Abstract
Selective immunoglobulin E (IgE) deficiency (IgED) is defined as serum levels of IgE more than or equal to 2 kIU/L and is associated with immune dysregulation and autoimmunity. This study aimed to investigate a prevalence of atherosclerotic cardiovascular disease (ASCVD) in population with IgED. Within the electronic patient record (EPR) database of Leumit Health Care Services (LHS) in Israel, data capture was performed using IBM Cognos 10.1.1 BI Report Studio software. The case samples were drawn from the full study population (n = 18,487), having any allergy-related symptoms and/or those requesting antiallergy medications and performed serum total IgE measurement during 2012 at LHS. All subjects aged more than or equal to 40 years old, with serum total IgE less than 2 kIU/L were included in case group. Control group was randomly sampled from the remained subjects, with a case-control ratio of 10 controls for each case (1:10). The comorbid cardiovascular diseases during less than or equal to 10 years before serum total IgE testing were identified and retrieved using specific International Classification of Diseases, 9th Revision, Clinical Modification diagnostic codes. There were 103 in case and 1030 subjects in control group. Compared with control group patients, the case group had significantly more arterial hypertension [34 (37.7%) versus 187 (18.2%), p < 0.001], ischemic heart disease (IHD) [26 (25.2%) versus 87 (8.4%), p < 0.001], carotid stenosis [5 (4.9%) versus 7 (0.7%), p = 0.003], cerebrovascular disease (CVD) [3 (2.9%) versus 5 (0.5%), p = 0.029], and peripheral vascular disease (PVD) [4 (3.9%) versus 9 (0.9%), p = 0.024]. IgED is associated with higher prevalence of arterial hypertension and ASCVD.
Keywords: Selective, IgE, deficiency, cardiovascular, ischemic, heart, arterial, hypertension
Immunoglobulin E (IgE) is best known for its pathological effects in allergic diseases and the beneficial role of IgE in host defense against parasitic infections, in particular, against helminth infections.1 Normal levels of IgE are highly variable within the population,2 and there have been few studies on patients with a low or undetectable serum total IgE. Selective IgE deficiency (IgED) is currently defined as a significant reduction in serum levels of IgE (less than or equal to 2 kIU/L) in a patient whose other immunoglobulin levels are normal or diminished (mixed IgED).3 Patients with IgE hypogammaglobulinemia were found to have an increased prevalence of multiple immunoglobulin deficits, autoimmune disease, and nonallergic reactive airway disease when compared with a population of patients with normal to elevated IgE levels.4
Recently, we described a relatively large group of patients with undetectable serum total IgE and found that IgE less than or equal to 2 kIU/L may serve as a marker of immune dysregulation and autoimmunity.5
IgE is thought to be potentially atherogenic through its proinflammatory influence on mast cells and platelets.6,7 Likewise, allergic inflammation can promote arterial cell apoptosis and atherogenesis.8 Nevertheless, previous findings on the relationship between IgE and atherosclerotic cardiovascular disease (ASCVD) are conflicting.9–11 Recently, one group found that atopy, as defined by allergen specific IgE, is inversely related to past myocardial infarction in the United States population.12
In this study, we tried to investigate a prevalence of ASCVD in our population with IgED.
MATERIALS AND METHODS
This study was conducted using the electronic patient record (EPR) database of Leumit Health Care Services (LHS), a health maintenance organization that covers approximately 720,000 residents of Israel.
LHS has implemented an EPR system that facilitates a database that includes comprehensive information on the insured population and resource use, such as demographic data, records of clinical visits, laboratory tests performed at a single centralized laboratory, and diagnostic codes using the International Classification of Diseases, 9th Revision, Clinical Modification. This database was used to obtain information on diagnoses and laboratory results by means of cross-linking data using a unique patient identifier. Data capture was performed using IBM Cognos 10.1.1 BI Report Studio software. Results of queries were downloaded into Microsoft Excel (version 14) spreadsheets for analysis. This study was approved by the LHS Institutional Review Committee.
Subjects
Inclusion Criteria.
