Abstract
Hypoxia in utero is a critical insult causing intrauterine growth restriction (IUGR). Adult offspring born with hypoxia-induced IUGR have impaired endothelium-dependent vascular function. We tested whether aerobic exercise improves IUGR-induced endothelial dysfunction. Pregnant Sprague–Dawley rats were exposed to control (21% oxygen) or hypoxic (11% oxygen) conditions from gestational day 15 to 21. Male and female offspring from normoxic and hypoxic (IUGR) pregnancies were randomized at 10 weeks of age to either an exercise-trained or sedentary group. Exercise-trained rats ran on a treadmill for 30 min at 20 m min−1, 5 deg gradient, 5 days week−1, for 6 weeks. Concentration–response curves to phenylephrine and methylcholine were performed in second order mesenteric and gastrocnemius muscle arteries, in the presence or absence of l-NAME (100 μm), MnTBAP (peroxynitrite scavenger; 10 μm), apamin (0.1 μm) and TRAM-34 (an intermediate-conductance calcium-activated potassium channel blocker; 10 μm), or indomethacin (5 μm). In adult male IUGR offspring, prenatal hypoxia had no effect on total vasodilator responses in either vascular bed. Aerobic exercise training in IUGR males, however, improved endothelium-derived hyperpolarization (EDH)-mediated vasodilatation in gastrocnemius muscle arteries. Female IUGR offspring had reduced NO-mediated vasodilatation in both vascular beds, along with decreased total vasodilator responses and increased prostaglandin-mediated vasoconstriction in gastrocnemius muscle arteries. In contrast to males, aerobic exercise training in IUGR female offspring had no effect on either vascular bed. Exercise may not prove to be a beneficial therapy for specific vascular pathways affected by prenatal hypoxia, particularly in female offspring.
Key points
Prenatal hypoxia, one of the most common consequences of complicated pregnancies, leads to intrauterine growth restriction (IUGR) and impairs later-life endothelium-dependent vascular function.
Early interventions are needed to ultimately reduce later-life risk for cardiovascular disease.
Aerobic exercise training has been shown to prevent cardiovascular diseases. Whether exercise can be used as an intervention to reverse the vascular phenotype of this susceptible population is unknown.
Aerobic exercise training enhanced endothelium-derived hyperpolarization-mediated vasodilatation in gastrocnemius muscle arteries in male IUGR offspring, and did not improve nitric oxide-mediated vasodilatation in IUGR offspring.
Understanding the mechanisms by which exercise impacts the cardiovascular system in a susceptible population and the consideration of sexual dimorphism is essential to define whether exercise could be used as a preventive strategy in this population.
Introduction
During a compromised pregnancy, the in utero environment can affect fetal growth and development resulting in intrauterine growth restriction (IUGR). Between 2 and 11% of all newborns are affected by IUGR (Olusanya, 2010), which is associated with an increased risk of neonatal morbidity (Wu et al. 2012) and mortality (Lawn et al. 2005). Importantly, IUGR has also been associated with an increased risk of chronic disease in adulthood; population-based studies have demonstrated that being born growth restricted is a risk factor for the development of ischaemic heart disease, insulin resistance, diabetes and stroke later in life (Barker et al. 1989; Jaquet et al. 2000; Class et al. 2014). Likewise, there is a large body of evidence that has demonstrated the association of fetal programming with adult chronic disease in a variety of animal models including dietary interventions, placental dysfunction, glucocorticoid administration, genetic models and hypoxia (reviewed in Santos & Joles, 2012; Giussani & Davidge, 2013).
Prenatal hypoxia is a critical insult causing IUGR in many pregnancy complications (Kingdom & Kaufmann, 1997). We have previously shown that IUGR following a prenatal hypoxic insult has been associated with decreased cardiac performance after ischaemia (Xu et al. 2006; Rueda-Clausen et al. 2009) and increased susceptibility to secondary stressors (e.g. ageing or a high fat diet) (Rueda-Clausen et al. ,). Furthermore, IUGR has been associated with vascular dysfunction in a variety of vascular beds, affecting both male and female offspring, characterized by increased arterial wall stiffness, reduced arterial diameter, altered collagen deposition, increased vasoconstrictor capacity, decreased endothelium-derived hyperpolarization (EDH) and decreased nitric oxide (NO)-mediated vasodilatation (Payne et al. 2003; Mazzuca et al. 2010; Morton et al. ,; Bourque et al. 2013).
Oxidative stress has been proposed as a mechanism for vascular dysfunction in IUGR (reviewed in Giussani & Davidge, 2013). Being born from a hypoxic pregnancy, independent of IUGR, has been associated with increased aortic nitrotyrosine levels (Giussani et al. 2012), which is a footprint of peroxynitrite, a powerful oxidant produced by the reaction of NO with superoxide (Beckman & Koppenol, 1996). Further, maternal treatment with antioxidants in hypoxic pregnancy rescued endothelial dysfunction in the offspring at adulthood (Giussani et al. 2012). Several other studies have shown that a vascular oxidant tone affecting NO bioavailability is functional in fetal life and that it can be modified by hypoxic conditions and by exposure to antioxidants or to agents that increase NO bioavailability (Thakor et al. 2010; Herrera et al. 2012; Kane et al. 2012). Thus both maternal and fetal treatments are important approaches for reducing the vascular effects of prenatal hypoxia exposure. Unfortunately, not all interventions can be administrated during pregnancy and therefore offspring who are at an increased risk of cardiovascular disease due to their prenatal environment will still require prevention or treatment options.
Aerobic exercise training has been shown to improve vascular function, and thus has been proposed as an intervention to prevent cardiovascular diseases. Exercise has been associated with an improvement of vascular function by increasing EDH-mediated vasodilatation in gastrocnemius muscle arteries from spontaneously hypertensive rats (Gündüz et al. 2011), increasing the expression of endothelial nitric oxide synthase in the aorta (Sessa et al. 1994), increasing NO-mediated vasodilatation in epicardial coronary arteries (Wang et al. 1993), reducing plasma endothelin-1 (Maeda et al. 2009) and decreasing reactive oxygen species (ROS) generation in aortic endothelial cells (Rush et al. 2003). Interestingly, however, in other studies it has been shown that in an animal model of vascular disease, STZ-diabetic rats, intensive exercise did not improve endothelium-dependent vasodilatation in the thoracic aorta (Zguira et al. 2013). Furthermore, Allen et al. demonstrated that exercise did not improve vascular function as assessed by brachial artery flow-mediated dilatation in subjects with type 2 diabetes and peripheral artery disease (Allen et al. 2014). Thus, exercise training may not be beneficial in conditions with a susceptible vascular pathology. Furthermore, the role of exercise in IUGR offspring with compromised vascular function has not yet been assessed.
Exercise training in growth-restricted animal models has been shown to improve metabolic function by suppressing glucose-stimulated insulin production and decreasing hepatic glucose production in female Sprague–Dawley rats (Garg et al. 2009). Moreover, Laker et al. have shown that exercise training at weaning increased relative islet surface area and β-cell mass in growth-restricted male Wistar–Kyoto rats (Laker et al. 2011). These findings suggest that exercise can improve the metabolic phenotype associated with intrauterine growth restriction; however, whether exercise can be used as an intervention to reverse the vascular phenotype of this susceptible population is unknown. Thus, the aim of our study was to determine whether aerobic exercise training could be used as an early intervention strategy to improve vascular function in IUGR offspring.
Methods
Ethical approval
All procedures in this study were approved by the University of Alberta Animal Welfare Committee, and were in accordance with the guidelines of the Canadian Council on Animal Care. Our protocols followed the ARRIVE guidelines for reporting in vivo experiments.
Animal model
We used a model of hypoxia-induced IUGR in which we have previously demonstrated that hypoxia not only decreased offspring body weight, crown–rump length and abdominal girth, but was also associated with long-term cardiovascular effects later in life (Xu et al. 2006; Rueda-Clausen et al. 2009; Morton et al. ,). Briefly, 3-month-old Sprague–Dawley (n = 26) rats (Charles River, Wilmington, MA, USA) were housed in the animal facility where room conditions were as follows: 35% humidity, 10 h:14 h light:dark cycle, and fed ad libitum with standard rodent chow. After acclimatization, rats were mated overnight. Upon confirmation of pregnancy (presence of sperm in a vaginal smear designated as gestational day (GD) 0), female rats were housed singly and then exposed to control (room air) or hypoxic (11% oxygen) conditions from GD 15 to 21. Previous findings have reported that during hypoxic conditions dams have a reduced food intake (Williams et al. 2005a; Camm et al. 2010). Since activity levels are also reduced in hypoxic conditions, it is likely that nutrient supply is appropriate to metabolic demand; however, it is noted that this model may consist of an interaction of nutrient restriction and hypoxia.
At the time of birth (GD 22), anthropometric parameters such as body weight, crown to rump length and abdominal girth were measured. Offspring from dams exposed to hypoxia are referred to as IUGR offspring and offspring from dams exposed to normoxia are referred to as control offspring. Litters were randomly reduced to eight pups (4 males and 4 females) to control access to maternal nutrition. Offspring were weaned at 3 weeks and the exercise intervention was started at 10 weeks of age. From each litter, two males and two females were randomly allocated to the exercise training group and two males and two females were assigned to a sedentary group. One male and one female from each group were used in a separate series of cardiac experiments and were not included in this report.
Exercise tolerance test
To determine if exercise capacity was different in control and IUGR offspring and to inform the prescription of exercise training, an exercise tolerance test was conducted. At 10 weeks of age, a subset of offspring (3 per group: control and IUGR, male and female) were familiarized with running on a motor-driven treadmill (Animal treadmill: Exer 3/6 Columbus Instruments, Columbus, OH, USA) for 10 min at 25 m min−1 and a 10 deg gradient for 4 days. After familiarization, each rat performed a progressive, incremental exercise test to fatigue. Testing began at a speed of 25 m min−1 and a 10 deg gradient for 3 min; treadmill speed was then increased to 40 m min−1 with the gradient held constant for 3 min. Subsequently, treadmill speed was increased progressively by 5 m min−1 every minute until rats were not able to continue running. Total exercise time, maximal treadmill speed and total distance run were recorded for each rat to determine maximal exercise capacity.
