Abstract
A compromised cardiac function is often seen in elderly cancer patients receiving doxorubicin therapy. The present study tested the hypothesis that acute intervention with resveratrol, a natural anti-oxidant found in grapes and red wine, reduces the cardiotoxicity of doxorubicin through restoration of sirtuin 1 (SIRT1) deacetylase activity, and attenuation of the catabolic/apoptotic pathways orchestrated by USP7, a p53 deubiquitinating protein, using young (aged 2 months) and old (aged 10 months) senescence-accelerated mice prone 8 (SAMP8). Animals were randomised to receive saline, doxorubicin, and doxorubicin in combination with resveratrol, in the presence or absence of SIRT1 inhibitors, sirtinol or EX527. Resveratrol alone, but not in combination with either of the SIRT1 inhibitors, suppressed the doxorubicin-induced impairment of cardiac systolic function in aged animals. Doxorubicin reduced SIRT1 deacetylase activity, and elevated proteasomal activity and USP7; it also increased the protein level of p300 and ubiquitinated proteins in hearts from aged SAMP8. These doxorubicin-induced alterations were prevented by resveratrol, whereas the protective action of resveratrol was antagonised by sirtinol and EX527. In young SAMP8 hearts, resveratrol attenuated the doxorubicin-induced increases in acetylation of Foxo1 and transactivation of MuRF-1, whereas these mitigations were not found after treatment with SIRT1 inhibitors. However, the protein contents of acetylated Foxo1 and MuRF-1 were not affected by any of the drugs studied in aged SAMP8 hearts. Resveratrol also ameliorated the augmentation of pro-apoptotic markers including p53, Bax, caspase 3 activity and apoptotic DNA fragmentation induced by doxorubicin in hearts from aged animals, whereas these reductions were diminished by combined treatment with SIRT1 inhibitors. These data demonstrate that resveratrol ameliorates doxorubicin-induced cardiotoxicity in aged hearts through the restoration of SIRT1 activity to attenuate USP7-related catabolic/pro-apoptotic signalling.
Key points
Doxorubicin induced functional deteriorations and elevations of USP7-related apoptotic/catabolic signalling in the senescent heart
Resveratrol protects against doxorubicin-induced alterations through the restoration of SIRT1 deacetylase activity
Introduction
Doxorubicin has been used clinically as an anti-cancer agent, although its use is known to be associated with cardiotoxicity. Studies have found an increase of mortality associated with the total accumulated dose of doxorubicin in chemotherapy cycles (Von Hoff et al. 1977). In particular, senior patients undergoing doxorubicin therapy have an additional risk of developing congestive heart failure (Von Hoff et al. 1977). Research has focussed on the design of doxorubicin analogues with reduced acute toxicity, although this has been confounded by a paralleled loss of anti-cancer efficacy (Weiss, 1992). Despite the fact that the 10-year survival of patients with doxorubicin-related cardiomyopathy was found to be pronounced after receiving heart transplantation (Lenneman et al. 2013), the co-administration of cardioprotective adjuvants may appear to represent a more effective approach for enhancing the therapeutic effects of doxorubicin.
Resveratrol, a natural anti-oxidant commonly found in grapes, red wine and berries, is a small-molecule activator of the longevity-related gene sirtuin 1 (SIRT1) (Howitz et al. 2003). Studies show that resveratrol (10 mg kg−1) reduces cell proliferation and increases cell cycle arrest induced by doxorubicin in tumour-bearing mice, and also reduces pathohistological damage to the hearts of doxorubicin-treated albino rats (Osman et al. 2013). Pretreatment with resveratrol enhances SIRT1 activity, as indicated by a reduced acetylation of histone H3, which may alleviate oxidative stress (measured as carboxy-H2DCFDA) in doxorubicin-treated cardiomyocytes (Danz et al. 2009). These results are in agreement with studies showing that dietary supplementation with resveratrol (20 mg kg−1) reduces markers of oxidative damage, including malondialdehyde and 4-hydroxyalkenals, as well as cardiac inflammatory infiltration after exposure of the heart to doxorubicin (Dudka et al. 2012).
Other compelling evidence suggests that resveratrol confers anti-apoptotic effects in other experimental models of doxorubicin-induced cardiotoxicity. Oral administration of resveratrol (20 mg kg−1 day−1) reduces the elevation of caspase 3 and fibrotic deposition in doxorubicin-exposed hearts (Arafa et al. 2014). Resveratrol (15 mg kg−1 day−1) also up-regulates SIRT1 and improves cardiac systolic function after doxorubicin exposure; there was also a concomitant reduction of the protein contents of acetylated p53 and Bax and the terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) index, suggesting that the intrinsic deacetylase activity may be involved in the mechanism of action of SIRT1 leading to the cardioprotective effects of resveratrol (Zhang et al. 2011). Other studies confirm that doxorubicin increases p53 and cleaved caspase 3 in cardiomyocytes, although these elevations were prevented by overexpression of AMP-activated protein kinase (AMPK), an activating signal of SIRT1 in mouse embryonic fibroblasts (Wang et al. 2012). Nonetheless, whether resveratrol would suppress apoptosis in the aged heart after doxorubicin treatment through the activation of SIRT1 deacetylase activity remains unknown.
It is generally assumed that activation of the catabolic pathway contributes to myocardial toxicity induced by doxorubicin. Foxo1 is a member of the Forkhead-transcription factor family, which regulates the expression of apoptotic and atrophic genes. Proteomic profiling in H9c2 cardiomyocytes revealed that cathepsin B, a transcriptional target of Foxo1, was increased by doxorubicin (Bao et al. 2012). In other studies, short-term exercise was shown to prevent doxorubicin-induced elevation of mRNA levels of Foxo1 and MuRF-1, which are atrophic factors of the heart (Kavazis et al. 2014), whereas a reduction of SIRT1 and its deacetylase activity is associated with increased pro-apoptotic signalling in cardiomyocytes of patients with advanced heart failure (Lu et al. 2014) and in senescent mice (Sin et al. 2014). USP7 is a p53-deubiquitinating enzyme (Li et al. 2002) that affects the accumulation of p53 in human osteosarcoma cells (Fang & Luna, 2013). It has been reported that p53, HAUSP and polyubiquitinated proteins, and the proteasomal chymotrypsin-like activity and cleavage of caspase 3 are all elevated in the hearts of patients with dilated cardiomyopathy compared to non-failing hearts (Birks et al. 2008). Taken together, these findings suggest that apoptotic and catabolic pathways may act in concert to mediate doxorubicin-induced myocardial toxicity. In the present study, we investigated whether doxorubicin causes a functional deterioration of cardiac function in senescence-accelerated mice prone 8 (SAMP8) through the suppression of SIRT1 deacetylase activity and subsequent up-regulation of Foxo1/USP7-related catabolic/apoptotic axis, and also whether resveratrol can protect against the associated decrease of SIRT1 deacetylase activity.