Inclusion criteria included all subjects, having any allergy-related symptoms and/or those requesting antiallergy medications and performed serum total IgE measurement during 2012 at LHS. The case samples were drawn from the full study population (n = 18,487). All subjects aged more than or equal to 40 years old, with serum total IgE less than 2 kIU/L were included in case group. Control group was randomly sampled from the remained subjects with a case-control ratio of 10 controls for each case (1:10). The randomization was performed using the Epi Info 6 software (Atlanta, GA) using simple random sampling. The comorbid cardiovascular diseases, diagnosed by the corresponding board-certificated physicians during less than or equal to 10 years before serum total IgE testing, were identified and retrieved from LHS electronic database using specific International Classification of Diseases, 9th Revision, Clinical Modification diagnostic codes.
Information on age, gender, and cigarette smoking status was obtained from the EPR database. Body mass index was calculated as weight (kg)/height (m).2 Diabetes was defined according the guidelines of the American Diabetes Association as fasting (8 hours) serum glucose more than or equal to 126 mg/dL, nonfasting serum glucose more than or equal to 200 mg/dL, or a self-reported current use of oral hypoglycemic medication or insulin. To define systolic and diastolic blood pressure, the average of the second and the third blood pressure readings after a five-minute rest in the sitting position was used.
Exclusion Criteria.
Exclusion criteria included selective IgA deficiency, common variable immunodeficiency, ataxia telangiectasia, human immunodeficiency virus/acquired immunodeficiency syndrome, chronic systemic corticosteroid therapy, or on any other form of immunosuppressive therapy during the four weeks before serum total IgE measurement.
Measurement of Serum Immunoglobulins
Serum samples were analyzed for total IgE levels using the immunometric assay by the Immulite 2000 system (Diagnostic Products Corporation; Global Siemens Healthcare, Erlangen, Germany). Total serum IgE ranged from 2 to 2000 IU/mL for the whole study population. Complete data for analysis of total serum IgE were available for 18,247 subjects. In all cases of serum total IgE less than or equal to 2 kIU/L, analyses of other serum immunoglobulins were routinely performed to exclude humoral immunodeficiency. Serum immunoglobulins (IgM, IgA, IgG, IgG1, IgG2, IgG3, and IgG4) were measured by nephelometry (BN II System; Dade Behring, Deerfield, IL).
Statistical Analyses
Differences in demographic and clinical characteristics between the groups were analyzed using χ2 or Fisher's exact test when appropriate. The multiple regression analyses adjusted for age, sex, smoking status, diabetes mellitus, hypertension, and hyperlipidemia (odds ratio [OR] with 95% confidence intervals) were performed. To deal with possible multiplicity issues, we applied a Bonferroni correction testing at a significance level of α(0.05)/k (number of tests) to lower the chance of a type 1 error. Analyses were conducted using the Statistical Package SPSS 16.0. (StatSoft, Inc., Tulsa, OK).
RESULTS
A total number of 18,487 subjects aged 4–71 years old were identified as having performed a serum total IgE test between January 1 and December 31, 2012. The main reason for measurement of total serum IgE in both case and control was having any allergy-related symptoms. There were 103 subjects aged more than or equal to 40 years old with serum total IgE less than 2 kIU/L. Control group included 1030 subjects with serum total IgE more than or equal to 2 kIU/L. The demographic and laboratory characteristics of the subjects are presented in Table 1.
Table 1.
