Abstract
Individuals value the opportunity to make choices and exert control over their environment. This perceived sense of agency has been shown to have broad influences on cognition, including preference, decision-making, and valuation. However, it is unclear whether perceived control influences memory. Using a combined behavioral and functional magnetic resonance imaging approach, we investigated whether imbuing individuals with a sense of agency over their learning experience influences novel memory encoding. Participants encoded objects during a task that manipulated the opportunity to choose. Critically, unlike previous work on active learning, there was no relationship between individuals' choices and the content of memoranda. Despite this, we found that the opportunity to choose resulted in robust, reliable enhancements in declarative memory. Neuroimaging results revealed that anticipatory activation of the striatum, a region associated with decision-making, valuation, and exploration, correlated with choice-induced memory enhancements in behavior. These memory enhancements were further associated with interactions between the striatum and hippocampus. Specifically, anticipatory signals in the striatum when participants are alerted to the fact that they will have to choose one of two memoranda were associated with encoding success effects in the hippocampus on a trial-by-trial basis. The precedence of the striatal signal in these interactions suggests a modulatory relationship of the striatum over the hippocampus. These findings not only demonstrate enhanced declarative memory when individuals have perceived control over their learning but also support a novel mechanism by which these enhancements emerge. Furthermore, they demonstrate a novel context in which mesolimbic and declarative memory systems interact.
Keywords: agency, choice, fMRI, hippocampus, memory, striatum
Introduction
Decades of research have shown that active learning benefits declarative memory (Prince, 2004). Recent work has begun to investigate the mechanisms guiding active learning, focusing primarily on understanding the contribution of executive processes, such as re-study decisions (Voss et al., 2011b; Wing et al., 2013; Yee et al., 2014). Relatively less is known about how other important factors, such as decision-making, valuation, and exploration, contribute to the benefits of active learning. One factor that differs between active and passive learning is an individuals' sense of agency over their environment. Active learning gives individuals the ability to make choices and exert control over learning experiences. However, the specific influence of agency on declarative memory, i.e., perceived control over learning, and its underlying neurophysiology has yet to be investigated.
Perceived agency has broad influences on cognition, including preference formation and decision-making. Individuals assign more value to items they actively choose compared with items selected for them (Izuma and Murayama, 2013) and alter memories in service of previously made decisions (Mather et al., 2000). Furthermore, agency modulates the neural circuitry underlying valuation and decision-making, i.e., the mesolimbic dopamine system. Specifically, an individual's perception of agency is associated with dorsomedial and ventrostriatal activation (Tricomi et al., 2004; Tanaka et al., 2008; Leotti and Delgado, 2011). Although these studies show that agency engages brain regions linked with the mesolimbic dopamine system, the influence of this engagement on declarative memory remains unclear.
In other domains, mesolimbic activation has been shown to facilitate declarative memory and modulate hippocampal activation (Shohamy and Adcock, 2010). In rodents, dopamine has been shown to facilitate putative cellular markers of memory formation (for review, see Lisman et al., 2011). In humans, behavioral contexts that engage the mesolimbic dopamine system facilitate declarative memory encoding (Murayama and Kitagami, 2014; Murty and Adcock, 2014). For example, striatal responses to reward cues predict successful encoding of incentivized information (Wittmann et al., 2005; Adcock et al., 2006). These studies support that mesolimbic activation benefits declarative memory; however, whether a similar mechanism could enhance memory during active learning in the absence of extrinsic rewards remains unknown.
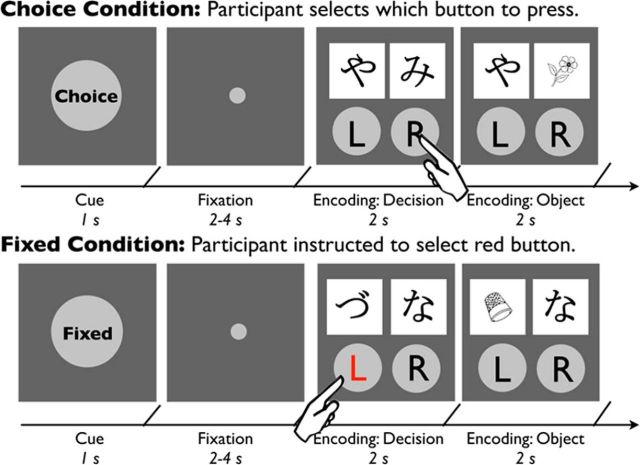
We investigate whether manipulating agency over a learning experience facilitates declarative memory. Participants performed an intentional encoding task in which they studied object images (Fig. 1). However, these images were presented on each trial underneath occluder screens. On each trial, participants made button presses to remove one of the occluders revealing the underlying object. We varied participants' agency over their learning experience by manipulating the opportunity to choose which screen was revealed on a trial-by-trial basis. In the choice condition, participants chose which of the two screens to remove, whereas in the fixed condition, participants were prompted to select a specific occluder. Critically, the memoranda on each trial were predetermined and trial unique; thus, participants' choices had no influence on the content of the memoranda because they were not choosing the objects but rather the occluder screens. We focused analyses to test whether neural signatures of active choice were associated with hippocampus-dependent memory.
Figure 1.
Overview of the choice encoding task. Participants had to press a button to remove occluder screens and reveal to-be encoded objects. In the choice condition (top), participants were able to decide which occluder screen to remove. In the fixed condition (bottom), participants were instructed to select the red button. Before the encoding phase, a condition cue appeared that informed the upcoming trial type. L, Left; R, right.
Materials and Methods
Participants.
For all studies, participants were recruited from the New York University and New York City communities. Informed consent was obtained from each participant in a manner approved by the University Committee on Activities Involving Human Subjects. For the fMRI study, 24 healthy, right-handed participants were paid $50 to participate. Two participants were excluded (one for failure to follow task instructions; one for poor neuroimaging data quality), yielding 22 participants (13 females; age range, 19–31 years; median, 22 years). For analyses including recognition memory, two additional participants were excluded because of a failure to complete the 24 h recognition memory test (12 females; age range, 19–31 years; median, 21.5 years). For the eye-tracking study, 16 healthy, right-handed participants were paid $25 to participate (nine females; age range, 18–35 years; median age, 23 years). One participant's eye-tracking data were non-usable because of equipment failure.
General procedure for the fMRI study.
Participants were first given instructions outside of the scanner. Once inside the scanner, participants performed a practice session, the pre-encoding rating task, four runs of the encoding task, and the post-encoding rating task. Twenty-four hours later, participants returned to a behavioral testing room and performed a recognition memory task.
Task details.
Before and after the encoding task, participants performed a rating task on the hiragana characters that would appear as the occluders. Each trial of the rating task consisted of the presentation of a single hiragana character in which participants had to indicate how much they liked each character on a scale of 1 to 5 (in which 1 was lowest rating and 5 was the highest rating; 80 characters; duration, 4 s; intertrial interval, 2–5 s). Sixty of the 80 most neutrally rated hiragana characters were then selected in pairs matched for preference to use as occluder screens during the encoding task.
