Abstract
Endothelial cells (ECs) exist in different microenvironments in vivo, including under different levels of shear stress in arteries versus veins. Standard stem cell differentiation protocols to derive ECs and EC-subtypes from human induced pluripotent stem cells (hiPSCs) generally use growth factors or other soluble factors in an effort to specify cell fate. In this study, a biomimetic flow bioreactor was used to subject hiPSC-derived ECs (hiPSC-ECs) to shear stress to determine the impacts on phenotype and upregulation of markers associated with an anti-thrombotic, anti-inflammatory, arterial-like phenotype. The in vitro bioreactor system was able to efficiently mature hiPSC-ECs into arterial-like cells in 24 hours, as demonstrated by qRT-PCR for arterial markers EphrinB2, CXCR4, Conexin40 and Notch1, as well protein-level expression of Notch1 intracellular domain (NICD). Furthermore, the exogenous addition of soluble factors was not able to fully recapitulate this phenotype that was imparted by shear stress exposure. The induction of these phenotypic changes was biomechanically mediated in the shear stress bioreactor. This biomimetic flow bioreactor is an effective means for the differentiation of hiPSC-ECs toward an arterial-like phenotype, and is amenable to scale-up for culturing large quantities of cells for tissue engineering applications.
Keywords: Human induced pluripotent stem cells (hiPSCs), endothelial cells, shear stress, bioreactor, arterial, vascular tissue engineering
1. Introduction
The vasculature is one of the earliest organ systems to develop in the embryo. Endothelial cells (ECs) line the entire vasculature and have critically important roles in maintaining a dynamic barrier between blood and interstitial compartments, maintaining vasomotor tone, regulating inflammation and thrombosis, and maintaining overall vascular homeostasis. ECs exhibit significant functional and phenotypic heterogeneity [1], and various stimuli, including biomechanical shear stress, can modulate their phenotype.
In order to establish a cellular platform for studying endothelial biology and generating functional vascularized tissue suitable for transplantation, a reliable EC source is needed. Currently, isolated endothelial cells from human tissue are the main cell source used to study basic endothelial biology. However, these primary cells rapidly lose phenotypic marker expression in vitro, and isolation of large numbers of cells for expansion is difficult. The advent of human induced pluripotent stem cells (hiPSCs) [2], which can be derived from a person’s own somatic cells and differentiated into virtually every cell type in the body, constitutes a powerful tool for vascular tissue regeneration. In addition, ECs derived from hiPSCs are unique in that they have never been exposed to blood flow, which makes them different from tissue-derived ECs, which are invariably exposed to flow during the lifetime of the tissue donor. Thus, immature and non-lineage-committed endothelial cells, derived from hiPSCs, may possess greater inherent plasticity as compared to primary ECs, such as human umbilical cord vein endothelial cells (HUVECs) or human aortic endothelial cells (HAECs).
To date, multiple groups have described the differentiation of ECs from hiPSCs [3–6]. These cells display both specific functions characteristic of primary ECs [3] and have been demonstrated to have therapeutic potential by promoting perfusion of ischemic tissues in a mouse model of peripheral arterial disease [7]. However, ECs were isolated from static culture based on general EC marker expression, including CD31 (platelet endothelial cell adhesion molecule-1 or PECAM-1), KDR (vascular endothelial growth factor receptor-2 or VEGFR2) and VE-cadherin (CD144), which are present on arterial, venous, and lymphatic subtypes of endothelium. This means that endothelium derived from hiPSCs may be heterogeneous in terms of their precise lineage [5]. We sought to determine the impact of shear using a biomimetic flow bioreactor on the lineage specification of ECs derived from hiPSCs, in order to gain a better understanding of differentiation pathways and to potentially enhance the suitability of cells for various research and therapeutic applications.
Studies have shown that shear stress, or the adrenomedullin/cAMP pathway [8], can activate Notch signaling [9], thereby causing endothelial maturation and full differentiation into the arterial phenotype. Further, shear stress regulates EC gene expression for proliferation and survival [10] as well as vasoactive and anti-thrombotic substances such as nitric oxide (NO), prostacyclin [11], and thrombomodulin [12]. Shear pre-conditioned ECs have been shown to maintain their anti-thrombotic surface in arterial tissue engineered vascular grafts (TEVGs) in vivo, possibly by releasing NO [13]. Thus, shear stress caused by hemodynamic fluid flow is a crucial regulator of vascular homeostasis and normal EC function.
Arterio-venous fate determination occurs concurrently with the onset of blood flow [14]. Distinct molecular markers signify the differences between arterial and venous ECs during normal vascular patterning [15]. Nevertheless, the vascular endothelium is plastic in nature, and shear stress caused by blood flow can modulate the expression of arterial and venous-specific genes [16]. However, this phenotypic plasticity is present only to a certain degree in mature primary (adult) ECs. It has been shown that venous markers on vein grafts are lost after placement in the arterial environment, but that arterial identity is not induced, suggesting an incomplete adaptation to the high-flow arterial environment [17]. However, ECs derived from stem cells (hESCs) have much more plasticity as compared to adult ECs, as they are able to effectively upregulate markers associated with an arterial phenotype [9].
In this study, we evaluated the impact of shear stress on the expression of venous and arterial markers in ECs that were derived from hiPSCs. We generated ECs from hiPSCs using a directed differentiation approach, and examined the impact of shear stress on the maturation of hiPSC-ECs toward a venous- or arterial-like phenotype using our flow bioreactor. We cultured hiPSC-ECs on a porous mesh inside a biomimetic bioreactor system that mimics blood flow through a vessel, imparting “arterial” or “venous” levels of shear stress on the cells. The activation of vasoprotective, anti-inflammatory markers KLF2 and KLF4 was assessed, as well as the angiogenic potential of hiPSC-EC that were cultured in the bioreactor as compared to human umbilical cord vein endothelial cells (HUVECs) and human arterial endothelial cells (HAECs) We then compared the effect of the addition of soluble factors that have been shown to impact arterial specification on the expression of these same markers. Our results showed that physiological levels of shear stress upregulates markers associated with a vasoprotective, arterial-like phenotype significantly better than soluble factors, thus demonstrating the importance of biomechanical flow on EC subtype specification.
2. Materials and Methods
2.1 Cultivation of human iPS cells (hiPSCs)
Previously described human iPSC (hiPSC) lines were utilized for all experiments [18, 19] and were maintained on Matrigel as described in prior publications [2, 19]. All hiPSCs expressed Oct4, Sox2, and Nanog as assessed by immunostaining (data not shown). These cells have normal karyotypes, express cell surface markers and genes that characterize pluripotent human ES cells, and maintain the developmental potential to differentiate into advanced derivatives of all three primary germ layers. Briefly, hiPSCs were propagated on hESC-qualified Matrigel (BD Bioscience) from passages 25–40 and maintained in mTeSR medium (Stemcell Technologies). Medium was replaced daily and hiPSC colonies were routinely passaged every 5–7 days by mechanical dissociation using dispase (Stemcell technologies). The hiPSC line C2 (neonatal foreskin) utilized here was provided by Dr. James A Thomson, Department of Anatomy, University of Wisconsin-Madison, Madison, WI and p-hiPSC line (human newborn fibroblasts) was provided by Dr. Yibing Qyang, Department of Medicine, Section of Cardiovascular Medicine, Yale University, New Haven, CT.
2.2 In vitro differentiation and isolation of endothelial cells from hiPSCs (hiPSC-ECs)
hiPSCs were differentiated into ECs via embryoid body formation using directed differentiation (Figure 1A, top) in a manner similar to previously published protocols [5, 6]. Briefly, embryoid bodies (EBs) were formed using dispase on hiPSC colonies for 15 minutes, until colonies lifted off plate, and were carefully collected into a 15 mL conical tube. After washing twice with phosphate buffered saline (PBS), EBs were plated at high density into ultra-low attachment 6-well plates (Corning, Inc.) and first differentiated to mesoderm using 20 ng/mL BMP-4 (R&D Systems) for 4 days in human embryoid medium (hEB), containing knockout DMEM (KO-DMEM, Life technologies) with 20% FBS (Hyclone), 1% NEAA, 1 mM L-glutamine and 0.5 mM 2-mercaptoethanol. At then end of 4 days, EB’s were attached to 0.67% gelatin-coated plates (1 well to 1 well ratio) and were cultured for an additional 10 days in the differentiation medium containing Vasculife VEGF medium (Lifeline Technologies) supplemented with 10% FBS and 50 ng/mL VEGF (Figure 2A–D) to specify vascular fate with medium changes every other day.
