Abstract Abstract
Peripheral pulmonary artery stenosis (PPAS) is an underrecognized condition in the adult population. PPAS can lead to pulmonary hypertension but is likely misdiagnosed as either idiopathic pulmonary arterial hypertension or chronic thromboembolic pulmonary hypertension. We retrospectively identified adult patients with PPAS either in its isolated form or related to other congenital defects from January 1998 to September 2012. We reviewed the patients’ clinical data by using our hospital electronic medical records and/or their paper charts. We identified 6 adult patients with PPAS with an age range of 16–56 years (1 woman and the rest men). Presenting signs and symptoms were thoracic murmurs, progressive dyspnea, and syncope. Three patients had Williams-Beuren syndrome. Pulmonary angiography showed that PPAS was predominantly located in main branches or lobar pulmonary arteries in 5 patients, while in 1 patient the arterial narrowing was at the level of the segmental pulmonary arteries. Right heart catheterization showed a mean pulmonary artery pressure (PAP) ranging from 35 to 60 mmHg. Balloon dilation was performed in all patients, predominantly in the lobar arteries, and it caused a decrease in mean PAP that ranged from 16% to 46% in 5 patients. In 1 patient the mean PAP did not decrease. All but 1 patient had follow-up echocardiograms at 1 year that showed stable echocardiographic findings. Pulmonary hypertension due to PPAS continues to presents a diagnostic challenge. Therefore, a high index of suspicion during the initial evaluation of pulmonary hypertension is essential for its prompt diagnosis and adequate treatment.
Keywords: pulmonary hypertension, peripheral pulmonary artery stenosis, diagnosis
Pulmonary hypertension (PH) is a condition characterized by an elevated pulmonary artery pressure (PAP) that can lead to right heart failure and, ultimately, death.1 PH is caused by a variety of diseases that are divided into 5 groups.2 These groups are pulmonary arterial hypertension (PAH; group 1), PH due to left heart diseases (group 2), PH owing to lung diseases and/or hypoxia (group 3), chronic thromboembolic pulmonary hypertension (CTEPH; group 4), and PH with unclear multifactorial mechanism (group 5).2
Peripheral pulmonary artery stenosis (PPAS) is well described in children3-5 but not in the adult population, where it is often underrecognized. PPAS was recently included in the latest classification of PH (Nice, 2013) as part of group 5 (PH with unclear multifactorial mechanism), under the name “segmental PH.”2 PPAS is a rare disease that is commonly misdiagnosed as idiopathic PAH or CTEPH.6-8 PPAS can present as an isolated congenital problem9 or, more commonly, as part of Williams-Beuren syndrome (WBS) or Alagille syndrome or after surgery for congenital heart disease involving pulmonary artery reconstruction.10 In addition, Takayasu arteritis and Behçet disease have been associated with pulmonary artery stenoses that required immunosuppressive therapy and pulmonary artery balloon angioplasty with or without stenting.11-15
Isolated cases of PPAS in adulthood have been seldom reported,7,8,16 a fact that can reflect a low prevalence of the disease in addition to underrecognition and misdiagnosis.16 These last two factors are of great relevance, since patients may not receive appropriate treatment (i.e., balloon or stent angioplasty) or the therapy might be inappropriate for PPAS (i.e., PH-specific therapies or pulmonary thromboendarterectomy).
In this study, we describe the experience of a large PH center in the diagnosis and management of PH related to PPAS in adult patients. Our aim was to determine the clinical presentation, treatment received, and prognosis of patients with PPAS seen at our institution. In addition, we have reviewed the literature on this topic and describe the diagnostic and treatment approach for this particular disease, emphasizing the characteristics that might help uncover this uncommon condition in patients with PH.
Methods
The Institutional Review Board at Cleveland Clinic approved this study (protocol no. 13-184) and waived the need for informed consent. We retrospectively queried the Congenital Heart Disease database at the Cleveland Clinic to identify cases of PPAS. We included adult patients with PPAS, either in its isolated form or related to other congenital defects, from January 1998 to September 2012. We reviewed the patients’ clinical data, using our hospital electronic medical records and/or their paper charts. We initially identified 9 subjects with PPAS, and of those 6 met the inclusion criteria because they were adults (≥16 years old).