After the first rating task, participants performed a choice encoding task to test for the effects of agency on declarative memory (Fig. 1). Each trial began with the presentation of a cue for 1 s indicating the condition (i.e., choice or fixed), followed by a fixation dot for 2–4 s and then an encoding phase for 4 s. The first half of the encoding phase, the decision component, consisted of the presentation of two occluder screens with a button centered beneath each occluder. Each occluder was labeled with a hiragana character, and each pair of occluders on a given trial presented hiragana characters matched for preference (see rating task). Participants were instructed to make a button press to remove one of the occluder screens within 2 s. Pairs of occluder hiragana characters were repeated six times in the same condition across the experiment, and the left/right position of each character was counterbalanced across trials. During the second half of the encoding phase, the object component, the selected occluder, was removed, and an underlying trial-unique object was revealed for 2 s. Participants were instructed to encode that object for a subsequent recognition memory test. In the choice condition, participants were given agency over which button they selected and thus which occluder screen was removed. In the fixed condition, participants were instructed to select the button that was highlighted with red text. Critically, unbeknownst to the participants, object images were predetermined by random selection. Individuals' choices had no effect on the content of what they were presented. A similar experimental paradigm has been used previously to investigate agency in the context of value-based decision-making (Leotti and Delgado, 2011, 2014). A fixation cross was presented between trials for 3–24 s. Trial onsets, cue-target intervals, and trial order were optimized using Optseq software (http://surfer.nmr.mgh.harvard.edu/optseq/). Trials in which participants failed to select a button or selected an incorrect button were removed from analysis (∼1% of trials). Participants completed 60 choice and 60 fixed trials (four 8-min runs). To help orthogonalize neural responses to the cue and encoding phases, participants were also presented with six cue-only trials consisting of only cues and fixation dots in the choice and fixed conditions. After encoding, participants performed 10 additional trials of encoding that included a modification to probe individuals' preference for the fixed versus choice condition. Following Leotti et al. (2011, 2014), on each trial, participants had 4 s to decide the condition of the upcoming trial (i.e., choice, fixed). After indicating their response, their chosen trial type appeared. Objects from these trials did not appear on the 24 h memory test. After completing this task, participants were removed from the scanner and completed a postscan questionnaire, which included a question about the perception of their encoding success in the fixed and choice conditions. Specifically, individuals indicated whether they “learned objects better in the choice or fixed condition” on a five point scale (1, more choice; 3, no difference; 5, more fixed).
After the encoding task, participants made preference ratings for Hiragana characters, allowing us to measure changes in choice-induced preference across conditions. We found that, in the choice, but not fixed, condition, participants showed greater preference for the characters they selected more often. Specifically, the number of times a character was selected linearly predicted changes in preferences ratings in the choice (p < 0.001), but not fixed, condition (p = 0.35). However, we did not find a within-subjects relationship between the number of times a character was selected previously and memory encoding success for underlying objects in either condition (p values >0.52). Given these findings, the number of times characters were repeated and preference changes were not considered in additional analyses.
At a 24 h delay, participants performed a self-paced, recognition task to test their memory for objects presented during the encoding task. Participants were shown an object image and had to first indicate whether they had previously seen the object image (yes/no and their confidence in their response, “very sure,” “pretty sure,” or “just guessing”). Test trials were self-paced and were followed by a 1 s intertrial interval. Participants completed 240 recognition memory trials: 60 objects from the choice condition, 60 objects from the fixed condition, and 120 novel/foil objects.
Behavioral analysis.
Recognition memory from the test phase was assessed by submitting the percentage of objects endorsed old to a paired t test with novelty status (old, new) as a within-subjects factor. Then object recognition was submitted to a paired t test with condition (choice, fixed) as a within-subjects factor. Reaction times (RTs) from the decision phase of the encoding task were submitted to a paired t test with condition (choice, fixed) as a within-subjects factor.
MRI data acquisition and preprocessing.
Functional imaging data were acquired on a Siemens Allegra 3T head-only scanner with a custom head coil (NM-011; Nova Medical) using an echo planar [echo planar imaging (EPI)] pulse sequence (echo time, 15 ms; flip angle, 82°; repetition time, 2000 ms; 34 contiguous slices; voxel size, 3 × 3 × 3 mm). Slices were positioned parallel to the anterior commissure–posterior commissure axis and included whole-brain coverage except for the most superior portions of the motor and parietal cortices. Each of the four functional runs consisted of 240 volumes. After collection of the functional runs, we collected a high-resolution T1-weighted anatomical scan (magnetization-prepared rapid-acquisition gradient echo sequence; voxel size, isotropic 1 mm) for use in spatial normalization.
Before fMRI preprocessing, data were inspected on custom software for head motion and scanning artifacts. Data were analyzed only if they exhibited <3.0 mm motion (absolute maximum). Slice acquisitions with isolated transient noise artifacts (i.e., scanner spiking) were replaced with interpolated data from neighboring time points. fMRI preprocessing was than performed using FEAT (for FMRIB fMRI Expert Analysis Tool) version 6.00 as implemented in FSL (for FMRIB Software Library) version 5.0.2.1. The first four scans of each run were discarded to allow for signal saturation. BOLD images were skull stripped using the Brain Extraction Tool (Smith, 2002). Images were then realigned within-run, intensity was normalized by a single multiplicative factor, spatially smoothed with a 5.0 mm full-width at half-maximum kernel, and subjected to a high-pass filter (Gaussian-weighted least-squares straight line fitting, with σ = 50.0 s). A relatively small smoothing kernel was selected because many of our areas of interest (i.e., striatum, hippocampus) are relatively small, and a larger smoothing kernel may have intermixed signals across discrete anatomical regions. Spatial normalization was performed using a two-step procedure on FMRIB (for Functional MRI of the Brain) Linear Registration Tool (Smith et al., 2004). First, mean EPIs from each run were coregistered to the high-resolution anatomical image. Then, the high-resolution anatomical image was normalized to the high-resolution standard space image in Montreal Neurological Institute (MNI) space using a nonlinear transformation with a 10 mm warp resolution, as implemented in FMRIB Non-Linear Registration Tool. All clusters are reported in MNI space with a voxel dimension of 2 × 2 × 2 mm.
General linear model: task-related activations.
To investigate differences in activation as a function of condition, we constructed first-level (within-run) general linear models (GLMs) that included four regressors that modeled choice cues, fixed cues, choice encoding, and fixed encoding. Cue regressors were modeled with event durations of 1 s and included cue-only trials. Encoding regressors were modeled with event durations of 4 s, collapsing over the decision and object phase. All regressors were convolved with a double-gamma hemodynamic response function. Using this GLM, individual maps of parameter estimates were generated for four contrasts of interest: (1) choice cue > baseline, (2) fixed cue > baseline, (3) choice > fixed cue, and (4) choice > fixed encoding. Second-level analyses for each of these contrasts (i.e., across runs, within subject) were modeled using a fixed-effect analysis. Parameter estimates from the choice cue > baseline and the fixed cue > baseline contrasts were extracted from the striatum to use in bivariate correlations with memory. To verify that the results from our choice > fixed encoding contrast were not driven by differences in RT, we verified the above analyses in a model that included regressors to account for trial-by-trial variation in RT.
GLM: memory success activations.
