Abstract
Advancing age as well as diseases such as diabetes are characterized by both increased large artery stiffness and impaired peripheral artery function. It has been hypothesized that greater large artery stiffness causes peripheral artery dysfunction; however, a cause-and-effect relationship has not previously been established. We used elastin heterozygote mice (Eln+/–) as a model of increased large artery stiffness without co-morbidities unrelated to the large artery properties. Aortic stiffness, measured by pulse wave velocity, was ∼35% greater in Eln+/– mice than in wild-type (Eln+/+) mice (P = 0.04). Endothelium-dependent dilatation (EDD), assessed by the maximal dilatation to acetylcholine, was ∼40% lower in Eln+/– than Eln+/+ mice in the middle cerebral artery (MCA, P < 0.001), but was similar between groups in the gastrocnemius feed arteries (GFA, P = 0.79). In the MCA, EDD did not differ between groups after incubation with the nitric oxide (NO) synthase inhibitor Nω-nitro-l-arginine methyl ester (P > 0.05), indicating that lower NO bioavailability contributed to the impaired EDD in Eln+/– mice. Superoxide production and content of the oxidative stress marker nitrotyrosine was higher in MCAs from Eln+/− compared with Eln+/+ mice (P < 0.05). In the MCA, after incubation with the superoxide scavenger TEMPOL, maximal EDD improved by ∼65% in Eln+/– (P = 0.002), but was unchanged in Eln+/+ mice (P = 0.17). These results indicate that greater large artery stiffness has a more profound effect on endothelial function in cerebral arteries compared with skeletal muscle feed arteries. Greater large artery stiffness can cause cerebral artery endothelial dysfunction by reducing NO bioavailability and increasing oxidative stress.
Key points
Increased large artery stiffness is a hallmark of arterial dysfunction with advancing age and is also present in other disease conditions such as diabetes. Increased large artery stiffness is correlated with resistance artery dysfunction in humans.
Using a mouse model of altered arterial elastin content, this is the first study to examine the cause-and-effect relationship between large artery stiffness and peripheral resistance artery function.
Our results indicate that mice with genetically greater large artery stiffness have impaired cerebral artery endothelial function, but generally preserved skeletal muscle feed artery endothelial function. The mechanisms for impaired cerebral artery endothelial function are reduced nitric oxide bioavailability and increased oxidative stress.
These findings suggest that interventions that target large artery stiffness may be important to reduce disease risk associated with cerebral artery dysfunction in conditions such as advancing age.
Introduction
Resistance artery dysfunction is a common feature of advancing age, as well as other diseases such as diabetes (De Vriese et al. 2000; Lakatta & Levy, 2003). A key feature of this dysfunction is impaired endothelial function, characterized by reduced endothelium-dependent dilatation (EDD), as occurs with ageing in cerebral arteries and skeletal muscle feed arteries (Woodman et al. 2002; Modrick et al. 2009; Walker et al. 2014). However, the mechanisms contributing to impaired EDD in resistance arteries are not completely understood.
It has been hypothesized that peripheral artery dysfunction can occur secondary to increased large artery stiffness (O'Rourke & Safar, 2005; Mitchell, 2008). With healthy ageing or other chronic disease states, there is a progressive stiffening of the large elastic arteries, attributed to an increased collagen content, reduced elastin content, increased collagen cross-linking and increased vascular tone (Lakatta & Levy, 2003). A stiffer aorta and carotid arteries can result in less pulse dampening, leading to greater pulsatility in peripheral resistance arteries that may induce arterial dysfunction and tissue impairment (O'Rourke & Safar, 2005; Mitchell, 2008). Indeed, greater aortic stiffness, as measured by pulse wave velocity, is related to elevated stroke risk, greater leukoaraiosis following stroke and lower scores on tests of memory function (Mattace-Raso et al. 2006; Mitchell et al. 2011; Webb et al. 2012), as well as impaired resistance artery function in the forearm (Mitchell et al. 2005). However, due to the difficulty of modulating large artery stiffness in vivo in the absence of disease, the cause-and-effect nature of this hypothesis has yet to be investigated. To study the effects of large artery stiffness in isolation from ageing or other disease phenotypes (e.g. diabetes), we used mice with an altered elastin content (elastin heterozygote, Eln+/–) that have greater aortic stiffness without other co-morbidities unrelated to the large artery properties (Wagenseil et al. 2005; Pezet et al. 2008).
