Out goes the old
It is well known that athletes have a low resting heart rate (bradycardia). The bradycardia can be moderate to severe: reports of heart rates of 40–60 beats min−1 in athletes are common (Boyett et al. 2013), and Jensen-Urstad et al. (1997) reported heart rates of <30 beats min−1 in elite athletes at night. Consistent with this, S. Sharma has studied 142 elite cyclists and rowers and observed heart rates over the range 30–70 beats min−1; the distribution of heart rates in the athletes (and for comparison in a normal population) is shown in Fig.1. The resting heart rate is easy to measure and it is clear that athletes use the resting heart rate as a measure of fitness and speak of it in terms of pride and bravado. This is despite evidence that veteran athletes are more likely to need an electronic heart pacemaker fitted in later life (Baldesberger et al. 2008). The bradycardia is widely believed to be the result of high vagal tone; this is a natural assumption because high vagal tone will reduce the heart rate. However, despite this widespread belief, efferent vagal nerve activity to the heart's pacemaker (the sinus node) has never been recorded. It is not obvious how it could be measured, because the vagus nerve carries afferent as well as efferent nerve fibres. Because of this, the scientific community uses what is assumed to be a surrogate of vagal nerve activity to the sinus node, heart rate variability. Heart rate variability is a beat-to-beat variability in the heart rate and is assumed to be the result of stochastic fluctuations in autonomic nerve activity to the sinus node and changes in heart rate variability are assumed to represent changes in this. PubMed lists more than 17,000 publications concerned with heart rate variability. Heart rate variability is higher in athletes (Aubert et al. 2003) and this is taken as evidence of high vagal tone in athletes, and this is then assumed to be responsible for the bradycardia. However, a causative link between autonomic nerve activity and heart rate variability has never been demonstrated (for reasons discussed above). Furthermore, we have recently analysed the biophysics underlying heart rate variability and we have shown that, regardless of whatever is responsible for heart rate variability, heart rate variability is a steep exponential function of heart rate (heart rate variability increases with a decrease in heart rate) and the majority of reported changes in heart rate variability can be explained by the concurrent change in heart rate (Monfredi et al. 2014). All published data concerning the increase in heart rate variability in athletes that we have analysed can be explained by the bradycardia in the athletes (Monfredi et al. 2014). To dissect the mechanisms underlying the low resting heart rate in athletes, investigators have blocked autonomic nerve activity to the heart by injection of blockers (frequently but not exclusively atropine and propranolol). We have reviewed these studies and in no study in which the evidence indicates that there was complete autonomic blockade was the bradycardia in athletes abolished (if the bradycardia is the result of high vagal tone it should not be present after block of vagal activity to the sinus node; Boyett et al. 2013). In fact, the bradycardia can be larger after complete autonomic blockade, such as in the study of Katona et al. (1982).
Figure 1. Distribution of resting heart rates in elite athletes and a normal population.
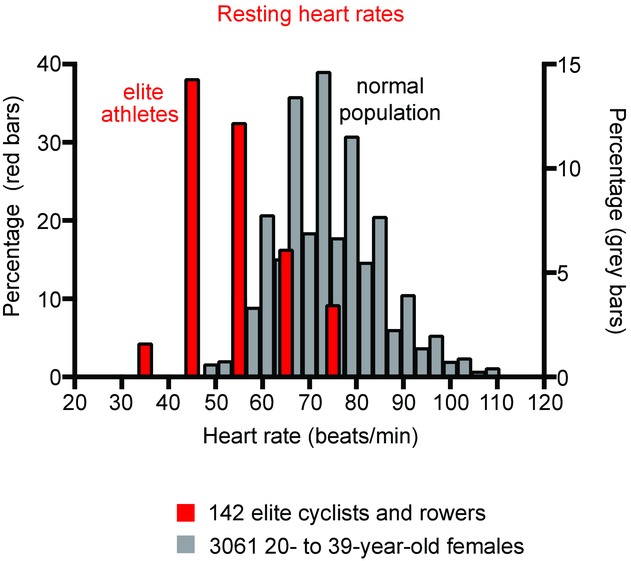
Red bars, histogram of resting heart rate of 142 elite cyclists and rowers (S. Sharma, unpublished observations). Grey bars, histogram of resting heart rate of 3061 20- to 39-year-old female subjects (Ostchega et al. 2011).