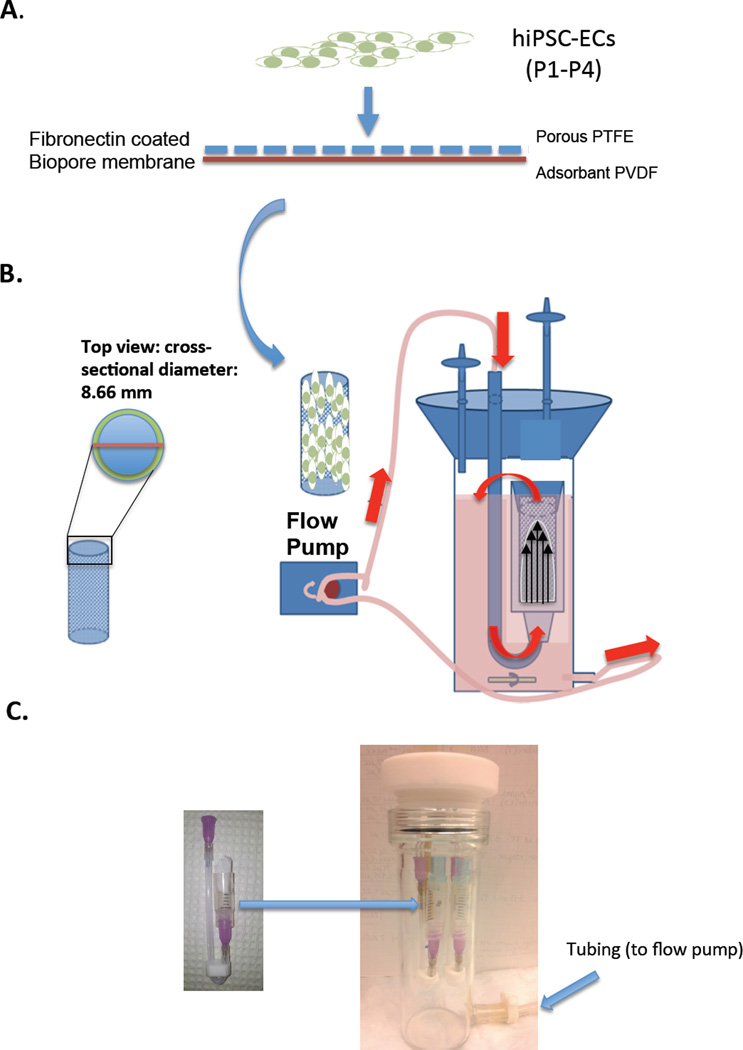
Figure 1.
(A) Schematic showing the seeding of hiPSC-ECs (7 × 104 cells/cm2) onto fibronectin-coated Biopore membranes (2.5 cm × 2.8 cm) and placement into cylindrical vessel chamber, indicated by the arrow (B) Schematic showing cross-section of the vessel chamber (OD: 8.66 mm) and assembled bioreactor with vessel chamber inside, connected to flow pump and medium flowing through tubing in direction of red arrows. (C) Physical model of vessel chamber with arrow pointing to placement in assembled glass bioreactor.
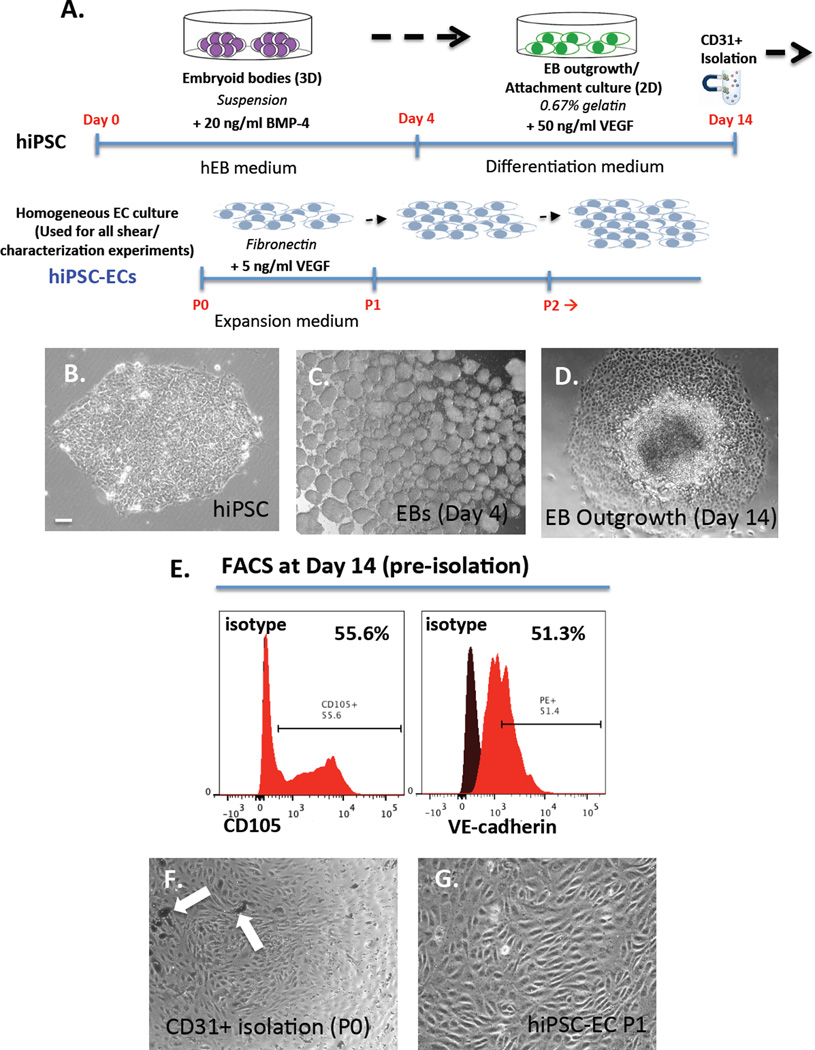
Figure 2.
(A) Schematic for directed differentiation protocol of hiPSCs to ECs in vitro in 14 days via embryoid body formation (top) and isolation/expansion of hiPSC-ECs (bottom). Phase contrast images of (B) typical hiPSC colony (C) cluster of EBs in suspension and (D) outgrowth of EBs in attachment culture at day 14 (E) FACS analysis of day 14 hiPSC-ECs (pre-isolation) showing 55.6% CD105+ cells and 51.3% VE-Cadherin+ cells (F) CD31+ magnetically isolated ECs with arrows pointing to magnetic beads and (G) isolated monolayer culture of hiPSC-ECs at passage 1 (P1). Scale bar = 10 µm.
Isolation of hiPSC-ECs: After 14 days of differentiation, cells were harvested using a CD31+ magnetic bead isolation kit to obtain a homogeneous population of hiPSC-ECs. Briefly, medium was aspirated, cells were washed with PBS and collagenase A/B 2 mg/mL was added for 30 minutes at 37°C. Cells were pipeted vigorously to break up clusters and were filtered through a 40 µm nylon mesh and centrifuged at 1000 RPM for 5 minutes. Then, the supernatant was aspirated and 5 mL of accutase (Stemcell technologies) was added for an additional 5 minutes to ensure a single cell suspension. To isolate ECs, a CD31 magnetic bead (Dynabeads, Invitrogen) isolation kit was used according to manufacturer's protocol. Briefly, approximately 2 million cells (per 6-well plate) were incubated with 20 µL of Dynabeads in PBS + 0.1% bovine serum albumin (BSA) for 20 minutes at 4°C with rocking, followed by placing tube in magnet and washing to remove non-CD31+ cells. After isolation, CD31+ hiPSC-ECs (passage 0, P0) were plated on fibronectin-coated plates (3 µg/cm2) in expansion medium containing Vasculife VEGF medium + 2% FBS + 5 ng/mL VEGF (Differentiation protocol, Figure 1A, bottom). Once confluent, cells were passaged routinely and plated at 10,000 cells/cm2. These isolated and expanded homogeneous hiPSC-ECs were used for all subsequent bioreactor seeding/shear experiments. Cells were routinely passaged and maintained CD31 and VE-cadherin marker expression as assessed by qPCR (Figure 3H). Doubling time for cells was approximately 24 hours and cells up to P4 were used for experiments.
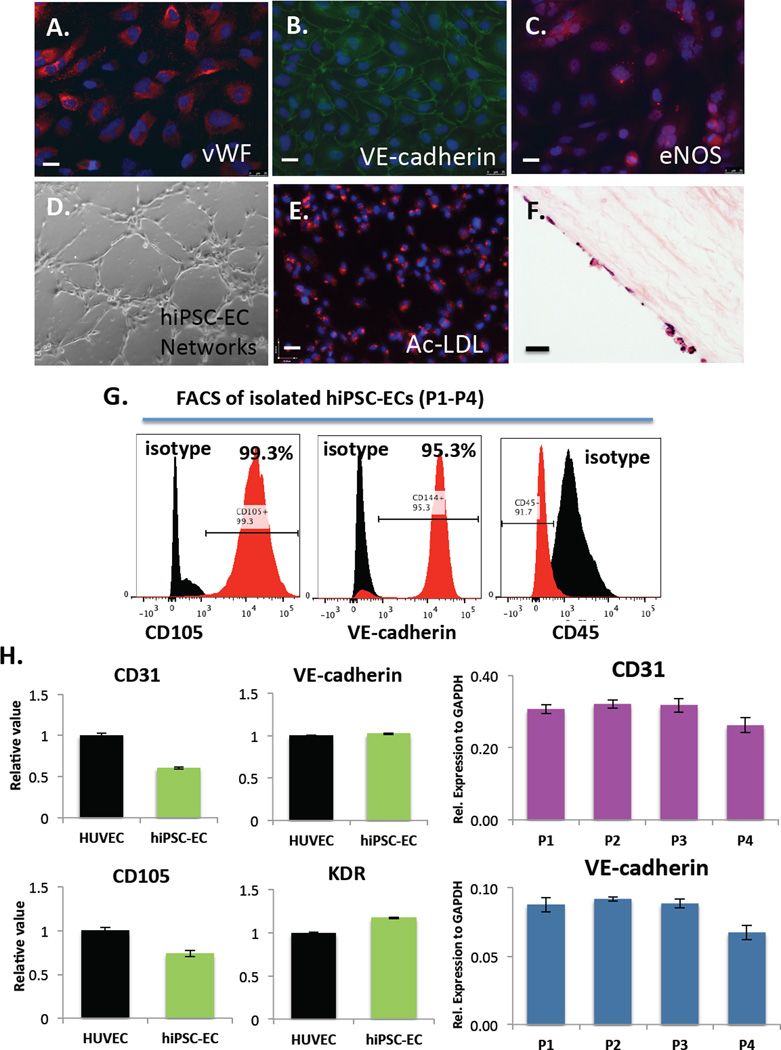
Figure 3.
