Abstract
Background
Oxygenation index (OI=mean airway pressure MAP × FiO2 × 100÷PaO2) is used to assess severity of hypoxic respiratory failure (HRF) and persistent pulmonary hypertension of the newborn (PPHN). An indwelling arterial line or arterial punctures are necessary to obtain PaO2 for calculation of OI. Oxygenation can be continuously and non-invasively assessed using pulse oximetry. The use of oxygen saturation index (OSI=MAP × FiO2 × 100÷SpO2) can be an alternate method of assessing severity of HRF.
Objective
To evaluate the correlation between OSI and OI in (a) neonates with HRF and (b) a lamb model of meconium aspiration syndrome.
Methods
Human neonates: A retrospective chart review of 74 ventilated late preterm/term neonates with indwelling arterial access and SpO2 values in the first 24 hours of life was conducted. OSI and OI were calculated and correlated.
Lambs: Arterial blood gases were drawn and preductal SpO2 was documented in 40 term newborn lambs with asphyxia and meconium aspiration. OI and OSI were calculated and correlated with pulmonary vascular resistance (PVR).
Results
Mean values of OSI & OI showed a correlation coefficient of 0.952 in neonates (mean value of 308 observations in 74 neonates) and 0.948 in lambs (mean value of 743 observations in 40 lambs). In lambs, with increasing PVR, there was a decrease in OI and OSI.
Conclusion
OSI correlates significantly with OI in infants with HRF. This noninvasive measure may be used to assess severity of HRF and PPHN in neonates without arterial access.
Keywords: oxygenation index, pulmonary vascular resistance, pulmonary hypertension
Introduction
Oxygenation index (OI) is commonly used to assess the severity of hypoxic respiratory failure (HRF) and persistent pulmonary hypertension of the newborn (PPHN) in neonatal intensive care units (NICU). This index is considered a better indicator of lung injury compared to the PaO2/FiO2 ratio as it includes mean airway pressure (MAP), an important determinant of oxygenation [1]. Both clinical practice guidelines and research trials evaluating the use of therapies for HRF and PPHN have adopted OI to define entry criteria and assess outcome [2-5]. It is a common practice to use OI to dictate management, such as, initiation of inhaled nitric oxide (iNO), need for surfactant and extracorporeal membrane oxygenation (ECMO). Oxygenation index is calculated by the following equation: mean airway pressure MAP (in cmH2O) × FiO2 × 100 ÷ PaO2. There are several disadvantages to the use of OI in the NICU. It is invasive and requires an indwelling arterial line or arterial puncture to obtain a blood gas sample. It is only intermittently measured when blood gases are obtained. The site of sampling is decided by the location of the arterial line (e.g., only postductal gases can be obtained if an umbilical arterial line is present and the postductal PaO2 might be considerably lower than the preductal PaO2 perfusing the heart and brain). Finally, it is not known if OI correlates with the severity of lung disease or pulmonary vascular resistance (PVR) in PPHN.
Recently, studies from pediatric critical care units have suggested the use of oxygen saturation index (OSI) [1] or saturation to FiO2 ratios [6] for assessing severity of HRF and acute respiratory distress syndrome or acute lung injury (ARDS/ALI) . We evaluated the correlation between OI and OSI (=MAP × FiO2 × 100 ÷ SpO2) in human neonates with HRF and lambs with meconium aspiration syndrome (MAS). We hypothesize that OI and OSI correlate well with each other but neither index correlates well with PVR in HRF/PPHN.
Methods
Neonates with HRF
A retrospective chart review was conducted in neonates born between September 2011 and May 2014 and admitted to the NICU at The Women and Children's Hospital of Buffalo. The study was approved by Children and Youth Institutional Review Board of the State University of New York at Buffalo. Data was extracted from electronic medical records. Infants ≥34 weeks gestation who required intubation and mechanical ventilation and had at least one arterial blood gas on the first day of life and a corresponding saturation value (Masimo Radical 7 pulse oximeter, Masimo Inc, Irvine CA) recorded simultaneously were included. Arterial blood gases in the first 24 hours of life and corresponding oxygen saturations were collected in 74 infants. Ventilator settings at the time of blood gas draw were recorded. OI and OSI were calculated using the formulae listed above.