And in comes the new
If the bradycardia is not the result of high vagal tone, what is the underlying mechanism? The sinus node is the ‘Cinderella’ of the heart and is under-studied as compared to the working myocardium and, therefore, when discussing possible causes of change in sinus node function the investigator has had little to guide them. Older literature therefore ascribes dysfunction of the sinus node in disease to ‘fibrosis’ with little or no justification (in much the same way that bradycardia in athletes has been ascribed to high vagal tone). However, this has changed in the last 10 years and it has been demonstrated that change or dysfunction of the sinus node in familial bradycardia (Milanesi et al. 2006), ageing (Hao, 2011; Yanni et al. 2011b), heart failure (Zicha et al. 2005; Yanni et al. 2011a), pulmonary hypertension (Yamanushi et al. 2010), atrial fibrillation (Yeh et al. 2009), metabolic syndrome (Albarado-Ibañez et al. 2013) and pregnancy (El Khoury et al. 2013) is the result of a remodelling of ion channels and related molecules in the sinus node. This is unsurprising because the function of the sinus node is electrical and as such depends on the expression of ion channels, etc. The most common reported cause of a bradycardia in these various conditions is a downregulation of the funny channel (HCN4) and the corresponding funny current, an important pacemaker mechanism (Zicha et al. 2005; Milanesi et al. 2006; Yeh et al. 2009; Yamanushi et al. 2010; El Khoury et al. 2013).
The arguments raised above prompted us to hypothesise that the bradycardia in athletes is the result of ion channel remodelling in the sinus node. We worked on rat and mouse models of athletic training (treadmill running for 3 months in the case of the rat and swim training for 1 month in the case of the mouse; D'Souza et al. 2014). There was a resting bradycardia in vivo in the trained animals that was still largely present after complete autonomic blockade. The spontaneous beating rate of the isolated (and therefore denervated) sinus node was also lower in the case of the trained animals. Analysis of tissue biopsies from the sinus node of the trained animals by quantitative PCR showed a widespread remodelling of ion channels and related molecules in the sinus node, including a downregulation of the important pacemaker channel HCN4. The corresponding funny current was also downregulated. In vivo and ex vivo, after block of the funny current, the heart rate (or spontaneous beating rate) of sedentary and trained mice was the same (or similar) and this suggests that the resting bradycardia in athletes (rats and mice at least) is the result of a downregulation of HCN4 and funny current. What is responsible for this change? Within the sinus node of the trained animals, we observed a downregulation of the transcription factor Tbx3, upregulation of another, NRSF, and upregulation of a micro-RNA, miR-1, and these changes are appropriate to explain the downregulation of HCN4. However, whatever is driving the changes in transcription factors and micro-RNA is unknown.
As well as sinus bradycardia, first degree heart block (slowing of atrioventricular node conduction), second degree heart block (intermittent heart block), and possibly third degree or complete heart block is more common among athletes especially at night (Northcote et al. 1989) and again this is attributed to high vagal tone (Maron & Pelliccia, 2006). However, once again this is doubtful (Stein et al. 2002) and we hypothesise that it is the result of a similar remodelling of ion channels etc. in the atrioventricular node. The popularity of sport in general, and of ultra-endurance events in particular, is increasing with >500 marathons held annually worldwide. This is expected to lead to a rise in the number of athletes with heart rhythm problems and a proper understanding of the underlying mechanisms will be central to tackling these issues.
Call for comments
Readers are invited to give their views on this and the accompanying CrossTalk articles in this issue by submitting a brief (250 word) comment. Comments may be submitted up to 6 weeks after publication of the article, at which point the discussion will close and the CrossTalk authors will be invited to submit a ‘Last Word’. Please email your comment to jphysiol@physoc.org.
Biography
 Alicia D'Souza is a postdoctoral research fellow at the University of Manchester following a PhD in cardiac physiology. She is also the recipient of the 2014–2015 International Society for Heart Research/Servier Research Fellowship. Dr D'Souza's current research examines the regulation of the cardiac conduction system in health and disease with special emphasis on the effect of exercise training on arrhythmogenesis. Sanjay Sharma is a cardiologist. He qualified in medicine in 1989 from Leeds University. He is currently Professor of Inherited Cardiac Diseases in Sports Cardiology at St George's Hospital, London. He specialises in sports cardiology, in particular pre-participation screening in athletes and the condition known as athlete's heart. Mark Boyett studied cardiac electrophysiology at University College London under the supervision of Brian Jewell and in 1977 was awarded his PhD. After a year as a Royal Society Overseas Fellow at the University of Berne, he moved to the University of Leeds. In 1982 he worked for the first time with Itsuo Kodama who introduced him to the wonders of the sinus node. Mark remained at Leeds for 26 years. In 2005 he moved to the University of Manchester as Professor of Cardiac Electrophysiology.