Patients were diagnosed with PPAS by selective pulmonary angiography during right heart catheterization, and all underwent balloon angioplasty. The hemodynamic and angiographic data were carefully reviewed before and after angioplasty in all these cases. We recorded the number and site of pulmonary vessels that were dilated. We collected data on echocardiographic determinations and quantitative nuclear lung perfusion before and after the interventions. In addition, we determined the survival status as of March 1, 2014, both by reviewing our records and by querying the US Social Security Death Index.
Results
We identified 6 adult patients with PPAS, with an age range of 16–56 years (Table 1). Five of the 6 were males, and 4 had other congenital abnormalities. Other congenital abnormalities were part of WBS in 3 patients. The 3 patients with WBS had cardiac murmurs that led to diagnosis of supravalvular aortic stenosis and PPAS. Two patients had progressive dyspnea, while the last presented with a thoracic murmur and a history of a recent syncope. Before balloon dilation all patients had dyspnea with activities.
Table 1.
Patient characteristics
| Characteristic | Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 |
|---|---|---|---|---|---|---|
| Age, years | 35 | 26 | 16 | 17 | 17 | 56 |
| Sex | M | M | F | M | M | M |
| Other congenital anomalies | Anomalous origin of left CA from main PA | None | WBS | WBS + partial anomalous venous return | WBS | None |
| Initial presentation | Progressive dyspnea | Thoracic murmur and syncope | Cardiac murmur | Cardiac murmur | Cardiac murmur | Progressive dyspnea and palpitations |
| Prior interventions | None | None | None | None | Open heart surgery for supravalvular aortic stenosis | None |
| TRJV, m/sa | 4.1 | 4.2 | Not obtainedb | 4.2 | NA | Not obtainedb |
| Qualitative RV functiona | Normal | Normal | Normal | Normal | NA | Normal |
| RV hypertrophya | Moderate | NA | Severe | Moderate | NA | Moderate |
| Location of PA stenosis | Proximal bilateral | Peripheral bilateral | Proximal bilateral | Proximal bilateral | Proximal bilateral | Proximal bilateral |
| Quantitative nuclear lung perfusion, % | ||||||
| Right | 51 | NA | 33 | 44 | NA | 76 |
| Left | 48 | NA | 67 | 56 | NA | 23 |
| Mean PA pressure, mmHg | 35 | 60 | 45 | 32 | 54 | 55 |
| PVR, Wood units | 5.6 | 11 | 9.7 | 3.8 | NA | 7.1 |
| CI, L/min/m2 | 2.9 | 2.6 | 2.1 | 2.9 | NA | 3.6 |
CA: coronary artery; CI: cardiac index; F: female; M: male; NA: not available; PA: pulmonary artery; PVR: pulmonary vascular resistance; RV: right ventricular; TR: tricuspid regurgitation; TRJV: tricuspid regurgitant jet velocity; WBS: Williams-Beuren syndrome.
Echocardiogram immediately before the intervention.
Unable to obtain, given trivial TR jet.
A nuclear quantitative perfusion scan was done in 4 patients, and in 2 it showed marked differences in radiotracer uptake between the lungs. PPAS was predominantly located in main branches or lobar pulmonary arteries in 5 patients, while in the remaining patient the arterial narrowing was at the level of the segmental pulmonary arteries. In all patients left and right pulmonary arteries were affected. Right heart catheterization showed a mean PAP ranging from 35 to 60 mmHg proximal to the sites of stenoses.
Balloon dilation was performed in all patients, predominantly in the lobar arteries (Table 2), and it caused a decrease in proximal mean PAP that ranged from 16% to 46% in 5 patients (Figs. 1, 2). In 1 patient there was a small (12%) increase in mean PAP (possibly related to substantial contrast dye administration, given that the patient had 7 vessels dilated, and his pulmonary artery occlusion pressure increased accordingly). Similarly, the ratio of right-to-left ventricular systolic pressure decreased in all but 1 patient (Table 2). The number of pulmonary arteries that were dilated by balloon angioplasty varied markedly between 2 to 19, depending on the patient’s anatomy and sites of stenoses. Segmental vessels, instead of lobar arteries, were targeted in the patient who underwent 19 balloon dilations. All patients had balloon angioplasties on left and right pulmonary arteries, in a similar number of vessels. In all patients, at least one of the balloon dilations showed an increase in the pulmonary artery luminal diameter of at least 50%. One patient developed reperfusion, a mild degree of pulmonary edema that improved with diuretics in the course of 24 hours. Patients experienced improvement of their symptoms in all cases.