Immunofluorescence analysis of EC markers in hiPSC-ECs after differentiation, isolation and expansion for 2–3 passages on fibronectin-coated plates shows expression of (A) von Willebrand Factor (vWF) (B) VE-cadherin and (C) eNOS. Functional assays demonstrate that hiPSC-ECs (D) form networks when cultured on Matrigel-coated plates for 24 hours (E) take up acetylated-LDL and (F) engraft and proliferate on decellularized human aorta slices in vitro as shown by H&E staining. Scale bars = 25 µm. (G) FACs analysis of isolated hiPSC-ECs at day 28 (P1) showing 99.3% CD105+ cells, 95.3% VE-cadherin+ cells and CD45- cells and (H) qRT-PCR analysis of EC gene expression (CD31, VE-cadherin, CD105 and KDR) in hiPSC-ECs compared to HUVECs (left) and marker expression of CD31 and VE-cadherin over multiple passages (P1–P4) in vitro (right). Values from three independent experiments from the triplicate PCR reactions for genes of interest were normalized against average GAPDH Ct values from the same cDNA sample. Fold change of GOI transcript levels between hiPSC-ECs equals 2-−ΔΔCt, where ΔCt=Ct(GOI) - Ct(GAPDH), and ΔΔCt=ΔCt(ATII) - ΔCt(ATII). (Bar indicates ± SEM and n = 3 independent experiments for qRT-PCR and flow cytometry).
2.3 Primary human endothelial cell culture
Pooled primary human umbilical cord vein endothelial cells (HUVECs) were obtained from the Yale University Vascular Biology and Therapeutics Tissue Culture Core Facility. Human aortic endothelial cells (HAECs) were purchased from PromoCell. Both cell lines were maintained in Vasculife VEGF medium (Lifeline Technologies). All cell lines were routinely passaged at 80% confluence every 3–4 days and used between passages 2–6.
2.4 Bioreactor design and assembly
The bioreactor used in this study is designed to simulate the flow of blood through a blood vessel lumen. The housing was custom-made (Yale University glass blower, Figure 1C) and consists of a borosilicate glass chamber 51 mm OD, 43 mm ID, capped with a threaded white PTFE cap (Figure 1B, 2C). Three holes, 7 mm, were bored through the cap, two for silicon tubing and one for a sterile air filter attachment port. A medium outlet port, 5 mm, is incorporated at the base. For closed-loop flow, Master-Flex L/S 16 tubing (Cole-Parmer ZW-06508-16) was used to attach to this base via a sealing luer-lock (Cole-Parmer ZW-06464-90) to male connectors on the cap. The lumens were 3 cc luer-lock syringes (BD 209657) cut at the 1.5 cc mark and each bioreactor housed two lumens. A peristaltic pump was used for flow (Cole Parmer).
Millipore’s Biopore Membrane (BGCM00010) was used as a cell support inside the lumens of the vessel chambers that were placed inside the bioreactors (Figure 1A, arrow). This membrane has been shown to be highly supportive of cell growth [20]. This membrane is bi-layer with the upper being composed of 50 µm thick polytetrafluorethylene (PTFE) with 0.4 µm pores for cell attachment and ingrowth. The lower layer is 200 µm thick hydrophilic polyvinyl (PVDF) with 0.22 µm pores, which is an absorbant layer that acts as a medium reservoir for nutrient transfer to the cell layer above. These membranes could be rolled and placed inside vessel chambers, which are 8.66 mm in diameter (Figure 1B and 1C, left side) and mimic the inner lumen of a blood vessel.
2.5 Application of shear stress to hiPSC-ECs in the bioreactor system
Prior to shear stress experiments, all bioreactor components were autoclaved. Biopore membranes were cut to size (2.5 cm× 2.8 cm) and immersed in sterile 70% ethanol for 20 minutes under UV light. The membranes were washed twice with 1× PBS and coated with fibronectin (3 µg/cm2) (Sigma-Aldrich) for hiPSC-EC seeding and with 0.1% gelatin for HUVEC or HAEC seeding. Early passage cells (P1–P4) were removed from tissue culture plates using accutase (Stemcell Technologies) and reseeded onto coated membranes (Figure 1A) at a concentration of 7 × 104 cells/cm2. The constructs were incubated at 37°C for 4 h to allow the cells to completely attach. Culture medium (Vasculife VEGF medium) was then added to completely submerge the constructs for one day to allow cells to firmly attach and proliferate on the membrane. The membranes were then carefully rolled into pre-sterilized vessel chambers (Figure 1B, curved arrow), which were sealed into the flow port to ensure the flow path was confined to passage through the vessel chamber. These were then attached to the tubing connectors in the cap. All tubing was pre-connected to allow for closed-loop flow, and 100 mL of culture medium was added to the bioreactor reservoir. The lines were purged of air, and the cap was tightly sealed and bioreactor system was connected to a peristaltic pump. Flow was started at 10 mL/min to allow cells to adapt to shear stress. Shear stress was estimated using Poisuelle’s equation τ= 4ηQ/πr3, where τ is the wall shear stress in dyne/cm2, η is the medium viscosity and estimated to be 0.8 cP [21], Q is volumetric flow rate which is calibrated by measuring the medium dispensed through lumen per minute (cm3/min), and r is the inner diameter of the lumen (0.8 cm). Flow was gradually increased to either low shear rates (venous) of approximately 5 dyne/cm2 or high shear rates (arterial) of approximately 10 dyne/cm2 and maintained at this level for 24 hours. After this exposure to shear for one day, cells were immediately harvested for further analysis.
2.6 Small molecule differentiation of hiPSC-ECs to arterial-like endothelial cells
We examined the effect of the Notch activator, 8-bromo-cAMP (cAMP) [8] and high levels of VEGF [22] on the phenotype of hiPSC-ECs in vitro. hiPSC-ECs were cultured under conditions described above, but with the addition of either 5 µM cAMP (Sigma-Aldrich), 50 ng/ml VEGF (R&D systems) or a combination of both cAMP and VEGF for 24 h. Cells were then harvested for qRT-PCR or Western blotting.
2.7 In vitro matrigel angiogenesis assay and acetylated-LDL uptake
To assess the angiogenic capacity of hiPSC-ECs in vitro, a thin layer of Matrigel (BD Bioscience) was used to coat the wells of a 48 well plate, followed by seeding with 5 × 104 cells directly in a minimal volume of medium. After 24 hours, wells were fixed in 4% paraformaldehyde and networks were imaged using a phase-contrast microscope. For the quantification of networks, WimTube software was used (Wimasis). For acetylated-LDL uptake, DiI-labeled acetylated-LDL (Invitrogen) was incubated with cells in serum-free medium (EBM2) for 4 hours. Cells were then washed twice with 1× PBS, fixed with 4% paraformaldehyde, and imaged using a Leica DMI6000 B fluorescence microscope.
2.8 Flow cytometry and immunohistochemistry analysis
Differentiated hiPSC-ECs were assessed by immunofluorescence staining and flow cytometry. For immunostaining, ECs were seeded into four-well chamber slides for 24 hours, washed with PBS and fixed in 4% paraformaldehyde in PBS for 20 min at room temperature (RT). Cells were then permeabilized and blocked for non-specific antigen binding with PBS + 10% FBS + 0.1% Triton X-100 for 1 hour at RT. The cells were then incubated with primary antibodies against PECAM-1 (CD31) (Abcam, ab28364), VE-Cadherin (CD144) (Santacruz, sc-6458), eNOS (Abcam, ab5589), vWF (Abcam, ab6994), overnight at 4°C. The next day, cells were washed three times with 1X PBS and incubated with corresponding secondary antibody (1:500) for 2 hours at RT. After washing three times with 1× PBS, mounting medium containing 4, 6-diamidino-2-phenylindole (DAPI) (Vectorlabs) was applied to slides to visualize nuclei and coverslips were placed to seal slides. The slides were visualized using a Leica DMI6000 B fluorescence microscope.