Methods
Experimental animals
Male SAMP8 were obtained from The Chinese University of Hong Kong. Animal husbandry was carried out as described previously (Sin et al. 2014). All experimental procedures were approved by the Animal Subjects Ethics Subcommittee of The Hong Kong Polytechnic University.
Experimental design
To investigate whether the cardioprotective effects of resveratrol is SIRT1-dependent, the present study used two commonly-adopted inhibitors of SIRT1: sirtinol and EX527. Mice aged 2 months (young) and 10 months (aged) were randomly assigned to receive saline control (SC), doxorubicin and vehicle (DV), doxorubicin + resveratrol (DR), or a combination of DR + sirtinol (DRS) or DR + EX527 (DRE) (Fig.1).
Figure 1. Schematic diagram illustrating the treatment protocol of SAMP8 of both age groups.
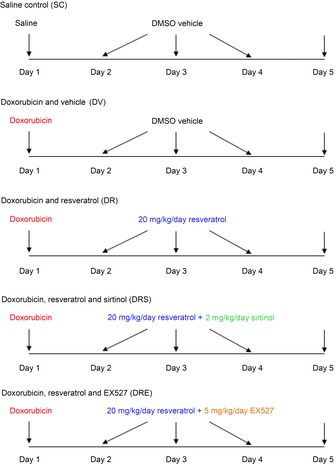
SAMP8 aged 2 months (young) and 10 months (aged) were randomly assigned to receive SC, DV, DR and DRS or DRE.
On day 1, animals were injected i.p. with doxorubicin at 18 mg kg−1 (DV) (Zhu et al. 2010), except that the saline control group (SC) receiving the corresponding amount of saline. The DR, DRS and DRE groups were administered resveratrol (20 mg kg−1 day−1) from day 2 to day 4 (Oktem et al. 2012), whereas the SC and DV groups were injected with vehicle (95% DMSO), accordingly. In the DRS and DRE groups, 2 mg kg−1 day−1 sirtinol (Yang et al. 2013) and 5 mg kg−1 day−1 EX527 (based on our dose-optimisation experiments), respectively, were administered via the i.p. route immediately after all doses of resveratrol. On day 5, all mice were subject to non-invasive echocardiography and sacrificed using an overdose of ketamine (Alfasan, Woerden, The Netherlands). Left ventricular tissues were quickly dissected for further analysis. The treatment protocol is illustrated in Fig.1.
Non-invasive echocardiography
Cardiac function was assessed by non-invasive echocardiography as described by the American Society of Echocardiography (Lang et al. 2006). Parameters, including left ventricle end-systolic dimension (LVESD), left ventricle end-diastolic dimension (LVEDD), anterior wall thickness, posterior wall thickness and heart rate, were measured under M-mode scanning with an Esaote MyLab 70 X-Vision Ultrasound System (Esaote, Genova, Italy). Systolic function measured as fractional shortening (FS) and ejection fraction (EF) was calculated by the equations for FS (%) = [(LVEDD – LVESD)/LVEDD] × 100 and EF (%) = Y + [(100 – Y) × 0.15], where Y = [(LVEDD2 – LVESD2)/LVEDD2] × 100. All results are reported as average values from three consecutive cardiac cycles.
Western blotting
Preparation of cytoplasmic protein extracts and the Bradford assay were performed as described previously (Sin et al. 2014). Briefly, 30 μg of protein was loaded on 10% polyacrylamide gels and separated by SDS-PAGE. The proteins were then transferred to PVDF membranes (Immobilon P; Millipore, Billerica, MA, USA) at 300 mA for 2 h in 1 × transfer buffer containing 10% methanol, except for p300, in which the 1 × transfer buffer contained 5% methanol. Equal loading and transfer efficiency were assured by Coomassie blue and Ponceau S red staining, respectively. The membranes were blocked in 5% non-fat milk in Tris-buffered saline with 0.1% Tween 20 (TBST) for 1 h at room temperature followed by overnight incubation at 4°C with the corresponding primary antibodies in TBST with 2% bovine serum albumin (Table1). The membranes were washed 3 times using TBST, followed by 1 h of incubation with the corresponding secondary antibodies at room temperature (Table1). Luminol reagent (NEL103001EA; Perkin Elmer, Waltham, MA, USA) was applied and chemiluminescent signals were captured using a 4000R Pro camera (Eastman Kodak, Rochester, NY, USA). All data were normalised to the signal of β-tubulin.
Table 1.
Antibodies used in Western blotting
| Antibody | Dilution factor | Source |
|---|---|---|
| SIRT1 rabbit polyclonal | 1:500 | sc15404; Santa Cruz Biotechnology (Santa Cruz, CA, USA) |
| p300 rabbit polyclonal | 1:100 | sc584; Santa Cruz Biotechnology |
| Ac-FKHR rabbit polyclonal | 1:200 | sc4943; Santa Cruz Biotechnology |
| FKHR rabbit polyclonal | 1:500 | sc11350; Santa Cruz Biotechnology |
| MAFbx rabbit polyclonal | 1:200 | sc33782; Santa Cruz Biotechnology |
| MuRF-1 rabbit polyclonal | 1:500 | sc32920; Santa Cruz Biotechnology |
| Ubiquitin mouse monoclonal | 1:1000 | 3936; Cell Signaling (Beverly, MA, USA) |
| USP7 rabbit polyclonal | 1:800 | 101648; Abcam (Cambridge, MA, USA) |
| p53 mouse monoclonal | 1:200 | sc56179; Santa Cruz Biotechnology |
| Bax rabbit polyclonal | 1:200 | sc493; Santa Cruz Biotechnology |
| β-tubulin mouse monoclonal | 1:2000 | T0198; Sigma-Aldrich (St Louis, MO, USA) |
| Mouse IgG | 1:3000 | 7076; Cell Signaling |
| Rabbit IgG | 1:3000 | 7074; Cell Signaling |
All antibodies used for Western blotting in the present study are listed in the order: origins of host, dilution factors and manufacturer.
