUCY2E or CNGA1 expression in conjunction with an increase in PDE6β retarded photoreceptor cell degeneration. However, this bipartite expression did not provide dramatically greater cell rescue efficacy than conventional monogenic therapies.
Discussion
Defects in the beta (β) subunit of PDE6 result in increased levels of the signaling molecules cGMP and Ca2+ in both the Pde6brd1 and Pde6bH620Q mouse models of RP.5,9,14 Such increase of intracellular cGMP and Ca2+ levels is toxic to photoreceptors.28 Based on this understanding of the biochemical pathogenesis, we developed a therapeutic strategy using a lentivirus vector to express wild-type PDE6β in Pde6bH620Q mice.5 Even though photoreceptor cell count and function showed some rescue, they were not completely restored to wild-type levels. In our previous study, a control vector (CMV::GFP) was used as a negative control to demonstrate that the rescue resulted from the expression of the specific proteins being introduced, rather than from the presence in the rods of a virally introduced promoter.5 As an alternative approach, we studied whether knocking down the expression of GUCY2E would reduce the high cGMP concentration in order to avoid Ca2+ overdose. Similarly, we tested whether Cnga1 knockdown could prevent cation entry; we expected this to have the added benefit of not affecting cGMP levels. Our results of these alternative studies demonstrated that shRNA knockdown of GUCY2E or CNGA1 retards photoreceptor cell degeneration.18 However, the ability of each of the three monopartite vectors to retard retinal degeneration in Pde6bH620Q mice was limited. Our novel bipartite lentiviral vectors were designed to improve photoreceptor function and survival by providing functional PDE6β and concurrently decreasing abnormal cGMP and Ca2+ levels.
Here we have shown that some combined gene therapies can overcome the limitations of single therapies. Two outcomes were used to evaluate the effect of the therapies: cell survival and photoreceptor function. Since both the monopartite and bipartite methods resulted in increased cell survival, we compared the preservation of function by each in order to determine the relative effectiveness of the strategies.
The bipartite H1::Gucy2e vector is an improvement over the monopartite shRNA-Gucy2e vector. Gucy2e knockdown showed weaker rescue of ERG responses,18 which is similar to the Gucy2e knockout phenotype.20 Although shRNA-Gucy2e produces cell survival, these photoreceptors do not express sufficient PDE6β to elicit a significant response to light. By simultaneously introducing the wild-type Pde6bβ and Gucy2e shRNA, we partially corrected this. Because PDE6β decreases the level of cGMP and GUCY2E ultimately increases the level of cGMP, the two changes induced by this vector should be combined to improve the function more than the monopartite virus.
The bipartite Opsin::Gucy2e vector produces a lower ERG rescue than Opsin::Pde6b alone. PDE6β expression in C57BL6 mouse transduced retinas was not significantly higher than endogenous levels. There may not be sufficient PDE6β to reverse the effect of shRNA-Gucy2e. This may be caused by preferential processing of the chimeric Pde6b/Gucy2e transcript by the shRNA processing pathway at the expense of Pde6b cDNA translation. Future designs for this strategy need to improve Pde6b cDNA expression from this vector.
H1::Cnga1 was designed to simultaneously diminish the number of CNG channels, thereby reducing the entry of Ca2+ into rods, and express wild-type PDE6β, increasing cGMP breakdown. Even though the retina showed morphological retention after H1::Cnga1 transduction, the ERG responses remained much lower than in mice transduced with either Pde6b or Cnga1 monopartite vectors.5,18 These data suggest that simultaneous expression of wild-type PDE6β and CNGA1 knockdown negatively affects Pde6bH620Q mutant photoreceptor physiology. The combination of a reduction in CNG channel numbers and an increase in PDE6β activity may reduce Ca2+ to levels insufficient for efficient phototransduction.