For flow cytometric analysis, cells were dissociated into single cell suspensions by incubation in accutase for 5 min. The dissociated cells were resuspended (2 × 105 cells) in 200 µl of blocking solution (PBS + 10% FBS) for 20 minutes, followed by incubation in flurophore-conjugated antibodies for 20 minutes at 4°C. After washing twice with PBS, cells were fixed in 4% paraformaldehyde for 15 minutes, washed once with PBS and resuspended in 200 µl PBS, and filtered through a 40 µm nylon mesh. The cell samples were analyzed using a BD-LSR II flow cytometer.
2.9 Real time quantitative RT-PCR Reaction
Real-time quantitative RT-PCR (qRT-PCR) was used to determine the expression of endothelial markers in differentiated cells from hiPSCs, cultured in the bioreactor and in static conditions. Adult human primary HUVECs and HAECs were used as controls. Briefly, total cellular RNA was prepared using the RNeasy Mini Kit following the manufacturer’s instructions. Single stranded cDNA was synthesized using the reverse transcription-PCR protocol of the First Strand cDNA Synthesis Kit from Invitrogen. Quantitative real-time PCR was performed using SYBR Green PCR Supermix (Bio-Rad). Concentrations of all primers were optimized before use. PCR conditions included an initial denaturation step of 4 minutes at 95°C, followed by 40 cycles of PCR consisting of 15 seconds at 95°C, 30 seconds at 60°C, and 30 seconds at 72°C. Each sample was run in triplicate. The comparative Ct value method using GAPDH as a housekeeping gene for an internal standard was employed to determine relative levels of gene expression. Ct values from the triplicate PCR reactions for a gene of interest (GOI) were normalized against average GAPDH Ct values from the same cDNA sample. Fold change of GOI transcript levels between sample A and sample B = 2−ΔΔCt, where ΔCt = Ct(GOI) – Ct(GAPDH) and ΔΔCt = ΔCt(A) –ΔCt(B). Primers were used in this study are listed in Table 1.
Table 1.
Sequences of primers used in qRT-PCR
| Gene | Length (bp) |
Forward Primer | Reverse Primer |
|---|---|---|---|
| CD31 | 134 | TGCAGTGGTTATCATCGGAGTG | CGTTGTTGGAGTTCAGAAGTG |
| CD144 | 80 | TCACCTGGTCGCCAATCC | AGGCCACATCTTGGGTTCCT |
| KDR | 90 | TGCCTCAGAAGAGCTGAAAAC | CACAGACTCCCTGCTTTTGCT |
| EphrinB2 | 107 | GTTCCGGCGTTTATTTCTG | CCACAGCTAAATCGTCACC |
| Notch1 | 132 | CACGCGGATTAATTTGCATC | TCTTGGCATACACACTCCG |
| Conexin40 | 198 | CTGCTAGGGAGTCACTGTACAC | CTGGTCAGGGTTCGAGAGAG |
| CXCR4 | 102 | CAC CGC ATC TGG AGA ACC A | GCC CAT TTC CTC GGT GTA GTT |
| COUP.TFII | 77 | GCCATAGTCCTGTTCACCTC | CTGAGACTTTTCCTGCAAGC |
| EphB4 | 88 | TGAAGAGGTGATTGGTGCAG | AGGCCTCGCTCAGAAACTCAC |
| KLF2 | 102 | ACTTTCGCCAGCCCGTGC | AGTCCAGCACGCTGTTGAG |
| KLF4 | 93 | CGAACCCACACAGGTGAGAA | TACGGTAGTGCCTGGTCAGTTC |
| GAPDH | 122 | GACAACAGCCTCAAGATCATCAG | ATGGCATGGACTGTGGTCATGAG |
2.10 Western blotting
One million cells (HUVEC, HAEC, hiPSC-EC) were lysed with RIPA Buffer (Invitrogen) and quantified by Bradford assay (Bio-Rad). 20 µg of protein was loaded on a 4–12% SDS-page gel (Bio-Rad) for Western blot analysis. Anti-eNOs (abcam ab5589) 1:1000, anti-Notch1 (abcam 8925) 1:1000, anti β-actin 1:3000 were used to probe for proteins of interest overnight at 4°C. Blots were washed and incubated with 1:5000 goat anti-rabbit HRP antibody for 1 hour and developed using ECL reagents and film (Kodak).
Statistical analyses
All experiments were repeated at least three times and each condition was analyzed in triplicate. Data are presented as the means ± SEM for quantitative variables. An unpaired Student t test was performed to evaluate whether the two groups were significantly different from each other. One-way variance (ANOVA) analysis was used to determine whether there was a significant difference in the gene expression among different test groups. A P value of p ≤ 0.05 (two-tailed) was considered statistically significant.
3. Results
Previous studies have described differentiation protocols, having various degrees of efficiency, that derive ECs from hiPSCs under static culture conditions. Differentiation is based on expression of general EC markers such as CD31, VE-Cadherin and KDR. In this study, we sought to mimic partially the in vivo flow conditions of a blood vessel, and developed a biomimetic flow bioreactor to subject hiPSC-ECs to shear stress to determine whether we could enrich for specific endothelial subtypes, including an anti-thrombotic, anti-inflammatory, arterial-like phenotype.
3.1. Differentiation and characterization of hiPSC to ECs in vitro
In order to derive ECs from hiPSCs, we used a step-wise differentiation protocol via mesoderm induction, followed by vascular specification in attachment culture using VEGF (Figure 2A). First, hiPSC colonies (Figure 2B) were formed into EBs in suspension (Figure 2C) for 4 days and then attached (EB outgrowth) for another 10 days (Figure 2D). At day 14, CD31+ cells (passage 0) were isolated from differentiation culture using magnetic beads (Figure 2F, arrows pointing to magnetic beads). These ECs were then passaged and expanded on fibronectin-coated plates in expansion medium with VEGF and subsequently utilized for experiments (Figure 2G). The differentiation protocol results in >50% CD105+CD144+ cells (Figure 2E) at day 14 (pre-isolation). After purification using magnetic beads and culture on fibronectin-coated plates, we characterized the hiPSC-ECs at approximately day 28 (2 weeks after isolation to allow for growth and expansion).
Immunohistochemistry revealed robust expression of vWF, VE-Cadherin and eNOS (Figure 3A–C). Functional assays demonstrated that hiPSC-ECS form networks when cultured on Matrigel (Figure 3D), take up acetylated-LDL (Figure 3E) and attach and proliferate on decellularized human aorta slices in vitro as seen by H&E staining (Figure 3F). We also observed high rates of positivity for CD105+ (99.3%) and VE-Cadherin+ (95.3%) and negative staining for hematopoietic marker CD45 as compared to isotype controls by flow cytometry (Figure 3G). Also, hiPSC-EC gene expression of CD31, CD144, CD105, and KDR was comparable to that of HUVECs as demonstrated by qRT-PCR (Figure 3H, left). Finally, hiPSC-ECs maintain gene expression levels of CD31 and VE-cadherin over multiple passages in vitro (Figure 3H, right).
Furthermore, Western blotting confirmed the protein level expression of eNOS and Notch1 (Figure 4A) in two separate hiPSC-EC lines, as compared to HUVECs and HAECs. (The p-hiPSC line gave the most robust differentiation and was used for all subsequent experiments. The differentiation and characterization of hiPSC line C2 is shown in Supplementary Figure 1A.) These results show that our differentiation protocol efficiently produces hiPSC-ECs, which express all mature and functional EC markers.
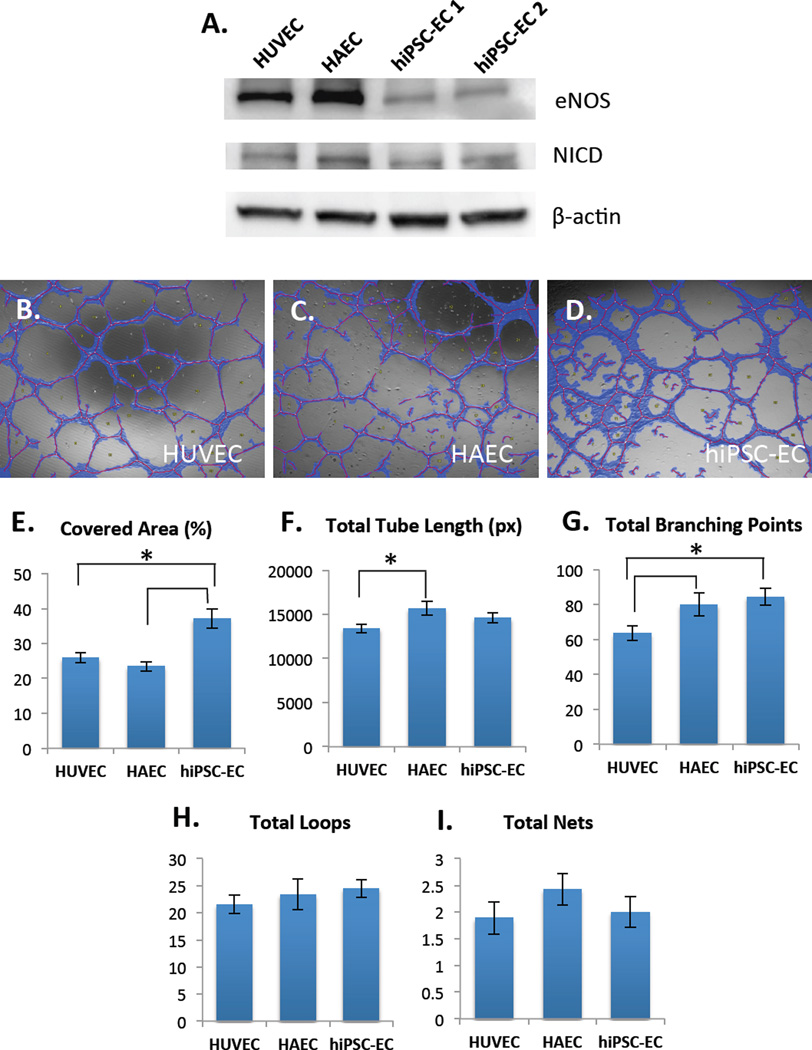
Figure 4.