Alicia D'Souza is a postdoctoral research fellow at the University of Manchester following a PhD in cardiac physiology. She is also the recipient of the 2014–2015 International Society for Heart Research/Servier Research Fellowship. Dr D'Souza's current research examines the regulation of the cardiac conduction system in health and disease with special emphasis on the effect of exercise training on arrhythmogenesis. Sanjay Sharma is a cardiologist. He qualified in medicine in 1989 from Leeds University. He is currently Professor of Inherited Cardiac Diseases in Sports Cardiology at St George's Hospital, London. He specialises in sports cardiology, in particular pre-participation screening in athletes and the condition known as athlete's heart. Mark Boyett studied cardiac electrophysiology at University College London under the supervision of Brian Jewell and in 1977 was awarded his PhD. After a year as a Royal Society Overseas Fellow at the University of Berne, he moved to the University of Leeds. In 1982 he worked for the first time with Itsuo Kodama who introduced him to the wonders of the sinus node. Mark remained at Leeds for 26 years. In 2005 he moved to the University of Manchester as Professor of Cardiac Electrophysiology.
Additional information
Competing interests
None declared.
References
- Albarado-Ibañez A, Avelino-Cruz JE, Velasco M, Torres-Jacome J. Hiriart M. Metabolic syndrome remodels electrical activity of the sinoatrial node and produces arrhythmias in rats. PLoS One. 2013;8:e76534. doi: 10.1371/journal.pone.0076534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aubert AE, Seps B. Beckers F. Heart rate variability in athletes. Sports Med. 2003;33:889–919. doi: 10.2165/00007256-200333120-00003. [DOI] [PubMed] [Google Scholar]
- Baldesberger S, Bauersfeld U, Candinas R, Seifert B, Zuber M, Ritter M, Jenni R, Oechslin E, Luthi P, Scharf C, Marti B. Attenhofer Jost CH. Sinus node disease and arrhythmias in the long-term follow-up of former professional cyclists. Eur Heart J. 2008;29:71–78. doi: 10.1093/eurheartj/ehm555. [DOI] [PubMed] [Google Scholar]
- Boyett MR, D'Souza A, Zhang H, Morris GM, Dobrzynski H. Monfredi O. Viewpoint: Is the resting bradycardia in athletes the result of remodeling of the sinoatrial node rather than high vagal tone? J Appl Physiol (1985) 2013;114:1351–1355. doi: 10.1152/japplphysiol.01126.2012. [DOI] [PubMed] [Google Scholar]
- D'Souza A, Bucchi A, Johnsen AB, Logantha SJ, Monfredi O, Yanni J, Prehar S, Hart G, Cartwright E, Wisloff U, Dobryznski H, DiFrancesco D, Morris GM. Boyett MR. Exercise training reduces resting heart rate via downregulation of the funny channel HCN4. Nat Commun. 2014;5:3775. doi: 10.1038/ncomms4775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El Khoury N, Mathieu S, Marger L, Ross J, El Gebeily G, Ethier N. Fiset C. Upregulation of the hyperpolarization-activated current increases pacemaker activity of the sinoatrial node and heart rate during pregnancy in mice. Circulation. 2013;127:2009–2020. doi: 10.1161/CIRCULATIONAHA.113.001689. [DOI] [PubMed] [Google Scholar]
- Hao X, Zhang Y, Zhang X, Nirmalan M, Davies L, Konstantinou D, Yin F, Dobrzynski H, Wang X, Grace A, Zhang H, Boyett M, Huang CL. Lei M. TGF-β1-mediated fibrosis and ion channel remodeling are key mechanisms in producing sinus node dysfunction associated with SCN5A deficiency and aging. Circ Arrhythm Electrophysiol. 2011;4:397–406. doi: 10.1161/CIRCEP.110.960807. [DOI] [PubMed] [Google Scholar]