To investigate task-related activations as a function of condition and memory success, we constructed first-level GLMs that included seven regressors that modeled choice cues, fixed cues, subsequently remembered choice encoding trials, subsequently forgotten choice encoding trials, subsequently remembered fixed encoding trials, subsequently forgotten fixed encoding trials, and a nuisance regressor. Trials in which the encoded object was endorsed confidently as old during recognition (very sure, pretty sure) were considered subsequently remembered, whereas trials in which the encoded object was endorsed as new were considered subsequently forgotten. Trials in which object memoranda were endorsed as old but with low confidence (just guessing) were included in the nuisance regressor. All regressors were convolved with a double-gamma hemodynamic response function. Using this GLM, individual maps of parameter estimates were generated for two contrasts of interest: (1) choice remembered > forgotten events and (2) fixed remembered > forgotten events. Second-level analyses for each of these contrasts (i.e., across runs but within subject) were modeled using fixed-effect analyses.
GLM: single-trial cue-evoked striatal responses.
GLM results identified a functional region of interest (ROI) in the left striatum that responded more to choice versus fixed cues (p < 0.05, whole-brain corrected; see Results). For use in subsequent parametric analyses (detailed below), we modeled and extracted single-trial parameter estimates of striatal cue-evoked responses. Using an iterative GLM approach, a separate GLM was constructed to model the signal of the striatum in response to each single-cue event (i.e., 120 GLMs per participant). Each model consisted of a regressor modeling the single-trial cue event of interest and nuisance regressors modeling all other events. Each GLM was then fit to an extracted time series from the striatum, and t values were calculated for the regressor of interest (a single-trial response of the striatum to a cue), yielding single-trial parameter estimates for each of the 120 cue events.
GLM: cue-to-encoding interactions.
To investigate interactions between cue-evoked striatal signals and encoding-evoked activation on a trial-by-trial basis, first-level parametric GLMs were constructed that investigated the parametric modulation of encoding events by cue-evoked left striatum responses as a function of condition and memory success (for similar analyses, see Murty et al., 2012; Murty and Adcock, 2014). Specifically, we wanted to investigate how striatal modulation of encoding varies as a function of condition and memory success. Parametric GLMs were constructed that included four parametric regressors and seven standard task-related regressors. For parametric regressors, each encoding event amplitude was weighted by the preceding cue-evoked striatal activation from the same trial (extracted from single-trial modeling and then demeaned within run). Thus, these parametric regressors reflect how predictive cue-evoked striatal activations are of encoding activations on a trial-by-trial basis. Parametric regressors modeled striatal modulations over choice and fixed encoding events, separately for subsequently remembered and forgotten events. Standard regressors were identical to those described above (GLM: memory success activations). Using this GLM, individual maps of parameter estimates were generated for three contrasts of interest: (1) striatal–cue interactions for remembered choice > remembered fixed events, (2) striatal–cue interactions for remembered > forgotten choice events, and (3) striatal–cue interactions for remembered > forgotten fixed events. Of note, this cue-encoding analysis is statistically independent to the bivariate striatal/memory correlations depicted in Figure 3. The bivariate correlation models univariate signals from the striatum time-locked to the cue, whereas this analysis uses multivariate signals from the hippocampus time-locked to the encoding phase (although it measures how this signal is modulated by cue-evoked striatal signals). Furthermore, the bivariate correlation investigates memory across participants, whereas this analysis investigates memory within participants. To look for statistical differences in bivariate correlations across conditions, we submitted our bivariate correlations to permutation-based testing. Specifically, we randomly shuffled the condition variable (choice, fixed) within participants and permuted our data 10,000 times to build a null distribution of differences in correlations across conditions.
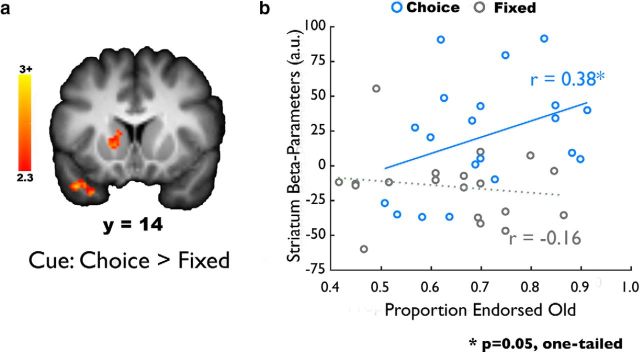
Figure 3.
Cue-evoked striatal activation correlates with choice memory. a, Choice cues were associated with greater left striatal activation compared with fixed cues. b, The striatal activation identified from the choice > fixed cue was used for an across-participants, brain-behavior correlation. Striatal activation to choice cues correlates with later object memory in the choice condition, although there is no relationship between striatal activation to fixed cues and object memory in the fixed condition. This relationship was significantly stronger than the correlation between striatal activation and memory in the fixed condition.
GLM: cue-evoked psychophysiological interactions.
To investigate whether striatal–hippocampal interactions before encoding contributed to memory success, we conducted a psychophysiological interaction (PPI) analyses with the striatum as the seed region. GLMs were constructed that included seven standard task-related regressors, one physiological regressor, and four PPI regressors. Task-related regressors modeled choice cues on subsequently remembered trials, choice cues on subsequently forgotten trials, fixed cues on subsequently remembered trials, fixed cues on subsequently forgotten trials, choice encoding trials, fixed encoding trials, and a nuisance regressor (i.e., trials in which individuals indicated just-guessing responses). The physiological regressor was a time course extracted from striatal clusters identified in the choice > fixed cue contrast. The PPI regressors multiplied the striatal physiological regressor against subsequently remembered and forgotten trials separately for the choice and fixed cues. Contrast of PPI regressors were compared for subsequently remembered versus forgotten trials for choice and fixed cues, respectively.
Group-level analysis.
We modeled group-level analyses using the mixed-effects analyses in FSL [FLAME 1 (for FMRIB Local Analysis of Mixed Effects)] and thresholded data to an overall α = 0.05 (familywise error rate) as calculated with AlphaSim tool in AFNI (for Automated Functional Neuro-Imaging; http://afni.nimh.nih.gov/afni/doc/manual/AlphaSim; height extant, p < 0.01, cluster extent > 77 voxels). ROI analyses were performed to probe interactions of the striatum with the hippocampus, which is involved with the encoding of object images. β parameters were extracted separately from a probabilistic atlas of the left and right hippocampus (Harvard–Oxford subcortical atlas, www.fmrib.ox.ac.uk/fsl/fslview/) using the probabilistic values as weights for the average.
Behavioral eye-tracking methods.
We ran an eye-tracking study in a separate behavioral cohort to investigate changes in looking time at occluder screens and memoranda across condition. The experimental protocol was identical to those described previously with the following exceptions. First, all phases of the task were conducted in a behavioral testing room. Second, memory recognition was tested both immediately and at a 24 h delay. Participants were tested on half of the memoranda during each recognition test. Finally, eye-tracking data were collected from participants during encoding. Eye movements were recorded at 1000 Hz with an infrared videographic camera equipped with a telephoto lens (Eyelink; SR Research). Eye-tracking data were collected from the left eye. Calibrations were performed before each encoding run. Before analysis, eye-tracking data were blink corrected by interpolation over the previous and subsequent 10 samples. Trials were only included if eye-tracking data were collected for >75% of the trial epoch. Looking time for each trial was calculated as (looking time in the visual ROI)/(total looking time at the screen). In other words, time periods when eye-tracking data were not collected were not considered in the looking-time measure.