(A) Western blot shows protein-level of expression of eNOS (top) and Notch1-intracellular domain (NICD) (middle) in HUVECs, HAECs and two different hiPSC-EC lines, hiPSC-EC1 and hiPSC-EC2 (under static conditions). β-actin was used as a loading control. Angiogenic properties of hiPSC-ECs (B) on Matrigel were compared to HUVECs (C) and HAECs (D) as assessed by Wimasis software. hiPSC-ECs have significantly higher covered area compared to HUVECs and HAECs (E). HAECs have higher total tube length compared to HUVECs, but not hiPSC-ECs (F). hiPSC-ECs and HAECs have significantly higher total branching points compared to HUVECs (G). There were no significant differences in total loops (H) and total nets (I). These results demonstrate certain intrinsic differences in angiogenic properties between venous, arterial and hiPSC-ECs (*p < 0.05, n=3 biological replicates, with 3 fields of view captured per sample).
3.2 Angiogenesis network formation
In order to understand the angiogenic potential of hiPSC-ECs as compared to HUVECs and HAECs, we cultured 5 × 104 cells on Matrigel for 24 h in 48 well plates in the same medium and fixed samples for analysis. Each condition was carried out in triplicate wells and three images were taken per well. We then quantified network formation using Wimtube (Wimasis) [23], which automates and standardizes image analysis to provide objective data processing (Figure 4B–D). We found a statistically significant difference in the covered area (roughly 15%) between hiPSC-ECs and HUVECs and HAECs (Figure 4E). We also found that HAECs have greater tube length as compared to HUVECs (Figure 4F). Further, both HAECs and hiPSC-ECs have a statistically significant higher total number of branching points as compared to HUVECs (Figure 4G). We found no statistically significant differences between the total number of loops and nets for the cell types (Figure 4H–I). Since it is generally assumed that tubule length and node complexity correlate with angiogenic potential [24], these results demonstrate intrinsic differences in angiogenic potential between venous, arterial and hiPSC-ECs, agreeing with literature that arterial lineage ECs form a more extensive capillary network in vivo [5].
3.3 Effect of shear on the mechanosensory complex
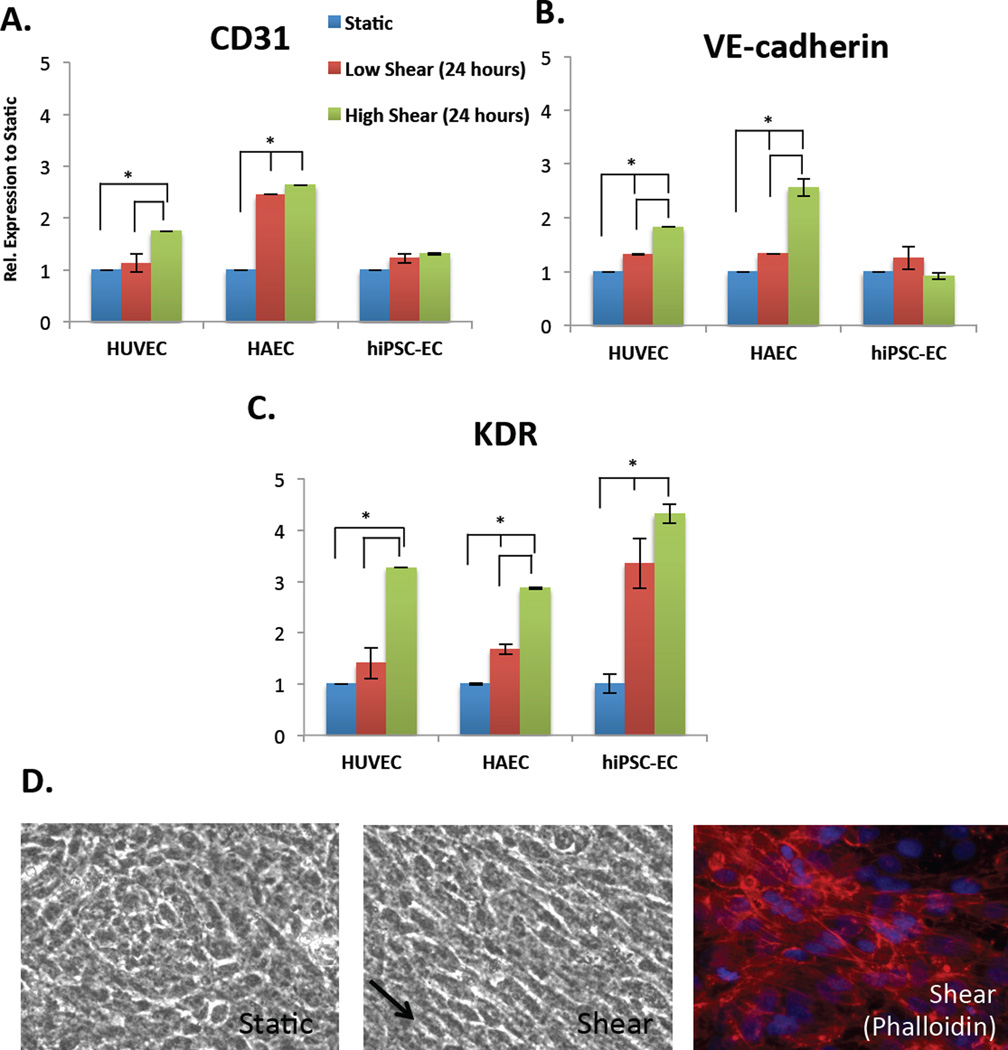
In order to test the effect of shear stress on the phenotype of hiPSC-ECs, cells were placed onto fibronectin-coated Biopore membranes and placed into a cylindrical vessel chamber (Figure 1A–B) that is constantly exposed to fluid from flowing medium. The cells on the porous membrane maintain proper cobblestone morphology, align in the direction of flow (arrow) and have elongated actin stress fibers (Figure 5D). The normal in vivo physiological levels of shear stress experienced by ECs varies widely, with arterial shear stress being in the range of 10–70 dyne/cm2 (1–7 Pa) and venous in the range of 1–6 dyne/cm2 (0.1–0.6 Pa) [25]. The mechanism by which ECs sense shear stress is not completely understood, as all the molecules involved in the mechanosensing process have not been completely identified [26]. However, important work has identified CD31 and KDR (VEGFR2) as essential for responsiveness to flow, with VE-Cadherin playing an important role as an adaptor [27]. Our data demonstrate that shear stress significantly increases CD31 and VE-Cadherin expression in HUVECs and HAECs, relative to static conditions, but shear stress does not have this effect in hiPSC-ECs (Figure 5A–B). However, shear stress significantly increases KDR expression in HUVECs and HAECs as well as in hiPSC-ECs, with higher shear inducing a larger response (Figure 5C). KDR expression was also significantly increased in hiPSC-ECs as compared to HUVECs and HAECs. While KDR also forms part of the essential mechanosensory complex for sensing shear stress in ECs, it is also an early, primitive EC progenitor marker that is present on mesodermal precursor cells in hESCs [28]. Thus, shear stress may be more likely to activate KDR expression in ECs derived from hiPSCs, which are less mature than primary ECs.
Figure 5.
The effect of shear stress (24 hours) on the gene expression, relative to static, of the mechanosensory complex comprised of (A) CD31, (B) VE-cadherin and (C) KDR in HUVECs, HAECs and hiPSC-ECs (left to right) as shown by qRT-PCR. Both low shear (red bar, 5 dyne/cm2) and high (green bar, 10 dyne/cm2) are normalized to static conditions (blue bar). All conditions are also normalized to internal house-keeping gene GAPDH. (D) Phase-contrast images of morphology of hiPSC-ECs on porous membrane in static conditions (left), alignment under shear (right, arrow pointing in direction of flow) and phalloidin showing actin stress fibers (*p < 0.05, n=3 biological replicates, n=3 technical replicates each, Student’s t-test for samples within groups).