- Jensen-Urstad K, Saltin B, Ericson M, Storck N. Jensen-Urstad M. Pronounced resting bradycardia in male elite runners is associated with high heart rate variability. Scand J Med Sci Sports. 1997;7:274–278. doi: 10.1111/j.1600-0838.1997.tb00152.x. [DOI] [PubMed] [Google Scholar]
- Katona PG, Mclean M, Dighton DH. Guz A. Sympathetic and parasympathetic cardiac control in athletes and nonathletes at rest. J Appl Physiol Respir Environ Exerc Physiol. 1982;52:1652–1657. doi: 10.1152/jappl.1982.52.6.1652. [DOI] [PubMed] [Google Scholar]
- Maron BJ. Pelliccia A. The heart of trained athletes: cardiac remodeling and the risks of sports, including sudden death. Circulation. 2006;114:1633–1644. doi: 10.1161/CIRCULATIONAHA.106.613562. [DOI] [PubMed] [Google Scholar]
- Milanesi R, Baruscotti M, Gnecchi-Ruscone T. DiFrancesco D. Familial sinus bradycardia associated with a mutation in the cardiac pacemaker channel. N Engl J Med. 2006;354:151–157. doi: 10.1056/NEJMoa052475. [DOI] [PubMed] [Google Scholar]
- Monfredi O, Lyashkov AE, Johnsen AB, Inada S, Schneider H, Wang R, Nirmalan M, Wisloff U, Maltsev VA, Lakatta EG, Zhang H. Boyett MR. Biophysical characterisation of the under-appreciated and important relationship between heart rate variability and heart rate. Hypertension. 2014;64:1334–1343. doi: 10.1161/HYPERTENSIONAHA.114.03782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northcote RJ, Canning GP. Ballantyne D. Electrocardiographic findings in male veteran endurance athletes. Br Heart J. 1989;61:155–160. doi: 10.1136/hrt.61.2.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ostchega Y, Porter KS, Hughes J, Dillon CF. Nwankwo T. Resting pulse rate reference data for children, adolescents, and adults: United States, 1999–2008. Natl Health Stat Report. 2011;41:1–16. [PubMed] [Google Scholar]
- Stein R, Medeiros CM, Rosito GA, Zimerman LI. Ribeiro JP. Intrinsic sinus and atrioventricular node electrophysiologic adaptations in endurance athletes. J Am Coll Cardiol. 2002;39:1033–1038. doi: 10.1016/s0735-1097(02)01722-9. [DOI] [PubMed] [Google Scholar]
- Yamanushi TT, Yanni J, Dobrzynski H, Kabuto H. Boyett MR. Changes in ion channel expression in right-sided congestive heart failure. J Mol Cell Cardiol. 2010;48:S73. (Suppl.) [Google Scholar]
- Yanni J, Tellez JO, Maczewski M, Mackiewicz U, Beresewicz A, Billeter R, Dobrzynski H. Boyett MR. Changes in ion channel gene expression underlying heart failure-induced sinoatrial node dysfun-ction. Circ Heart Fail. 2011a;4:496–508. doi: 10.1161/CIRCHEARTFAILURE.110.957647. [DOI] [PubMed] [Google Scholar]
- Yanni J, Tellez JO, Maczewski M, Sutyagin PV, Mackiewicz U, Atkinson A, Inada S, Beresewicz A, Billeter R, Dobrzynski H. Boyett MR. Ageing-dependent remodelling of ion channel and Ca2+ clock genes underlying sinoatrial node pacemaking. Exp Physiol. 2011b;96:1163–1178. doi: 10.1113/expphysiol.2011.057752. [DOI] [PubMed] [Google Scholar]
- Yeh YH, Burstein B, Qi XY, Sakabe M, Chartier D, Comtois P, Wang Z, Kuo CT. Nattel S. Funny current downregulation and sinus node dysfunction associated with atrial tachyarrhythmia: a molecular basis for tachycardia-bradycardia syndrome. Circulation. 2009;119:1576–1585. doi: 10.1161/CIRCULATIONAHA.108.789677. [DOI] [PubMed] [Google Scholar]
- Zicha S, Fernández-Velasco M, Lonardo G, L'Heureux N. Nattel S. Sinus node dysfunction and hyperpolarization-activated (HCN) channel subunit remodeling in a canine heart failure model. Cardiovasc Res. 2005;66:472–481. doi: 10.1016/j.cardiores.2005.02.011. [DOI] [PubMed] [Google Scholar]