Exercise intervention
At 10 weeks of age, male and female offspring from hypoxic and normoxic pregnancies were randomized to an exercise training group. Offspring were progressively habituated to motor-driven treadmill running during 5 consecutive days and then exercised for 30 min at 20 m min−1, 5 deg gradient, 5 days week−1, for 6 weeks. This exercise protocol was adapted from Jendzjowsky & DeLorey (2011). Rats were encouraged to run with a jet of air applied to the hindquarters. Offspring in the sedentary group were exposed to the same room environment for the same period of time as the training group.
Food intake, body weight, echo MRI
Food intake and body weight were calculated weekly from 10 to 16 weeks of age. At 15 weeks of age, whole body composition was measured in conscious offspring using EchoMRI analysis (Houston, TX, USA). The percentage of fat and lean tissue relative to body weight was calculated for each animal.
Vascular function
At 16 weeks of age, and 24 h after the last bout of exercise, offspring were anaesthetized with a single dose (1.5 ml) of inhaled isoflurane and killed by exsanguination. Vascular function was assessed using wire myography from sedentary and exercise, control and IUGR, male and female offspring. Since the vascular assessments were conducted 24 h after the last bout of exercise, we were not able to assess females at a particular day of their oestrous cycle. Second order mesenteric arteries and first or second order arteries from the medial head of the gastrocnemius muscle (defined as first or second branches of the feed artery that traverses the superficial portion of the muscle) were isolated and dissected in ice-cold physiological saline solution (in mm: 10 Hepes, 5.5 glucose, 1.56 CaCl2, 4.7 KCl, 142 NaCl, 1.17 MgSO4, 1.18 KH2PO4, pH 7.4). Mesenteric arteries were used to assess the effect of aerobic exercise training on resistance arteries important for blood pressure regulation. Gastrocnemius muscle arteries were used to assess the effect of exercise training on a vascular segment from a muscle group recruited during treadmill exercise training.
Arteries were mounted on two 40 μm wires attached to a wire myograph (DMT, Copenhagen, Denmark) to allow isometric tension recordings. Vessels were normalized and functional endothelial and smooth muscle integrity were checked as previously described (Morton et al. 2010). A cumulative concentration–response curve (CCRC) to phenylephrine (PE, 0.001–100 μm for mesenteric arteries and 0.00001–100 μm for gastrocnemius muscle arteries) was performed to determine the EC80 (concentration producing 80% of the maximum response (Emax) for the vasoconstrictor). Responses were normalized to artery length. For both vascular beds assessed, the maximum contractile response of smooth muscle cells was tested using a high potassium solution at the end of the protocol (in mm: 10 Hepes, 5.5 glucose, 4.9 CaCl2, 124 KCl, 24 NaCl, 2.4 MgSO4, 1.18 KH2PO4, pH 7.4).
We have previously shown that mesenteric arteries from IUGR offspring have a reduced NO component of vasodilatation while maintaining EDH, with no contribution of prostaglandins to vasodilatation (Morton et al. 2010). Therefore, we tested the mechanisms by which NO-induced vasodilatation was reduced in IUGR and whether this could be improved by exercise. To investigate vascular responses to the endothelium-dependent vasodilator methylcholine (MCh, 0.00001–3 μm), a CCRC was performed following preconstriction with the EC80 concentration of PE. CCRCs to MCh and PE were performed in mesenteric arteries with separate baths used to incubate the arteries with the following inhibitors: l-NAME (Calbiochem, Germany; 100 μm) to inhibit NO synthase activity, or Mn(III)tetrakis(4-benzoic acid)porphyrin chloride (MnTBAP, Calbiochem, Germany; 10 μm) to scavenge peroxynitrite (Batinić-Haberle et al. 2009).
Our primary question in any vascular bed is to address the main mechanisms of vasodilatation. Prior to our study, the mechanisms of vasodilatation had not been investigated in gastrocnemius muscle arteries of IUGR offspring. Therefore, CCRCs to MCh and PE were performed in gastrocnemius muscle arteries with separate baths used to incubate the arteries with the following inhibitors: l-NAME (100 μm), a combination of apamin (0.1 μm) and 1-[(2-chlorophenyl)diphenylmethyl]-1H-pyrazole (TRAM-34; 10 μm) to block small-conductance calcium-activated potassium (SKCa) channels and intermediate-conductance calcium-activated potassium (IKCa) channels, or indomethacin, a cyclooxygenase inhibitor (5 μm). Unless otherwise stated all drugs were purchased from Sigma (St. Louis, MO, USA).
Corticosterone assay
After offspring were anaesthetized and killed, a blood sample was collected by venipuncture of the inferior vena cava. Serum corticosterone levels were determined according to the manufacturer's instructions using a commercially available, colorimetric competitive enzyme immunoassay (Enzo Life Sciences, Inc., Farmingdale, NY, USA).
Statistical analyses
The data were presented as mean ± standard error of the mean (SEM). The Shapiro–Wilk test was used to assess normality of continuous data. Body weights, crown–rump length, abdominal girth, total exercise time, maximal treadmill speed and total distance run were analysed using two-sample t tests or a Mann–Whitney test when data were not normally distributed. This study had a two-way ANOVA design where the effect of being born growth restricted and the effect of aerobic exercise training were determined. Therefore, food intake, body weight, body composition and corticosterone levels were tested using a two-way ANOVA followed by a Bonferroni post hoc test. Offspring were chosen for each experimental procedure such that no two animals in one procedure were from the same litter. Seven to thirteen offspring were used in each experiment.
The effect of l-NAME, MnTBAP, apamin + TRAM-34, or indomethacin on vasoconstriction and vasodilatation was compared within each study group using a one-way ANOVA followed by a Dunnett's post hoc test analysis of pEC50 (negative log of effective concentration producing 50% of the maximum response) and Emax data.
The contribution of NO, ROS, EDH or prostaglandins to vasodilatation was further compared between study groups by assessment of the Δ of the vasodilatation area under the curve (AUC) with or without inhibitors. The effect of being born growth restricted and the effect of aerobic exercise training were tested using a two-way ANOVA followed by a Bonferroni post hoc test.
Female and male offspring data were analysed separately due to phenotypical differences. Statistical significance was defined as P < 0.05. All data were analysed using GraphPad Prism 5 statistical software (GraphPad Software, Inc., La Jolla, CA, USA).
Results
Animal model
At the time of birth, male and female offspring from dams exposed to hypoxia had a lower body weight, similar crown–rump length and lower abdominal girth than offspring born from dams in normoxic conditions suggesting asymmetric growth in both sexes. However, the crown–rump length to abdominal girth ratio only reached significance in the male IUGR offspring suggesting a more subtle phenotype in the females (Table1).
Table 1.
Morphological characteristics at birth of male and female offspring born from normoxic (control) dams or dams exposed to hypoxia (IUGR)
| Parameter | Control male offspring | IUGR male offspring | Control female offspring | IUGR female offspring |
|---|---|---|---|---|
| Body weight (g) | 7.2 ± 0.1 | 6.2 ± 0.2*** | 6.7 ± 0.1 | 5.9 ± 0.1*** |
| Crown–rump length (mm) | 45.2 ± 0.5 | 44.1 ± 0.5 | 44.1 ± 0.6 | 43.1 ± 0.5 |
| Abdominal girth (mm) | 47.7 ± 0.5 | 44.8 ± 0.7** | 46.4 ± 0.6 | 44.2 ± 0.8* |
| CRL/ABG ratio | 0.94 ± 0.006 | 0.97 ± 0.007** | 0.94 ± 0.006 | 0.95 ± 0.01 |
Data presented as mean ± SEM. CRL, crown–rump length; ABG, abdominal girth. *P < 0.05, **P < 0.01 and ***P < 0.0001 vs. control offspring.
Exercise tolerance test
There were no differences regarding time and speed reached at the end of the test between control offspring and IUGR offspring in either male (time: 8.8 ± 0.4 min control vs. 9.6 ± 1.8 min IUGR, P = 0.7; speed: 56.7 ± 1.6 m min−1 control vs. 58.3 ± 9.2 m min−1 IUGR, P = 0.7) or female offspring (time: 9.2 ± 1.3 min control vs. 10.4 ± 1.3 min IUGR, P = 1.0; speed: 56.7 ± 4.4 m min−1 control vs. 68.6 ± 3.3 m min−1 IUGR, P = 0.7). Given the similar exercise capacity in all groups, the experimental exercise training intervention performed represented the same absolute work rate and relative exercise intensity in both control and IUGR groups.
Food intake, body composition and body weight
During the entire 6 weeks of the protocol, control male offspring had a lower food consumption compared to IUGR male offspring (P < 0.0001). Aerobic exercise, however, did not have an effect on food consumption in either male control or IUGR offspring. While aerobic exercise training decreased the percentage of fat tissue in male control offspring only (P = 0.01), body weight was not different between male control and IUGR, sedentary or exercised offspring (Table2).
Table 2.