Results
Behavioral results
At the 24 h memory test, participants showed significant differences across choice, fixed, and novel foil objects (F(19) = 2.76, p = 0.004). Post hoc t tests revealed significant memory for objects studied in both the choice and fixed condition (choice > novel foils, t(19) = 10.26, p < 0.001; fixed > novel foils, t(19) = 8.12, p < 0.001). Furthermore, objects in the choice condition were significantly better remembered than those studied in the fixed condition (t(19) = 3.54, p = 0.002; Fig. 2; Table 1), demonstrating that the opportunity to choose results in enhanced declarative memory. Choice-induced memory benefits remained significant when limiting the analysis to highest confidence memory (very sure response; t(19) = 2.35, p = 0.03). To determine whether these behavioral enhancements were consistent across the entire encoding session, we submitted our recognition memory data to repeated-measures ANOVA with condition (choice, fixed) and time of encoding (first half, second half) as within-subjects factors. We found a main effect of condition (F(1) = 12.94, p = 0.002), no significant effect of time (F(1) = 1.69, p = 0.21), and no time × condition interaction (F(1) = 0.09, p = 0.77), suggesting that memory enhancements were stable throughout the entire encoding session.
Figure 2.
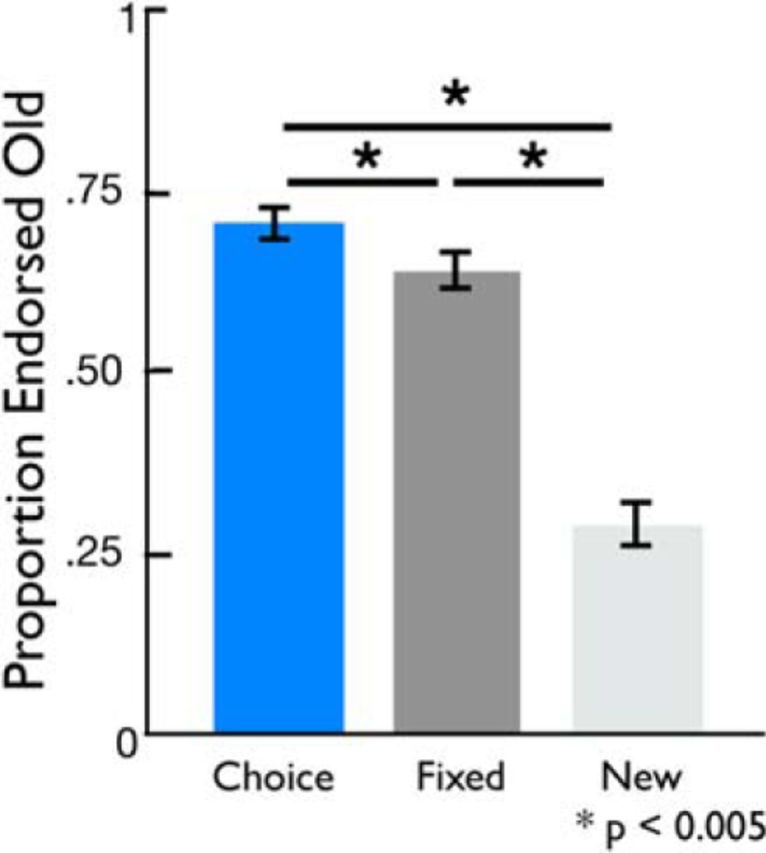
Choice influences declarative memory. At a 24 h test, object recognition was better for old objects presented in the choice compared with the fixed condition. Objects from both conditions were endorsed as old more often than novel foils.
Table 1.
Main effect of condition during the cue phase
| Region | x | y | z | Z | k |
|---|---|---|---|---|---|
| Cue: choice > fixed | |||||
| Left anterior temporal pole | −42 | 20 | −34 | 3.19 | 131 |
| Right anterior temporal pole | 40 | 24 | −32 | 3.9 | 90 |
| Left striatum | −16 | 14 | 2 | 2.84 | 77 |
| Cue: fixed > choice | |||||
| No significant activations |
x, y, and z are MNI coordinates, Z is peak z-score, and k is cluster size.
During the encoding scan, RTs to select occluder screens during the decision phase were faster in the fixed versus choice condition [choice, 1.08 ± 0.04 s (mean ± SE); fixed, 0.77 ± 0.03 s; t(21) = 10.97, p < 0.001]. Importantly, however, the object memoranda always appeared for a fixed amount of time in both experimental conditions (2 s). Furthermore, RTs to select an occluder screen were not related to subsequent memory performance either within subjects (p > 0.20) or across subjects (p > 0.25).
On day 1 after encoding, participants indicated their relative preference for condition types and also indicated whether they believed they were more successful at encoding in either condition. Preference was measured by looking at choice behavior in a short encoding task in which participants could choose the condition of the upcoming trials (choice, fixed), whereas perceived encoding success was measured using self-report in which participants indicated whether they learned objects better in the choice or fixed condition on a five point scale (1, more choice; 3, no difference; 5, more fixed). Despite significant enhancements in memory in the choice condition, participants did not indicate any differences in perceived encoding success across or a preference for condition type (p values >0.80). Furthermore, neither of these measures predicted memory enhancements across participants (p values >0.35).
fMRI results: anticipation of choice and memory
We computed a whole-brain contrast to identify regions exhibiting differential BOLD activity during choice and fixed trials. During the presentation of cues before any memoranda were presented, the choice cue compared with the fixed cue was associated with greater BOLD activation in the left striatum and bilateral anterior temporal lobes (p < 0.05, whole-brain corrected; Fig. 3; Table 1). The reverse contrast (fixed > choice cue) did not reveal any significant activations. To investigate whether individual differences in these striatal anticipatory signals were related to behavior, we regressed left striatal activation (defined from the contrast of choice > fixed cues) against memory performance across participants. We found that, during choice cues, left striatal activation was significantly related to memory for objects presented in the choice condition (simple regression, r = 0.38, p = 0.05, one-tailed). Permutation-based testing and robust regression replicated the results of the simple regression. However, there was no such relationship between left striatal activation and object memory in the fixed condition (r = −0.16; permutation-based testing, r = −0.001; robust regression of the striatum on memory, β = −0.03; all p values >0.40). Finally, a direct comparison revealed that the correlation between striatal activation and subsequent memory was significantly greater in the choice versus fixed condition (permutation-based testing, p = 0.04), suggesting that this relationship was unique to the choice condition. There was no relationship between anticipatory anterior temporal lobe activation and memory performance (p > 0.39).
fMRI: choice-related activations during encoding
The above findings focused on cue effects during the opportunity to choose. We next examined brain activation differences between the two conditions present during encoding (i.e., when object memoranda were revealed). During the object-encoding phase, choice was associated with greater activation in a broad network of regions, including the orbitofrontal cortex, lateral frontal cortex, hippocampus, and midbrain (overlapping with the ventral tegmental area, as defined by Murty et al., 2014). The opposite contrast (fixed > choice encoding) revealed activation in the bilateral frontal medial cortex, subgenual cingulate cortex, and left angular gyrus (p < 0.05, whole-brain corrected; Table 2). Differences in activation across conditions remained significant when covarying out trial-by-trial variation in RTs.