Lamb protocol
This study was approved by the Institutional Animal Care and Use Committee (IACUC) at State University of New York at Buffalo. Forty time-dated pregnant ewes (139-142 days gestation; Term 145 days) (Newlife Pastures, Attica NY) were sedated, intubated and ventilated with 2% isoflurane. Jugular and carotid lines were placed on the right side for access, drawing preductal blood gases and blood pressure monitoring. Catheters were placed in the main pulmonary artery and left atrium for pressure monitoring as previously described [7, 8]. Masimo Rad 7 pulse oximeter was attached to the right forelimb to monitor preductal SpO2. Fetal lambs were asphyxiated by umbilical cord occlusion and meconium was instilled into their endotracheal tube as previously described (Lakshminrusimha et al, Pediatric Research, in press).The lambs were then delivered, ventilated and blood gases were obtained at 30 minutes of age and then every 15 minutes until 6 hours. The preductal SpO2 was monitored and recorded continuously. OI, OSI & PVR were calculated. PVR was calculated by the following formula: PVR = (mean pulmonary arterial pressure – mean left atrial pressure) ÷ left pulmonary blood flow corrected for body weight.
Statistical Analysis
OI and OSI for different saturation ranges were analyzed using correlation coefficients (Pearson correlation) and calculated with the use of linear regression techniques. Average OI and OSI values for each lamb and neonate were used to represent repeated measures. Data were analyzed by mixed effect model (LME model) with SAS 9.0 (SAS Institute, Cary NC). [9]
Results
Correlation between OI and OSI
Human neonates with HRF
Data were collected from 74 neonates admitted to NICU with HRF between September 2011 and May 2014. A total of 308 observations with simultaneous SpO2 (either preductal or postductal), ventilator settings and ABG were recorded during the first 24 hours of age prior to a blood transfusion. Mean OI and OSI were calculated for each neonate and showed a correlation coefficient of 0.952 (p < 0.0001, Figure 1A). The scatter-plot with all the observations is shown in figure 1B.
Figure 1.
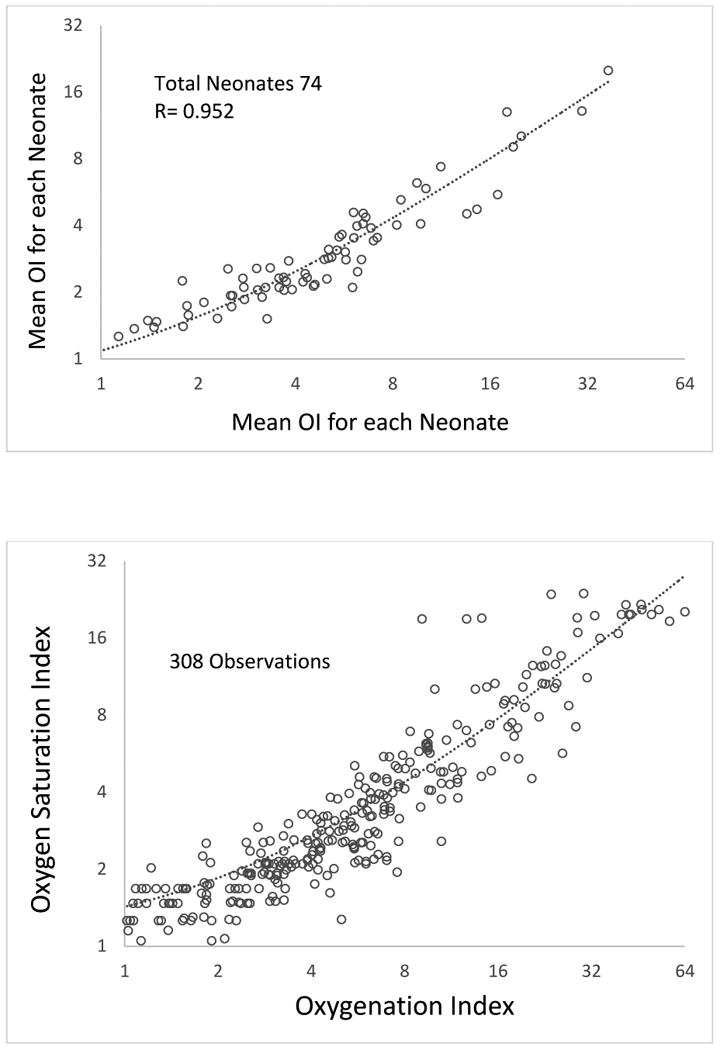
A. Scatter-plot showing mean OI and mean OSI in late preterm and term neonates with hypoxic respiratory failure during the first 24 hours of age. The axis for OI and OSI are on a logarithmic scale to a base of 2.