Clinical and laboratory characteristics of the subjects aged more than or equal to 40 years old
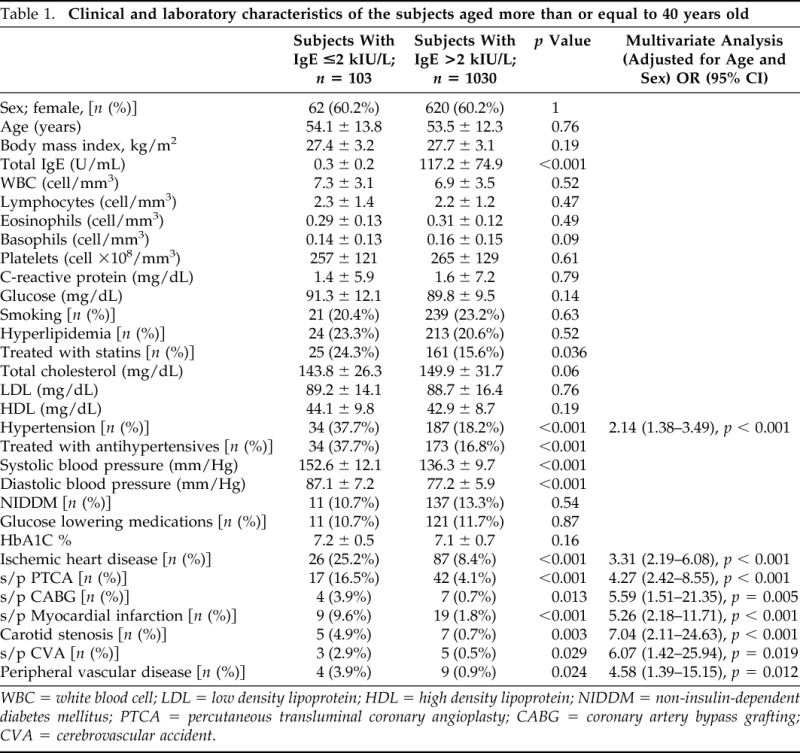
WBC = white blood cell; LDL = low density lipoprotein; HDL = high density lipoprotein; NIDDM = non-insulin-dependent diabetes mellitus; PTCA = percutaneous transluminal coronary angioplasty; CABG = coronary artery bypass grafting; CVA = cerebrovascular accident.
IgED and Cardiovascular Risk Factors
The case group was characterized by higher rate of arterial hypertension [34 (37.7%) versus 187 (18.2%), p < 0.001] than control group. Both systolic (152.6 ± 12.1 mm/Hg) and diastolic (87.1 ± 7.2 mm/Hg) blood pressure was higher in case group than in control group (136.3 ± 9.7 mm/Hg; p < 0.001 and 77.2 ± 5.9 mm/Hg; p < 0.001, respectively). In multivariate analyses adjusted for age, sex, smoking status, diabetes mellitus, hypertension, and hyperlipidemia, IgED was a positive predictor for arterial hypertension with OR 2.14 (1.38–3.49), p < 0.001.
There were no differences in the prevalence of hyperlipidemia, diabetes, and smoking status between the study groups. No differences in the blood levels of total cholesterol, low density lipoprotein, high density lipoprotein, C-reactive protein, and glucose levels were observed between case and control groups (Table 1).
IgED and Cardiovascular Diseases
Compared with control group patients, the case group had significantly more ischemic heart disease (IHD) [26 (25.2%) versus 87 (8.4%), p < 0.001], carotid stenosis [5 (4.9%) versus 7 (0.7%), p = 0.003], cerebrovascular disease (CVD) [3 (2.9%) versus 5 (0.5%), p = 0.029], and peripheral vascular disease (PVD) [4 (3.9%) versus 9 (0.9%), p = 0.024]. More case group patients underwent percutaneous transluminal coronary angioplasty and coronary artery bypass grafting than those from the control group (Table 1).
IgED as a Positive Predictor of ASCVD
IgED (being in the case group) was significantly associated with having ASCVD. IgED was a positive predictor for IHD [OR 3.31 (2.19–6.08), p < 0.001], carotid stenosis [OR 7.04 (2.11–24.63), p < 0.001], CVD [OR 6.07 (1.42–25.94), p = 0.019], and PVD [OR 4.58 (1.39–15.15), p = 0.012].
DISCUSSION
The study describes the prevalence of cardiovascular diseases in patients with IgED. Although the prevalence of arterial hypertension (18.2%) in the control group was roughly similar to the national prevalence of hypertension (20.7%) in Israel,13 the prevalence of hypertension in our patients with IgED was 37.7%. Moreover, the case group was characterized by higher systolic and diastolic blood pressure. It is hard to explain the observed high prevalence of arterial hypertension in IgED. The concept that immune system plays an important role in pathophysiology of hypertension is not new, but most of the previous studies explored a role cellular and innate immunity in the development of the disease.14 There are no published studies investigating an association of adaptive humoral immunity with hypertension. It is also controversial whether chronic low-grade inflammation caused by allergic diseases can predispose to hypertension.15 We previously observed an improvement of blood pressure control in hypertensive patients with allergic rhinitis after pharmacologic attenuation of allergic inflammation.16 The presence of subnormal levels of IgE impairs an ability of mast cells to respond normally to antigens and induce Th2 development; as a result, Th1 and Th17 responses predominate, leading to corresponding immunopathology.17 Several studies suggested that Th1 cells are important in mineralocorticoid-induced and angiotensin II-induced animal models of hypertension.18 Further basic or clinical studies are needed to clarify a role of humoral immunity and IgE in human arterial hypertension.