3.4 Effect of shear on arterial marker expression
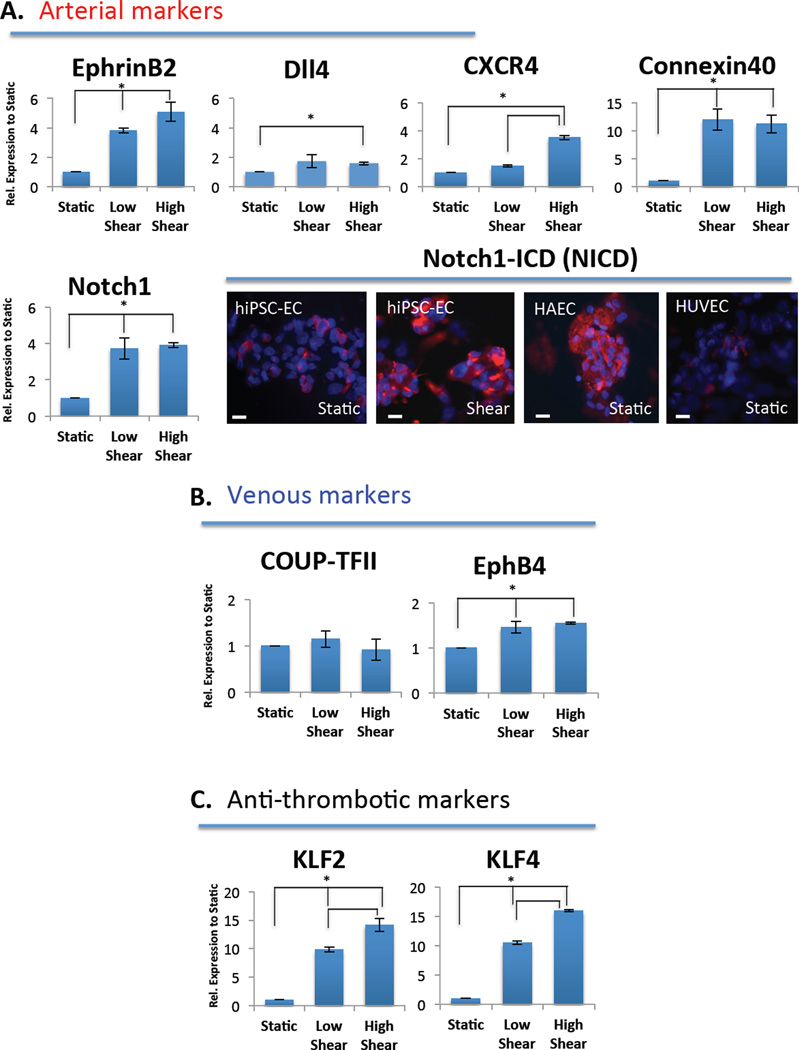
Shear stress is known to regulate arterial marker expression, including EphrinB2 and activation of the Notch signaling pathway [29]. As such, we assessed whether hiPSC-ECs that were cultured in the flow bioreactor for 24 hours are able to upregulate markers associated with this phenotype. We found that low and high shear triggered a 4- and 5-fold increase in EphrinB2 expression levels, respectively, over static. CXCR4 expression was also increased approximately 4-fold by shear as assessed by RT-PCR (Figure 6A). Since these are definitive and primitive arterial EC markers, respectively, the shear stress caused by flow significantly increased the expression of the markers associated with the arterial phenotype. Additionally, we looked at connexin40 expression, which is a gap junction molecule that provides a means of signaling between ECs that is widely distributed in the endothelium of large arteries [30]. We found that both low and high shear stress caused a significant upregulation (12-fold) of connexin40 in hiPSC-ECs over static conditions, indicating flow promotes gap junction formation. This is in line with literature showing that maximal induction of connexin40 expression occurs between 6 and 10 dyne/cm2 [31].
Figure 6.
qPCR demonstrating the effect of shear stress, low = 5 dyne/cm2 and high = 10 dyne/cm2, on the expression of (A) arterial gene markers, including EphrinB2, Dll4, CXCR4, Conexin40 and Notch1. Immunostaining of Notch1-ICD (NICD) demonstrating staining that is increased with shear stress in hiPSC-ECs (middle) as compared to static hiPSC-ECs (left), confirming activation of Notch at the protein level. Control static HAECs and HUVECs are also included (B) venous marker genes COUP-TFII and EphB4 remain relatively unchanged and (C) anti-thrombotic markers KLF2 and KLF4 are significantly increased with both low and high shear exposure. Both low and high shear conditions are relative to static and normalized to internal house-keeping gene GAPDH (n=3, asterisks indicate p < 0.05).
Finally, we looked at the major signaling pathway in inducing an arterial phenotype, the Notch pathway. We found Notch1 to be significantly upregulated in response to shear (Figure 6A) both at the mRNA level (low and high shear) and at the protein level as assessed by immunostaining for the activated form of Notch1 or Notch1-intracellular domain (NICD) (Figure 6A, bottom right panel). It is possible that in our system using immature hiPSC-ECs, which have never been exposed to physiological flow, relatively low shear is sufficient to have a significant effect on Notch1 signaling. However, the Notch ligand Dll4 was not considerably affected by flow (Figure 6A). Furthermore, shear did not appreciably affect the expression of venous markers COUPT-FII and EphB4 (Figure 6B). Taken together, these data show that shear stress can activate the Notch signaling pathway as indicated by increased Notch1 mRNA and increased activated form of Notch1, but that this increase did not result from an increase in gene expression of the Notch ligand Dll4. The increased Notch signaling may cause a downstream upregulation of EphrinB2 expression and other arterial markers, including CXCR4 and conexin40, while not appreciably affecting venous marker expression (*p< 0.05, n=3 biological replicates for each experiment).
3.5 Effect of shear on anti-thrombotic marker expression
Laminar shear stress has been shown to confer antithrombotic, anti-adhesive, and anti-inflammatory effects to endothelium, as well as to enhance endothelial survival [32]. An important indicator of the anti-thrombotic and anti-inflammatory effects of laminar shear stress on ECs is the expression of the transcription factors kruppel-like factors 2 and 4 (KLF2 and KLF4). These factors have been shown to be differentially upregulated in cultured hiPSC-ECs under “atheroprotective” high shear stress waveforms [3]. These markers were both significantly upregulated in both low and high shear conditions, with approximately 10 and 15 fold change increases over static conditions, respectively (Figure 6C). Thus, the upregulation of these markers is indicative of a functional vasoprotective phenotype that is typical of large artery endothelium under laminar shear stress.
3.6 Effects of soluble factors added to static culture, as compared to shear stress effects
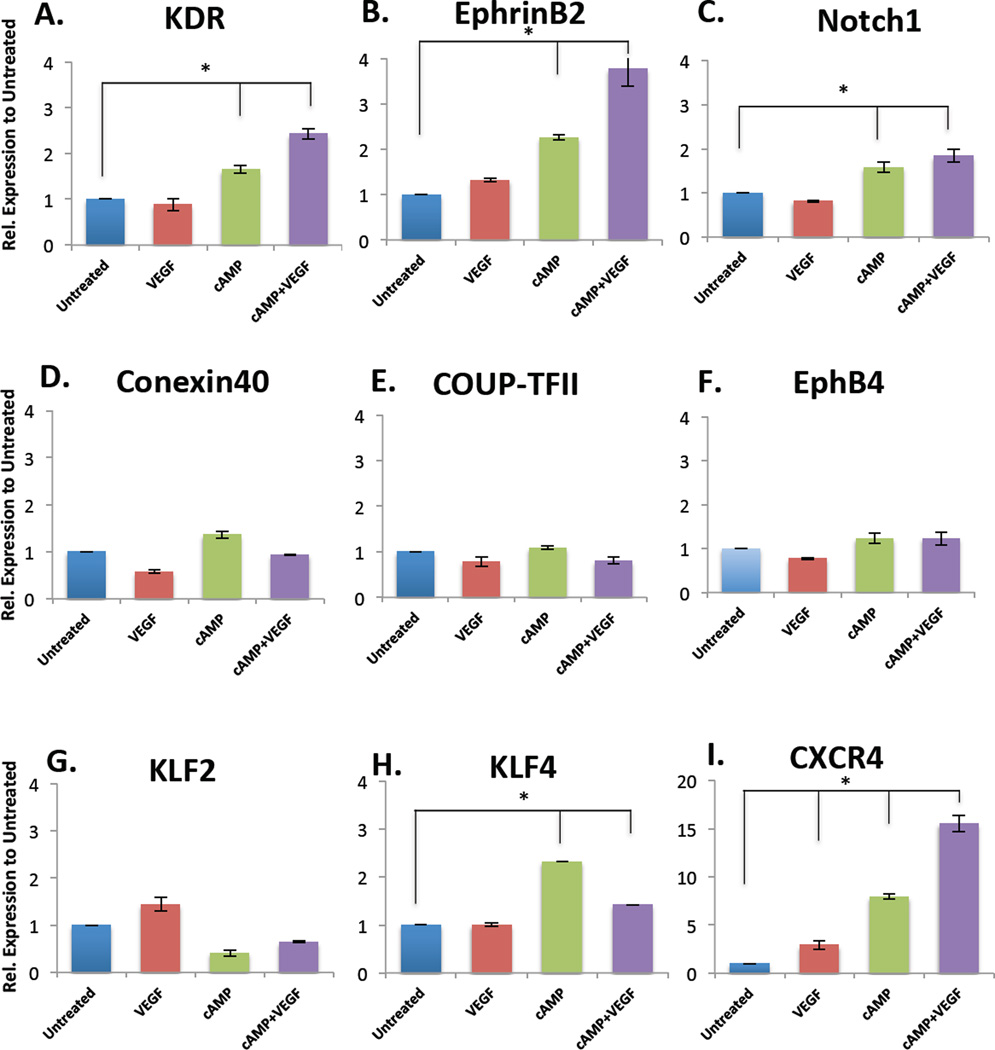
We also evaluated the impact of exogenously adding the soluble factors - VEGF and adrenomedullin or 8bromo-cAMP (abbreviated cAMP) - to the culture. We evaluated addition of high levels of VEGF (50 ng/ml), and cAMP (5 µM), either separately or in combination, to cultured hiPSC-derived ECs. There were significantly increased expression levels of KDR (Figure 7A), EphrinB2 (Figure 7B) and Notch1 (Figure 7C) after exposure to either cAMP alone, or to cAMP + VEGF, as compared to untreated controls. The combination of cAMP and VEGF had the most significant impact on EphrinB2 expression, which is consistent with previously published work showing that together, these soluble factors enhance arterial marker gene expression in hESC-derived ECs [8]. In contrast, addition of the soluble factors had no significant effect on the expression of the venous markers COUP-TFII and EphB4 (Figure 7E–F). This is an expected response, since cAMP activates Notch signaling and subsequent downstream EphrinB2 expression, which reciprocally mediates EphB4. Also, soluble factors did not significantly increase conexin40 (Figure 7D) or KLF2 (Figure 7G) expression, with only minor increases in KLF4 expression (Figure 7H). Finally, compared to shear stress, only CXCR4 expression is significantly increased (~5 fold) with the addition of cAMP+VEGF (Figure 7I, Supplementary Figure 2D). These findings indicate, generally, that a simple combination of VEGF and cAMP are not sufficient to replicate the arterial differentiation specification that is conferred by mechanical shear stress. While there are some modest increases in some arterial marker gene expression with the addition of soluble factors, they largely do not recapitulate the arterial-like phenotype that is gained after hiPSC-ECs are exposed to shear stress (Supplementary Figure 2D). This implicates the importance of biomechanical stimuli such as shear stress in committing ECs to their fate during development.