Table 2.
Intervention performed and results
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
|---|---|---|---|---|---|---|
| Intervention | Balloon dilation of lobar PAs | Balloon dilation of segmental PAs | Balloon dilation of lobar PAs | Balloon dilation of main PAs | Balloon dilation of main PAs | Balloon dilation of lobar PAs |
| Number of vessels dilated | 2 | 19 | 5 | 7 | 2 | 5 |
| Right | 1 | 10 | 2 | 4 | 1 | 2 |
| Left | 1 | 9 | 3 | 3 | 1 | 3 |
| Mean PA pressure after intervention, mmHg (% change) | 27 (−23) | 41 (−32) | 38 (−16) | 36 (+12) | 29 (−46) | 43 (−22) |
| RV/LV systolic pressure, % | ||||||
| Before intervention | 61 | 80 | 75 | 71 | 57 | 74 |
| After intervention | 50 | 55 | 50 | 79 | 44 | 57 |
| Quantitative nuclear lung perfusion after intervention, % | ||||||
| Right | 55 | NA | 42 | 53 | NA | 63 |
| Left | 45 | NA | 58 | 47 | NA | 37 |
| TRJV, m/sa | 3.5 | NA | Not obtainedb | 3.6 | 2 | 3 |
| Qualitative RV functiona | Normal | NA | Normal | Mild | Normal | Normal |
| RV hypertrophya | Moderate | NA | Mild | NA | NA | Mild |
| Survival status | Alive | Deadc | Alive | Alive | Alive | Alive |
LV: left ventricular; NA: not available; PA: pulmonary artery; RV: right ventricular; TR: tricuspid regurgitatiom; TRJV: tricuspid regurgitant jet velocity.
Echocardiogram performed a year after the intervention.
Unable to obtain, given trivial TR jet.
Unclear cause of death; patient was followed elsewhere during the 6 years after he was seen in the clinic.
Figure 1.
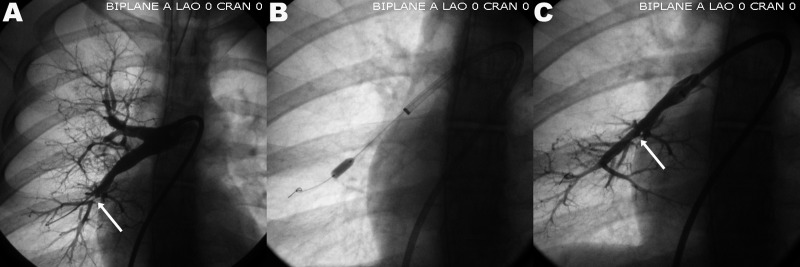
Balloon angioplasty of the right lobar pulmonary artery in a patient with peripheral pulmonary artery stenosis. A, Pulmonary artery stenosis; B, balloon dilation; C, final result after dilation. White arrows point to the area of pulmonary artery stenosis before (A) and after (C) balloon dilation. CRAN: cranial; LAO: left anterior oblique.
Figure 2.