Although our lentivirus vectors did produce some rescue, there are several improvements that must be achieved before these treatments can be translated from the lab to the clinic. These include efficient transduction of photoreceptors; early and appropriate expression of wild-type PDE6β (or shRNAs); prevention of vector silencing; and prevention of host immune responses to transduced cells. For example, we have previously shown low photoreceptor transduction rates using lentiviral vectors expressing LacZ,5 in agreement with a report of 5% photoreceptor transduction using other LacZ-expressing viral vectors.29 However, as shRNAs and siRNAs can be transported between cells through gap junctions,30,31 the degree of rescue may spread beyond the infected cells. In unpublished serial sections of rescued retinas, a majority of the sections proximal to the injection site contain rescued photoreceptors.
Another question to be addressed is which viral vector system might provide a more effective treatment. Most recently, Jing Pang et al.32 used AAV8 (Y733F) to achieve significant long-term rescue of the Pde6brd10 mutant phenotype for over six months, which is greater than the rescue achieved with previously tested AAV vectors. The high viral titer and photoreceptor transduction rates of AAV8 vectors may preclude the necessity for a combined therapy approach.
Acknowledgments
The research was funded by: Burroughs-Wellcome Program in Biomedical Sciences Fellow, Charles Culpeper Scholarship, Foundation Fighting Blindness, Hirschl Trust, Schneeweiss Stem Cell Fund, Joel Hoffmann Foundation, Jonas Family Fund, Crowley Research Fund, Jahnigen/Hartford/American Geriatrics Society, Eye Surgery Fund, Bernard Becker-Association of University Professors in Ophthalmology-Research to Prevent Blindness (RPB), Fight for Sight (FFS) and EY018213. C-SL is the Homer McK Rees Scholar.
Footnotes
Author contributions: SHT, RJD, C-SL and JT participated in the design of the study; RJD, JT, JS-P and CWH conducted the experiments; JT, JS-P, RJD, KVW, JDS and SHT interpreted the results; and JT, JS-P, RJD, KVW and SHT wrote the manuscript.
References
- 1.McLaughlin ME, Ehrhart TL, Berson EL, Dryja TP. Mutation spectrum of the gene encoding the beta subunit of rod phosphodiesterase among patients with autosomal recessive retinitis pigmentosa. Proc Natl Acad Sci USA. 1995;92:3249–53. doi: 10.1073/pnas.92.8.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bird AC. Retinal photoreceptor dystrophies: the LI. Edward Jackson Memorial Lecture. Am J Ophthalmol. 1995;119:543–62. doi: 10.1016/s0002-9394(14)70212-0. [DOI] [PubMed] [Google Scholar]
- 3.Hartong DT, Berson EL, Dryja TP. Retinitis pigmentosa. Lancet. 2006;368:1795–809. doi: 10.1016/S0140-6736(06)69740-7. [DOI] [PubMed] [Google Scholar]
- 4.Daiger SP, Bowne SJ, Sullivan LS. Perspective on genes and mutations causing retinitis pigmentosa. Arch Ophthalmol. 2007;125:151–8. doi: 10.1001/archopht.125.2.151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Davis RJ, Tosi J, Janisch KM, Kasanuki JM, Wang NK, Kong J, Tsui I, Cilluffo M, Woodruff ML, Fain GL, Lin CS, Tsang SH. Functional rescue of degenerating photoreceptors in mice homozygous for a hypomorphic cGMP phosphodiesterase 6 allele (Pde6bH620Q) Invest Ophthalmol Vis Sci. 2008;49:5067–76. doi: 10.1167/iovs.07-1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Barhoum R, Martinez-Navarrete G, Corrochano S, Germain F, Fernandez-Sanches L, de la Rosa EJ, de la Villa P, Cuenca N. Functional and structural modifications during retinal degeneration in the rd10 mouse. Neuroscience. 