Another interesting finding of our study is a high prevalence of ASCVD in patients with IgED. We also found that IgED had significant positive relationships to IHD, CVD, PVD, and carotid stenosis, clinical forms of ASCVD. In human atherosclerotic lesions, IgE and its receptor Fc ε receptor 1 α are localized into macrophage and mast cell-rich areas.19 In atherosclerosis-prone apolipoprotein E-deficient mice, the absence of Fc ε receptor 1 α dramatically reduced atherosclerotic lesion sizes.8 Blood levels of IgE, one of the key mast cell activators, are elevated in hyperlipidemia19 and acute coronary syndromes.20 With atherosclerotic plaques progression, mast cell numbers increase in human coronary arteries,21 carotid arteries,22 and aorta.23 Several small human population studies showed a positive association between total serum IgE levels and IHD.24,25 In contrast, plasma total IgE levels did not show any correlation with disease progression or mast cell numbers in other studies.26 Interestingly, lower IgE levels were observed in patients that used statins, possibly by affecting the antibody production by B cells.27 Additionally, B-cell depletion was associated with a marked reduction in T-cell accumulation in atherosclerotic lesions, as well as with signs of decreased activation of effector T cells.28
Conversely, experimental data indicate that ASCVD is Th1 biased, and reduced levels of IgE can further enhance Th1 polarization.29 Future studies in animal models are warranted to determine whether IgE depletion may enhance Th1-biased immune activation and aggravate atherogenesis. Currently, it is unknown whether Th2-biased immunomodulation can reduce atherosclerosis. Nevertheless, in human autopsy study, we found that Opisthorchis felineus chronic helminthic infection is associated with a significant attenuation of atherosclerosis.30
As mentioned previously, IgED is characterized by chronic inflammation, immune dysregulation, and autoimmunity.4,5 It is accepted that systemic autoimmune diseases are similarly characterized by chronic inflammation and immune dysregulation. Therefore, some investigators postulate an autoimmune nature for atherosclerosis.31
In recent years, there is no longer any doubt that atherosclerosis shares numerous autoimmune pathways.32 The excessive cardiovascular events observed in patients with IgED are not fully explained by classic risk factors described since the Framingham heart study. We speculate that in IgED and ASCVD several shared pathophysiological pathways may possibly converge into a common proatherogenic phenotype.33 Although IgED is characterized by a high degree of ASCVD, this association may be a surrogate marker for a genetic trait leading to the development of premature vascular damage in this population.
Previously, some small studies demonstrated association of IgA deficiency with IgE hypogammaglobulinemia.34–36 Unlike to the patients, having combined IgA-IgED in these studies, our patients had selective IgED. We did not find any previous study exploring epidemiologic link between IgA deficiency and cardiovascular diseases. Nevertheless, the possible association of ASCVD with selective IgA deficiency has to be addressed in upcoming surveys.
Several limitations of the study need to be addressed. First, only those having allergy-related symptoms and/or those requesting antiallergy medications were checked for serum total IgE levels. This potential selection bias can impact the study results. Second, as a retrospective study, it may suffer from information bias, because of possible inaccurate clinical records, loss to follow-up, and missing data. Because the analysis was based on a retrospective review of EPR, some factors of potential relevance to cardiovascular risk were not obtained: family history of heart disease, exercise status, and differentiation of ex-smokers from current smokers.
Recently, the United States Food and Drug Administration announced that its review of safety studies suggested a slightly increased risk of arterial thrombotic events among patients being treated with Xolair (omalizumab).37 This observation might have several implications for both research and medical practice. Future basic and clinical studies will be needed to get a clearer picture of IgE role in the pathophysiology of ASCVD.