B: Scatter-plot showing all observations of OI and OSI in late preterm and term neonates with hypoxic respiratory failure during the first 24 hours of age. The axis for OI and OSI are on a logarithmic scale to a base of 2.
Lambs with MAS and HRF
Data were obtained from 40 term lambs with asphyxia, meconium aspiration and PPHN. For all SpO2 values, mean OI & OSI for each lamb showed a correlation of 0.948 (p< 0.0001, Figure 2A). The scatter-plot with all the individual observations is shown in figure 2B.
Figure 2.
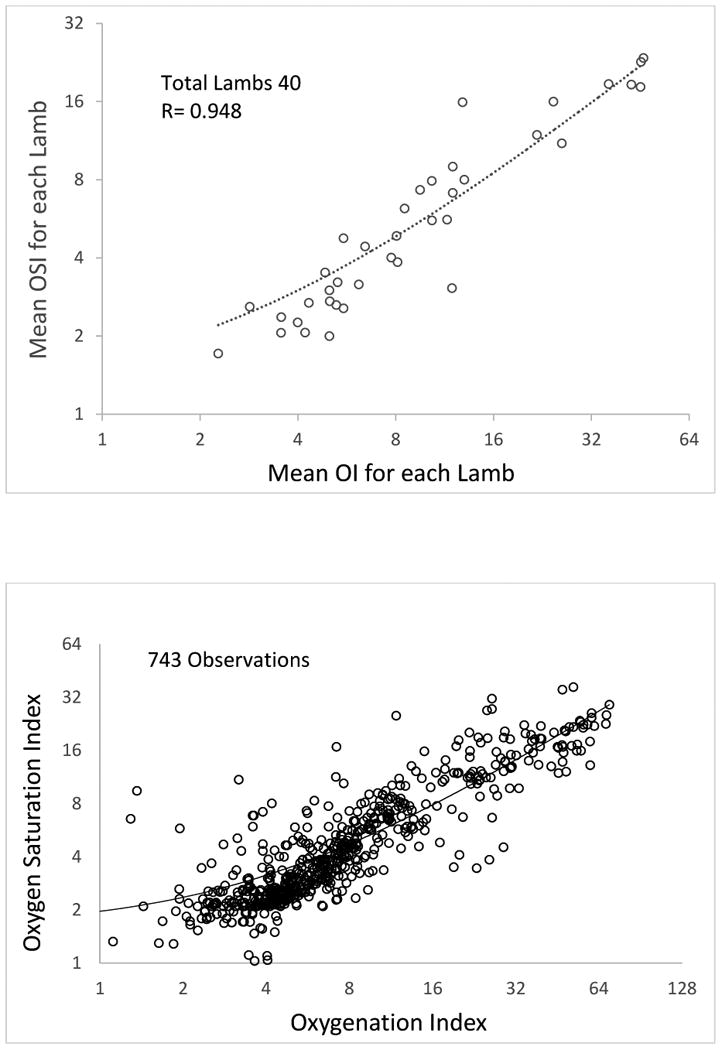
A. Scatter-plot showing mean OI and mean OSI in term lambs with asphyxia and meconium aspiration and PPHN. The axis for OI and OSI are on a logarithmic scale to a base of 2.
B. Scatter-plot showing all observations of OI and OSI in term lambs with asphyxia and meconium aspiration and PPHN. The axis for OI and OSI are on a logarithmic scale to a base of 2.
Mathematical relationship between OI and OSI
For all SpO2 values, the slope of the trend line of the correlation between OI and OSI were represented by the following equation: OI= 1.95 × OSI -0.5. We simplified this equation for practical purposes to OI=2×OSI.
Ability to predict OI using OSI
We subsequently tested the ability of OSI to predict OI of ≥ 10, ≥ 15, ≥ 20 and ≥ 25. For various mean OI values the corresponding mean OSI values based on the equation OI=2×OSI showed good sensitivity and specificity and a high negative predictive value (Table 1).