SIRT1 deacetylation assay
Deacetylase activity of SIRT1 was assessed by a fluorometric method in accordance with the manufacturer's instructions (Cyclex, Nagoya, Japan). Each reaction was initiated by the addition of 5 μl of protease inhibitor-free cardiac protein extracts with thorough mixing. Fluorescence intensity was measured by a microplate fluorometer (Infinite F200; Tecan, Männedorf, Switzerland). All readings were normalised to the respective protein concentrations.
Proteasome activity assay
Proteasomal activity was measured based on the release of fluorescent 7-amino-4-methylcoumarin from 7-amino-4-methylcoumarin-tagged peptide substrate in the presence of proteolytic activity. All procedures were carried out in accordance with the manufacturer's instructions (K245-100; Biovision, Milpitas, CA, USA).
Caspase 3 activity assay
Protease activity of caspase 3 was assessed by a fluorometric approach involving the use of the caspase 3 substrate, DEVD-AFC (1007–200; Biovision). All procedures were the same as those described previously (Teng et al. 2011).
Cell death enzyme-linked immunosorbent assay
A cell death enzyme-linked immunosorbent assay (Roche Diagnostics, Indianapolis, IN, USA) was used to determine apoptotic DNA fragmentation in accordance with the manufacturer's instructions.
Statistical analysis
Statistical analyses were conducted using SPSS, version 21.0 (IBM Corp., Armonk, NY, USA). A normality test was performed to examine data distributions. All data are expressed as the mean ± SEM. Comparisons were made using one-way ANOVA followed by Tukey's post hoc tests. P < 0.05 was considered statistically significant.
Results
Cardiac systolic function
Representative M-mode echocardiographic images of treatment groups are shown in Fig.2A. Cardiac systolic functioning measured as FS and EF was 22% and 16% lower in aged compared to young SAMP8, respectively (Fig.2B and C). Doxorubicin significantly reduced FS in both age groups, although the absolute percentage change observed in aged mice appeared to be less prominent (young DV vs. aged DV: 17% vs. 9%) (Fig.2B); a similar pattern was observed with the EF parameter (young DV vs. aged DV: 12% vs. 9%) (Fig.2C). Resveratrol attenuated significantly the doxorubicin-induced reductions of FS and EF in both age groups, whereas its cardioprotective effects were abolished by the co-administration of either of the SIRT1 inhibitors, sirtinol or EX527 (Fig.2B and C). Except for LVESD, all other cardiac parameters were not significantly different among groups (Fig.2D–H).
Figure 2. Resveratrol attenuated the doxorubicin-induced impairment of cardiac function in aged SAMP8.
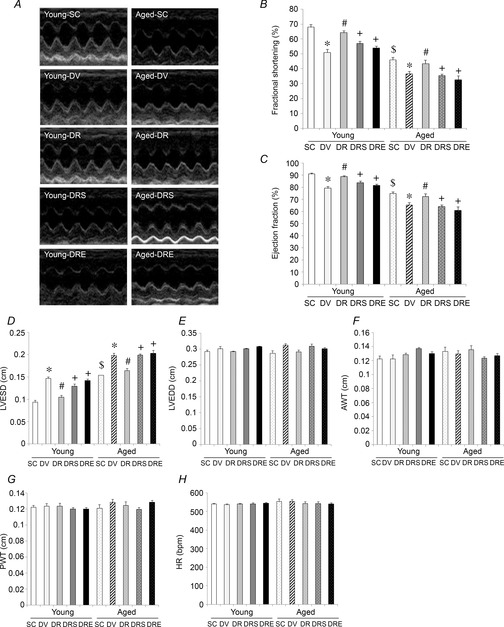
Non-invasive echocardiography was performed upon completion of the treatment protocol. Representative echocardiographic images are shown (A). FS (B) and EF (C) were calculated according to cardiac parameters including LVESD (D) and LVEDD (E). Anterior wall thickness (AWT), posterior wall thickness (PWT) and heart rate (HR) were also measured in all mice (F–H). *P < 0.05, doxorubicin effect; #P < 0.05, resveratrol effect; +P < 0.05, inhibitor effect; $P < 0.05, ageing effect. Mice were assigned to SC, DV, DR and DRS or DRE groups.