The negative consequences of increased pulsatility in resistance arteries are probably due to increased uniaxial circumferential stress on the vascular tissue (Anwar et al. 2012). Increased circumferential stress in cultured endothelial cells leads to increased reactive oxygen species production (Cheng et al. 1998). In arteries, increased reactive oxygen species can impair EDD by reducing the bioavailability of the key vasodilator nitric oxide (NO), through the reaction of superoxide with NO to produce peroxynitrite (Le Brocq et al. 2008). However, whether increased large artery stiffness leads to increased resistance artery oxidative stress or a reduced contribution of NO to EDD is unknown.
Using Eln+/– mice as a model of greater large artery stiffness without co-morbidity except those related to the large artery effects, we hypothesized that Eln+/– mice would have impaired EDD in cerebral (middle cerebral artery, MCA) and skeletal muscle (gastrocnemius feed artery, GFA) resistance arteries compared with their wild-type littermates (Eln+/+). We further hypothesized that impaired MCA and GFA EDD would result from less NO-mediated dilatation, but no differences in prostaglandin-mediated dilatation. In addition, we hypothesized that reductions in NO-mediated dilatation would be associated with increased arterial oxidative stress and superoxide-mediated suppression of EDD.
Methods
Animals
Young male mice with a heterozygote deletion of exon 1 of the elastin gene (Eln+/–) and their wild-type littermates (Eln+/+) housed at the University of Utah were used for studies (Li et al. 1998). All mice were 5–8 months of age at the time of study. Mice were killed by exsanguination under inhaled isoflurane. An additional set of mice (12 male, one female) 5–6 months of age housed at Washington University were used for catheter measurement of blood pressure. All mice were housed in an animal care facility on a 12/12 h light–dark cycle at 24°C. All animal procedures conformed to the Guide to the Care and Use of Laboratory Animals (version 8, revised 2011) and were approved by the Institutional Animal Care and Use Committees at the University of Utah and Washington University.
Arterial blood pressure and stiffness
Systolic blood pressure was assessed non-invasively in the conscious state by determining the tail blood volume with a volume pressure recording sensor and an occlusion tail-cuff (CODA System, Kent Scientific, Torrington, CT, USA) as previously described in detail (Feng et al. 2008; Donato et al. 2013). Blood pressure and heart rate recordings were made in a quiet and warm (room temperature ∼24°C) environment. Mice were placed in restrainers on a heating unit and given 15–20 min to acclimatize and reach a steady tail skin temperature of 30–35°C. Each session consisted of 5–10 acclimatization measures and documentation of stable values, followed by 20 experimental measures. Measures with aberrant movement/behaviour or inadequate tail volume/flow values were excluded and remaining values were used to calculate mean values for each animal (Feng et al. 2008; Donato et al. 2013).
Systolic blood pressure, diastolic blood pressure and pulse pressure were measured via a catheter (Millar Instruments, Houston, TX, USA) as previously described (Wagenseil et al. 2005). Briefly, the catheter was inserted into the right common carotid artery of anaesthetized mice (i.p. injection of 100 mg ml−1 ketamine and 20 mg ml−1 xylazine) and pressures were recorded for 15 min.
Aortic pulse wave velocity (PWV) was measured as described previously (Reddy et al. 2005; Donato et al. 2013; Henson et al. 2014). Briefly, anaesthetized (2% inhaled isoflurane) mice were secured in a supine position on a heated board (approximately 35°C) to maintain body temperature. Velocities were measured with 20 MHz Doppler probes (Indus Instruments, Webster, TX, USA) at the transverse aortic arch and approximately 4 cm distal at the abdominal aorta simultaneously and collected using WinDAQ Pro + software (DataQ Instruments, Akron, OH, USA). After velocities were collected, a precise measure of the distance between the probes was obtained using a scientific caliper and recorded. Absolute pulse arrival times were indicated by the sharp upstroke, or foot, of each velocity waveform analysed with WinDAQ Waveform Browser (DataQ Instruments). Aortic PWV was then calculated as the quotient of the separation distance and difference in absolute arrival times.
Ex vivo artery stiffness of the MCA and GFA was assessed by the passive response to increasing pressure in isolated arteries (Lesniewski et al. 2009). Briefly, MCAs and GFAs were incubated in Ca2+-free physiological saline solution (PSS) for 1 h. Lumen diameter and medial wall thickness were measured in response to increases in intraluminal pressure from 5 to 100 cmH2O (3.7–73.5 mmHg) in 5 cmH2O increments.