Table 1. Sensitivity, specificity, positive predictive value (PPV) and negative predictive value (NPV) of OSI values to predict the likelihood of OI whose values are twice that of OSI in neonates with hypoxic respiratory failure (HRF) and lambs with meconium aspiration syndrome (MAS).
| Sensitivity | Specificity | PPV | NPV | |
|---|---|---|---|---|
| Neonates with HRF | ||||
| OI≥10 & OSI ≥5 | 0.80 | 0.97 | 0.80 | 0.97 |
| OI≥15 & OSI ≥7.5 | 0.83 | 1 | 1 | 0.99 |
| OI≥20 & OSI ≥10 | 1 | 0.99 | 0.75 | 1 |
| OI≥25 & OSI ≥12.5 | 1 | 0.99 | 0.67 | 1 |
| Lambs with MAS | ||||
| OI≥10 & OSI ≥5 | 0.94 | 0.92 | 0.88 | 0.96 |
| OI≥15 & OSI ≥7.5 | 1 | 0.88 | 0.67 | 1 |
| OI≥20 & OSI ≥10 | 1 | 0.97 | 0.89 | 1 |
| OI≥25 & OSI ≥12.5 | 0.71 | 0.94 | 0.71 | 0.94 |
OI, OSI and PVR
Finally, we compared mean PVR measurement in each lamb with MAS and HRF. Increasing OI and OSI and decreasing SpO2 were associated with increased PVR (figure 3). However, both OI and OSI do not correlate well with PVR (correlation coefficient = -0.044 and -0.060 respectively). Preductal SpO2 showed better correlation with PVR (correlation coefficient = 0.4).
Figure 3.
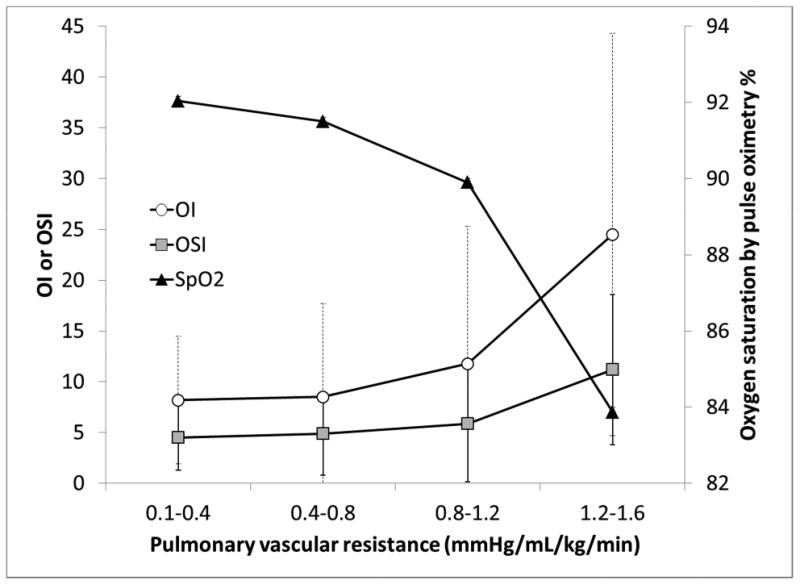
Graph depicting trends of OI, OSI and oxygen saturations in relation to pulmonary vascular resistance (PVR).
Discussion
The results from this study demonstrate that OSI calculated by substituting SpO2 obtained by pulse oximetry for PaO2 can be as accurate as OI in assessing severity of HRF in human neonates and lambs with MAS. As expected, neither OI nor OSI demonstrated good correlation with PVR. We have previously shown better correlation between PaO2 and SpO2 to PVR in a lamb model of PPHN induced by antenatal ductal ligation – a model without significant parenchymal lung disease [8]. We speculate that MAP may have different effects on PVR based on the degree of parenchymal lung disease limiting the ability of OI/OSI to predict PVR.
The high negative predictive value (table 1) and the linear nature of the association between OI and OSI in the clinically relevant range of OI (between 4 and 32) make OSI a valuable clinical tool to assess severity of HRF in the NICU. However, there are several limitations to this study. Location of the pulse oximeter probe (preductal or postductal) will influence OSI and should be taken into consideration. A standardized approach of using preductal SpO2 may improve the usefulness of OSI. The relationship between SpO2 and PaO2 (the oxygen dissociation curve) is altered by the type of hemoglobin (fetal vs. adult – which may be altered after a transfusion), pH, temperature etc. and influence the validity of OSI. Lastly, because of the shape of the oxygen dissociation curve, extremely high and very low SpO2 values do not correlate well with PaO2. For example, an infant with a PaO2 of 65 mmHg (and SpO2 of 98%) on 100% inspired oxygen and 15 cmH2O MAP has an OI of 23 and OSI of 15.3. If this patient receives inhaled nitric oxide and PaO2 improves to 165 mmHg (with SpO2 of 100%), his/her OI will decrease to 9 but OSI will remain at 15. The OSI value may theoretically be improved by weaning FiO2 and avoiding extremely high SpO2.