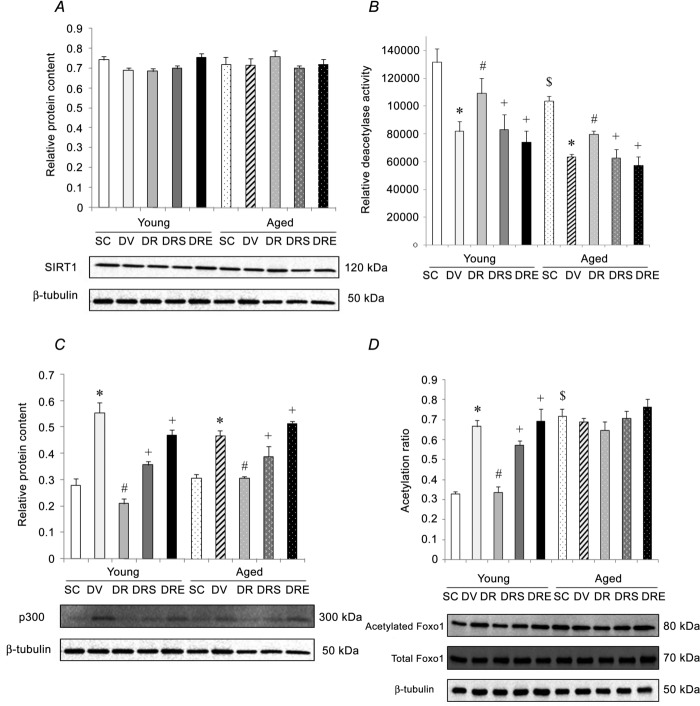
SIRT1-p300-Foxo1 axis
The protein level of SIRT1 was not significantly different in hearts obtained from both age groups and was not affected by drug treatment (Fig.3A). Conversely, the heart deacetylase activity of SIRT1 was 21% lower in aged- compared to young-SAMP8 (Fig.3B). Compared to saline-treated controls, the activity of SIRT1 in the heart was reduced significantly by 38% in young doxorubicin-treated SAMP8 and by 37% in aged doxorubicin-treated SAMP8 (Fig.3B). Resveratrol antagonised significantly the reductions of SIRT1 activity induced by doxorubicin in hearts from both young and aged SAMP8 (Fig.3B). Cardiac SIRT1 activity in mice co-treated with doxorubicin, resveratrol and sirtinol, or EX527, was not significantly different from their age-matched littermates treated with doxorubicin alone (Fig.3B). The protein content of p300 acetylase was not significantly different between the hearts of saline-treated animals of young and aged SAMP8 (Fig.3C). Doxorubicin elevated significantly the protein level of p300 by 98% and 52%, in hearts obtained from young and aged SAMP8, respectively, whereas these increases were antagonised by resveratrol (Fig.3C). However, the resveratrol-induced reductions of p300 in both age groups were attenuated significantly by both SIRT1 inhibitors (Fig.3C). In the saline controls, the acetylation status of Foxo1 was 118% higher in hearts from aged SAMP8 compared to young SAMP8 (Fig.3D). Doxorubicin elevated significantly the protein content of acetylated Foxo1 by 102% in the hearts obtained from young SAMP8 (Fig.3D). This doxorubicin-induced increase was blunted significantly by resveratrol alone but not by resveratrol in combination with sirtinol or EX527 (Fig.3D). Acetylation of Foxo1 was not affected by any interventions in the hearts of aged SAMP8 (Fig.3D).
Figure 3. Doxorubicin-induced cardiotoxicity involved disruption of SIRT1 but not Foxo1.
The protein level of SIRT1 was not different among all groups. A, doxorubicin, but not in combination with resveratrol, reduced further the deacetylase activity of SIRT1 in the aged heart. B, the protein content of p300 was not affected by ageing but was elevated by doxorubicin in both groups. C, doxorubicin did not increase further the acetylation status of Foxo1 in the aged heart regardless of resveratrol treatment. D, *P < 0.05, doxorubicin effect; #P < 0.05, resveratrol effect; +P < 0.05, inhibitor effect; $P < 0.05, ageing effect. Mice were assigned to SC, DV, DR and DRS or DRE groups.
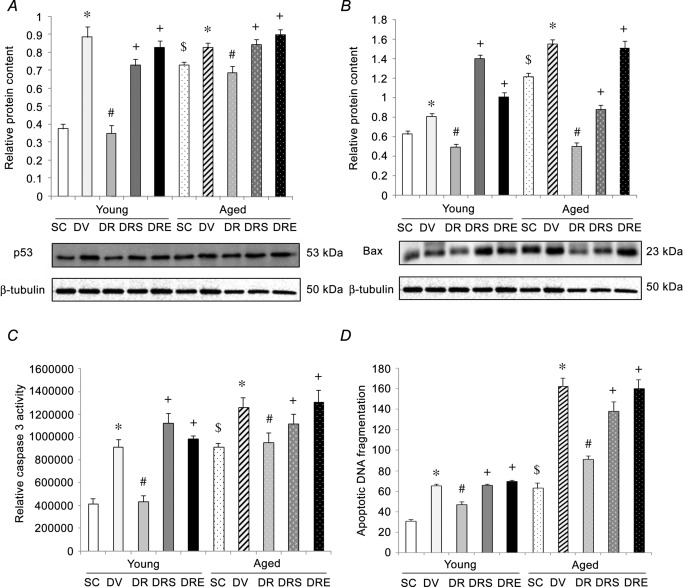
Catabolic markers
The protein expression of MAFbx was not significantly different among treatment or age groups (Fig.4A). However, the protein level of MuRF-1 was 185% higher in hearts obtained from aged SAMP8 compared to young SAMP8 (Fig.4B). Doxorubicin elevated significantly the MuRF-1 by 249% in the hearts of young SAMP8, and this increase was antagonised significantly by resveratrol alone but not when used in conjunction with sirtinol or EX527 (Fig.4B). The protein level of MuRF-1 in the hearts of aged SAMP8 was not modified by any of the treatments (Fig.4B). Basal protein ubiquitination was not different between hearts obtained from saline-treated young and aged SAMP8 (Fig.4C). Doxorubicin elevated significantly the level of ubiquitinated proteins in the heart by 131% and 105% in young and aged SAMP8, respectively (Fig.4C). These doxorubicin-induced increases were antagonised by resveratrol alone but not by resveratrol in combination with sirtinol or EX527 (Fig.4C). Basal proteasomal activity and the protein level of USP7 were 105% and 85% higher in the hearts from aged SAMP8 compared to young SAMP8, respectively (Fig.4D and E). In young SAMP8, proteasomal activity and the protein level of USP7 were significantly increased by 145% and 321%, respectively, in response to doxorubicin treatment; these elevations were significantly antagonised by resveratrol alone but not by resveratrol in combination with sirtinol or EX527 (Fig.4D and E). Moreover, doxorubicin also elevated proteasomal activity and the protein level of USP7 in hearts obtained from aged SAMP8 by 110% and 417%, respectively (Fig.4D and E); these changes were abolished by resveratrol alone but not by resveratrol in combination with sirtinol or EX527 (Fig.4D and E).
Figure 4. Resveratrol reduced the elevation of catabolic markers in doxorubicin-challenged aged hearts.