2008;155:698–713. doi: 10.1016/j.neuroscience.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 7.Gargini C, Terzibasi E, Mazzoni F, Strettoi E. Retinal organization in the retinal degeneration 10 (rd10) mutant mouse: a morphological and ERG study. J Comp Neurol. 2007;500:222–38. doi: 10.1002/cne.21144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sancho-Pelluz J, Arango-Gonzalez B, Kustermann S, Romero FJ, van Veen T, Zrenner E, Ekström P, Paquet-Durand F. Photoreceptor cell death mechanisms in inherited retinal degeneration. Mol Neurobiol. 2008;38:253–69. doi: 10.1007/s12035-008-8045-9. [DOI] [PubMed] [Google Scholar]
- 9.Farber DB, Lolley RN. Cyclic guanosine monophosphate: elevations in degenerating photoreceptor cells of the C3H mouse retina. Science. 1974;186:449–51. doi: 10.1126/science.186.4162.449. [DOI] [PubMed] [Google Scholar]
- 10.Chang B, Hawes NL, Hurd RE, Davisson MT, Nusinowitz S, Heckenlively JR. Retinal degeneration mutants in the mouse. Vision Res. 2002;42:517–25. doi: 10.1016/s0042-6989(01)00146-8. [DOI] [PubMed] [Google Scholar]
- 11.Chang B, Hawes NL, Pardue MT, German AM, Hurd RE, Davisson MT, Nusinowitz S, Rengarajan K, Boyd AP, Sidney SS, Phillips MJ, Stewart RE, Chaudhury R, Nickerson JM, Heckenlively JR, Boatright JH. Two mouse retinal degenerations caused by missense mutations in the beta-subunit of rod cGMP phosphodiesterase gene. Vision Res. 2007;47:624–33. doi: 10.1016/j.visres.2006.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Punzo C, Cepko C. Cellular responses to photoreceptor death in the rd1 mouse model of retinal degeneration. Invest Ophthalmol Vis Sci. 2007;48:849–57. doi: 10.1167/iovs.05-1555. [DOI] [PubMed] [Google Scholar]
- 13.Bowes C, Li T, Danciger M, Baxter LC, Applebury ML, Farber DB. Retinal degeneration in the rd mouse is caused by a defect in the beta subunit of rod cGMP-phosphodiesterase. Nature. 1990;347:677–80. doi: 10.1038/347677a0. [DOI] [PubMed] [Google Scholar]
- 14.Doonan F, Donovan M, Cotter TG. Activation of multiple pathways during photoreceptor apoptosis in the rd mouse. Invest Ophthalmol Vis Sci. 2005;46:3530–8. doi: 10.1167/iovs.05-0248. [DOI] [PubMed] [Google Scholar]
- 15.Fain GL, Lisman JE. Light, Ca2+, and photoreceptor death: new evidence for the equivalent-light hypothesis from arresting knockout mice. Invest Ophthalmol Vis Sci. 1999;40:2770–2. [PubMed] [Google Scholar]
- 16.Lisman J, Fain G. Support for the equivalent light hypothesis for RP. Nat Med. 1995;1:1254–5. doi: 10.1038/nm1295-1254. [DOI] [PubMed] [Google Scholar]
- 17.Bainbridge JW, Tan MH, Ali RR. Gene therapy progress and prospects: the eye. Gene Ther. 2006;13:1191–7. doi: 10.1038/sj.gt.3302812. [DOI] [PubMed] [Google Scholar]
- 18.Tosi J, Davis RJ, Wang NK, Naumann M, Lin CS, Tsang SH. shRNA knockdown of guanylate cyclase 2e or cyclic nucleotide gated channel alpha 1 increases photoreceptor survival in a cGMP phosphodiesterase mouse model of retinitis pigmentosa. J Cell Mol Med. 2011;15:1778–87. doi: 10.1111/j.1582-4934.2010.01201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hart AW, McKie L, Morgan JE, Gautier P, West K, Jackson IJ, Cross SH. Genotype-phenotype correlation of mouse pde6b mutations. Invest Ophthalmol Vis Sci. 2005;46:3443–50. doi: 10.1167/iovs.05-0254. [DOI] [PubMed] [Google Scholar]