Food intake, body weight and body composition from male control and IUGR, sedentary and exercised offspring
| Control | Control | IUGR | IUGR | ||||
|---|---|---|---|---|---|---|---|
| sedentary | exercised | sedentary | exercised | 2-way ANOVA | |||
| Parameter | offspring | offspring | offspring | offspring | IUGR | Exercise | Interaction |
| Food intake (g week−1) | |||||||
| Week 1 | 34.2 ± 0.9 | 33.4 ± 1.6 | 63.2 ± 2.4 | 59.9 ± 5.8 | <0.0001 | 0.35 | 0.44 |
| Week 2 | 34.1 ± 2.9 | 32.1 ± 1.6 | 68.5 ± 4.9 | 59.7 ± 6.1 | <0.0001 | 0.28 | 0.49 |
| Week 3 | 35.7 ± 1.4 | 32.6 ± 0.9 | 66.2 ± 2.3 | 61.2 ± 5.7 | <0.0001 | 0.22 | 0.76 |
| Week 4 | 33.8 ± 0.6 | 34.7 ± 2.1 | 68.2 ± 6.2 | 60.8 ± 7.4 | <0.0001 | 0.52 | 0.42 |
| Week 5 | 34.9 ± 1.9 | 32.1 ± 1.4 | 64.2 ± 2.9 | 60.8 ± 5.6 | <0.0001 | 0.40 | 0.93 |
| Week 6 | 33.6 ± 3.1 | 29.9 ± 2.9 | 67.1 ± 3.2 | 71.9 ± 4.3 | <0.0001 | 0.23 | 0.88 |
| Body weight (g) | |||||||
| Week 1 | 542.8 ± 16.9 | 547.6 ± 16.3 | 514.5 ± 13.1 | 546.2 ± 16.9 | 0.10 | 0.19 | 0.62 |
| Week 2 | 562.1 ± 16.4 | 557.2 ± 14.0 | 544.7 ± 14.6 | 558.0 ± 19.0 | 0.61 | 0.79 | 0.58 |
| Week 3 | 583.9 ± 18.4 | 569.2 ± 13.8 | 541.3 ± 27.4 | 572.8 ± 22.0 | 0.34 | 0.68 | 0.26 |
| Week 4 | 604.0 ± 19.5 | 581.6 ± 16.8 | 578.2 ± 19.6 | 586.8 ± 21.0 | 0.59 | 0.72 | 0.43 |
| Week 5 | 622.3 ± 19.8 | 601.5 ± 16.6 | 597.9 ± 22.3 | 596.4 ± 22.5 | 0.47 | 0.68 | 0.53 |
| Week 6 | 640.1 ± 21.0 | 613.3 ± 15.9 | 612.4 ± 21.4 | 609.0 ± 26.2 | 0.46 | 0.48 | 0.58 |
| Body composition (% of body weight after 6 weeks of aerobic exercise) | |||||||
| Fat tissue | 12.2 ± 1.2 | 8.4 ± 0.3* | 12.5 ± 0.6 | 11.4 ± 1.2 | 0.09 | 0.01 | 0.18 |
| Lean tissue | 71.0 ± 2.0 | 71.1 ± 1.6 | 72.7 ± 2.3 | 72.4 ± 1.4 | 0.71 | 0.17 | 0.76 |
Data presented as mean ± SEM. *P < 0.05 vs. control sedentary after a Bonferroni post hoc test.
In female offspring, there were no differences regarding food consumption or body weight gain (Table3). Being born growth restricted, however, was associated with an increased percentage of fat tissue (P = 0.02) and aerobic exercise training increased the percentage of lean tissue in both control and IUGR offspring (Table3, P = 0.03).
Table 3.
Food intake, body weight and body composition from female control and IUGR, sedentary and exercised offspring
| Control | Control | IUGR | IUGR | ||||
|---|---|---|---|---|---|---|---|
| sedentary | exercised | sedentary | exercised | 2-way ANOVA | |||
| Parameter | offspring | offspring | offspring | offspring | IUGR | Exercise | Interaction |
| Food intake (g week–1) | |||||||
| Week 1 | 22.2 ± 0.8 | 21.5 ± 0.7 | 21.4 ± 1.8 | 19.9 ± 0.9 | 0.22 | 0.38 | 0.68 |
| Week 2 | 22.3 ± 1.1 | 23.2 ± 0.9 | 21.3 ± 0.9 | 20.9 ± 0.9 | 0.09 | 0.82 | 0.53 |
| Week 3 | 21.7 ± 0.3 | 22.5 ± 0.6 | 21.9 ± 1.1 | 21.6 ± 1.2 | 0.71 | 0.78 | 0.58 |
| Week 4 | 21.1 ± 0.6 | 21.9 ± 0.7 | 22.3 ± 1.5 | 21.5 ± 1.3 | 0.68 | 0.99 | 0.47 |
| Week 5 | 20.9 ± 3.9 | 22.1 ± 3.7 | 21.7 ± 2.0 | 22.7 ± 1.4 | 0.78 | 0.66 | 0.96 |
| Week 6 | 20.3 ± 1.5 | 22.8 ± 0.5 | 22.7 ± 0.9 | 23.3 ± 1.1 | 0.12 | 0.09 | 0.34 |
| Body weight (g) | |||||||
| Week 1 | 310.2 ± 5.7 | 310.0 ± 5.4 | 307.7 ± 7.1 | 315.4 ± 11.4 | 0.40 | 0.56 | 0.12 |
| Week 2 | 317.5 ± 6.4 | 317.2 ± 6.5 | 309.9 ± 6.5 | 321.8 ± 13.9 | 0.87 | 0.51 | 0.50 |
| Week 3 | 325.9 ± 7.2 | 324.9 ± 6.4 | 317.4 ± 6.6 | 327.6 ± 13.8 | 0.75 | 0.61 | 0.63 |
| Week 4 | 332.6 ± 6.7 | 341.8 ± 6.4 | 325.3 ± 7.5 | 330.7 ± 13.7 | 0.52 | 0.67 | 0.86 |
| Week 5 | 341.7 ± 8.4 | 343.4 ± 7.7 | 329.4 ± 8.9 | 338.0 ± 12.2 | 0.39 | 0.64 | 0.65 |
| Week 6 | 346.3 ± 8.4 | 352.9 ± 6.6 | 339.4 ± 10.7 | 346.5 ± 14.6 | 0.85 | 0.84 | 0.64 |
| Body composition (% of body weight after 6 weeks of aerobic exercise) | |||||||
| Fat tissue | 11.6 ± 1.2 | 9.4 ± 0.6 | 13.2 ± 0.9 | 12.3 ± 1.0 | 0.02 | 0.11 | 0.52 |
| Lean tissue | 73.4 ± 1.1 | 75.6 ± 0.6 | 72.2 ± 0.8 | 73.9 ± 0.7 | 0.10 | 0.03 | 0.73 |
Data presented as mean ± SEM.
Serum corticosterone concentration
Being born growth restricted was not associated with an increase in serum corticosterone levels in male offspring (61.6 ± 6.8 ng ml−1 sedentary control vs. 58.4 ± 7.6 ng ml−1 sedentary IUGR; P = 0.67). Aerobic exercise training tended to decrease serum corticosterone levels in both male control and IUGR offspring (45.4 ± 8.2 ng ml−1 exercised control and 41.9 ± 8.8 ng ml−1 exercised IUGR; P = 0.0503).
In female offspring, neither being born growth restricted nor performing aerobic exercise training had an effect on serum corticosterone levels (60.7 ± 7.9 ng ml−1 sedentary control; 55.7 ± 6.3 ng ml−1 exercised control; 58.0 ± 7.7 ng ml−1 sedentary IUGR; 59.7 ± 4.7 ng ml−1 exercised IUGR; P = 0.8).
Mesenteric arteries: PE-induced vasoconstriction
Compared to controls, being born growth restricted did not modify PE-induced vasoconstriction in either male (P = 0.07) or female offspring (P = 0.6). In addition, compared to sedentary animals, aerobic exercise training had no effect on maximal vasoconstriction to PE: male control offspring (Emax: 9.9 ± 0.4 mN mm−1 sedentary vs. 10.8 ± 0.7 mN mm−1 exercised), male IUGR offspring (Emax: 9.1 ± 0.7 mN mm−1 sedentary vs. 9.4 ± 0.7 mN mm−1 exercised), female control offspring (Emax: 7.4 ± 0.4 mN mm−1 sedentary vs. 8.1 ± 0.6 mN mm−1 exercised) or female IUGR offspring (Emax: 7.5 ± 0.3 mN mm−1 sedentary vs. 8.4 ± 0.5 mN mm−1 exercised).
In males, there were no changes regarding maximal vasoconstriction to PE in the presence or absence of l-NAME or MnTBAP in any of the groups (data not shown). In females, the presence of l-NAME increased maximal PE-induced vasoconstriction only in control sedentary offspring (Emax: 7.4 ± 0.4 mN mm−1 no inhibitor vs. 9.3 ± 0.4 mN mm−1l-NAME, P < 0.05). Vascular sensitivity to PE, however, was unchanged following the addition of l-NAME or MnTBAP to mesenteric arteries from all groups (data not shown).
Mesenteric arteries: vasoconstriction to high potassium solution
In male and female offspring, neither IUGR nor aerobic exercise training affected maximum vasoconstriction to high potassium solution (data not shown).
Mesenteric arteries: MCh-induced vasodilatation in male offspring
In male offspring, there was an interaction effect (P < 0.04) on MCh-induced vasodilatation, whereby exercise increased vasodilatation in control but decreased responses in IUGR offspring (Fig.1A).
Figure 1. CCRCs to methylcholine in mesenteric arteries from male and female, control and IUGR, sedentary and exercised offspring.