Table 2.
Main effect of condition during the encoding phase
| Region | x | y | z | Z | k |
|---|---|---|---|---|---|
| Encoding: choice > fixed | |||||
| Bilateral lateral occipital, precuneous, fusiform, inferior temporal, and middle temporal cortices | 16 | −72 | 50 | 5.15 | 20020 |
| Right insula, orbitofrontal, inferior frontal, middle frontal, and precentral cortices | 36 | 24 | 4 | 5.25 | 2980 |
| Left insula, orbitofrontal, inferior frontal, middle frontal, and precentral cortices | −34 | 18 | −6 | 4.83 | 2875 |
| Bilateral paracingulate, anterior cingulate, and supplementary motor cortices | 4 | 16 | 44 | 4.63 | 1260 |
| Bilateral thalamus, medial temporal lobe, and hippocampus | −12 | −18 | 10 | 4.09 | 598 |
| Bilateral posterior and middle cingulate cortices | −4 | −30 | 26 | 4.13 | 4.61 |
| Bilateral brainstem | 2 | −26 | −20 | 4.13 | 212 |
| Left middle frontal and superior frontal cortices and precentral gyrus | −24 | −2 | 48 | 3.44 | 172 |
| Left hippocampus and parahippocampal cortex | −28 | −8 | −32 | 3.04 | 89 |
| Encoding: fixed > choice | |||||
| Bilateral frontal medial and subgenual cingulate cortices | 6 | 54 | −2 | 3.83 | 749 |
| White matter | −16 | −44 | 16 | 3.79 | 481 |
| White matter | 22 | −40 | 20 | 3.28 | 210 |
| Lateral occipital cortex | −36 | −76 | 38 | 3.01 | 82 |
| Angular gyrus and lateral occipital cortex | −56 | −56 | 22 | 3.12 | 78 |
x, y, and z are MNI coordinates, Z is peak z-score, and k is cluster size.
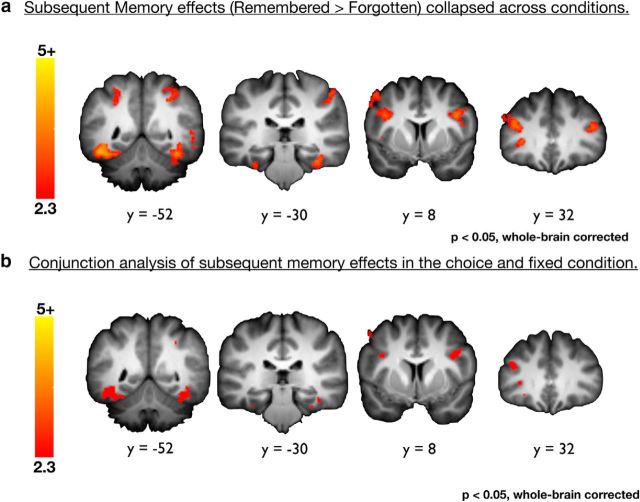
Despite the large differences in overall activation during encoding, subsequent memory effects were evident for both choice and fixed conditions in similar regions. Specifically, a network of regions, including the perirhinal cortex, a region known to mediate object encoding (Davachi, 2006; Staresina et al., 2011), displayed significant subsequent memory effects (remembered > forgotten) during the encoding phase (Fig. 4a; Table 3). These effects were equally robust in both the choice and fixed conditions (Fig. 4b). Thus, no differential subsequent memory effects between conditions were evident when considering activation only during object viewing [choice (remembered > forgotten) > fixed (remembered > forgotten)]. These results remained nonsignificant even when investigating anatomical ROIs in the hippocampus (left hippocampus, t(19) = 1.4, p = 0.18; right hippocampus, t(19) = 1.11, p = 0.28) suggesting that, on average, choice did not have a direct relationship on which brain regions were important for successful object encoding.
Figure 4.
Encoding-success activations are equivalent across conditions. a, Activations that predicted successful memory encoding during the encoding phase collapsed across conditions. b, Conjunction analysis of activations that predict successful memory encoding during the encoding phase in the choice and fixed conditions, respectively.
Table 3.
Regions showing a subsequent memory effect collapsed across condition (remembered > forgotten trials)
| Region | x | y | z | Z | k |
|---|---|---|---|---|---|
| Left inferior temporal gyrus and lateral occipital cortex | −44 | −50 | −14 | 4.34 | 2473 |
| Right lateral occipital and fusiform cortices | 34 | −70 | 34 | 4.09 | 2332 |
| Left middle frontal gyrus, precentral gyrus, and inferior frontal gyrus | −50 | 6 | 52 | 3.58 | 503 |
| Left middle frontal gyrus, inferior frontal gyrus, and frontal pole | −40 | 30 | 24 | 4.18 | 439 |
| Right lateral occipital cortex | 34 | −90 | 0 | 3.35 | 279 |
| Right precentral gyrus, inferior frontal gyrus, and middle frontal gyrus | 42 | 10 | 28 | 3.8 | 277 |
| Bilateral paracingulate and cingulate cortices | −4 | 16 | 48 | 3.16 | 133 |
| Left insula and putamen | −32 | 4 | 0 | 2.88 | 96 |
| Right middle frontal gyrus, frontal pole, and inferior frontal gyrus | 44 | 34 | 20 | 3.31 | 92 |
| Right supramarginal gyrus and postcentral gyrus | 54 | −30 | 54 | 3.08 | 83 |
x, y, and z are MNI coordinates, Z is peak z-score, and k is cluster size.