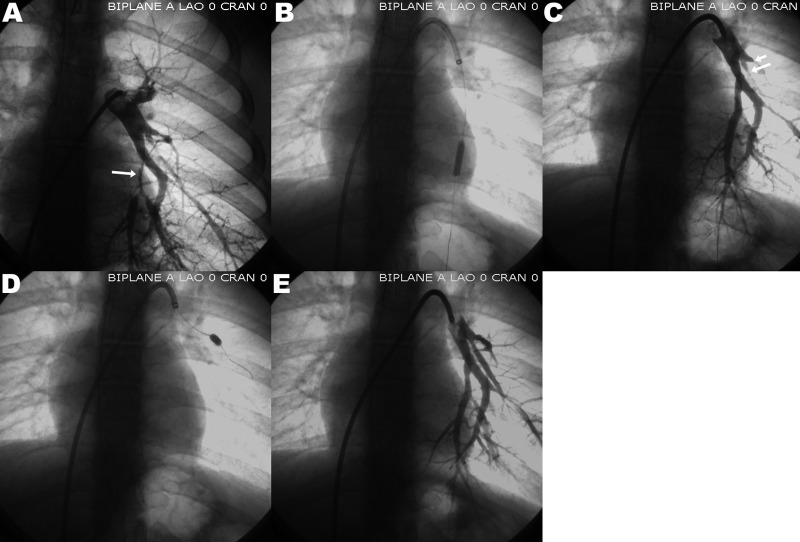
Balloon angioplasty of two segmental left lower-lobe pulmonary arteries in a patient with peripheral pulmonary artery stenosis. White arrows point to the areas of pulmonary artery stenosis. A, Segmental pulmonary artery stenosis; B, balloon dilation of the stenosis; C, result after dilation; D, balloon dilation of 2 segmental stenoses; E, final results after both balloon dilations. CRAN: cranial; LAO: left anterior oblique.
All but 1 patient had follow-up echocardiograms at 1 year that showed stable echocardiographic findings. Four patients had follow-up quantitative nuclear lung perfusion scans that for the most part revealed a more even distribution of the radiotracer (Table 2). One patient who was followed at another institution died of an unknown cause 6 years after he was last seen in our clinic. During our follow-up no patient required reintervention for PPAS.
Discussion
In this case series, we described the characteristics of adult PH patients with PPAS. These patients were recognized because they had cardiac murmurs or progressive dyspnea in association with congenital syndromes associated with PPAS, prior balloon dilation of the pulmonary arteries, or asymmetric quantitative nuclear lung perfusion. It is possible that some patients with PPAS who did not have the aforementioned presentation could have received a misdiagnosis of idiopathic PAH. In our cohort, it is unlikely that PPAS patients were mislabeled as CTEPH patients, because our center performs pulmonary thromboendarterectomies; hence, these PH patients undergo an extensive evaluation of the pulmonary circulation before their surgery. In all our patients with PPAS, pulmonary angiography revealed multiple bilateral, nonuniform stenoses in lobar or segmental/subsegmental pulmonary arteries. Pulmonary balloon angioplasty generally improved the vessel diameter, decreased the proximal PAP, and, more importantly, was associated with symptomatic improvement.
PPAS is frequently a vascular manifestation of the WBS,17,18 which is an autosomal dominant disorder that has an estimated incidence of 1 in 10,000 live births.19 WBS is due to a microdeletion of a portion of chromosome 7q11.23,20 which includes parts of the elastin gene and leads to reduced elastin synthesis and increased proliferation of vascular smooth muscle cells and fibroblasts.21 Vascular involvement is common (60%–80% of affected individuals) in WBS and is characterized by narrowing of medium- and large-sized elastic arteries due to thickening of the media,21,22 which leads to supravalvular aortic stenosis and PPAS. PPAS is sometimes severe and bilateral, involving the main pulmonary arteries as well as the branching points of the lobar vessels. Interestingly, spontaneous improvement of PPAS over time may occur in these patients, particularly when the stenoses are not severe.23-25 Therefore, catheter-based interventions are reserved for those with significantly elevated right ventricular (RV) pressure and/or symptoms.
Isolated PPAS continues to be underrecognized, although it was first reported in 1938.26 The progressive and nonuniform segmental vascular obstruction of the pulmonary circulation is typically associated with progressive dyspnea and fatigue in the second decade of life.8 The segmental stenosis can be single or multiple and can be confined to the main pulmonary arteries or to the peripheral segmental branches.8 Depending on the location of the stenosis, lung perfusion imaging may reveal segmental defects or unbalanced quantitative perfusion of the lungs, but because of its lack of specificity, diagnosis typically requires direct arterial imaging by contrast-enhanced computed tomography, magnetic resonance angiography, or catheter-based arteriography. Pulmonary arteriography remains the gold standard for the diagnosis of PPAS.