Footnotes
The authors have no conflicts of interest to declare pertaining to this article
REFERENCES
- 1. Sutton BJ, Gould HJ. The human IgE network. Nature 366:421–428, 1993. [DOI] [PubMed] [Google Scholar]
- 2. Peat JK, Toelle BG, Dermand J, et al. Serum IgE levels, atopy, and asthma in young adults: Results from a longitudinal cohort study. Allergy 51:804–810, 1996. [PubMed] [Google Scholar]
- 3. Pate MB, Smith JK, Chi DS, Krishnaswamy G. Regulation and dysregulation of immunoglobulin E: A molecular and clinical perspective. Clin Mol Allergy 8:3, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Smith JK, Krishnaswamy GH, Dykes R, et al. Clinical manifestations of IgE hypogammaglobulinemia. Ann Allergy Asthma Immunol 78:313–318, 1997. [DOI] [PubMed] [Google Scholar]
- 5. Magen E, Schlesinger M, David M, et al. Selective IgE deficiency, immune dysregulation, and autoimmunity. Allergy Asthma Proc 35:e27–e33, 2014. [DOI] [PubMed] [Google Scholar]
- 6. Ishizaka T, Ishizaka K. Activation of mast cells for mediator release through IgE receptors. Prog Allergy 34:188–235, 1984. [PubMed] [Google Scholar]
- 7. Joseph M, Capron A, Ameisen JC, et al. The receptor for IgE on blood platelets. Eur J Immunol 16:306–312, 1986. [DOI] [PubMed] [Google Scholar]
- 8. Wang J, Cheng X, Xiang MX, et al. IgE stimulates human and mouse arterial cell apoptosis and cytokine expression and promotes atherogenesis in Apoe−/− mice. J Clin Invest 121:3564–3577, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Sinkiewicz W, Błazejewski J, Bujak R, et al. Immunoglobulin E in patients with ischemic heart disease. Cardiol J 15:122–128, 2008. [PubMed] [Google Scholar]
- 10. Korkmaz ME, Oto A, Saraçlar Y, et al. Levels of IgE in the serum of patients with coronary arterial disease. Int J Cardiol 31:199–204, 1991. [DOI] [PubMed] [Google Scholar]
- 11. Langer RD, Criqui MH, Feigelson HS, et al. IgE predicts future nonfatal myocardial infarction in men. J Clin Epidemiol 49:203–209, 1996. [DOI] [PubMed] [Google Scholar]
- 12. Jaramillo R, Cohn RD, Crockett PW, et al. Relationship between objective measures of atopy and myocardial infarction in the United States. J Allergy Clin Immunol. J Allergy Clin Immunol 131:405–411.e11, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Amad S, Rosenthal T, Grossman E. The prevalence and awareness of hypertension among Israeli Arabs. J Hum Hypertens 10(suppl. 3):S31–S33, 1996. [PubMed] [Google Scholar]
- 14. Harrison DG. The immune system in hypertension. Trans Am Clin Climatol Assoc 125:130–138; discussion 138–140, 2014. [PMC free article] [PubMed] [Google Scholar]
- 15. Li C, Cheung CL, Cheung TT, et al. Hay fever and hypertension in the US adult population. Clin Exp Hypertens 36:206–210, 2014. [DOI] [PubMed] [Google Scholar]
- 16. Magen E, Yosefy C, Viskoper RJ, Mishal J. Treatment of allergic rhinitis can improve blood pressure control. J Hum Hypertens 20:888–893, 2006. [DOI] [PubMed] [Google Scholar]
- 17. Kawakami T, Kitaura J. Mast cell survival and activation by IgE in the absence of antigen: A consideration of the biologic mechanisms and relevance. J Immunol 175:4167–4173, 2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Harrison DG, Guzik TJ, Lob HE, et al. Inflammation, immunity, and hypertension. Hypertension 57:132–140, 2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Kovanen PT, Mänttäri M, Palosuo T, et al. Prediction of myocardial infarction in dyslipidemic men by elevated levels of immunoglobulin classes A, E, and G, but not M. Arch Intern Med 158:1434–1439, 1998. [DOI] [PubMed] [Google Scholar]
- 20. Szczeklik A, Sladek K, Szczerba A, Dropinski J. Serum immunoglobulin E response to myocardial infarction. Circulation 77:1245–1249, 1988. [DOI] [PubMed] [Google Scholar]