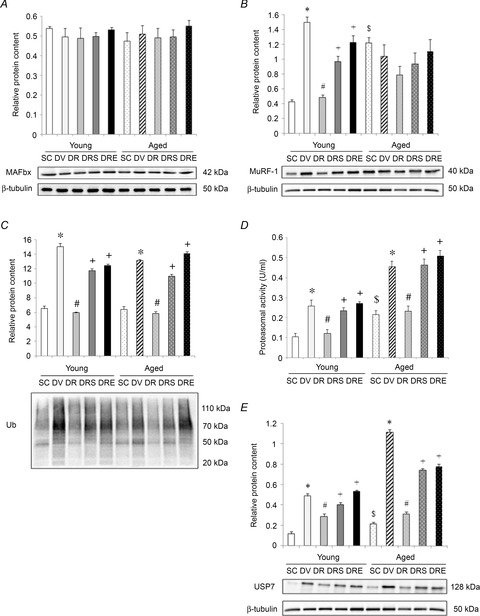
The protein level of MAFbx was neither affected by age, nor any interventions. A, the protein abundance of MuRF-1 exhibited a similar changing pattern as acetylation of Foxo1. B, resveratrol mitigated the doxorubicin-induced elevation of protein ubiquitination in the heart of both age groups. C, augmentation of proteasomal activity (D) and protein content of USP7 (E) by doxorubicin in aged heart was blunted after resveratrol treatment. *P < 0.05, doxorubicin effect; #P < 0.05, resveratrol effect; +P < 0.05, inhibitor effect; $P < 0.05, ageing effect. Mice were assigned to SC, DV, DR and DRS or DRE groups.
Apoptotic signalling
The basal protein expression of pro-apoptotic factors p53 and Bax was significantly higher in hearts obtained from aged SAMP8 compared to young SAMP8, by 95% and 94%, respectively; the activity of caspase 3 and apoptotic DNA fragmentation were also 123% and 104% significantly higher, respectively, in hearts obtained from aged SAMP8 (Fig.5). Doxorubicin significantly increased the levels of p53 and Bax, caspase 3 activity and apoptotic DNA fragmentation by 135%, 29%, 122% and 111%, respectively, in the hearts of young SAMP8 compared to their age-matched saline controls (Fig.5). Doxorubicin also increased significantly the protein levels of p53 and Bax, caspase 3 activity and apoptotic DNA fragmentation in the aged heart by 13%, 28%, 38% and 157%, respectively; these elevations were antagonised by resveratrol (Fig.5). Sirtinol and EX527 significantly reversed the suppression of all apoptotic markers induced by co-treatment with doxorubicin and resveratrol in both age groups (Fig.5).
Figure 5. Resveratrol reduced apoptotic activation in aged hearts in response to doxorubicin exposure.
Resveratrol blunted the doxorubicin-induced augmentation of protein p53 (A) and Bax (B), caspase 3 activity (C), and apoptotic DNA fragmentation (D) in the aged heart. *P < 0.05, doxorubicin effect; #P < 0.05, resveratrol effect; +P < 0.05, inhibitor effect; $P < 0.05, ageing effect. Mice were assigned to SC, DV, DR and DRS or DRE groups.
Discussion
Cardioprotection by resveratrol requires SIRT1
Alteration of SIRT1 expression/activity has been implicated in various cardiac pathologies. The level of SIRT1 appears to be reduced in the left atrium of patients with advanced heart failure (Lu et al. 2014), whereas inhibition of SIRT1 by sirtinol was shown to reduce the improvement of cardiac contractility induced by resveratrol in a rat model of traumatic haemorrhage (Jian et al. 2012); sirtinol also blocks resveratrol-induced reductions of infarct size in mice exposed to ischaemia–reperfusion injury (Shalwala et al. 2014). These former studies support the hypothesis that SIRT1 is required to mediate the cardioprotective effects of resveratrol. Although the molecular mechanism of action underlying the attenuation of doxorubicin-induced cardiac contractile abnormalities including bradycardia and QTc interval prolongation in response to resveratrol administration remains to be identified (Rezk et al. 2006), these promising physiological outcomes could be attributable to the activation of SIRT1 deacetylase. Importantly, the present study shows that cardiac systolic function and SIRT1 deacetylase activity were reduced concomitantly in the aged heart, an observation consistent with our previous study (Sin et al. 2014), and we also provide new evidence showing that resveratrol prevents doxorubicin-induced cardiotoxicity in aged hearts through the restoration of SIRT1 deacetylase activity.
Resveratrol inhibits catabolic machinery in a SIRT1-dependent manner
A growing body of evidence suggests that doxorubicin-induced cardiotoxicity may involve activation of the catabolic pathway. It has been reported that doxorubicin prevents the accumulation of endogenous proteasomal substrates, c-Jun and β-catenin, in neonatal rat cardiomyocytes (Liu et al. 2008) and increased carbonylation and degradation of cardiac myosin binding protein C in HL-1 cardiomyocytes and spontaneously hypertensive rat cardiomyocytes (Aryal et al. 2014). By contrast, the reduction of the cardiomyocyte surface area induced by doxorubicin was blunted by treatment with the proteasomal inhibitor, MG132 (Yamamoto et al. 2008). Although doxorubicin was shown to increase protein ubiquitination, an observation paralleled by elevation of protein abundance of MAFbx and MuRF-1 in the heart (Sishi et al. 2013), the present study further demonstrates that resveratrol prevents the up-regulation of protein ubiquitination induced by doxorubicin in hearts from old-age mice. Indeed, previous studies have shown that resveratrol inhibits the total protein degradation induced by phorbol ester in C2C12 myotubes (Wyke & Tisdale 2006) and proteasomal chymotrypsin-like activity in cultured murine macrophages (Qureshi et al. 2012). In the present study, we demonstrate that inhibition of SIRT1 by sirtinol or EX527 reversed the resveratrol-induced suppression of protein ubiquitination and proteasomal activity in the aged heart challenged with doxorubicin. These novel data strengthen the notion that SIRT1 is required to mediate the anti-catabolic effects of resveratrol with respect to reducing the myocardial toxicity of doxorubicin in the aged heart.