Stress was calculated as:
where P is pressure in dyne cm–2, D is lumen diameter and WT is wall thickness.
Strain was calculated as:
where Di is the initial starting diameter.
Data for each artery were fit to the curve:
where σi is the initial starting stress (5 cmH2O) and β is the slope of tangential elastic modulus versus stress. A higher β represents a stiffer artery (Baumbach & Hajdu, 1993; Dunn & Gardiner, 1997; Izzard et al. 2006).
Glucose tolerance test (GTT)
Glucose tolerance was assessed by an intraperitoneal GTT as described previously (Donato et al. 2012). Briefly, mice were fasted for 2 h in the morning before baseline blood glucose was measured using a Precision Xceed Pro Glucose Analyser in whole blood collected via a tail nick (∼5 μl). Glucose (2000 mg kg−1) was administered by intraperitoneal injection, and blood glucose was monitored at 15, 30, 45, 60 and 90 min after the injection.
Histology and immunofluorescence
Thoracic aortas, GFAs and MCAs were saved in optimal cutting temperature (OCT) solution, frozen and sliced into 8 μm sections. Each mouse aorta, GFA or MCA had 3–4 sections per slide, which were averaged. Slides were batched to have equal numbers of each group. Elastin images were captured by the autofluorescence in the FIT-C channel on slides mounted with Prolong Gold with DAPI (Life Technologies, Grand Island, NY, USA) using a Zeiss Axio Imager AX10 and ZEN 2011 software (Briones et al. 2003; Gonzalez et al. 2005). Elastin content was analysed by the mean grey intensity over the arterial wall using Image J software (NIH, Bethesda, MD, USA). In addition, GFA and MCA sections were stained for elastin via Verhoeff–Van Gieson stain and aorta sections were stained for collagen via Picrosirius Red as previously described (Donato et al. 2013; Henson et al. 2014). For measurement of superoxide, MCA sections were rehydrated in PBS, and then incubated at 37°C for 30 min with 2 μm dihydroethidium (DHE) solution in a dark humid chamber. Slides were rinsed and mounted with Prolong Gold with DAPI. For nitrotyrosine quantification, MCA sections were fixed, incubated with primary antibody for nitrotyrosine (Millipore, Billerica, MA, USA) and Alexa Fluor 647 (Life Technologies) secondary antibody, and were mounted with Prolong Gold with DAPI. One section on each slide did not receive primary antibody (no primary control). For DHE and nitrotyrosine, arteries were imaged with an Olympus Fluoview FV1000 (Tokyo, Japan) confocal microscope. To limit inclusion of perivascular DHE or nitrotyrosine content, a projection of the Z slices only containing elastic lamina was used for analysis. DHE and nitrotyrosine contents were analysed as the mean grey intensity within the artery wall using ImageJ software. For nitrotyrosine, to account for background fluorescence, values were calculated as: (average intensity for that mouse) – (intensity of the no primary control).
EDD: effect of NO, prostaglandins and oxidative stress
Measurements of EDD and endothelial-independent dilatation (EID) in isolated MCAs and GFAs studied ex vivo were performed using a method previously described in detail (Donato et al. 2009, 2011; Lesniewski et al. 2009). Briefly, mice were killed by exsanguination via cardiac puncture while under isoflurane anaesthesia. GFAs and MCAs were excised and placed in a myograph chamber (DMT Inc., Aarhus, Denmark) with PSS that contained 145.0 mm NaCl, 4.7 mm KCl, 2.0 mm CaCl2, 1.17 mm MgSO4, 1.2 mm NaH2PO4, 5.0 mm glucose, 2.0 mm pyruvate, 0.02 mm EDTA, 3.0 mm MOPS buffer and 1 g/100 ml BSA, pH 7.4 at 37°C, cannulated onto glass micropipettes and secured with nylon (11-0) suture. Once cannulated, arteries were warmed to 37°C, pressurized and allowed to equilibrate for ∼1 h. All arteries were submaximally preconstricted with phenylephrine (2–6 μm to obtain 20–40% preconstriction), and increases in luminal diameter in response to increasing concentrations of the endothelium-dependent dilator, acetylcholine (ACh: 1×10−9 to 1×10−4 m in MCA, 1×10−10 to 1×10−4 m in GFA), and endothelium-independent dilator, sodium nitroprusside (SNP: 1×10−10 to 1×10−4 m), were determined. Responses to ACh were repeated in the presence of the NO synthase inhibitor Nω-nitro-l-arginine methyl ester (l-NAME: 0.1 mm, 30 min incubation) to determine the contribution of NO to dilatation and in the presence of the cyclooxygenase inhibitor indomethacin (0.1 mm, 60 min incubation) to determine the contribution of prostaglandins to dilatation. To determine the superoxide- (oxidative stress) mediated suppression of EDD, responses to ACh were measured following a 60 min incubation in the presence of the superoxide scavenger TEMPOL (1 mm) (Zhang et al. 2003; Qamirani et al. 2005; Didion et al. 2006) in different MCA segments from those initially incubated with l-NAME and indomethacin. Measurements of ACh responses were repeated in TEMPOL-treated arteries after addition of l-NAME.