It is conceivable that many subjects who would meet entry criteria for enrollment in randomized clinical trials for HRF and PPHN are not enrolled due to the inability to obtain serial ABGs within the window defined in the inclusion criteria [1]. In addition, if the bedside provider fails to obtain an ABG and calculate OI, the severity of HRF or PPHN and the need for intervention (for example transfer from a non-ECMO NICU to an ECMO center) may potentially be missed. With advancement in pulse oximetry technology in recent years, this non-invasive and measure of systemic oxygenation has become the fifth vital sign[10]. We speculate that developing software to integrate MAP, FiO2 and SpO2 data and display OSI in patients on mechanical ventilation will enable bedside providers to promptly assess changing severity of HRF and PPHN.
References
- 1.Thomas NJ, Shaffer ML, Willson DF, Shih MC, Curley MA. Defining acute lung disease in children with the oxygenation saturation index. Pediatric critical care medicine : a journal of the Society of Critical Care Medicine and the World Federation of Pediatric Intensive and Critical Care Societies. 2010;11(1):12–17. doi: 10.1097/PCC.0b013e3181b0653d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Inhaled nitric oxide in full-term and nearly full-term infants with hypoxic respiratory failure. The Neonatal Inhaled Nitric Oxide Study Group. The New England journal of medicine. 1997;336(9):597–604. doi: 10.1056/NEJM199702273360901. [DOI] [PubMed] [Google Scholar]
- 3.Clark RH, Kueser TJ, Walker MW, Southgate WM, Huckaby JL, Perez JA, Roy BJ, Keszler M, Kinsella JP. Low-dose nitric oxide therapy for persistent pulmonary hypertension of the newborn. Clinical Inhaled Nitric Oxide Research Group. The New England journal of medicine. 2000;342(7):469–474. doi: 10.1056/NEJM200002173420704. [DOI] [PubMed] [Google Scholar]
- 4.Golombek SG, Young JN. Efficacy of inhaled nitric oxide for hypoxic respiratory failure in term and late preterm infants by baseline severity of illness: a pooled analysis of three clinical trials. Clin Ther. 2010;32(5):939–948. doi: 10.1016/j.clinthera.2010.04.023. [DOI] [PubMed] [Google Scholar]
- 5.Konduri GG, Solimano A, Sokol GM, Singer J, Ehrenkranz RA, Singhal N, Wright LL, Van Meurs K, Stork E, Kirpalani H, et al. A randomized trial of early versus standard inhaled nitric oxide therapy in term and near-term newborn infants with hypoxic respiratory failure. Pediatrics. 2004;113(3 Pt 1):559–564. doi: 10.1542/peds.113.3.559. [DOI] [PubMed] [Google Scholar]
- 6.Khemani RG, Patel NR, Bart RD, 3rd, Newth CJ. Comparison of the pulse oximetric saturation/fraction of inspired oxygen ratio and the PaO2/fraction of inspired oxygen ratio in children. Chest. 2009;135(3):662–668. doi: 10.1378/chest.08-2239. [DOI] [PubMed] [Google Scholar]
- 7.Lakshminrusimha S, Russell JA, Steinhorn RH, Swartz DD, Ryan RM, Gugino SF, Wynn KA, Kumar VH, Mathew B, Kirmani K, et al. Pulmonary hemodynamics in neonatal lambs resuscitated with 21%, 50%, and 100% oxygen. Pediatric research. 2007;62(3):313–318. doi: 10.1203/PDR.0b013e3180db29fe. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lakshminrusimha S, Swartz DD, Gugino SF, Ma CX, Wynn KA, Ryan RM, Russell JA, Steinhorn RH. Oxygen concentration and pulmonary hemodynamics in newborn lambs with pulmonary hypertension. Pediatric research. 2009;66(5):539–544. doi: 10.1203/PDR.0b013e3181bab0c7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Roy A. Estimating correlation coefficient between two variables with repeated observations using mixed effects model. Biometrical Journal. 2006;2(48):286–301. doi: 10.1002/bimj.200510192. [DOI] [PubMed] [Google Scholar]
- 10.Mower WR, Sachs C, Nicklin EL, Baraff LJ. Pulse oximetry as a fifth pediatric vital sign. Pediatrics. 1997;99(5):681–686. doi: 10.1542/peds.99.5.681. [DOI] [PubMed] [Google Scholar]