Anti-apoptotic effects of resveratrol require SIRT1
Many studies support the concept that SIRT1 may alleviate cardiomyocyte apoptosis in response to doxorubicin challenge. It has been reported that, in doxorubicin-treated cardiomyocytes, the protein level of p53 is elevated with concomitant reduction in activity of AMPK, an upstream activating signal of SIRT1 (Wang et al. 2012), whereas transfection with SIRT1-expressing adenovirus reduced the increased cleavage of caspase 3 induced by transcriptional silencing of Nkx2.5 (Zheng et al. 2013). These observations support studies reporting that resveratrol increases the activities of SIRT1 and SOD2 in doxorubicin-treated cardiomyocytes (Danz et al. 2009), whereas overexpression of SIRT1 blunted the elevation of cleaved caspase 3 and the number of TUNEL-positive nuclei induced by doxorubicin in the heart (Zheng et al. 2013). By contrast, the specific SIRT1 inhibitor, EX527, abolished the sesamin-induced elevation of SOD2 in H9c2 cardiomyocytes challenged with doxorubicin (Su et al. 2014). In agreement with a previous study showing that resveratrol enhanced the activity of SIRT1 and ventricular function and attenuated p53-related apoptotic signalling in the doxorubicin-treated heart (Zhang et al. 2011), our data further show that resveratrol prevents the augmentation of p53, Bax, caspase 3 activity and apoptotic DNA fragmentation induced by doxorubicin in the aged heart. It is noteworthy that these alterations were diminished by the co-administration with sirtinol or EX527, suggesting that the anti-apoptotic effects of resveratrol are SIRT1-dependent.
Orchestration of catabolic and apoptotic pathway involves USP7 but not Foxo1
Considering that daunorubicin-induced increases in E3 ligases including MAFbx and MuRF-1 are associated with reduced phosphorylation (Sishi et al. 2012), it is plausible that doxorubicin would elevate the acetylation and activity of Foxo1. However, there is also evidence that Foxo1 may not exhibit a similar changing pattern with its transcriptional targets. Previous studies have reported that tumour necrosis factor treatment increased the mRNA content of MAFbx but had no effects on the expression and localisation activity of Foxo1 (Moylan et al. 2008). However, the transcript level of Foxo1, but not those of MAFbx and MuRF-1, is elevated in the vastus lateralis muscle of patients with systolic heart failure (Forman et al. 2014). Intriguingly, the results of the present study showed that a concomitant increase of acetylated Foxo1 and MuRF-1 induced by doxorubicin treatment was observed only in the hearts of young but not aged mice. Although the mechanisms underlying this age-related difference are not clear, our data suggest that acetylation of Foxo1 might not be required to mediate the doxorubicin-induced cardiotoxicity. In agreement with a recent study showing that reducing the expression of p53-deubiquitinating enzyme, USP7 (Li et al. 2002), blocked the elevation of p53 in human U2OS cells treated with supervillin dsRNA (Fang & Luna 2013), our data demonstrate that doxorubicin (but not in combination with resveratrol) elevated further the protein level of USP7 in aged hearts. Furthermore, our data reveal that doxorubicin increased concomitantly the protein content of USP7, protein ubiquitination, proteasomal activity and apoptotic markers in the aged heart. These novel findings are consistent with those reported in dilated cardiomyopathy (Birks et al. 2008). Moreover, additional evidence indicates that resveratrol enhances deubiquitination and the up-regulation of p53 through USP10 (Oi et al. 2014). Although it is not known whether this would represent a cardioprotective mechanism of resveratrol the present findings suggest that alleviation of apoptotic signalling induced by resveratrol in doxorubicin-challenged aged hearts may, at least in part, be mediated by USP7 through the activation of SIRT1 deacetylase (Fig.6).
Figure 6. Proposed mechanism of resveratrol to protect against doxorubicin-induced cardiotoxicity in the aged heart.
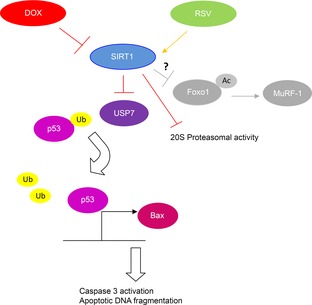
Resveratrol diminished the doxorubicin-induced cardiotoxicity in aged hearts through the restoration of SIRT1 deacetylase activity. This led to the inhibition of USP7, a p53-deubiquitinating enzyme, followed by a reduced expression of p53 and Bax, which subsequently de-activated the apoptotic pathway. Enhancement of SIRT1 activity by resveratrol also mitigated the augmentation of proteasomal activity induced by doxorubicin treatment. In young mice, resveratrol repressed the elevation of catabolic signalling measured as acetylated Foxo1 and MuRF-1 induced by doxorubicin. However, it is not known why the acetylation of Foxo1 and transactivation of MuRF-1 were unaffected by pharmacological interventions in aged hearts.
Conclusions
The present study reports novel data demonstrating that resveratrol antagonised doxorubicin-induced cardiotoxicity in the aged heart through the restoration of SIRT1 deacetylase activity and suppression of the USP7-associated catabolic/apoptotic pathway. This conclusion is supported by data showing that the resveratrol-induced alterations were diminished after inhibition of SIRT1. In addition, the finding that doxorubicin increased the acetylation of Foxo1 in young hearts but not in aged hearts warrants additional research to uncover the molecular mechanisms accounting for this age-related difference. Nevertheless, the present study sheds light on the possibility that modulation of the SIRT1-USP7 axis may represent a possible therapeutic target of resveratrol and other candidates of cardioprotective adjuvants in elderly patients undergoing doxorubicin therapy.
Acknowledgments
The authors would like to acknowledge animal husbandry support received from the Centralised Animal Facilities of The Hong Kong Polytechnic University and the Laboratory Services Centre of The Chinese University of Hong Kong.
Glossary
- AMPK
AMP-activated protein kinase
- DR
doxorubicin + resveratrol
- DRE
doxorubicin + resveratrol + EX527
- DRS
doxorubicin + resveratrol + sirtinol
- DV
doxorubicin and vehicle
- EF
ejection fraction
- FS
fractional shortening
- LVEDD
left ventricle end-diastolic dimension
- LVESD
left ventricle end-systolic dimension
- SAMP8
senescence-accelerated mice prone 8
- SC
saline control
- SIRT1
sirtuin 1
- TBST
Tris-buffered saline with 0.1% Tween 20
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labelling
Additional information
Competing interests
The authors declare that they have no competing interests.
Author contributions
T.K.S., B.T.T., B.Y.Y., S.P.Y., L.W.C., C.S.W. and P.M.S designed the experiments. T.K.S., B.T.T., M.Y., J.A.R. and P.M.S. were involved in the collection, analysis and interpretation of data. T.K.S. and P.M.S. drafted and critically revised the manuscript for important intellectual content. All authors approved the final version submitted for publication.
Funding
This study was supported by The Hong Kong Polytechnic University (RPTL).