Gene expression
mRNA expression for superoxide dismutase (SOD) isoforms was measured in lysed MCAs by quantitative RT-PCR using the Quantitect Reverse Transcription kit (Qiagen, Valencia, CA, USA) and FastStart SYBR Green Master Mix (Roche Diagnostics, Roche Applied Science, Indianapolis, IN, USA) according to the manufacturers’ protocols. Fold change in mRNA expression was calculated as the fold difference in expression of target mRNA to 18S rRNA for each animal (2−(target CT – 18S CT); 18 s rRNA QuantiTect Primer Assay; Qiagen). 18S rRNA was used as a housekeeping gene transcript to control for tissue concentration in samples. Sod1 mRNA primers were: forward, AACCAGTTGTGTTGTCAGGAC; reverse, CCACCATG-TTTCTTAGAGTGAGG. Sod2 mRNA primers were: fortward, CAGACCTGCCTTACGACTATGG; reverse, CTCGGTGGCGTTGAGATTGTT. Sod3 mRNA primers were: forward, CCTTCTTGTTCTACGGCTTGC; reverse, TCGCCTATCTTCTCAACCAGG.
Statistics
For animal and artery characteristics, group differences were determined by t-test for independent samples. Histology, immunofluorescence and gene expression measures were normalized to the mean of the Eln+/+ group. For all dose responses, group differences were determined by repeated-measures ANOVA. A least significant difference (LSD) post hoc test was used for preplanned comparisons where appropriate. EC50 values were calculated by fitting each dose response to a four-parameter logistic equation. EC50 values for the MCA response to ACh in the presence of l-NAME were not calculated as these responses do not fit a logistic equation. Data are presented as mean ± SEM. Significance was set at P < 0.05.
Results
Animal characteristics
Eln+/– and Eln+/+ mice did not differ in age (6.6 ± 0.3 and 6.7 ± 0.3 months, P = 0.83), body mass (32.1 ± 0.8 and 30.7 ± 0.8 g, P = 0.27), heart rate (662 ± 11 and 656 ± 17 b.p.m., P = 0.73,) fasting glucose (153 ± 5 and 161 ± 5 mg dl−1, P = 0.33) or glucose tolerance (area under the curve (AUC) during glucose tolerance test, 19,654 ± 1359 vs. 20,307 ± 904 mg dl−1, P = 0.70). In tail cuff measurements in conscious mice, Eln+/– mice had greater systolic blood pressure compared with Eln+/+ mice (134 ± 4 vs. 112 ± 5 mmHg, P = 0.001). In a cohort of mice from Washington University, intracarotid measurement in anaesthetized mice indicated greater systolic blood pressure (158 ± 5 vs. 121 ± 6 mmHg, P = 0.001), diastolic blood pressure (102 ± 7 vs. 84 ± 5 mmHg, P = 0.04) and pulse pressure (56 ± 9 vs. 36 ± 3 mmHg, P = 0.03) in Eln+/– compared with Eln+/+ mice (n = 6–7 per group).
Large artery properties
Eln+/– mice had ∼35% higher aortic PWV, indicating greater large artery stiffness, compared with Eln+/+ mice (P = 0.04, Fig.1A). Heart rates during PWV testing were not significantly different between groups (Eln+/–: 400 ± 9 b.p.m. vs. Eln+/+: 422 ± 11 b.p.m., P = 0.12). In the aorta, elastin content was lower in Eln+/– compared with Eln+/+ mice (P = 0.008, Fig.1D), but collagen content was similar between groups (Eln+/–: 0.92 ± 0.15 AU vs. Eln+/+: 1.00 ± 0.12 AU, P = 0.70).