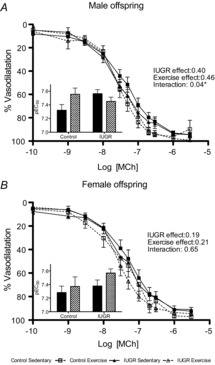
Percentage vasodilatation to methylcoline (MCh) in mesenteric arteries from males (A; n = 8–13) and females (B; n = 8–13). Groups include: control sedentary offspring (continuous lines, filled squares), control exercised offspring (dashed lines, open squares), IUGR sedentary offspring (continuous lines, filled triangles), and IUGR exercised offspring (dashed lines, open triangles). Data are presented as mean ± SEM and summarized as pEC50 in the inset bar graph figures: sedentary offspring (filled bars) and exercised offspring (hatched bars). *P < 0.05 for a statistically significant interaction between aerobic exercise training and IUGR in male offspring.
l-NAME decreased mesenteric artery sensitivity to MCh (Fig.2A), demonstrating that there was an NO contribution to vasodilatation in all male groups (Table4). Maximal vasodilatation was also decreased in the presence of l-NAME in control sedentary and IUGR exercised offspring (Fig.2A; Table4). Based on analysis of the ΔAUC for all male groups, neither being born IUGR nor exercise affected the NO contribution to vasodilatation in male offspring (Fig.2B).
Figure 2. CCRCs to methylcholine in mesenteric arteries from male control and IUGR, sedentary and exercised offspring in the presence or absence of l-NAME or MnTBAP.
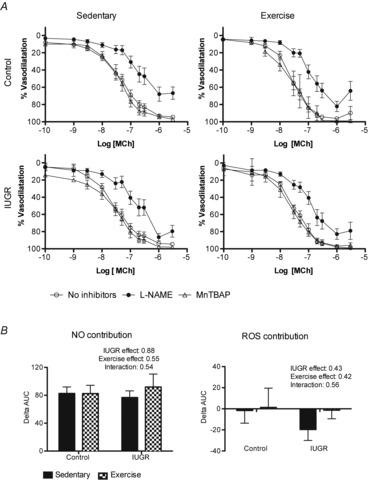
A, percentage vasodilatation to MCh in mesenteric arteries from males (n = 7–13). Responses to methylcholine in control and IUGR animals in sedentary or exercised groups are shown in the absence of inhibitors (open circles), in the presence of l-NAME (filled circles), or in the presence of MnTBAP (open triangles). B, summary data of the ΔAUC in vessels incubated with no inhibitor or l-NAME, equivalent to the contribution of NO to the vasodilator response to MCh, and in vessels incubated with no inhibitor or MnTBAP, equivalent to the contribution of ROS to the vasodilator response to MCh. Data are presented as mean ± SEM.
Table 4.
Mesenteric artery summary data (pEC50 and Emax) of vasodilatation to MCh from male and female, control and IUGR, sedentary and exercised offspring
| pEC50 | Emax (%) | ||||||
|---|---|---|---|---|---|---|---|
| Male offspring | No inhibitors | l-NAME | MnTBAP | No inhibitors | l-NAME | MnTBAP | |
| Control sedentary | 7.32 ± 0.08 | 6.8 ± 0.14** | 7.36 ± 0.08 | 93.16 ± 1.76 | 68.04 ± 8.28** | 94.24 ± 2.40 | |
| Control exercised | 7.56 ± 0.06 | 7.01 ± 0.1** | 7.47 ± 0.2 | 96.26 ± 1.39 | 82.27 ± 5.96 | 99.03 ± 0.44 | |
| IUGR sedentary | 7.56 ± 0.09 | 6.76 ± 0.15*** | 7.43 ± 0.11 | 93.76 ± 2.08 | 86.54 ± 4.25 | 97.73 ± 0.81 | |
| IUGR exercised | 7.45 ± 0.06 | 6.98 ± 0.11** | 7.59 ± 0.09 | 98.01 ± 2.56 | 82.87 ± 6.15* | 96.81 ± 1.58 | |
| Female offspring | |||||||
| Control sedentary | 7.28 ± 0.09 | 6.76 ± 0.06* | 7.49 ± 0.26 | 91.29 ± 3.31 | 86.09 ± 5.07 | 98.92 ± 0.83 | |
| Control exercised | 7.37 ± 0.14 | 6.55 ± 0.12*** | 7.55 ± 0.09 | 96.47 ± 1.81 | 82.57 ± 6.24 | 99.11 ± 0.39 | |
| IUGR sedentary | 7.37 ± 0.08 | 6.39 ± 0.51 | 7.49 ± 0.14 | 90.75 ± 3.13 | 71.75 ± 9.07 | 96.06 ± 2.51 | |
| IUGR exercised | 7.57 ± 0.06 | 6.88 ± 0.16** | 7.38 ± 0.12 | 93.64 ± 2.26 | 83.19 ± 7.21 | 97.26 ± 1.28 | |
Data presented as mean ± SEM. *P < 0.05, **P < 0.01, ***P < 0.001 vs. artery with no inhibitor after Dunnett's post hoc test.
The addition of MnTBAP did not affect sensitivity or maximal vasodilatation of mesenteric arteries to MCh in any of the groups (Fig.2A; Table4). Moreover, analysis of ΔAUC did not show a significant group effect of either phenotype or exercise on MnTBAP treatment (Fig.2B).
Mesenteric arteries: MCh-induced vasodilatation in female offspring
Neither phenotype nor aerobic exercise training had an effect on vasodilator responses to MCh in female offspring (Fig.1B).
In female offspring, a significant reduction in sensitivity to MCh following the addition of l-NAME was present in control sedentary and exercised offspring as well as IUGR exercised offspring (Fig.3A, Table4). Maximal vasodilatation, however, was not reduced in the presence of l-NAME in any female group (Table4). Analysis of the ΔAUC demonstrated that there was a significant interaction of phenotype and exercise suggesting that aerobic exercise training only improved NO-mediated vasodilatation in control offspring (P = 0.03; Fig.3B).
Figure 3. CCRCs to methylcholine in mesenteric arteries from female control and IUGR, sedentary and exercised offspring in the presence or absence of l-NAME or MnTBAP.
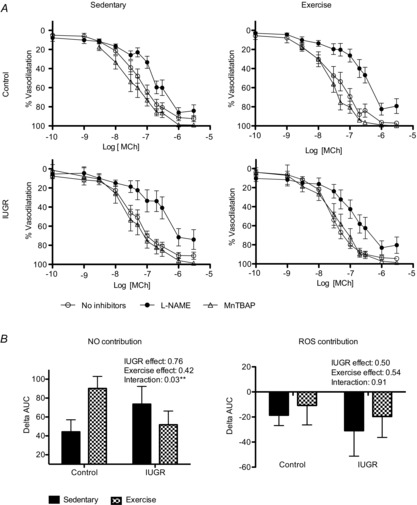
A, percentage vasodilatation to MCh in mesenteric arteries from females (n = 7–13). Responses to methylcholine in control and IUGR animals in sedentary or exercised groups are shown in the absence of inhibitors (open circles), in the presence of l-NAME (filled circles), or in the presence of MnTBAP (open triangles). B, summary data of the ΔAUC in vessels incubated with no inhibitor or l-NAME, equivalent to the contribution of NO to the vasodilator response to MCh, and in vessels incubated with no inhibitor or MnTBAP, equivalent to the contribution of ROS to the vasodilator response to MCh. Data are presented as mean ± SEM. **P < 0.01 for a statistically significant interaction between aerobic exercise training and IUGR in the presence of l-NAME.
MnTBAP did not affect vascular sensitivity to MCh or maximal vasodilatation in either sedentary or exercised, control or IUGR female offspring (Fig.3A; Table4). ΔAUC analysis demonstrated that neither aerobic exercise training nor being born IUGR had an effect on the contribution of ROS to vasodilatation (Fig.3B).
Gastrocnemius muscle arteries: PE-induced vasoconstriction
There were no changes regarding sensitivity to PE or maximal PE-induced vasoconstriction in the presence or absence of l-NAME, indomethacin or apamin + TRAM-34 in any of the groups (male and female, control and IUGR, sedentary and exercised; data not shown).
Gastrocnemius muscle arteries: vasoconstriction to high potassium solution
In male and female offspring, neither IUGR nor aerobic exercise training affected maximum vasoconstriction to high potassium solution (data not shown).
Gastrocnemius muscle arteries: MCh-induced vasodilatation in male offspring
In male offspring, there was an interaction of being born IUGR and aerobic exercise training (P = 0.04) whereby maximal vasodilatation to MCh tended to be increased following exercise in IUGR offspring (Emax; 76 ± 4.9% sedentary control vs. 61.9 ± 9.2% exercised control; 71.22 ± 8.1% sedentary IUGR vs. 89.9 ± 6.8% exercised IUGR).
Following the addition of l-NAME, a decrease in the MCh Emax demonstrated that NO contributed to vasodilatation in gastrocnemius muscle arteries from control sedentary offspring, but not IUGR sedentary offspring. The variability in the IUGR offspring data, however, was greater and thus careful interpretation of the data is required (Fig.4A, Table5). Interestingly, NO significantly contributed to vasodilatation in both control and IUGR groups following exercise (P < 0.01, and P < 0.001; Table5). In addition we found that EDH contributed to vasodilatation in gastrocnemius muscle arteries from control sedentary offspring (P < 0.05) as well as IUGR exercised offspring (P < 0.001, Table5). There was no significant contribution of prostaglandins to vasodilatation in any male group. In addition, sensitivity to MCh was unaltered by any inhibitor in control or IUGR, sedentary or exercised offspring (Table5).
Figure 4. CCRCs to methylcholine of gastrocnemius muscle arteries from male and female, control, IUGR, sedentary and exercised offspring in the presence or absence of l-NAME, apamin + TRAM-34, or indomethacin.
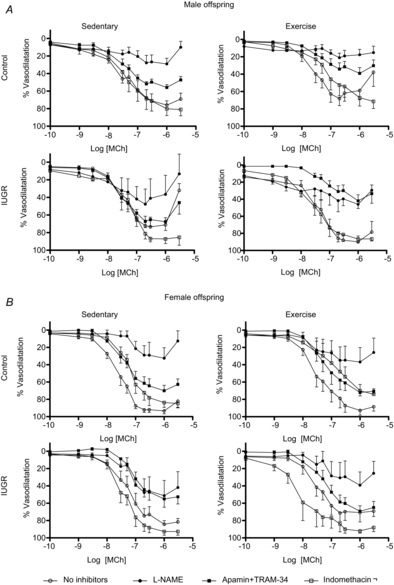
Percentage vasodilatation to MCh in gastrocnemius muscle arteries from males (A, n = 4–8) and females (B, n = 4–7). Responses to methylcholine in control and IUGR animals in sedentary or exercised groups are shown in the absence of inhibitors (open circles), in the presence of l-NAME (filled circles), in the presence of apamin + TRAM-34 (filled squares), or in the presence of indomethacin (open squares). Data are presented as mean ± SEM.