References
- Arafa MH, Mohammad NS, Atteia HH. Abd-Elaziz HR. Protective effect of resveratrol against doxorubicin-induced cardiac toxicity and fibrosis in male experimental rats. J Physiol Biochem. 2014;70:701–711. doi: 10.1007/s13105-014-0339-y. [DOI] [PubMed] [Google Scholar]
- Aryal B, Jeong J. Rao VA. Doxorubicin-induced carbonylation and degradation of cardiac myosin binding protein C promote cardiotoxicity. Proc Natl Acad Sci U S A. 2014;111:2011–2016. doi: 10.1073/pnas.1321783111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bao GY, Wang HZ, Shang YJ, Fan HJ, Gu ML, Xia R, Qin Q. Deng AM. Quantitative proteomic study identified cathepsin B associated with doxorubicin-induced damage in H9c2 cardiomyocytes. Biosci Trends. 2012;6:283–287. [PubMed] [Google Scholar]
- Birks EJ, Latif N, Enesa K, Folkvang T, Luong le A, Sarathchandra P, Khan M, Ovaa H, Terracciano CM, Barton PJ, Yacoub MH. Evans PC. Elevated p53 expression is associated with dysregulation of the ubiquitin-proteasome system in dilated cardiomyopathy. Cardiovasc Res. 2008;79:472–480. doi: 10.1093/cvr/cvn083. [DOI] [PubMed] [Google Scholar]
- Danz ED, Skramsted J, Henry N, Bennett JA. Keller RS. Resveratrol prevents doxorubicin cardiotoxicity through mitochondrial stabilization and the Sirt1 pathway. Free Radic Biol Med. 2009;46:1589–1597. doi: 10.1016/j.freeradbiomed.2009.03.011. [DOI] [PubMed] [Google Scholar]
- Dudka J, Gieroba R, Korga A, Burdan F, Matysiak W, Jodlowska-Jedrych B, Mandziuk S, Korobowicz E. Murias M. Different effects of resveratrol on dose-related Doxorubicin-induced heart and liver toxicity. Evid Based Complement Alternat Med. 2012;2012:606183. doi: 10.1155/2012/606183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fang Z. Luna EJ. Supervillin-mediated suppression of p53 protein enhances cell survival. J Biol Chem. 2013;288:7918–7929. doi: 10.1074/jbc.M112.416842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman DE, Daniels KM, Cahalin LP, Zavin A, Allsup K, Cao P, Santhanam M, Joseph J, Arena R, Lazzari A, Schulze PC. Lecker SH. Analysis of skeletal muscle gene expression patterns and the impact of functional capacity in patients with systolic heart failure. J Card Fail. 2014;20:422–430. doi: 10.1016/j.cardfail.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howitz KT, Bitterman KJ, Cohen HY, Lamming DW, Lavu S, Wood JG, Zipkin RE, Chung P, Kisielewski A, Zhang LL, Scherer B. Sinclair DA. Small molecule activators of sirtuins extend Saccharomyces cerevisiae lifespan. Nature. 2003;425:191–196. doi: 10.1038/nature01960. [DOI] [PubMed] [Google Scholar]
- Jian B, Yang S, Chaudry IH. Raju R. Resveratrol improves cardiac contractility following trauma-hemorrhage by modulating Sirt1. Mol Med. 2012;18:209–214. doi: 10.2119/molmed.2011.00365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kavazis AN, Smuder AJ. Powers SK. Effects of short-term endurance exercise training on acute doxorubicin-induced FoxO transcription in cardiac and skeletal muscle. J Appl Physiol (1985) 2014;117:223–230. doi: 10.1152/japplphysiol.00210.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang RM, Bierig M, Devereux RB, Flachskampf FA, Foster E, Pellikka PA, Picard MH, Roman MJ, Seward J, Shanewise J, Solomon S, Spencer KT, St John Sutton M. Stewart W. Recommendations for chamber quantification. Eur J Echocardiogr. 2006;7:79–108. doi: 10.1016/j.euje.2005.12.014. [DOI] [PubMed] [Google Scholar]
- Lenneman AJ, Wang L, Wigger M, Frangoul H, Harrell FE, Silverstein C, Sawyer DB. Lenneman CG. Heart transplant survival outcomes for adriamycin-dilated cardiomyopathy. Am J Cardiol. 2013;111:609–612. doi: 10.1016/j.amjcard.2012.10.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, Chen D, Shiloh A, Luo J, Nikolaev AY, Qin J. Gu W. Deubiquitination of p53 by HAUSP is an important pathway for p53 stabilization. Nature. 2002;416:648–653. doi: 10.1038/nature737. [DOI] [PubMed] [Google Scholar]
- Liu J, Zheng H, Tang M, Ryu YC. Wang X. A therapeutic dose of doxorubicin activates ubiquitin-proteasome system-mediated proteolysis by acting on both the ubiquitination apparatus and proteasome. Am J Physiol Heart Circ Physiol. 2008;295:H2541–H2550. doi: 10.1152/ajpheart.01052.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu TM, Tsai JY, Chen YC, Huang CY, Hsu HL, Weng CF, Shih CC. Hsu CP. Downregulation of Sirt1 as aging change in advanced heart failure. J Biomed Sci. 2014;21:57. doi: 10.1186/1423-0127-21-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moylan JS, Smith JD, Chambers MA, McLoughlin TJ. Reid MB. TNF induction of atrogin-1/MAFbx mRNA depends on Foxo4 expression but not AKT-Foxo1/3 signaling. Am J Physiol Cell Physiol. 2008;295:C986–C993. doi: 10.1152/ajpcell.00041.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oi N, Yuan J, Malakhova M, Luo K, Li Y, Ryu J, Zhang L, Bode AM, Xu Z, Lou Z. Dong Z. Resveratrol induces apoptosis by directly targeting Ras-GTPase-activating protein SH3 domain-binding protein 1. Oncogene. 2014 doi: 10.1038/onc.2014.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oktem G, Uysal A, Oral O, Sezer ED, Olukman M, Erol A, Akgur SA. Bilir A. Resveratrol attenuates doxorubicin-induced cellular damage by modulating nitric oxide and apoptosis. Exp Toxicol Pathol. 