Figure 1. Artery stiffness and elastin content.

Stiffness of the aorta (A), measured by in vivo pulse wave velocity, and of the middle cerebral artery (MCA, B) and gastrocnemius feed artery (GFA, C) assessed by the passive stress strain relationship calculated from pressure and diameter (n = 9–14 per group). Elastin content was measured by autofluorescence for the thoracic aorta (D), MCA (E) and GFA (F) (n = 5–12 per group). For representative images shown below, green: elastin (autofluorescence), blue: nuclei (DAPI). Scale bars: 100 μm for aorta, 10 μm for MCA and GFA. *P < 0.05 vs. Eln+/+. Values are mean ± SEM.
Cerebral and skeletal muscle feed artery properties
Elastin content in the MCA and GFA was similar between Eln+/+ and Eln+/– mice as measured by autofluorescence (all P > 0.05, Fig.1E, F) and Verhoeff–Van Gieson stain (all P > 0.05, data not shown). Autofluorescence images and Verhoeff–Van Gieson staining show only one elastic lamina for the MCA and GFA in all mice. In addition, maximal diameter and wall thickness of pressurized MCAs and GFAs were similar between groups (P > 0.05, Table1). The passive stiffness of the MCA did not differ between groups, as indicated by no differences in the pressure–diameter curves (P > 0.05, Fig.1B) or β (stiffness parameter, P = 0.57, Table1). However, stiffness of the GFA was greater in Eln+/– compared with Eln+/+ mice, as indicated by a larger β (P < 0.01, Table1) and group differences in the pressure–diameter curve (P < 0.05, Fig.1C).
Table 1.
Middle cerebral artery and gastrocnemius feed artery characteristics
| MCA | GFA | |||
|---|---|---|---|---|
| Eln+/+ | Eln+/– | Eln+/+ | Eln+/– | |
| Maximal diameter (μm) | 158 ± 4 | 160 ± 5 | 207 ± 4 | 203 ± 4 |
| Wall thickness (μm) | 10 ± 1 | 10 ± 1 | 18 ± 2 | 17 ± 1 |
| Intrinsic stiffness (β) | 10.4 ± 0.9 | 9.6 ± 0.9 | 7.8 ± 0.5 | 9.6 ± 0.5* |
| EC50 (log m) | ||||
| ACh | −7.6 ± 0.3 | −7.5 ± 0.2 | −8.2 ± 0.3 | −7.3 ± 0.4* |
| ACh + l-NAME | — | — | −6.9 ± 0.5 | −7.8 ± 0.5 |
| ACh + l-NAME + INDO | — | — | −6.8 ± 0.5 | −7.3 ± 0.2 |
| ACh + TEMPOL | −6.8 ± 0.4 | −6.8 ± 0.4 | — | — |
| SNP | −6.3 ± 0.4 | −6.2 ± 0.4 | − 8.3 ± 0.3 | −7.6 ± 0.1* |
| Preconstriction (%) | ||||
| ACh | 29 ± 2 | 28 ± 2 | 35 ± 3 | 30 ± 3 |
| ACh + l-NAME | 37 ± 6 | 33 ± 5 | 36 ± 3 | 38 ± 4 |
| ACh + l-NAME + INDO | 38 ± 5 | 45 ± 5 | 45 ± 5 | 46 ± 5 |
| ACh + TEMPOL | 29 ± 4 | 28 ± 3 | — | — |
| ACh + TEMPOL + l-NAME | 34 ± 7 | 36 ± 4 | — | — |
| SNP | 39 ± 4 | 42 ± 5 | 44 ± 6 | 48 ± 7 |
Values are mean ± SEM. MCA, middle cerebral artery; GFA, gastrocnemius feed artery; ACh, acetylcholine; l-NAME, NG-nitro-l-arginine methyl ester; INDO, indomethacin; SNP, sodium nitroprusside. *P < 0.05 vs. Eln+/+ within artery.