Treatment for PPAS includes surveillance, for those asymptomatic patients with preserved RV function and symmetric pulmonary blood flow, and balloon angioplasty,27,28 cutting balloon angioplasty,29 stent implantation,30 or surgery,31 for those with symptoms of right heart failure, substantial elevation of RV pressures, marked asymmetry in pulmonary blood flow (<25% of the total flow to a single lung), severe pulmonary regurgitation, or worsening hemodynamics or RV function.9 Recurrent stenosis is not uncommon, as it occurs in approximately 35% of successfully dilated vessels.32,33 Stent placement in the central pulmonary arteries has been performed with success,34 but stenting of small segmental arteries, particularly in patients with congenital PPAS, has been less successful.9,35,36 In recent years, surgical intervention for PPAS has been associated with low mortality and a reduction of the RV/aortic pressure ratio in half, a hemodynamic improvement that has been maintained during follow-up.31 However, surgery is limited to proximal pulmonary artery lesions; distal branches are difficult for the surgeon to repair.9,37
In summary, it is important to differentiate patients with PPAS from the more common groups of subjects with CTEPH6,7 or idiopathic PAH.8 It is necessary to consider PPAS in patients with prior pulmonary artery balloon dilations, certain genetic syndromes such as WBS, or prior surgeries for congenital heart disease; when the lung perfusion scan shows segmental perfusion defects; or when the PAP drops as the catheter advances through the pulmonary circulation.37 Further investigation, including contrast-enhanced computed tomography, magnetic resonance angiography, or pulmonary angiography, is needed to confirm the diagnosis.8
Conclusions. PH due to PPAS continues to present a diagnostic challenge. Therefore, a high index of suspicion during the initial evaluation of PH is essential for its prompt diagnosis and adequate treatment, since PPAS entails pulmonary artery balloon angioplasty instead of PH-specific therapies.
Sources of Support: ART is supported by a KL2 grant (TR000440) from the National Center for Research Resources, a component of the National Institutes of Health (NIH), and by NIH Roadmap for Medical Research.
Conflict of Interest: None declared.
References
- 1.McLaughlin VV, Archer SL, Badesch DB, Barst RJ, Farber HW, Lindner JR, Mathier MA, et al. ACCF/AHA 2009 expert consensus document on pulmonary hypertension: a report of the American College of Cardiology Foundation Task Force on Expert Consensus Documents and the American Heart Association: developed in collaboration with the American College of Chest Physicians, American Thoracic Society, Inc., and the Pulmonary Hypertension Association. Circulation 2009;119(16):2250–2294. [DOI] [PubMed]
- 2.Simonneau G, Gatzoulis MA, Adatia, I, Celermajer D, Denton C, Ghofrani A, Gomez Sanchez MA, et al. Updated clinical classification of pulmonary hypertension. J Am Coll Cardiol 2013;62(25 suppl.):D34–D41. [DOI] [PubMed]
- 3.Franch RH, Gay BB Jr. Congenital stenosis of the pulmonary artery branches: a classification, with postmortem findings in two cases. Am J Med 1963;35(4):512–529. [DOI] [PubMed]
- 4.Gentles TL, Lock JE, Perry SB. High pressure balloon angioplasty for branch pulmonary artery stenosis: early experience. J Am Coll Cardiol 1993;22(3):867–872. [DOI] [PubMed]
- 5.Eldredge WJ, Tingelstad JB, Robertson LW, Mauck HP, McCue CM. Observations on the natural history of pulmonary artery coarctations. Circulation 1972;45(2):404–409. [DOI] [PubMed]
- 6.Claudio MP, Barrocas M, Pifarré R, Neville WE, Meadows WR, Sharp JT. Peripheral pulmonary artery stenosis secondary to chronic pulmonary thromboembolic disease. Am J Cardiol 1970;25(4):495–500. [DOI] [PubMed]
- 7.Kreutzer J, Landzberg MJ, Preminger TJ, Mandell VS, Treves ST, Reid LM, Lock JE. Isolated peripheral pulmonary artery stenoses in the adult. Circulation 1996;93(7):1417–1423. [DOI] [PubMed]