- 20.Baehr W, Karan S, Maeda T, Luo DG, Li S, Bronson JD, Watt CB, Yau KW, Frederick JM, Palczewski K. The function of guanylate cyclase 1 and guanylate cyclase 2 in rod and cone photoreceptors. J Biol Chem. 2007;282:8837–47. doi: 10.1074/jbc.M610369200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rao MK, Wilkinson MF. Tissue-specific and cell type-specific RNA interference in vivo. Nat Protoc. 2006;1:1494–501. doi: 10.1038/nprot.2006.260. [DOI] [PubMed] [Google Scholar]
- 22.Xia XG, Zhou H, Samper E, Melov S, Xu Z. Pol II-expressed shRNA knocks down Sod2 gene expression and causes phenotypes of the gene knockout in mice. PLoS Genet. 2006;2:e10. doi: 10.1371/journal.pgen.0020010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Tsang SH, Burns ME, Calvert PD, Gouras P, Baylor DA, Goff SP, Arshavsky VY. Role of the target enzyme in deactivation of photoreceptor G protein in vivo. Science. 1998;282:117–21. doi: 10.1126/science.282.5386.117. [DOI] [PubMed] [Google Scholar]
- 24.Tsang SH, Chen J, Kjeldbye H, Li WS, Simon MI, Gouras P, Goff SP. Retarding photoreceptor degeneration in Pdegtm1/Pdegtm1 mice by an apoptosis suppressor gene. Invest Ophthamol Vis Sci. 1997;38:943–50. [PubMed] [Google Scholar]
- 25.Tsang SH, Woodruff ML, Jun L, Mahajan V, Yamashita CK, Pedersen R, Lin CS, Goff SP, Rosenberg T, Larsen M, Farber DB, Nusinowitz S. Transgenic mice carrying the H258N mutation in the gene encoding the beta-subunit of phosphodiesterase-6 (PDE6B) provide a model for human congenital stationary night blindness. Hum Mutat. 2007;28:243–54. doi: 10.1002/humu.20425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hood DG, Birch DG. Rod phototransduction in retinitis pigmentosa: estimation and interpretation of parameters derived from the rod a-wave. Invest Ophthalmol Vis Sci. 1994;35:2948–61. [PubMed] [Google Scholar]
- 27.Hood DC, Birch DG. A computational model of the amplitude and implicit time of the b-wave of the human ERG. Vis Neurosci. 1992;8:107–26. doi: 10.1017/s0952523800009275. [DOI] [PubMed] [Google Scholar]
- 28.Fox DA, Poblenz AT, He L. Calcium overload triggers rod photoreceptor apoptotic cell death in chemical-induced and inherited retinal degenerations. Ann N Y Acad Sci. 1999;893:282–5. doi: 10.1111/j.1749-6632.1999.tb07837.x. [DOI] [PubMed] [Google Scholar]
- 29.Kong J, Kim SR, Binley K, Pata I, Doi K, Mannik J, Zernant-Rajang J, Kan O, Iqball S, Naylor S, Sparrow JR, Gouras P, Allikmets R. Correction of the disease phenotype in the mouse model of Stargardt disease by lentiviral gene therapy. Gene Ther. 2008;15:1311–20. doi: 10.1038/gt.2008.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Valiunas V, Polosina YY, Miller H, Potapova IA, Valiuniene L, Doronin S, Mathias RT, Robinson RB, Rosen MR, Cohen IS, Brink PR. Connexin-specific cell-to-cell transfer of short interfering RNA by gap junctions. J Physiol. 2005;568:459–68. doi: 10.1113/jphysiol.2005.090985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Wolvetang EJ, Pera MF, Zuckerman KS. Gap junction mediated transport of shRNA between human embryonic stem cells. Biochem Biophys Res Commun. 2007;363:610–5. doi: 10.1016/j.bbrc.2007.09.035. [DOI] [PubMed] [Google Scholar]
- 32.Jing Pang J, Dai X, Boye SE, Barone I, Boye SL, Mao S, Everhart D, Dinculescu A, Liu L, Umino Y, Lei B, Chang B, Barlow R, Strettoi E, Hauswirth WW. Long-term retinal function and structure rescue using capsid mutant AAV8 vector in the rd10 mouse, a model of recessive retinitis pigmentosa. Mol Ther. 2011;19:234–42. doi: 10.1038/mt.2010.273. [DOI] [PMC free article] [PubMed] [Google Scholar]