MCA endothelium-dependent dilatation: contribution of NO and prostaglandins
In the MCA, the maximal response to ACh was ∼40% lower in Eln+/– compared with Eln+/+ mice (max.: P < 0.001; response: P = 0.02, Fig.2A, B), but there were no differences in sensitivity to ACh between groups (EC50, P = 0.86, Table1). Incubation with l-NAME decreased the response to ACh in both Eln+/– and Eln+/+ MCAs (all P < 0.05) and ACh responses were not different between groups in the presence of l-NAME (max.: P = 0.92, response: P = 0.72, Fig.2A, B). In MCAs from both groups, incubation with indomethacin and l-NAME together led to a ∼150% increase in ACh-induced dilatation compared with l-NAME alone (all P < 0.05) and these responses were not different between groups (max.: P = 0.53, response: P = 0.26, Fig.2B). In the MCA, the dose–response and sensitivity to SNP did not differ between groups (max.: P = 0.80, response: P = 0.99, EC50: P = 0.87, Fig.2C, Table1). There was no difference in MCA preconstriction between groups for any condition (Table1).
Figure 2. Middle cerebral artery (MCA) endothelium-dependent and -independent dilation.
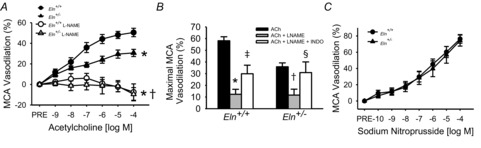
A, MCA endothelium-dependent dilatation to ACh in the absence or presence of l-NAME measured by pressure myography in Eln+/+ and Eln+/– mice (n = 16 per group, significance marks denote a time by group interaction or main effect for group from repeated-measures ANOVAs). B, maximal MCA dilatation to ACh in the absence and presence of l-NAME alone and in combination with the cyclooxygenase inhibitor indomethacin (INDO) in Eln+/+ and Eln+/– mice (n = 6–7 per group). C, MCA endothelium-independent dilatation to sodium nitroprusside in Eln+/+ and Eln+/– mice (n = 10–12 per group). *P < 0.05 vs. Eln+/+ ACh alone, †P < 0.05 vs. Eln+/– ACh alone, ‡P < 0.05 vs. Eln+/+ ACh with l-NAME, §P < 0.05 vs. Eln+/– ACh with l-NAME. Values are mean ± SEM.
GFA endothelium-dependent dilatation: contribution of NO and prostaglandins
In the GFA, the maximal dilatation and dose–response to ACh did not differ between Eln+/+ and Eln+/– mice (max.: P = 0.79, response: P = 0.33, Fig.3A). However, the GFAs from Eln+/– mice were less sensitive to ACh than GFAs from Eln+/+ mice (EC50, P = 0.03, Table1). l-NAME pre-incubation reduced maximal ACh dilatation by ∼35% in GFAs from Eln+/– and by ∼25% in GFAs from Eln+/+ (all P < 0.05, Fig.3A, B). In Eln+/– GFAs, the ACh response during incubation with indomethacin and l-NAME together was not different from incubation with l-NAME alone (max.: P = 0.60, response: P = 0.57, Fig.3B). In contrast, in Eln+/+ GFAs, incubation with indomethacin and l-NAME together reduced ACh response compared with l-NAME alone (max.: P = 0.01, response: P = 0.007, Fig.3B). Sensitivity to ACh did not differ between groups during incubation with l-NAME alone or with indomethacin (P > 0.05, Table1). In the GFA, the dose–response and maximal response to SNP did not differ between groups (max.: P = 0.83, response: P = 0.22, Fig.3C), but Eln+/– GFAs were less sensitive to SNP compared with Eln+/+ GFAs (P = 0.05, Table1). There was no difference in GFA preconstriction between groups for any condition (Table1).
Figure 3. Gastrocnemius feed artery (GFA) endothelium-dependent and -independent dilation).

A, GFA endothelium-dependent dilatation to ACh in the absence or presence of l-NAME measured by pressure myography in Eln+/+ and Eln+/– mice (n = 12–14 per group, significance marks denote a time by group interaction or main effect for group from repeated-measures ANOVAs). B, maximal GFA dilatation to ACh in the absence and presence of l-NAME alone and in combination with the cyclooxygenase inhibitor indomethacin (INDO) in Eln+/+ and Eln+/– mice (n = 10 per group). C, GFA endothelium-independent dilatation to sodium nitroprusside in Eln+/+ and Eln+/– mice (n = 10–12 per group). *P < 0.05 vs. Eln+/+ ACh alone, †P < 0.05 vs. Eln+/– ACh alone, ‡P < 0.05 vs. Eln+/+ ACh with l-NAME. Values are mean ± SEM.