- 21. Kaartinen M, Penttilä A, Kovanen PT. Accumulation of activated mast cells in the shoulder region of human coronary atheroma, the predilection site of atheromatous rupture. Circulation 90:1669–1678, 1994. [DOI] [PubMed] [Google Scholar]
- 22. Jeziorska M, McCollum C, Woolley DE. Mast cell distribution, activation, and phenotype in atherosclerotic lesions of human carotid arteries. J Pathol 182:115–122, 1997. [DOI] [PubMed] [Google Scholar]
- 23. Atkinson JB, Harlan CW, Harlan GC, Virmani R. The association of mast cells and atherosclerosis: A morphologic study of early atherosclerotic lesions in young people. Hum Pathol 25:154–159, 1994. [DOI] [PubMed] [Google Scholar]
- 24. Erdogan O, Altun A, Gul C, Ozbay G. C-reactive protein and immunoglobulin-E response to coronary artery stenting in patients with stable angina. Jpn Heart J 44:593–600, 2003. [DOI] [PubMed] [Google Scholar]
- 25. Shahzad F, Tawwab S, Afzal N. Association of interleukin-4 and IgE levels with LDL oxidation in atherosclerosis. Iran J Immunol 7:109–116, 2010. [PubMed] [Google Scholar]
- 26. Willems S, van der Velden D, Quax PH, et al. Circulating immunoglobulins are not associated with intraplaque mast cell number and other vulnerable plaque characteristics in patients with carotid artery stenosis. PLoS One 9:e88984, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Krauth MT, Majlesi Y, Sonneck K, et al. Effects of various statins on cytokine-dependent growth and IgE-dependent release of histamine in human mast cells. Allergy 61:281–288, 2006. [DOI] [PubMed] [Google Scholar]
- 28. Ait-Oufella H, Herbin O, Bouaziz JD, et al. B cell depletion reduces the development of atherosclerosis in mice. J Exp Med 207:1579–1587, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Verhoef CM, van Roon JA, Vianen ME, et al. Mutual antagonism of rheumatoid arthritis and hay fever; a role for type 1/type 2 T cell balance. Ann Rheum Dis 57:275–280, 1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Magen E, Bychkov V, Ginovker A, Kashuba E. Chronic Opisthorchis felineus infection attenuates atherosclerosis - an autopsy study. Int J Parasitol 43:819–824, 2013. [DOI] [PubMed] [Google Scholar]
- 31. Matsuura E, Atzeni F, Sarzi-Puttini P, et al. Is atherosclerosis an autoimmune disease? BMC Med 12:47, 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Packard RR, Lichtman AH, Libby P. Innate and adaptive immunity in atherosclerosis. Semin Immunopathol 31:5–22, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kahlenberg JM, Kaplan MJ. Mechanisms of premature atherosclerosis in rheumatoid arthritis and lupus. Annu Rev Med 64:249–263, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Stites DP, Ishizaka K, Fudenberg HH. Serum IgE concentrations in hypogammaglobulinemia and selective IgA deficiency. Studies on patients and family members. Clin Exp Immunol 10:391–397, 1972. [PMC free article] [PubMed] [Google Scholar]
- 35. Kanok JM, Steinberg P, Cassidy JT, et al. Serum IgE levels in patients with selective IgA deficiency. Ann Allergy 41:22–23, 1978. [PubMed] [Google Scholar]
- 36. Levy Y, Nakum A, Segal N, et al. The association of selective IgA deficiency and IgE hypogammaglobulinemia. Allergy 60:836–838, 2005. [DOI] [PubMed] [Google Scholar]
- 37. United States Food and Drug Administration. FDA Drug Safety Communication: FDA approves label changes for asthma drug Xolair (omalizumab), including describing slightly higher risk of heart and brain adverse events. http://www.fda.gov/Drugs/DrugSafety/ucm414911.htm?source=govdelivery&utm_medium=email&utm_source=govdelivery Accessed September 29, 2014.