Figure 7.
qPCR demonstrating the effect of soluble factors VEGF (50 ng/ml), cAMP (5 µM), either separately or in combination for 24 hours, on the gene expression levels of (A) KDR (B) EphrinB2 (C) Notch1 (D) Conexin40 (E) COUP-TFII (F) EphB4 (G) KLF2 (H) KLF4 (I) CXCR4. All conditions are relative to untreated controls and normalized to GAPDH (n = 3, *p<0.05, between untreated and respective condition).
4. Discussion
The endothelium is constantly subjected to numerous microenvironmental factors, both chemical and mechanical, making it a highly dynamic cell type that is imbued with a phenotypic plasticity. In the large vessels, including arteries, veins and the lymphatic system, the prevalent mechanical force is shear stress caused by blood (or lymph) flow. In this study, we generated ECs from hiPSCs and attempted to mimic physiological shear stress in a biomimetic flow bioreactor in order to evaluate the impact on maturation of these cells.
Current differentiation protocols have largely isolated heterogeneous populations of ECs based on general markers such as CD31, VE-Cadherin and KDR [4–6]. These cells are poorly characterized for their response to flow. Further, the in vitro systems designed to subject ECs to shear stress are mostly 2D systems that culture cells in monolayers [3, 33]. These systems are generally parallel plate flow chambers, in which EC monolayers are exposed to fluid flow through a small channel, or are cone and plate flow chambers, which expose ECs to shear under a rotating cone [34]. While highly tunable and validated by computational models, these methods are not without flaws. In addition to complexity of setup and use, the velocity gradients experienced by cells in the systems may be non-constant, as cells in the center are subjected to different forces from the cells at the edges, leading to inconsistences and experimental variation.
We developed a 3D biomimetic flow bioreactor which, when connected to a flow pump, perfuses medium through the center of a tube, similar to blood flowing through a blood vessel. By rolling a porous membrane on which hiPSC-ECs are cultured and placing it into a vessel chamber inside of a glass bioreactor, we were able to expose cells to constant physiological flow to promote the maturation of hiPSC-ECs toward an arterial-like phenotype. hiPSC-ECs cultured in this manner had expression of arterial markers EphrinB2, CXCR4, conexin40, and Notch1 as well as activated Notch1-ICD (NICD), while venous markers remained largely unchanged. Further, these cells had significantly upregulated KLF2 and KLF4 gene expression under flow, which is indicative of an “atheroprotective,” anti-thrombotic and anti-inflammatory functional phenotype [32]. However, these phenotypic characteristics were not fully recapitulated by the addition of soluble factors that have been previously reported to promote an arterial phenotype in hESCs, indicating that the biomechanical forces may be a more potent means of inducing an arterial-like phenotype in pluripotent stem cell-derived ECs.
The creation of large-scale cell culture systems that subject ECs and other cell types to physiological forces are becoming increasingly important [20]. Our bioreactor system provides a promising alternative to current methods of in vitro flow chambers and has the added advantage of scale-up potential by maturing millions of cells. The parts required to set up the bioreactor are autoclavable and readily available to most research laboratories. The ability to scale up functionally mature arterial hiPSC-ECs will be especially useful for the endothelialization of tissue engineered arterial grafts [35]. Such grafts could be primed to experience the high shear stress encountered in high-pressure arterial circulation, and would potentially be more resistant to acute thrombosis, inflammation and negative vascular remodeling than vein grafts when placed into arterial circulation. Further applications of the functionally mature cells would be for the improvement of tissue perfusion by generating robust, perfusion-competent microvessels that could be used as a therapy for a wide range of diseases, including chronic limb ischemia. By exploiting the biomechanical microenvironments that ECs are subjected to in vivo, both in normal and pathological states, we may create better systems that recapitulate those states for functional maturation and disease modeling, respectively. However, it remains to be seen whether cessation of flow alters the functional behavior of arterial hiPSC-ECs either in vitro or in vivo. Our system is one example of a system that can functionally mature large quantities of hiPSC-ECs for vascular tissue engineering applications.
5. Conclusion
Our study demonstrates that the use of a biomimetic flow bioreactor can be used to subject ECs derived from hiPSCs to shear stress, and to modulate hiPSC-EC phenotype and upregulate the markers associated with an arterial-like state. Our data show that the use of the flow bioreactor, in which cells are cultured on a membrane and exposed to flow similar to in vivo physiological conditions is significantly better than the use of soluble factors. An important advantage of using this bioreactor system is that it reduces or eliminates the need for exogenous addition of costly growth factors to culture medium. This system is amenable to scale-up and allows for maturation of large quantities of subtype-specific ECs for use in therapeutic or research applications in regenerative medicine.
Supplementary Material
ACKNOWLEDGEMENTS
This work was supported by Yale University, and by R01 HL083895-06 (to LEN), and by NIH 5U01HL110967-03 (to MG). The authors would also like to thank Dr. Karen Hirschi, Department of Medicine, Section of Cardiovascular Medicine, Yale University, for her valuable input for experiments.
Abbreviations
- hiPSC
human induced pluripotent stem cells
- hiPSC-EC
human iPSC-derived endothelial cells
- HUVEC
human umbilical cord vein endothelial cells
- HAEC
human aortic endothelial cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURE STATEMENT: L.E.N. has a financial interest in Humacyte, Inc, a regenerative medicine company. Humacyte did not fund these studies, and Humacyte did not affect the design, interpretation, or reporting of any of the experiments herein.