- 8.Kushner T, Halperin JL, Nair AP, Fuster V, Love BA. Peripheral pulmonary artery stenosis masquerading as pulmonary hypertension: a diagnostic and therapeutic challenge. Vasc Med 2012;17(4):235–238. [DOI] [PubMed]
- 9.Trivedi KR, Benson LN. Interventional strategies in the management of peripheral pulmonary artery stenosis. J Interv Cardiol 2003;16(2):171–188. [DOI] [PubMed]
- 10.Inglessis I, Landzberg MJ. Interventional catheterization in adult congenital heart disease. Circulation 2007;115(12):1622–1633. [DOI] [PubMed]
- 11.Luo Q, Zhang HL, Liu ZH, Xiong CM, Ni XH. Percutaneous transluminal angioplasty and stenting for pulmonary stenosis due to Takayasu’s arteritis: clinical outcome and four-year follow-up. Clin Cardiol 2009;32(11):639–643. [DOI] [PMC free article] [PubMed]
- 12.Pelage JP, El Hajjam M, Lagrange C, Chinet T, Vieillard-Baron A, Chagnon S, Lacombe P. Pulmonary artery interventions: an overview. Radiographics 2005;25(6):1653–1667. [DOI] [PubMed]
- 13.Toledano K, Guralnik L, Lorber A, Ofer A, Yigla M, Rozin A, Markovits D, Braun-Moscovici Y, Balbir-Gurman A. Pulmonary arteries involvement in Takayasu’s arteritis: two cases and literature review. Semin Arthritis Rheum 2011;41(3):461–470. [DOI] [PubMed]
- 14.Fei Y, Li X, Lin S, Song X, Wu Q, Zhu Y, Gao X, et al. Major vascular involvement in Behçet’s disease: a retrospective study of 796 patients. Clin Rheumatic 2013;32(6):845–852. [DOI] [PubMed]
- 15.Seyahi E, Melikoglu M, Akman C, Hamuryudan V, Ozer H, Hatemi G, Yurdakul S, Tuzun H, Oz B, Yazici H. Pulmonary artery involvement and associated lung disease in Behçet disease: a series of 47 patients. Medicine (Baltimore) 2012;91(1):35–48. [DOI] [PubMed]
- 16.Amano H, Tanabe N, Sakao S, Umekita H, Sugiura T, Kitazono S, Kitazono M, Kuroda F, Kasahara Y, Tatsumi K. A case of isolated peripheral pulmonary artery branch stenosis associated with multiple pulmonary artery aneurysms. Intern Med 2010;49(17):1895–1899. [DOI] [PubMed]
- 17.Williams JC, Barratt-Boyes BG, Lowe JB. Supravalvular aortic stenosis. Circulation 1961;24(6):1311–1318. [DOI] [PubMed]
- 18.Beuren AJ, Schulze C, Eberle P, Harmjanz D, Apitz J. The syndrome of supravalvular aortic stenosis, peripheral pulmonary stenosis, mental retardation and similar facial appearance. Am J Cardiol 1964;13(4):471–483. [DOI] [PubMed]
- 19.Pober BR, Johnson M, Urban Z. Mechanisms and treatment of cardiovascular disease in Williams-Beuren syndrome. J Clin Invest 2008;118(5):1606–1615. [DOI] [PMC free article] [PubMed]
- 20.Ewart AK, Morris CA, Atkinson D, Jin W, Sternes K, Spallone P, Stock AD, Leppert M, Keating MT. Hemizygosity at the elastin locus in a developmental disorder, Williams syndrome. Nat Genet 1993;5(1):11–16. [DOI] [PubMed]
- 21.Urbán Z, Riazi S, Seidl TL, Katahira J, Smoot LB, Chitayat D, Boyd CD, Hinek A. Connection between elastin haploinsufficiency and increased cell proliferation in patients with supravalvular aortic stenosis and Williams-Beuren syndrome. Am J Hum Genet 2002;71(1):30–44. [DOI] [PMC free article] [PubMed]
- 22.Li DY, Faury G, Taylor DG, Davis EC, Boyle WA, Mecham RP, Stenzel P, Boak B, Keating MT. Novel arterial pathology in mice and humans hemizygous for elastin. J Clin Invest 1998;102(10):1783–1787. [DOI] [PMC free article] [PubMed]
- 23.Giddins NG, Finley JP, Nanton MA, Roy DL. The natural course of supravalvar aortic stenosis and peripheral pulmonary artery stenosis in Williams’s syndrome. Br Heart J 1989;62(4):315–319. [DOI] [PMC free article] [PubMed]
- 24.Wren C, Oslizlok P, Bull C. Natural history of supravalvular aortic stenosis and pulmonary artery stenosis. J Am Coll Cardiol 1990;15(7):1625–1630. [DOI] [PubMed]
- 25.Wessel A, Pankau R, Kececioglu D, Ruschewski W, Bürsch JH. Three decades of follow-up of aortic and pulmonary vascular lesions in the Williams-Beuren syndrome. Am J Med Genet 1994;52(3):297–301. [DOI] [PubMed]
- 26.Oppenheimer EH. Partial atresia of main branches of pulmonary artery occurring in infancy and accompanied by calcification of pulmonary artery and aorta. Bull Johns Hopkins Hosp 1938;63:261–282.