Table 5.
Gastrocnemius muscle artery summary data (pEC50 and Emax) of vasodilatation to MCh from male and female, control and IUGR, sedentary and exercised offspring
| pEC50 | Emax (%) | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| No inhibitors | l-NAME | Apamin + Tram-34 | Indomethacin | No inhibitors | l-NAME | Apamin + Tram-34 | Indomethacin | ||
| Male offspring | |||||||||
| Control sedentary | 7.51 ± 0.16 | 7.73 ± 0.63 | 7.52 ± 0.09 | 7.37 ± 0.18 | 76.03 ± 4.89 | 29.09 ± 6.48*** | 55.96 ± 3.19* | 80.25 ± 7.66 | |
| Control exercised | 7.73 ± 0.26 | 7.73 ± 0.6 | 7.44 ± 0.15 | 7.05 ± 0.23 | 58.88 ± 10.68 | 17.72 ± 6.25** | 39.16 ± 6.72 | 67.17 ± 9.63 | |
| IUGR sedentary | 7.60 ± 0.14 | 8.04 ± 0.61 | 7.63 ± 0.14 | 7.21 ± 0.08 | 71.22 ± 8.12 | 36.6 ± 21.65 | 67.65 ± 0.81 | 87.17 ± 5.28 | |
| IUGR exercised | 7.37 ± 0.09 | ND | 7.19 ± 0.17 | 7.57 ± 0.13 | 89.92 ± 6.82 | 46.06 ± 4.87*** | 42.03 ± 7.13*** | 86.63 ± 4.17 | |
| Female offspring | |||||||||
| Control sedentary | 7.61 ± 0.07 | 7.11 ± 0.18* | 7.45 ± 0.08 | 7.23 ± 0.08 | 93.4 ± 3.47 | 32.61 ± 12.25*** | 70.13 ± 6.33 | 83.84 ± 5.67 | |
| Control exercised | 7.53 ± 0.12 | 7.59 ± 0.42 | 7.29 ± 0.13 | 6.87 ± 0.34 | 92.8 ± 5.49 | 36.85 ± 16.61** | 72.10 ± 3.97 | 70.04 ± 7.17 | |
| IUGR sedentary | 7.29 ± 0.11 | 7.22 ± 0.19 | 7.1 ± 0.1 | 7.43 ± 0.08 | 84.34 ± 4.15 | 51.39 ± 17.62 | 55.16 ± 9.54 | 92.81 ± 4.24 | |
| IUGR exercised | 7.56 ± 0.13 | 7.21 ± 0.30 | 7.28 ± 0.11 | 8.1 ± 0.22 | 70.79 ± 10.87 | 39.26 ± 15.26 | 69.3 ± 6.24 | 92.11 ± 2.48 | |
Data presented as mean ± SEM. ND, not determined. *P < 0.05, **P < 0.01, ***P < 0.001 vs. artery with no inhibitor after Dunnett's post hoc test.
Analysis of the ΔAUC showed that neither phenotype nor aerobic exercise training had an effect on either NO-mediated vasodilatation (Fig.5A) or prostaglandin-mediated vascular responses (Fig.5C). Further, EDH-mediated vasodilatation was increased only in IUGR exercised male offspring (Fig.5B).
Figure 5. Nitric oxide, EDH and prostaglandin contribution to gastrocnemius muscle artery vasodilatation in male control and IUGR, sedentary and exercised offspring.
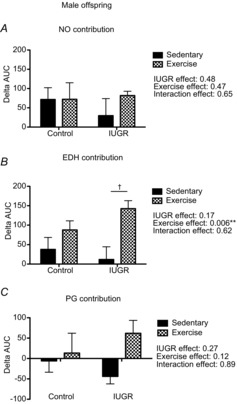
Summary data of vasodilator responses to MCh in gastrocnemius muscle arteries (n = 4–8). A, summary data of the ΔAUC in vessels incubated with no inhibitor or with l-NAME, equivalent to the contribution of NO to vasodilator responses to MCh. B, summary data of the ΔAUC in vessels incubated with no inhibitor or with apamin + TRAM-34, equivalent to the contribution of EDH to vasodilatation. C, summary data of the ΔAUC in vessels incubated with no inhibitor or with indomethacin, equivalent to the contribution of prostaglandins (PG) to vasodilatation. Data are presented as mean ± SEM; sedentary offspring (filled bars), exercised offspring (hatched bars). **P < 0.01 for a statistically significant exercise effect. †P < 0.05 vs. sedentary IUGR offspring after a Bonferroni post hoc test.
Gastrocnemius muscle arteries: MCh-induced vasodilatation in female offspring
Being born growth restricted impaired maximal vasodilatation to MCh in female offspring (Emax; 93.1 ± 0.3% control vs. 84.34 ± 4.15% IUGR, P = 0.02). Performing aerobic exercise did not improve vasodilatation in either control or IUGR female offspring.
In control sedentary females, NO contributed to vasodilatation [Fig.4B, ΔpEC50 (no inhibitors vs. l-NAME) P < 0.05, ΔEmax (no inhibitors vs. l-NAME) P < 0.001; Table5]. Following exercise, a contribution of NO to vasodilatation was still observed in control offspring [ΔEmax (no inhibitors vs. l-NAME) P < 0.001; Table5]. While neither EDH nor prostaglandins were found to significantly contribute to vasodilatation in either sedentary or exercised control offspring, the variability of the data may have precluded the detection of more minor contributions of these vasodilator pathways. In IUGR sedentary or exercised female offspring, there were no significant changes in sensitivity to MCh or maximal vasodilatation after the addition of any inhibitor (Fig.4B; Table5). As observed in arteries from male IUGR offspring, however, the variability of the data was greater than in control groups complicating the interpretation.
Analysis of the ΔAUC demonstrated that being born growth restricted was associated with a reduced NO vasodilator component (P = 0.007, Fig.6A), an increased involvement of prostaglandin-mediated vasoconstriction (P = 0.002, Fig.6C) and had no effect on EDH-mediated vasodilatation (Fig.6B).
Figure 6. Nitric oxide, EDH and prostaglandin contribution to gastrocnemius muscle artery vasodilatation in female, control and IUGR, sedentary and exercised offspring.
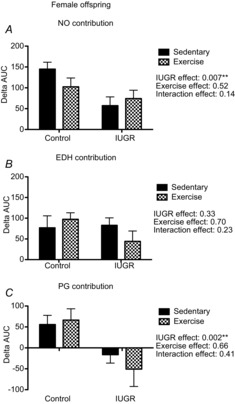
Summary data of vasodilator responses to MCh in gastrocnemius muscle arteries (n = 4–7). A, summary data of the ΔAUC in vessels incubated with no inhibitor or with l-NAME, equivalent to the contribution of NO to the vasodilator responses to MCh. B, summary data of the ΔAUC in vessels incubated with no inhibitor or with apamin + TRAM-34, equivalent to the contribution of EDH to vasodilatation. C, summary data of the ΔAUC in vessels incubated with no inhibitor or with indomethacin, equivalent to the contribution of prostaglandins (PG) to vasodilatation. Data are presented as mean ± SEM; sedentary offspring (filled bars), exercised offspring (hatched bars). Being born growth restricted was associated with a reduced NO vasodilator component, an increased involvement of prostaglandin-mediated vasoconstriction, and had no effect on EDH-mediated vasodilatation. **P < 0.01 for a statistically significant IUGR effect.
Discussion
Many strategies have been proposed to prevent cardiovascular diseases; however, despite large volumes of research that have attempted to discover cardiovascular protective mechanisms, exercise is one of the most practical and effective preventive treatments that has been found to date (Powers et al. 2008). To our knowledge, this is the first study to investigate the vascular effects of aerobic exercise in offspring born growth restricted. Using a hypoxia-induced IUGR model, we found that female IUGR offspring had reduced NO-mediated vasodilatation in both mesenteric and gastrocnemius muscle arteries while in male IUGR offspring NO-mediated vasodilatation was reduced only in the gastrocnemius muscle arteries. Furthermore, we demonstrated that female IUGR offspring had a decreased vasodilator response in gastrocnemius muscle arteries and there was an increase in prostaglandin-mediated vasoconstriction. Interestingly, while in control offspring aerobic exercise training increased vasodilator responses in mesenteric arteries from males and enhanced NO-modulation in mesenteric arteries from females; in IUGR offspring, aerobic exercise training only improved EDH-mediated vasodilatation in gastrocnemius muscle arteries from males.
A hypoxic insult during the last third of pregnancy has been associated with vascular dysfunction in the offspring. Prenatal hypoxia in rats has been associated with increased fetal aortic thickness (Camm et al. 2010; Giussani et al. 2012), and with increased vascular peroxynitrite generation (Giussani et al. 2012), which can lead to endothelial dysfunction. In addition, it has been shown in postnatal day 1 rats that there was an enhanced femoral vasoconstrictor response to phenylephrine while this response in the carotid arteries was decreased, demonstrating that neonatal vascular adaptations following a hypoxic insult are vascular bed specific and may be pivotal in increasing blood flow to vital organs such as the brain while reducing blood flow to other organs (Williams et al. 2005a). In both male and female IUGR offspring as adults, the contribution of NO to mesenteric artery vasodilatation was reduced (Williams et al. 2005b; Morton et al. ,; Giussani et al. 2012). Only female IUGR offspring demonstrated an enhanced myoendothelial gap junction/EDH-mediated vasodilatation in mesenteric arteries as a possible compensatory mechanism to maintain vascular function (Morton et al. 2010). In the current study, EDH-mediated vasodilatation was not significantly enhanced in gastrocnemius muscle arteries from either male or female IUGR offspring.