fMRI: interactions between cue-evoked striatal activity and hippocampus-dependent encoding
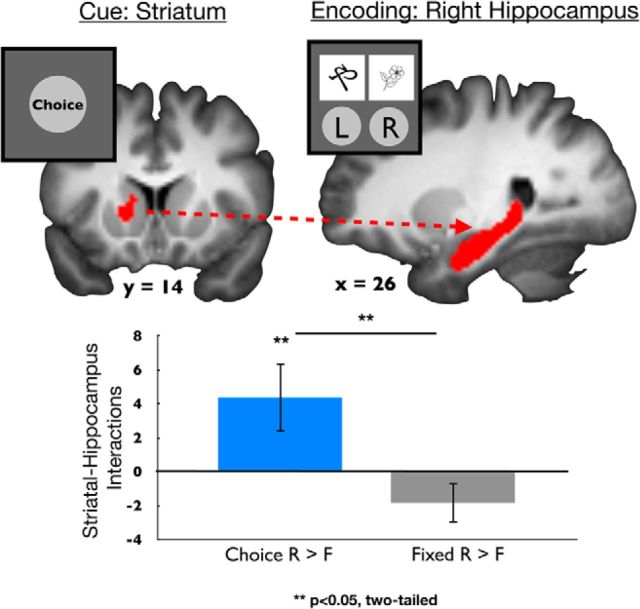
Our previous analysis revealed that, across participants, anticipatory striatal activation during the choice cue is related to memory encoding for the subsequently presented object in the choice, but not the fixed, condition. However, it is unlikely that this is a direct relationship because it is known that the striatum cannot support declarative memory on its own (Poldrack and Packard, 2003). Thus, we next asked whether there was a trial-by-trial relationship between cue-evoked striatal activity and object-evoked subsequent memory effects. Thus, we used a parametric analysis that queried the relationship between cue-evoked striatal activation and subsequent activation during the encoding phase in an anatomically defined ROI in the hippocampus, a region known to be critical in episodic memory formation (Davachi, 2006; Ranganath, 2010). Specifically, we used single-trial parameter estimates of cue-evoked striatal responses to predict activation during the subsequent encoding phase of the task. We found that cue-evoked striatal activity was associated with right hippocampal activation during the encoding of successfully remembered objects in the choice versus fixed condition (right hippocampus, t(19) = 2.55, p = 0.02), with a similar trend in the left hippocampus (left hippocampus, t(19) = 1.88, p = 0.08). Furthermore, these interactions were specific to successfully encoded items, such that the correlation between the striatum and right hippocampus was greater for successful memory encoding (i.e., remembered > forgotten) in the choice condition (t(19) = 2.16, p = 0.04; Fig. 5) but not the fixed condition (t(19) = −1.56, p = 0.14; Fig. 5). A direct comparison of this encoding success effect showed that these interactions were significantly greater in the choice condition compared with the fixed condition (choice > fixed, t(19) = 2.41, p = 0.03). In other words, during the choice condition, better subsequent memory was seen for objects presented on trials in which there was greater cue-to-encoding coupling between the striatum at the cue and right hippocampus during the subsequent encoding phase. These effects were directionally similar but nonsignificant in the left hippocampus (left hippocampus: choice, t(19) = 1.67, p = 0.11; fixed, t(19) = −0.13, p = 0.89; choice > fixed, t(19) = 1.57, p = 0.13). To further address the role of hippocampal laterality, we submitted encoding-success connectivity to a repeated-measures ANOVA with condition (choice, fixed) and laterality (left, right) as within-subject factors. This analyses revealed a significant effect of condition (F(1) = 4.30, p = 0.05), no significant effect of laterality (F(1) = 2.57, p = 0.17), and a significant laterality × condition interaction (F(1) = 33.74, p = 0.04). Whole-brain exploratory analysis did not reveal any additional regions outside of the hippocampus that demonstrated this pattern of results.
Figure 5.
Cue-evoked striatal activation interacts with the hippocampus during successful memory encoding. On a trial-by-trial basis, correlations between cue-evoked activation in the left (L) striatum and encoding-related activation in the right (R) hippocampus was associated with successful memory encoding in the choice, but not fixed, condition. R, Remembered trials; F, forgotten trials.
Finally, we asked whether striatal–hippocampal interactions were temporally specific to cue-evoked striatal activation and encoding-evoked hippocampus activation or whether striatal–hippocampal coupling during the presentation of the cue alone predicted successful memory encoding. Thus, we conducted a PPI analyses of striatal–hippocampal coupling during cue presentations and found that coupling between the striatum and hippocampus at the time of presentation of the cue did not predict memory in either the choice (p values >0.33) or the fixed (p values >0.56) conditions.
Eye tracking: viewing time of occluder screens and memoranda
One alternative explanation for our behavioral effects is that participants may spend a longer time viewing the memoranda presented on choice trials simply out of curiosity or investment. Thus, although memoranda were always presented for 2 s, it is possible that actual viewing times differed in a systematic manner. To rule this out, we conducted an additional eye-tracking study in a separate behavioral cohort (n = 16; see Materials and Methods). Critically, we replicated the behavioral effect. At the 24 h memory test, participants showed significant differences across choice, fixed, and novel foil objects (F(19) = 3.97, p < 0.001). Post hoc t tests revealed significant memory for objects studied in both the choice and fixed conditions (choice > novel foils, t(19) = 12.94, p < 0.001; fixed > novel foils, t(19) = 10.13, p < 0.001). Furthermore, objects in the choice condition were significantly better remembered than those studied in the fixed condition (t(19) = 2.93, p = 0.01). Eye-tracking data were analyzed to calculate the percentage of time participants were viewing both the occluder screens and the underlying revealed objects. During the decision period when participants were viewing the occluder screens only but no memoranda were visible on the screen, the time viewing the occluder screens was longer in the choice versus fixed condition [choice, 81.7 ± 1.52% (mean ± SEM); fixed, 78.6 ± 2.3%; difference, t(14) = 2.17, p = 0.05], which is expected given the response demands of the choice condition. Critically, however, during the encoding period (when memoranda appeared on the screen), viewing time of the revealed objects was greater in the fixed versus control condition [choice, 66.4 ± 3.3% (mean ± SEM); fixed, 78.6 ± 2.8%; difference, t(14) = 4.01, p = 0.001]. Thus, these results suggest that the amount of time viewing object memoranda (i.e., a simple attentional account) does not account for the memory enhancements seen in the choice condition because looking times were greater in the fixed condition.
Discussion
Active learning has long-lasting positive benefits for declarative memory, but the neural mechanisms guiding these enhancements remain an open area of research. Here, we provide evidence for a novel mechanism by which memory encoding during active learning is enhanced, by imbuing individuals with a sense of agency over their learning experience. We found that giving individuals the opportunity to make a simple choice during encoding enhanced subsequent memory, even when those choices did not influence the content of the memoranda. Using human neuroimaging, we provide evidence that memory enhancements were related to neural measures of choice-related processes. Specifically, fMRI data revealed that choice-induced memory enhancements were associated positively with anticipatory activation in the left striatum. Furthermore, trial-by-trial interactions between the striatum and the hippocampus were associated with successful encoding only when individuals had to the opportunity to choose. Together, these findings support a model in which an individual's perceived sense of agency during learning engages interactions between striatal activation and hippocampal-dependent encoding to facilitate active-learning memory benefits.
Previous work has shown that control over the content of what is studied and when it is studied can modulate long-term memory (Voss et al., 2011a, 2011b). The interpretation of these results is that memory enhancements are related to the ability to control the content and structure of the learning experience, such as the ability to revisit previously studied items. Here, we offer a novel, complementary mechanism that supports these enhancements by demonstrating that the perceived sense of agency, independent of actual control over the content of memoranda, contributes to the mnemonic benefits of active learning. Our data show that long-term memory (at 24 h) is enhanced for objects revealed in the choice condition during which participants are choosing the occluder screens but not the memoranda revealed underneath those screens. In addition to the content selection being matched, in the current design, all features of encoding across the choice and fixed conditions were well controlled such that motor demands, potential viewing time of memoranda, and experimental timing were matched. Furthermore, in a separate eye-tracking study, we replicated our behavioral findings and demonstrated that memory enhancements could not be explained simply by participants viewing the object memoranda for greater periods of time in the choice condition. These findings suggest that mechanisms beyond simple attention to object memoranda support the influence of agency on declarative memory.