Author contributions
LEN designed the research and wrote the manuscript; AS differentiated the hiPSCs, performed most of the analyses and wrote the manuscript; MG performed PCR and helped with data analysis; AVL performed western blotting; JM assisted with bioreactor set-up; YQ provided technical guidance and critical feedback
References
- 1.Nolan DJ, Ginsberg M, Israely E, Palikuqi B, Poulos MG, James D, et al. Molecular signatures of tissue-specific microvascular endothelial cell heterogeneity in organ maintenance and regeneration. Developmental Cell. 2013;26(2):204–219. doi: 10.1016/j.devcel.2013.06.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Takahashi K, Tanabe K, Ohnuki M, Narita M, Ichisaka T, Tomoda K, et al. Induction of pluripotent stem cells from adult human fibroblasts by defined factors. Cell. 2007;131(5):861–872. doi: 10.1016/j.cell.2007.11.019. [DOI] [PubMed] [Google Scholar]
- 3.Adams WJ, Zhang Y, Cloutier J, Kuchimanchi P, Newton G, Sehrawat S, et al. Functional vascular endothelium derived from human induced pluripotent stem cells. Stem Cell Reports. 2013;1(2):105–113. doi: 10.1016/j.stemcr.2013.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Li Z, Hu S, Ghosh Z, Han Z, Wu JC. Functional characterization and expression profiling of human induced pluripotent stem cell- and embryonic stem cell-derived endothelial cells. Stem cells and development. 2011;20(10):1701–1710. doi: 10.1089/scd.2010.0426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rufaihah AJ, Huang NF, Kim J, Herold J, Volz KS, Park TS, et al. Human induced pluripotent stem cell-derived endothelial cells exhibit functional heterogeneity. Am J Transl Res. 2013;5(1):21–35. [PMC free article] [PubMed] [Google Scholar]
- 6.White MP, Rufaihah AJ, Liu L, Ghebremariam YT, Ivey KN, Cooke JP, et al. Limited Gene Expression Variation in Human Embryonic Stem Cell and Induced Pluripotent Stem Cell-Derived Endothelial Cells. Stem Cells. 2013;31(1):92–103. doi: 10.1002/stem.1267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rufaihah AJ, Huang NF, Jamé S, Lee JC, Nguyen HN, Byers B, et al. Endothelial cells derived from human iPSCS increase capillary density and improve perfusion in a mouse model of peripheral arterial disease. Arterioscler Thromb Vasc Biol. 2011;31(11):e72–e79. doi: 10.1161/ATVBAHA.111.230938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Yurugi-Kobayashi T, Itoh H, Schroeder T, Nakano A, Narazaki G, Kita F, et al. Adrenomedullin/cyclic AMP pathway induces Notch activation and differentiation of arterial endothelial cells from vascular progenitors. Arterioscler Thromb Vasc Biol. 2006;26(9):1977–1984. doi: 10.1161/01.ATV.0000234978.10658.41. [DOI] [PubMed] [Google Scholar]
- 9.Masumura T, Yamamoto K, Shimizu N, Obi S, Ando J. Shear Stress Increases Expression of the Arterial Endothelial Marker EphrinB2 in Murine ES Cells via the VEGF-Notch Signaling Pathways. Arterioscler Thromb Vasc Biol. 2009;29(12):2125–2131. doi: 10.1161/ATVBAHA.109.193185. [DOI] [PubMed] [Google Scholar]
- 10.Akimoto S, Mitsumata M, Sasaguri T, Yoshida Y. Laminar Shear Stress Inhibits Vascular Endothelial Cell Proliferation by Inducing Cyclin-Dependent Kinase Inhibitor p21Sdi1/Cip1/Waf1. Circulation Research. 2000;86(2):185–190. doi: 10.1161/01.res.86.2.185. [DOI] [PubMed] [Google Scholar]
- 11.Boo YC, Sorescu G, Boyd N, Shiojima I, Walsh K, Du J, et al. Shear Stress Stimulates Phosphorylation of Endothelial Nitric-oxide Synthase at Ser1179 by Akt-independent Mechanisms Role of Protein Kinase A. J Biol Chem. 2002;277(5):3388–3396. doi: 10.1074/jbc.M108789200. [DOI] [PubMed] [Google Scholar]
- 12.Li Y-SJ, Haga JH, Chien S. Molecular basis of the effects of shear stress on vascular endothelial cells. Journal of Biomechanics. 2005;38(10):1949–1971. doi: 10.1016/j.jbiomech.2004.09.030. [DOI] [PubMed] [Google Scholar]
- 13.Quint C, Kondo Y, Manson RJ, Lawson JH, Dardik A, Niklason LE. Decellularized tissue-engineered blood vessel as an arterial conduit. Proceedings of the National Academy of Sciences. 2011 doi: 10.1073/pnas.1019506108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chong DC, Koo Y, Xu K, Fu S, Cleaver O. Stepwise arteriovenous fate acquisition during mammalian vasculogenesis. Dev Dyn. 2011;240(9):2153–2165. doi: 10.1002/dvdy.22706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang HU, Chen Z-F, Anderson DJ. Molecular Distinction and Angiogenic Interaction between Embryonic Arteries and Veins Revealed by ephrin-B2 and Its Receptor Eph-B4. Cell. 1998;93(5):741–753. doi: 10.1016/s0092-8674(00)81436-1. [DOI] [PubMed] [Google Scholar]
- 16.le Noble F, Moyon D, Pardanaud L, Yuan L, Djonov V, Matthijsen R, et al. Flow regulates arterial-venous differentiation in the chick embryo yolk sac. Development. 2004;131(2):361–375. doi: 10.1242/dev.00929. [DOI] [PubMed] [Google Scholar]
- 17.Kudo FA, Muto A, Maloney SP, Pimiento JM, Bergaya S, Fitzgerald TN, et al. Venous Identity Is Lost but Arterial Identity Is Not Gained During Vein Graft Adaptation. Arterioscler Thromb Vasc Biol. 2007;27(7):1562–1571. doi: 10.1161/ATVBAHA.107.143032. [DOI] [PubMed] [Google Scholar]
- 18.Kim D, Kim C-H, Moon J-I, Chung Y-G, Chang M-Y, Han B-S, et al. Generation of Human Induced Pluripotent Stem Cells by Direct Delivery of Reprogramming Proteins. Cell Stem Cell. 2009;4(6):472–476. doi: 10.1016/j.stem.2009.05.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, et al. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells. Science. 2007;318(5858):1917–1920. doi: 10.1126/science.1151526. [DOI] [PubMed] [Google Scholar]
- 20.Ghaedi M, Mendez JJ, Bove PF, Sivarapatna A, Raredon MSB, Niklason LE. Alveolar epithelial differentiation of human induced pluripotent stem cells in a rotating bioreactor. Biomaterials. 2014;35(2):699–710. doi: 10.1016/j.biomaterials.2013.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lee EJ, Niklason LE. A Novel Flow Bioreactor for In Vitro Microvascularization. Tissue Engineering Part C: Methods. 2010;16(5):1191–1200. doi: 10.1089/ten.tec.2009.0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yamamizu K, Matsunaga T, Uosaki H, Fukushima H, Katayama S, Hiraoka-Kanie M, et al. Convergence of Notch and B-catenin signaling induces arterial fate in vascular progenitors. J Cell Biol. 2010;189(2):325–338. doi: 10.1083/jcb.200904114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Khoo CP, Micklem K, Watt SM. A Comparison of Methods for Quantifying Angiogenesis in the Matrigel Assay In Vitro. Tissue Eng Part C Methods. 2011;17(9):895–906. doi: 10.1089/ten.tec.2011.0150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rae PC, Kelly RDW, Egginton S, John JCS. Angiogenic potential of endothelial progenitor cells and embryonic stem cells. Vascular Cell. 2011;3(1) doi: 10.1186/2045-824X-3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wragg JW, Durant S, McGettrick HM, Sample KM, Egginton S, Bicknell R. Shear Stress Regulated Gene Expression and Angiogenesis in Vascular Endothelium. Microcirculation. 2014;21(4):290–300. doi: 10.1111/micc.12119. [DOI] [PubMed] [Google Scholar]
- 26.Fujiwara K. Platelet endothelial cell adhesion molecule-1 and mechanotransduction in vascular endothelial cells. Journal of Internal Medicine. 2006;259(4):373–380. doi: 10.1111/j.1365-2796.2006.01623.x. [DOI] [PubMed] [Google Scholar]
- 27.Tzima E, Irani-Tehrani M, Kiosses WB, Dejana E, Schultz DA, Engelhardt B, et al. A mechanosensory complex that mediates the endothelial cell response to fluid shear stress. Nature. 2005;437(7057):426–431. doi: 10.1038/nature03952. [DOI] [PubMed] [Google Scholar]
- 28.Nourse MB, Halpin DE, Scatena M, Mortisen DJ, Tulloch NL, Hauch KD, et al. VEGF Induces Differentiation of Functional Endothelium From Human Embryonic Stem Cells Implications for Tissue Engineering. Arterioscler Thromb Vasc Biol. 2010;30(1):80–89. doi: 10.1161/ATVBAHA.109.194233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Jones EAV. Mechanical factors in the development of the vascular bed. Respiratory Physiology & Neurobiology. 2011;178(1):59–65. doi: 10.1016/j.resp.2011.03.026. [DOI] [PubMed] [Google Scholar]
- 30.Haefliger J-A, Nicod P, Meda P. Contribution of connexins to the function of the vascular wall. Cardiovasc Res. 2004;62(2):345–356. doi: 10.1016/j.cardiores.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 31.Vorderwülbecke BJ, Maroski J, Fiedorowicz K, Silva-Azevedo LD, Marki A, Pries AR, et al. Regulation of endothelial connexin40 expression by shear stress. American Journal of Physiology - Heart and Circulatory Physiology. 2012;302(1):H143–H152. doi: 10.1152/ajpheart.00634.2011. [DOI] [PubMed] [Google Scholar]
- 32.Atkins GB, Jain MK. Role of Krüppel-Like Transcription Factors in Endothelial Biology. Circulation Research. 2007;100(12):1686–1695. doi: 10.1161/01.RES.0000267856.00713.0a. [DOI] [PubMed] [Google Scholar]
- 33.Wang C, Baker BM, Chen CS, Schwartz MA. Endothelial Cell Sensing of Flow Direction. Arterioscler Thromb Vasc Biol. 2013;33(9):2130–2136. doi: 10.1161/ATVBAHA.113.301826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Hsieh H-J, Liu C-A, Huang B, Tseng AHH, Wang DL. Shear-induced endothelial mechanotransduction: the interplay between reactive oxygen species (ROS) and nitric oxide (NO) and the pathophysiological implications. J Biomed Sci. 2014;21(1) doi: 10.1186/1423-0127-21-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Niklason LE, Gao J, Abbott WM, Hirschi KK, Houser S, Marini R, et al. Functional arteries grown in vitro. Science. 1999;284(5413):489–493. doi: 10.1126/science.284.5413.489. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.