2012;64:471–479. doi: 10.1016/j.etp.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Osman AM, Al-Harthi SE, AlArabi OM, Elshal MF, Ramadan WS, Alaama MN, Al-Kreathy HM, Damanhouri ZA. Osman OH. Chemosensetizing and cardioprotective effects of resveratrol in doxorubicin- treated animals. Cancer Cell Int. 2013;13:52. doi: 10.1186/1475-2867-13-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qureshi AA, Guan XQ, Reis JC, Papasian CJ, Jabre S, Morrison DC. Qureshi N. Inhibition of nitric oxide and inflammatory cytokines in LPS-stimulated murine macrophages by resveratrol, a potent proteasome inhibitor. Lipids Health Dis. 2012;11:76. doi: 10.1186/1476-511X-11-76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rezk YA, Balulad SS, Keller RS. Bennett JA. Use of resveratrol to improve the effectiveness of cisplatin and doxorubicin: study in human gynecologic cancer cell lines and in rodent heart. Am J Obstet Gynecol. 2006;194:e23–26. doi: 10.1016/j.ajog.2005.11.030. [DOI] [PubMed] [Google Scholar]
- Shalwala M, Zhu SG, Das A, Salloum FN, Xi L. Kukreja RC. Sirtuin 1 (SIRT1) activation mediates sildenafil induced delayed cardioprotection against ischemia-reperfusion injury in mice. PLoS One. 2014;9:e86977. doi: 10.1371/journal.pone.0086977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sin TK, Yu AP, Yung BY, Yip SP, Chan LW, Wong CS, Ying M, Rudd JA. Siu PM. Modulating effect of SIRT1 activation induced by resveratrol on Foxo1-associated apoptotic signalling in senescent heart. J Physiol. 2014;592:2535–2548. doi: 10.1113/jphysiol.2014.271387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sishi BJ, Bester DJ, Wergeland A, Loos B, Jonassen AK, van Rooyen J. Engelbrecht AM. Daunorubicin therapy is associated with upregulation of E3 ubiquitin ligases in the heart. Exp Biol Med (Maywood) 2012;237:219–226. doi: 10.1258/ebm.2011.011106. [DOI] [PubMed] [Google Scholar]
- Sishi BJ, Loos B, van Rooyen J. Engelbrecht AM. Doxorubicin induces protein ubiquitination and inhibits proteasome activity during cardiotoxicity. Toxicology. 2013;309:23–29. doi: 10.1016/j.tox.2013.04.016. [DOI] [PubMed] [Google Scholar]
- Su S, Li Q, Liu Y, Xiong C, Li J, Zhang R, Niu Y, Zhao L, Wang Y. Guo H. Sesamin ameliorates doxorubicin-induced cardiotoxicity: involvement of Sirt1 and Mn-SOD pathway. Toxicol Lett. 2014;224:257–263. doi: 10.1016/j.toxlet.2013.10.034. [DOI] [PubMed] [Google Scholar]
- Teng BT, Tam EW, Benzie IF. Siu PM. Protective effect of caspase inhibition on compression-induced muscle damage. J Physiol. 2011;589:3349–3369. doi: 10.1113/jphysiol.2011.209619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Hoff DD, Rozencweig M, Layard M, Slavik M. Muggia FM. Daunomycin-induced cardiotoxicity in children and adults. A review of 110 cases. Am J Med. 1977;62:200–208. doi: 10.1016/0002-9343(77)90315-1. [DOI] [PubMed] [Google Scholar]
- Wang S, Song P. Zou MH. Inhibition of AMP-activated protein kinase alpha (AMPKalpha) by doxorubicin accentuates genotoxic stress and cell death in mouse embryonic fibroblasts and cardiomyocytes: role of p53 and SIRT1. J Biol Chem. 2012;287:8001–8012. doi: 10.1074/jbc.M111.315812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RB( The anthracyclines: will we ever find a better doxorubicin? Semin Oncol. 1992;19:670–686. [PubMed] [Google Scholar]
- Wyke SM. Tisdale MJ. Induction of protein degradation in skeletal muscle by a phorbol ester involves upregulation of the ubiquitin-proteasome proteolytic pathway. Life Sci. 2006;78:2898–2910. doi: 10.1016/j.lfs.2005.11.014. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y, Hoshino Y, Ito T, Nariai T, Mohri T, Obana M, Hayata N, Uozumi Y, Maeda M, Fujio Y. Azuma J. Atrogin-1 ubiquitin ligase is upregulated by doxorubicin via p38-MAP kinase in cardiac myocytes. Cardiovasc Res. 2008;79:89–96. doi: 10.1093/cvr/cvn076. [DOI] [PubMed] [Google Scholar]
- Yang Y, Duan W, Lin Y, Yi W, Liang Z, Yan J, Wang N, Deng C, Zhang S, Li Y, Chen W, Yu S, Yi D. Jin Z. SIRT1 activation by curcumin pretreatment attenuates mitochondrial oxidative damage induced by myocardial ischemia reperfusion injury. Free Radic Biol Med. 2013;65:667–679. doi: 10.1016/j.freeradbiomed.2013.07.007. [DOI] [PubMed] [Google Scholar]
- Zhang C, Feng Y, Qu S, Wei X, Zhu H, Luo Q, Liu M, Chen G. Xiao X. Resveratrol attenuates doxorubicin-induced cardiomyocyte apoptosis in mice through SIRT1-mediated deacetylation of p53. Cardiovasc Res. 2011;90:538–545. doi: 10.1093/cvr/cvr022. [DOI] [PubMed] [Google Scholar]
- Zheng W, Lu YB, Liang ST, Zhang QJ, Xu J, She ZG, Zhang ZQ, Yang RF, Mao BB, Xu Z, Li L, Hao DL, Lu J, Wei YS, Chen HZ. Liu DP. SIRT1 mediates the protective function of Nkx2.5 during stress in cardiomyocytes. Basic Res Cardiol. 2013;108:364. doi: 10.1007/s00395-013-0364-y. [DOI] [PubMed] [Google Scholar]
- Zhu J, Zhang J, Xiang D, Zhang Z, Zhang L, Wu M, Zhu S, Zhang R. Han W. Recombinant human interleukin-1 receptor antagonist protects mice against acute doxorubicin-induced cardiotoxicity. Eur J Pharmacol. 2010;643:247–253. doi: 10.1016/j.ejphar.2010.06.024. [DOI] [PubMed] [Google Scholar]