The present study also demonstrated that exposure to hypoxia in utero was associated with an enhanced prostaglandin-mediated vasoconstriction in gastrocnemius muscle arteries in IUGR female offspring. The mechanisms that have been associated with increased prostaglandin-mediated vasoconstriction include: upregulation of prostaglandin H synthase 1 and 2 (PGHS 1 and 2); activation of PGHS by ROS and enhanced activation of thromboxane receptors. Interestingly, increased prostaglandin-mediated vasoconstriction has been associated with conditions of vascular pathologies such as hypertension (Vanhoutte et al. 2005) and diabetes (Matsumoto et al. 2007) as well as ageing (Stewart et al. 2000). The increased PGHS-dependent constriction observed in our study of young female adults exposed to prenatal hypoxia suggests the development of vascular pathology prior to overt disease.
Our data suggest that the mechanisms that lead to endothelial dysfunction in IUGR offspring differ according to sex, with a greater negative impact on female IUGR offspring compared to male IUGR offspring. Contrary to our findings, Ozaki et al. found that both hypertension and vascular dysfunction in the offspring of protein-restricted dams was predominant among male offspring (Ozaki et al.
2001). In a model of uteroplacental insufficiency fetal programming, however, female offspring exhibited increased arterial wall stiffness in uterine and renal arteries in the absence of altered vascular reactivity in either mesenteric or femoral vascular beds (Mazzuca et al. 2012). Further, previous findings from our laboratory have determined that in aged female IUGR offspring NO-mediated, flow-induced vasodilatation was reduced and there was a predominant EDH-mediated vasodilator component compared to male IUGR offspring (Morton et al. 2011). In addition, with ageing the mechanisms involved in preserving EDH-mediated vasodilatation were different between sexes; a decrease in the contribution of myoendothelial gap junctions to vasodilatation has been found in aged male IUGR offspring compared to aged female IUGR offspring (Morton et al. 2010). It has also been reported that a hypoxic insult during pregnancy increased myogenic tone in mesenteric arteries in only male IUGR offspring (Hemmings et al. 2005). We can conclude that the type and severity of the insult that create a phenotype, the vascular bed assessed, as well as the sex of the offspring all play a role in the presentation of vascular dysfunction later in life following a compromised pregnancy.
Aerobic exercise training has been shown to improve vascular function by increasing NO bioavailability (reviewed in Green et al. 2004) and by reducing ROS (reviewed in Campos et al. 2013). Our findings in control female offspring showed that aerobic exercise training enhanced NO-mediated vasodilatation without affecting the total vasodilatation response in mesenteric arteries. This could be due to a reduction in other vasodilatory pathways such as EDH or prostaglandins; however, there is also the potential for redundancy of vasodilator mechanisms.
Our results regarding ROS did not demonstrate improved vasodilatation with a peroxynitrite scavenger. The impairment of NO-mediated vasodilatation observed in female IUGR offspring, therefore, was not likely to be secondary to a reduction in NO bioavailability via scavenging by superoxide and might instead be due to a decrease in NO production. Further experiments are needed in order to assess this observation.
In accordance with our findings, it has previously been demonstrated that exercise training was associated with an increase in EDH-mediated vasodilatation in animal models of disease such as hypertension (Yen et al. 1995; Gündüz et al. 2011) and diabetes (Minami et al. 2002). The mechanism by which this improvement may occur is not completely understood and remains under investigation. Exercise training, however, has been shown to increase whole cell potassium current activation and improve functional gating of calcium-activated potassium channels without affecting protein expression in coronary arteries from diabetic dyslipidaemic pigs (Mokelke et al. 2003). In addition, Milkau et al. determined that EDH-mediated vasodilatation was crucial for active hyperaemia, and was mediated through the activation of endothelial SKCa channels and spread along the vascular wall by connexin 40 endo–endo and myo–endo gap junctions (Milkau et al. 2010). Upregulation of SKCa and connexin 40 or improvement of their function could, therefore, be involved in the beneficial effect of aerobic exercise training in the vasculature observed in male IUGR offspring.
Aerobic exercise as an intervention in rodents has been suggested to chronically activate stress responses (Arida et al. 2004; Leasure & Jones, 2008). Moreover, it has been shown that being born growth restricted was associated with increased mineralocorticoid mRNA expression in the hippocampus and increased free corticosterone in plasma after a 30 min restraint stress test (Lesage et al. 2002). In the present study we found that neither exposure to hypoxia in utero nor aerobic exercise training was associated with changes in corticosterone levels. These findings suggest that aerobic exercise training as an intervention in a hypoxia-induced IUGR animal model is not a secondary stressor.
A variety of training protocols (voluntary vs. forced exercise; frequency; intensity and time) have been utilized to investigate physiological adaptations to exercise. Training-mediated changes in vascular function appear to be sensitive to the intensity and volume of exercise training (Green et al. 2011; Jendzjowsky & DeLorey, 2013). Our protocol was based on findings from Jendzjowsky & DeLorey (2011) where the authors calculated that the given exercise protocol corresponded to approximately 50% of maximal exercise tolerance in rodents. Moreover, the chosen training protocol is in alignment with the American College of Sports Medicine guidelines for exercise testing and prescription (ACSM, 2013) which recommend 150 min week−1 of moderate intensity aerobic activity to delay premature mortality and reduce the risk of chronic diseases (including cardiovascular diseases). In the present study, exercise tolerance was not different between control and IUGR offspring and therefore exercise training was completed at the same absolute work rate and relative intensity in all groups. Thus, group and sex differences in the vascular responses to exercise training are not attributable to differences in the training stimulus.
We proposed aerobic exercise as an intervention to prevent the development of endothelial dysfunction later in life in young offspring born from dams exposed to hypoxia. Aerobic exercise as an intervention, however, has also been studied earlier in development (i.e. during pregnancy) to improve both maternal and offspring outcomes. Specifically, Vega et al. have shown in an animal model of maternal obesity that aerobic exercise improved metabolic function in the dams while it decreased leptin and triglyceride serum levels, and decreased fat deposition in male offspring (Vega et al. 2013). Maternal aerobic exercise training had no effect on female offspring. It has been proposed that the plasticity of any physiological system declines with age. Furthermore, ageing accumulates inadequate responses to new environmental challenges (Hanson et al. 2011). Thus, intervention strategies should focus not only on the timing, but also on the insult creating a phenotype and which population may benefit from the approach.
Our findings have demonstrated that hypoxia during the last third of pregnancy affects offspring birth weight and abdominal girth in both male and female offspring. The IUGR offspring, however, underwent a rapid growth phase and their body weights were not different compared to control offspring by the beginning of the aerobic exercise intervention at ten weeks of age.
In accordance with previous studies (Desai et al. 2007; Fukami et al. 2012), our findings show that male IUGR offspring had an increased appetite. Moreover, we found a disparity between increased appetite and weight gain in male control and IUGR offspring. Interestingly, fat tissue accumulation was not different among the groups, suggesting that in male IUGR offspring there could be metabolic alterations associated with this phenomenon. It has already been established that IUGR is associated with a reduction in proteins associated with nutrient absorption and transport as well as energy metabolism in the fetal gut, predisposing the gut to metabolic defects during gestation and neonatal periods (Wang et al. 2014). These alterations might make them prone to obesity later in life. Although in our study IUGR offspring weight was not increased compared to control offspring, we have previously described that IUGR offspring have an increased susceptibility to develop intra-abdominal fat deposition, insulin resistance, impaired glucose tolerance and dyslipidaemia with a high fat diet compared to control offspring (Rueda-Clausen et al. 2011). Since female IUGR offspring had a higher percentage of fat tissue compared to female control offspring we suggest that the catch-up growth observed in female offspring was due to fat tissue accumulation, suggesting a greater metabolic efficiency.
In conclusion, exposure to hypoxia in utero was associated with decreased NO-mediated vasodilatation in female offspring in both mesenteric and gastrocnemius muscle arteries. An increase in prostaglandin-mediated vasoconstriction in gastrocnemius muscle arteries was also observed in female IUGR offspring. Our data suggest that exercise enhanced NO-mediated vasodilatation in female control offspring but not IUGR offspring. Furthermore, exercise improved EDH-mediated vasodilatation only in male IUGR offspring. Results from the present study highlight that understanding the mechanisms by which exercise impacts specific vascular beds in a susceptible population is essential. Exercise may not prove to be a beneficial instrument for specific vascular pathways affected by prenatal hypoxia, particularly in female offspring.
Glossary
- AUC
area under the curve
- CCRC
cumulative concentration–response curve
- EDH
endothelium-derived hyperpolarization
- Emax
maximum response
- GD
gestational day
- IUGR
intrauterine growth restriction
- MCh
methylcholine
- NO
nitric oxide
- PE
phenylephrine
- ROS
reactive oxygen species
- SKCa
small-conductance calcium-activated potassium channels
Additional information
Competing interests
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Author contributions
L.M.R. contributed to the conception and design of the experiments, collection of the data, analysis and interpretation of the data, and drafting the article. J.S.M. contributed to the conception and design of the experiments, collection of the data, analysis and interpretation of the data, and revising the manuscript critically. R.K. contributed to the collection of the data, interpretation of the data, and revising the manuscript critically. D.S.D. contributed to the conception and design of the experiments, interpretation of the data, and revising the manuscript critically. S.T.D. contributed to the conception and design of the experiments, analysis and interpretation of the data, and revising the manuscript critically. The experiments were carried out in the laboratory of S.T.D at the University of Alberta. All authors approved the final version of the manuscript. All the people listed as authors qualify for authorship.