First, fMRI data revealed that the opportunity to choose was associated with greater anticipatory activation in the striatum. Striatal activation has long been associated with many behavioral processes that are likely relevant to active learning processes in our current task, including response selection (Balleine and O'Doherty, 2010), choice-induced preference (Sharot et al., 2009; Izuma et al., 2010; Fujiwara et al., 2013; Cockburn et al., 2014), perceived agency (Leotti and Delgado, 2011, 2014), goal-directed learning (Wise, 2004), and valuation (Bartra et al., 2013). We extend these results by showing that anticipatory striatal activations when participants know they will have the opportunity to choose are significantly correlated with individual differences in memory in the choice, but not the fixed, condition. Future research will have to specify how the variety of behavioral phenomena associated previously with striatal activation, such as response selection, goal-directed decision-making, and valuation, contribute to our memory benefits. Second, our fMRI results also revealed that successful memory encoding during the choice condition was associated with interactions between the striatum and hippocampus. Specifically, memory encoding was more successful on trials in which there was greater coupling between cue-evoked striatal activation and subsequent encoding-related hippocampal activation. In other words, striatal activity was associated with hippocampal activity to a greater extent during the successful encoding of objects from the choice versus fixed condition. Of note, this coupling was measured across different stages of the trial such that activation of the striatum temporally preceded that in the hippocampus. Interestingly, the temporal dynamics of these findings are in line with proposed models of how the mesolimbic dopamine and declarative memory systems may interact during encoding. Specifically, multiple prominent models propose that engagement of the mesolimbic dopamine system can “prepare” the hippocampus for successful declarative memory encoding by both modulating hippocampal physiology and enhancing synaptic plasticity (Shohamy and Adcock, 2010; Lisman et al., 2011). Thus, the relationship between cue-evoked striatal activation and subsequent hippocampal encoding effects are consistent with the notion that striatal activity may serve as a precursor to mediating successful encoding effects. Previous neuroimaging studies have demonstrated that functional coupling between the striatum and hippocampus can support declarative memory both during recombining word pairs (Sadeh et al., 2011) and rewarded decision-making (Wimmer et al., 2014). Thus, our findings further support that these two systems can interact to support successful memory encoding and extend these findings by demonstrating a temporal sequence of striatal activation preceding hippocampal activation to support declarative memory encoding. Furthermore, these results offer a novel context in which these systems may interact specifically during simple, non-rewarded, decision-making. Interpretive caution is warranted here because the current study measured fMRI activation, which cannot measure changes in dopaminergic tone. However, it is important to note that the dopaminergic midbrain (i.e., the ventral tegmental area) also showed greater activation during choice versus fixed encoding. Thus, future studies using pharmacological manipulations or positron emission tomography imaging will be needed to confirm the role of dopamine in guiding choice-induced memory enhancements.
In addition to the striatal–hippocampal effects noted above, we also found widespread differences in brain activation during the encoding period of the choice versus fixed condition. Specifically, we found that greater activation was evident in the ventral visual stream, dorsolateral prefrontal cortex, insula, medial prefrontal cortex, and midbrain on choice trials compared with fixed trials. Interestingly, a recent study investigating active learning also found greater univariate BOLD activations in many of these regions, including the medial prefrontal cortex, ventral visual stream, and midbrain (Voss et al., 2011b), as well as greater hippocampal connectivity with the precuneus, dorsolateral prefrontal cortex, and medial temporal lobe. The replication of greater engagement throughout this network lends support for a critical role of these regions during active learning. In this previous study and in our study, the univariate activations in these regions were not differentially related to the mnemonic benefits of active learning. Future research will need to identify the role of these regions during encoding to better understand the mechanisms supporting active-learning benefits.
Although the current results highlight a role for agency in the mnemonic benefits of active learning, previous work has shown that giving individuals actual control over their learning environment, such as what and when they encode, also leads to declarative memory benefits (Kornell and Metcalfe, 2006; Voss et al., 2011b). However, active learning likely enhances memory through multiple mechanisms, and understanding those mechanisms is critical to developing better applications of this knowledge. For example, the above described study on active control of memory encoding demonstrated that memory benefits were associated with hippocampal–cortical interactions (Voss et al., 2011b), a mechanism distinct from the hippocampal–striatal interactions we describe. Follow-up work also showed that this memory benefit was found specifically for items that were spontaneously “revisited,” suggesting that meta-memory-based selection of specific memoranda was likely responsible for memory benefits under volitional learning conditions. However, our findings suggest that active learning may also rely on processes that shift individuals' orientation to learning as opposed to the content of learning. For example, in our paradigm, the content and potential viewing time of memoranda was fixed, but the simple perception of control over their learning experience enhanced individuals' memory. In line with this interpretation, recent evidence has demonstrated that removing the ability to select what to study and allowing participants to solely select when to view memoranda is sufficient to enhance memory (Markant et al., 2014). Importantly, the current paradigm assessed whether a perceived sense of agency, in isolation, was sufficient to enhance memory by controlling for other factors that influence true agency, such as allowing participants to select when to study. Remarkably, we see that even the simplest form of choice can have a lasting consequence on memory, and, thus, together with previous work on active learning, motivates investigation of how different mechanisms may interact during active learning to support memory benefits.
In conclusion, our findings demonstrate enhanced declarative memory when individuals have control over their learning environment. Furthermore, we provide evidence for anticipatory activation of the striatum and interactions between the striatum and hippocampus. With respect to the active learning literature, these findings present a novel mechanism that supports active learning benefits (the manipulation of individuals perceived sense of agency) and highlight the potential role of the mesolimbic dopamine system in guiding active learning benefits. Furthermore, this enhancement in learning is initiated by a simple, non-invasive manipulation, changing an individual's sense of control over his or her learning experiences. Thus, our results highlight a mechanism of enhancing memory that may be applicable to a variety of contexts, including implementation in educational settings and remediation for memory-disorder populations.
Footnotes
This work was supported by National Institutes of Health Grant RO1 MH074692 (L.D.) and National Institutes of Health Grant F32 DA036361 (V.P.M.). We thank Clayton Curtis and Wayne Mackey for their assistance on the eye-tracking study.