- 27.Kan JS, Marvin WJ Jr., Bass JL, Muster AJ, Murphy J. Balloon angioplasty–branch pulmonary artery stenosis: results from the Valvuloplasty and Angioplasty of Congenital Anomalies Registry. Am J Cardiol 1990;65(11):798–801. [DOI] [PubMed]
- 28.Rothman A, Perry SB, Keane JF, Lock JE. Early results and follow-up of balloon angioplasty for branch pulmonary artery stenoses. J Am Coll Cardiol 1990;15(5):1109–1117. [DOI] [PubMed]
- 29.Sugiyama H, Veldtman GR, Norgard G, Lee KJ, Chaturvedi R, Benson LN. Bladed balloon angioplasty for peripheral pulmonary artery stenosis. Catheter Cardiovasc Interv 2004;62(1):71–77. [DOI] [PubMed]
- 30.Hwang B, Lee PC, Fu YC, Jan SL, Kao CC, Wang PY, Lien CH, Weng ZC, Meng CCL. Transcatheter implantation of intravascular stents for postoperative residual stenosis of peripheral pulmonary artery stenosis. Angiology 2004;55(5):493–498. [DOI] [PubMed]
- 31.Monge MC, Mainwaring RD, Sheikh AY, Punn R, Reddy VM, Hanley FL. Surgical reconstruction of peripheral pulmonary artery stenosis in Williams and Alagille syndromes. J Thorac Cardiovasc Surg 2013;145(2):476–481. [DOI] [PubMed]
- 32.Bush DM, Hoffman TM, Del Rosario J, Eiriksson H, Rome JJ. Frequency of restenosis after balloon pulmonary arterioplasty and its causes. Am J Cardiol 2000;86(11):1205–1209. [DOI] [PubMed]
- 33.Hoshina M, Tomita H, Kimura K, Ono Y, Yagihara T, Echigo S. Factors determining peripheral pulmonary artery stenosis remodeling in children after percutaneous transluminal balloon angioplasty. Circ J 2002;66(4):345–348. [DOI] [PubMed]
- 34.Shaffer KM, Mullins CE, Grifka RG, O’Laughlin MP, McMahon W, Nihill MR. Intravascular stents in congenital heart disease: short- and long-term results from a large single-center experience. J Am Coll Cardiol 1998;31(3):661–667. [DOI] [PubMed]
- 35.Khono K, Tamai A, Kobayashi T, Senzaki H. Effects of stent implantation for peripheral pulmonary artery stenosis on pulmonary vascular hemodynamics and right ventricular function in a patient with repaired tetralogy of Fallot. Heart Vessels 2011;26(6):672–676. [DOI] [PubMed]
- 36.Vranicar M, Teitel DF, Moore P. Use of small stents for rehabilitation of hypoplastic pulmonary arteries in pulmonary atresia with ventricular septal defect. Catheter Cardiovasc Interv 2002;55(1):78–82. [DOI] [PubMed]
- 37.Rhodes JF, Hijazi ZM, Sommer RJ. Pathophysiology of congenital heart disease in the adult, part II: simple obstructive lesions. Circulation 2008;117(9):1228–1237. [DOI] [PubMed]