Funding
S. T. Davidge is a Canada Research Chair in Maternal and Perinatal Cardiovascular Health. The Davidge laboratory receives funding from the Canadian Institutes of Health Research (CIHR, grant numbers: 118160540; 0020054), the Women and Children's Health Research Institute (WCHRI), Heart and Stroke Foundation of Canada (HSF, grant number: 0000936) and Alberta Innovates Health Solutions (AIHS). L. M. Reyes is an AIHS-supported graduate student (grant number: 0014195). D. S. DeLorey receives funding from the Natural Sciences and Engineering Research Council of Canada (NSERC, grant number: 353634).
References
- ACSM. ACSM's Guidelines for Exercise Testing and Prescription. 9th edn. Lippincott Williams & Wilkins, Philadelphia, PA; 2013. [Google Scholar]
- Allen JD, Stabler T, Kenjale AA, Ham KL, Robbins JL, Duscha BD, Kraus WE. Annex BH. Diabetes status differentiates endothelial function and plasma nitrite response to exercise stress in peripheral arterial disease following supervised training. J Diabetes Complications. 2014;28:219–225. doi: 10.1016/j.jdiacomp.2013.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arida RM, Scorza CA, da Silva AV, Scorza FA. Cavalheiro EA. Differential effects of spontaneous versus forced exercise in rats on the staining of parvalbumin-positive neurons in the hippocampal formation. Neurosci Lett. 2004;364:135–138. doi: 10.1016/j.neulet.2004.03.086. [DOI] [PubMed] [Google Scholar]
- Barker DJ, Winter PD, Osmond C, Margetts B. Simmonds SJ. Weight in infancy and death from ischaemic heart disease. Lancet. 1989;2:577–580. doi: 10.1016/s0140-6736(89)90710-1. [DOI] [PubMed] [Google Scholar]
- Batinić-Haberle I, Cuzzocrea S, Rebouças JS, Ferrer-Sueta G, Mazzon E, Di Paola R, Radi R, Spasojević I, Benov L. Salvemini D. Pure MnTBAP selectively scavenges peroxynitrite over superoxide: comparison of pure and commercial MnTBAP samples to MnTE-2-PyP in two models of oxidative stress injury, an SOD-specific Escherichia coli model and carrageenan-induced pleurisy. Free Radic Biol Med. 2009;46:192–201. doi: 10.1016/j.freeradbiomed.2008.09.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman JS. Koppenol WH. Nitric oxide, superoxide, and peroxynitrite: the good, the bad, and ugly. Am J Physiol Cell Physiol. 1996;271:C1424–C1437. doi: 10.1152/ajpcell.1996.271.5.C1424. [DOI] [PubMed] [Google Scholar]
- Bourque SL, Gragasin FS, Quon AL, Mansour Y, Morton JS. Davidge ST. Prenatal hypoxia causes long-term alterations in vascular endothelin-1 function in aged male, but not female, offspring. Hypertension. 2013;62:753–758. doi: 10.1161/HYPERTENSIONAHA.113.01516. [DOI] [PubMed] [Google Scholar]
- Camm EJ, Hansell JA, Kane AD, Herrera EA, Lewis C, Wong S, Morrell NW. Giussani DA. Partial contributions of developmental hypoxia and undernutrition to prenatal alterations in somatic growth and cardiovascular structure and function. Am J Obstet Gynecol. 2010;203:495.e424–495.e434. doi: 10.1016/j.ajog.2010.06.046. [DOI] [PubMed] [Google Scholar]
- Campos JC, Gomes KM. Ferreira JC. Impact of exercise training on redox signaling in cardiovascular diseases. Food Chem Toxicol. 2013;62:107–119. doi: 10.1016/j.fct.2013.08.035. [DOI] [PubMed] [Google Scholar]
- Class QA, Rickert ME, Lichtenstein P. D'Onofrio BM. Birth weight, physical morbidity, and mortality: A population-based sibling-comparison study. Am J Epidemiol. 2014;179:550–558. doi: 10.1093/aje/kwt304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desai M, Gayle D, Han G. Ross MG. Programmed hyperphagia due to reduced anorexigenic mechanisms in intrauterine growth-restricted offspring. Reprod Sci. 2007;14:329–337. doi: 10.1177/1933719107303983. [DOI] [PubMed] [Google Scholar]
- Fukami T, Sun X, Li T, Desai M. Ross MG. Mechanism of programmed obesity in intrauterine fetal growth restricted offspring: paradoxically enhanced appetite stimulation in fed and fasting states. Reprod Sci. 2012;19:423–430. doi: 10.1177/1933719111424448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garg M, Thamotharan M, Oak SA, Pan G, Maclaren DC, Lee PW. Devaskar SU. Early exercise regimen improves insulin sensitivity in the intrauterine growth-restricted adult female rat offspring. Am J Physiol Endocrinol Metab. 2009;296:E272–E281. doi: 10.1152/ajpendo.90473.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA, Camm EJ, Niu Y, Richter HG, Blanco CE, Gottschalk R, Blake EZ, Horder KA, Thakor AS, Hansell JA, Kane AD, Wooding FB, Cross CM. Herrera EA. Developmental programming of cardiovascular dysfunction by prenatal hypoxia and oxidative stress. PloS One. 2012;7:e31017. doi: 10.1371/journal.pone.0031017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giussani DA. Davidge ST. Developmental programming of cardiovascular disease by prenatal hypoxia. J Dev Orig Health Dis. 2013;4:328–337. doi: 10.1017/S204017441300010X. [DOI] [PubMed] [Google Scholar]
- Green DJ, Maiorana A, O'Driscoll G. Taylor R. Effect of exercise training on endothelium-derived nitric oxide function in humans. J Physiol. 2004;561:1–25. doi: 10.1113/jphysiol.2004.068197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green DJ, Spence A, Halliwill JR, Cable NT. Thijssen DH. Exercise and vascular adaptation in asymptomatic humans. Exp Physiol. 2011;96:57–70. doi: 10.1113/expphysiol.2009.048694. [DOI] [PubMed] [Google Scholar]
- Gündüz F, Koçer G, Ulker S, Meiselman HJ, Başkurt OK. Sentürk UK. Exercise training enhances flow-mediated dilation in spontaneously hypertensive rats. Physiol Res. 2011;60:589–597. doi: 10.33549/physiolres.932166. [DOI] [PubMed] [Google Scholar]
- Hanson M, Godfrey KM, Lillycrop KA, Burdge GC. Gluckman PD. Developmental plasticity and developmental origins of non-communicable disease: theoretical considerations and epigenetic mechanisms. Prog Biophys Mol Biol. 2011;106:272–280. doi: 10.1016/j.pbiomolbio.2010.12.008. [DOI] [PubMed] [Google Scholar]
- Hemmings DG, Williams SJ. Davidge ST. Increased myogenic tone in 7-month-old adult male but not female offspring from rat dams exposed to hypoxia during pregnancy. Am J Physiol Heart Circ Physiol. 2005;289:H674–H682. doi: 10.1152/ajpheart.00191.2005. [DOI] [PubMed] [Google Scholar]
- Herrera EA, Kane AD, Hansell JA, Thakor AS, Allison BJ, Niu Y. Giussani DA. A role for xanthine oxidase in the control of fetal cardiovascular function in late gestation sheep. J Physiol. 2012;590:1825–1837. doi: 10.1113/jphysiol.2011.224576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaquet D, Gaboriau A, Czernichow P. Levy-Marchal C. Insulin resistance early in adulthood in subjects born with intrauterine growth retardation. J Clin Endocrinol Metab. 2000;85:1401–1406. doi: 10.1210/jcem.85.4.6544. [DOI] [PubMed] [Google Scholar]
- Jendzjowsky NG. DeLorey DS. A prospective evaluation of non-interval- and interval-based exercise training progressions in rodents. Appl Physiol Nutr Metab. 2011;36:723–729. doi: 10.1139/h11-092. [DOI] [PubMed] [Google Scholar]
- Jendzjowsky NG. DeLorey DS. Short-term exercise training augments 2-adrenoreceptor-mediated sympathetic vasoconstriction in resting and contracting skeletal muscle. J Physiol. 2013;591:5221–5233. doi: 10.1113/jphysiol.2013.257626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kane AD, Herrera EA, Hansell JA. Giussani DA. Statin treatment depresses the fetal defence to acute hypoxia via increasing nitric oxide bioavailability. J Physiol. 2012;590:323–334. doi: 10.1113/jphysiol.2011.217968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingdom JC. Kaufmann P. Oxygen and placental villous development: origins of fetal hypoxia. Placenta. 1997;18:613–621. doi: 10.1016/s0143-4004(97)90000-x. discussion 623–626. [DOI] [PubMed] [Google Scholar]
- Laker RC, Gallo LA, Wlodek ME, Siebel AL, Wadley GD. McConell GK. Short-term exercise training early in life restores deficits in pancreatic β-cell mass associated with growth restriction in adult male rats. Am J Physiol Endocrinol Metab. 2011;301:E931–E940. doi: 10.1152/ajpendo.00114.2011. [DOI] [PubMed] [Google Scholar]
- Lawn JE, Cousens S. Zupan J. 4 million neonatal deaths: When? Where? Why. Lancet. 2005;365:891–900. doi: 10.1016/S0140-6736(05)71048-5. Lancet Neonatal Survival Steering Team ( [DOI] [PubMed] [Google Scholar]
- Leasure JL. Jones M. Forced and voluntary exercise differentially affect brain and behavior. Neuroscience. 2008;156:456–465. doi: 10.1016/j.neuroscience.2008.07.041. [DOI] [PubMed] [Google Scholar]