References
- Adcock RA, Thangavel A, Whitfield-Gabrieli S, Knutson B, Gabrieli JDE. Reward-motivated learning: mesolimbic activation precedes memory formation. Neuron. 2006;50:507–517. doi: 10.1016/j.neuron.2006.03.036. [DOI] [PubMed] [Google Scholar]
- Balleine BW, O'Doherty JP. Human and rodent homologies in action control: corticostriatal determinants of goal-directed and habitual action. Neuropsychopharmacology. 2010;35:48–69. doi: 10.1038/npp.2009.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartra O, McGuire JT, Kable JW. The valuation system: a coordinate-based meta-analysis of BOLD fMRI experiments examining neural correlates of subjective value. Neuroimage. 2013;76:412–427. doi: 10.1016/j.neuroimage.2013.02.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cockburn J, Collins AGE, Frank MJ. A reinforcement learning mechanism responsible for the valuation of free choice. Neuron. 2014;83:551–557. doi: 10.1016/j.neuron.2014.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davachi L. Item, context and relational episodic encoding in humans. Curr Opin Neurobiol. 2006;16:693–700. doi: 10.1016/j.conb.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Fujiwara J, Usui N, Park SQ, Williams T, Iijima T, Taira M, Tsutsui K, Tobler PN. Value of freedom to choose encoded by the human brain. J Neurophysiol. 2013;110:1915–1929. doi: 10.1152/jn.01057.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuma K, Murayama K. Choice-induced preference change in the free-choice paradigm: a critical methodological review. Front Psychol. 2013;4:41. doi: 10.3389/fpsyg.2013.00041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Izuma K, Matsumoto M, Murayama K, Samejima K, Sadato N, Matsumoto K. Neural correlates of cognitive dissonance and choice-induced preference change. Proc Natl Acad Sci U S A. 2010;107:22014–22019. doi: 10.1073/pnas.1011879108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornell N, Metcalfe J. Study efficacy and the region of proximal learning framework. J Exp Psychol Learn Mem Cogn. 2006;32:609–622. doi: 10.1037/0278-7393.32.3.609. [DOI] [PubMed] [Google Scholar]
- Leotti LA, Delgado MR. The inherent reward of choice. Psychol Sci. 2011;22:1310–1318. doi: 10.1177/0956797611417005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leotti LA, Delgado MR. The value of exercising control over monetary gains and losses. Psychol Sci. 2014;25:596–604. doi: 10.1177/0956797613514589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lisman J, Grace AA, Duzel E. A neoHebbian framework for episodic memory; role of dopamine-dependent late LTP. Trends Neurosci. 2011;34:536–547. doi: 10.1016/j.tins.2011.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markant D, DuBrow S, Davachi L, Gureckis TM. Deconstructing the effect of self-directed study on episodic memory. Mem Cognit. 2014;42:1211–1224. doi: 10.3758/s13421-014-0435-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, Shafir E, Johnson MK. Misremembrance of options past: source monitoring and choice. Psychol Sci. 2000;11:132–138. doi: 10.1111/1467-9280.00228. [DOI] [PubMed] [Google Scholar]
- Murayama K, Kitagami S. Consolidation power of extrinsic rewards: Reward cues enhance long-term memory for irrelevant past events. J Exp Psychol Gen. 2014;143:15–20. doi: 10.1037/a0031992. [DOI] [PubMed] [Google Scholar]
- Murty VP, Adcock RA. Enriched encoding: reward motivation organizes cortical networks for hippocampal detection of unexpected events. Cereb Cortex. 2014;24:2160–2168. doi: 10.1093/cercor/bht063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, Labar KS, Adcock RA. Threat of punishment motivates memory encoding via amygdala, not midbrain, interactions with the medial temporal lobe. J Neurosci. 2012;32:8969–8976. doi: 10.1523/JNEUROSCI.0094-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murty VP, Shermohammed M, Smith DV, Carter RM, Huettel SA, Adcock RA. Resting state networks distinguish human ventral tegmental area from substantia nigra. Neuroimage. 2014;100:580–589. doi: 10.1016/j.neuroimage.2014.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poldrack RA, Packard MG. Competition among multiple memory systems: converging evidence from animal and human brain studies. Neuropsychologia. 2003;41:245–251. doi: 10.1016/S0028-3932(02)00157-4. [DOI] [PubMed] [Google Scholar]
- Prince M. Does active learning work? A review of the research. J Eng Educ. 2004;93:223–231. doi: 10.1002/j.2168-9830.2004.tb00809.x. [DOI] [Google Scholar]
- Ranganath C. A unified framework for the functional organization of the medial temporal lobes and the phenomenology of episodic memory. Hippocampus. 2010;20:1263–1290. doi: 10.1002/hipo.20852. [DOI] [PubMed] [Google Scholar]
- Sadeh T, Shohamy D, Levy DR, Reggev N, Maril A. Cooperation between the hippocampus and the striatum during episodic encoding. J Cogn Neurosci. 2011;23:1597–1608. doi: 10.1162/jocn.2010.21549. [DOI] [PubMed] [Google Scholar]
- Sharot T, De Martino B, Dolan RJ. How choice reveals and shapes expected hedonic outcome. J Neurosci. 2009;29:3760–3765. doi: 10.1523/JNEUROSCI.4972-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shohamy D, Adcock RA. Dopamine and adaptive memory. Trends Cogn Sci. 2010;14:464–472. doi: 10.1016/j.tics.2010.08.002. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TEJ, Johansen-Berg H, Bannister PR, De Luca M, Drobnjak I, Flitney DE, Niazy RK, Saunders J, Vickers J, Zhang Y, De Stefano N, Brady JM, Matthews PM. Advances in functional and structural MR image analysis and implementation as FSL. Neuroimage. 2004;23(Suppl 1):S208–S219. doi: 10.1016/j.neuroimage.2004.07.051. [DOI] [PubMed] [Google Scholar]
- Staresina BP, Duncan KD, Davachi L. Perirhinal and parahippocampal cortices differentially contribute to later recollection of object- and scene-related event details. J Neurosci. 2011;31:8739–8747. doi: 10.1523/JNEUROSCI.4978-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka SC, Balleine BW, O'Doherty JP. Calculating consequences: brain systems that encode the causal effects of actions. J Neurosci. 2008;28:6750–6755. doi: 10.1523/JNEUROSCI.1808-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tricomi EM, Delgado MR, Fiez JA. Modulation of caudate activity by action contingency. Neuron. 2004;41:281–292. doi: 10.1016/S0896-6273(03)00848-1. [DOI] [PubMed] [Google Scholar]
- Voss JL, Gonsalves BD, Federmeier KD, Tranel D, Cohen NJ. Hippocampal brain-network coordination during volitional exploratory behavior enhances learning. Nat Neurosci. 2011a;14:115–120. doi: 10.1038/nn.2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voss JL, Warren DE, Gonsalves BD, Federmeier KD, Tranel D, Cohen NJ. Spontaneous revisitation during visual exploration as a link among strategic behavior, learning, and the hippocampus. Proc Natl Acad Sci U S A. 2011b;108:E402–E409. doi: 10.1073/pnas.1100225108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wimmer GE, Braun EK, Daw ND, Shohamy D. Episodic memory encoding interferes with reward learning and decreases striatal prediction errors. J Neurosci. 2014;34:14901–14912. doi: 10.1523/JNEUROSCI.0204-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing EA, Marsh EJ, Cabeza R. Neural correlates of retrieval-based memory enhancement: an fMRI study of the testing effect. Neuropsychologia. 2013;51:2360–2370. doi: 10.1016/j.neuropsychologia.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Dopamine, learning and motivation. Nat Rev Neurosci. 2004;5:483–494. doi: 10.1038/nrn1406. [DOI] [PubMed] [Google Scholar]
- Wittmann BC, Schott BH, Guderian S, Frey JU, Heinze HJ, Düzel E. Reward-related FMRI activation of dopaminergic midbrain is associated with enhanced hippocampus-dependent long-term memory formation. Neuron. 2005;45:459–467. doi: 10.1016/j.neuron.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Yee LTS, Warren DE, Voss JL, Duff MC, Tranel D, Cohen NJ. The hippocampus uses information just encountered to guide efficient ongoing behavior. Hippocampus. 2014;24:154–164. doi: 10.1002/hipo.22211. [DOI] [PMC free article] [PubMed] [Google Scholar]