- Lesage J, Dufourny L, Laborie C, Bernet F, Blondeau B, Avril I, Breant B. Dupouy JP. Perinatal malnutrition programs sympathoadrenal and hypothalamic-pituitary-adrenal axis responsiveness to restraint stress in adult male rats. J Neuroendocrinol. 2002;14:135–143. doi: 10.1046/j.0007-1331.2001.00753.x. [DOI] [PubMed] [Google Scholar]
- Maeda S, Sugawara J, Yoshizawa M, Otsuki T, Shimojo N, Jesmin S, Ajisaka R, Miyauchi T. Tanaka H. Involvement of endothelin-1 in habitual exercise-induced increase in arterial compliance. Acta Physiol (Oxf) 2009;196:223–229. doi: 10.1111/j.1748-1716.2008.01909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsumoto T, Kakami M, Noguchi E, Kobayashi T. Kamata K. Imbalance between endothelium-derived relaxing and contracting factors in mesenteric arteries from aged OLETF rats, a model of Type 2 diabetes. Am J Physiol Heart Circ Physiol. 2007;293:H1480–H1490. doi: 10.1152/ajpheart.00229.2007. [DOI] [PubMed] [Google Scholar]
- Mazzuca MQ, Tare M, Parkington HC, Dragomir NM, Parry LJ. Wlodek ME. Uteroplacental insufficiency programmes vascular dysfunction in non-pregnant rats: compensatory adaptations in pregnancy. J Physiol. 2012;590:3375–3388. doi: 10.1113/jphysiol.2012.230011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzuca MQ, Wlodek ME, Dragomir NM, Parkington HC. Tare M. Uteroplacental insufficiency programs regional vascular dysfunction and alters arterial stiffness in female offspring. J Physiol. 2010;588:1997–2010. doi: 10.1113/jphysiol.2010.187849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milkau M, Kohler R. de Wit C. Crucial importance of the endothelial K+ channel SK3 and connexin40 in arteriolar dilations during skeletal muscle contraction. FASEB J. 2010;24:3572–3579. doi: 10.1096/fj.10-158956. [DOI] [PubMed] [Google Scholar]
- Minami A, Ishimura N, Harada N, Sakamoto S, Niwa Y. Nakaya Y. Exercise training improves acetylcholine-induced endothelium-dependent hyperpolarization in type 2 diabetic rats, Otsuka Long-Evans Tokushima fatty rats. Atherosclerosis. 2002;162:85–92. doi: 10.1016/s0021-9150(01)00685-2. [DOI] [PubMed] [Google Scholar]
- Mokelke EA, Hu Q, Song M, Toro L, Reddy HK. Sturek M. Altered functional coupling of coronary K+ channels in diabetic dyslipidemic pigs is prevented by exercise. J Appl Physiol (1985) 2003;95:1179–1193. doi: 10.1152/japplphysiol.00972.2002. [DOI] [PubMed] [Google Scholar]
- Morton JS, Rueda-Clausen CF. Davidge ST. Mechanisms of endothelium-dependent vasodilation in male and female, young and aged offspring born growth restricted. Am J Physiol Regul Integr Comp Physiol. 2010;298:R930–R938. doi: 10.1152/ajpregu.00641.2009. [DOI] [PubMed] [Google Scholar]
- Morton JS, Rueda-Clausen CF. Davidge ST. Flow-mediated vasodilation is impaired in adult rat offspring exposed to prenatal hypoxia. J Appl Physiol (1985) 2011;110:1073–1082. doi: 10.1152/japplphysiol.01174.2010. [DOI] [PubMed] [Google Scholar]
- Olusanya BO. Intrauterine growth restriction in a low-income country: Risk factors, adverse perinatal outcomes and correlation with current WHO Multicenter Growth Reference. Early Hum Dev. 2010;86:439–444. doi: 10.1016/j.earlhumdev.2010.05.023. [DOI] [PubMed] [Google Scholar]
- Ozaki T, Nishina H, Hanson MA. Poston L. Dietary restriction in pregnant rats causes gender-related hypertension and vascular dysfunction in offspring. J Physiol. 2001;530:141–152. doi: 10.1111/j.1469-7793.2001.0141m.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Payne JA, Alexander BT. Khalil RA. Reduced endothelial vascular relaxation in growth-restricted offspring of pregnant rats with reduced uterine perfusion. Hypertension. 2003;42:768–774. doi: 10.1161/01.HYP.0000084990.88147.0C. [DOI] [PubMed] [Google Scholar]
- Powers SK, Quindry JC. Kavazis AN. Exercise-induced cardioprotection against myocardial ischemia-reperfusion injury. Free Radic Biol Med. 2008;44:193–201. doi: 10.1016/j.freeradbiomed.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Rueda-Clausen CF, Dolinsky VW, Morton JS, Proctor SD, Dyck JR. Davidge ST. Hypoxia-induced intrauterine growth restriction increases the susceptibility of rats to high-fat diet-induced metabolic syndrome. Diabetes. 2011;60:507–516. doi: 10.2337/db10-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rueda-Clausen CF, Morton JS. Davidge ST. Effects of hypoxia-induced intrauterine growth restriction on cardiopulmonary structure and function during adulthood. Cardiovasc Res. 2009;81:713–722. doi: 10.1093/cvr/cvn341. [DOI] [PubMed] [Google Scholar]
- Rush JW, Turk JR. Laughlin MH. Exercise training regulates SOD-1 and oxidative stress in porcine aortic endothelium. Am J Physiol Heart Circ Physiol. 2003;284:H1378–H1387. doi: 10.1152/ajpheart.00190.2002. [DOI] [PubMed] [Google Scholar]
- Santos MS. Joles JA. Early determinants of cardiovascular disease. Best Pract Res Clin Endocrinol Metab. 2012;26:581–597. doi: 10.1016/j.beem.2012.03.003. [DOI] [PubMed] [Google Scholar]
- Sessa WC, Pritchard K, Seyedi N, Wang J. Hintze TH. Chronic exercise in dogs increases coronary vascular nitric oxide production and endothelial cell nitric oxide synthase gene expression. Circ Res. 1994;74:349–353. doi: 10.1161/01.res.74.2.349. [DOI] [PubMed] [Google Scholar]
- Stewart KG, Zhang Y. Davidge ST. Aging increases PGHS-2-dependent vasoconstriction in rat mesenteric arteries. Hypertension. 2000;35:1242–1247. doi: 10.1161/01.hyp.35.6.1242. [DOI] [PubMed] [Google Scholar]
- Thakor AS, Herrera EA, Serón-Ferré M. Giussani DA. Melatonin and vitamin C increase umbilical blood flow via nitric oxide-dependent mechanisms. J Pineal Res. 2010;49:399–406. doi: 10.1111/j.1600-079X.2010.00813.x. [DOI] [PubMed] [Google Scholar]
- Vanhoutte PM, Feletou M. Taddei S. Endothelium-dependent contractions in hypertension. Br J Pharmacol. 2005;144:449–458. doi: 10.1038/sj.bjp.0706042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vega CC, Reyes-Castro LA, Bautista CJ, Larrea F, Nathanielsz PW. Zambrano E. Exercise in obese female rats has beneficial effects on maternal and male and female offspring metabolism. Int J Obes (Lond) 2013 doi: 10.1038/ijo.2013.150. (in press; DOI: 10.1038/ijo.2013.150 ) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang J, Wolin MS. Hintze TH. Chronic exercise enhances endothelium-mediated dilation of epicardial coronary artery in conscious dogs. Circ Res. 1993;73:829–838. doi: 10.1161/01.res.73.5.829. [DOI] [PubMed] [Google Scholar]
- Wang X, Lin G, Liu C, Feng C, Zhou H, Wang T, Li D, Wu G. Wang J. Temporal proteomic analysis reveals defects in small-intestinal development of porcine fetuses with intrauterine growth restriction. J Nutr Biochem. 2014;25:785–795. doi: 10.1016/j.jnutbio.2014.03.008. [DOI] [PubMed] [Google Scholar]
- Williams SJ, Campbell ME, McMillen IC. Davidge ST. Differential effects of maternal hypoxia or nutrient restriction on carotid and femoral vascular function in neonatal rats. Am J Physiol Regul Integr Comp Physiol. 2005a;288:R360–R367. doi: 10.1152/ajpregu.00178.2004. [DOI] [PubMed] [Google Scholar]
- Williams SJ, Hemmings DG, Mitchell JM, McMillen IC. Davidge ST. Effects of maternal hypoxia or nutrient restriction during pregnancy on endothelial function in adult male rat offspring. J Physiol. 2005b;565:125–135. doi: 10.1113/jphysiol.2005.084889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu G, Imhoff-Kunsch B. Girard AW. Biological mechanisms for nutritional regulation of maternal health and fetal development. Paediatr Perinat Epidemiol. 2012;26(Suppl. 1):4–26. doi: 10.1111/j.1365-3016.2012.01291.x. [DOI] [PubMed] [Google Scholar]
- Xu Y, Williams SJ, O'Brien D. Davidge ST. Hypoxia or nutrient restriction during pregnancy in rats leads to progressive cardiac remodeling and impairs postischemic recovery in adult male offspring. FASEB J. 2006;20:1251–1253. doi: 10.1096/fj.05-4917fje. [DOI] [PubMed] [Google Scholar]
- Yen MH, Yang JH, Sheu JR, Lee YM. Ding YA. Chronic exercise enhances endothelium-mediated dilation in spontaneously hypertensive rats. Life Sci. 1995;57:2205–2213. doi: 10.1016/0024-3205(95)02127-5. [DOI] [PubMed] [Google Scholar]
- Zguira MS, Vincent S, Le Douairon Lahaye S, Malarde L, Tabka Z. Saïag B. Intense exercise training is not effective to restore the endothelial NO-dependent relaxation in STZ-diabetic rat aorta. Cardiovasc Diabetol. 2013;12:32. doi: 10.1186/1475-2840-12-32. [DOI] [PMC free article] [PubMed] [Google Scholar]