Oxidative stress
DHE fluorescence intensity, a marker of superoxide, was 36% higher (P = 0.05, Fig.4A) and nitrotyrosine, a marker of protein nitration as by peroxynitrite, was 41% higher (P = 0.04, Fig.4B) in MCAs from Eln+/– compared with Eln+/+ mice. Incubation with the superoxide scavenger TEMPOL improved ACh response by 64% in Eln+/– MCAs (max.: P = 0.002, response: P = 0.008), but did not affect Eln+/+ MCAs (max.: P = 0.17, response: P = 0.17, Fig.5A). Incubation with l-NAME in the presence of TEMPOL reduced ACh-mediated dilatation for both groups (all P < 0.05) and the ACh responses in the presence of TEMPOL and l-NAME together did not differ between groups (max.: P = 0.97, response: P = 0.65, Fig.5B). MCA gene expression did not differ between Eln+/– and Eln+/+ mice for Sod1 (1.05 ± 0.14 vs. 1.00 ± 0.12 AU, P = 0.39), Sod2 (0.94 ± 0.10 vs. 1.00 ± 0.13 AU, P = 0.36) or Sod3 (1.00 ± 0.13 vs. 1.00 ± 0.14 AU, P = 0.49) (all n = 10–11 per group).
Figure 4. Middle cerebral artery (MCA) oxidative stress.

MCA DHE fluorescence intensity (A, n = 5 per group) and nitrotyrosine content determined by immunofluorescence (B, n = 6–9 per group) in Eln+/+ and Eln+/– mice. *P < 0.05 vs. Eln+/+. For the representative image shown to the right, red: DHE or nitrotyrosine; green: elastin (autofluorescence); blue: nuclei (DAPI).
Figure 5. Effect of oxidative stress on middle cerebral artery (MCA) dilation.

A, MCA dilatation to ACh in the absence or presence of superoxide scavenger TEMPOL measured by pressure myography in Eln+/+ and Eln+/– mice. B, MCA maximal dilatation to ACh in the absence of presence of TEMPOL alone or in combination with l-NAME (n = 5–16 per group, significance marks denote a time by condition interaction or main effect for condition from repeated-measures ANOVAs). *P < 0.05 vs. Eln+/+ ACh alone, †P < 0.05 vs. Eln+/– ACh alone, ‡P < 0.05 vs. Eln+/+ ACh with TEMPOL, §P < 0.05 vs. Eln+/– ACh with TEMPOL. Values are mean ± SEM.
Discussion
In the present study, we used Eln+/– mice as a model of increased large artery stiffening without co-morbidity except those resulting from the large artery effects. Indeed, we confirmed that these mice have greater large artery stiffness and demonstrated that they do not have altered stiffness in cerebral arteries. We found that Eln+/– mice have impaired cerebral artery EDD resulting from superoxide-dependent reduction in NO bioavailability. However, despite slightly stiffer skeletal muscle feed arteries in Eln+/–, we found no difference in maximal EDD between groups. Thus, it appears that cerebral artery endothelial function is impaired with increased large artery stiffening, while skeletal muscle feed arteries may be largely protected from the negative consequences of large artery stiffening.
Elastin deletion as a model of large artery stiffness
The elastin (Eln) loss of function mutation was originally created as a model of supravalvular aortic stenosis, which results from functional haploinsufficiency of the elastin gene in humans (Li et al. 1998). Complete deletion of the elastin gene (Eln−/−) results in mice that do not survive more than 5 days after birth (Li et al. 1998). However, mice with one elastin gene (Eln+/–) are viable and were previously demonstrated to have altered large artery mechanical properties (Faury et al. 2003; Wagenseil et al. 2005; Pezet et al. 2008). In the present study, we found that Eln+/– mice have a decreased aortic elastin content without changes to aortic collagen content, as seen previously (Li et al. 1998; Faury et al. 2003). In the aorta, Eln+/– mice also have additional elastic laminae present and greater elastin fragmentation (Faury et al. 2003; Pezet et al. 2008). We found increased aortic stiffness, as indicated by greater PWV, in the Eln+/– compared with Eln+/+ mice. This indicates greater in vivo stiffness of the aorta in this model and is similar to ex vivo studies that found greater aortic incremental stiffness (elastic modulus) in aortas from Eln+/– mice (Faury et al. 2003). These features of the aorta in Eln+/– mice, specifically greater stiffness, decreased elastin content and increased elastin fragmentation, are similar to the changes in the aorta seen with advancing age (Pezet et al. 2008; Donato et al. 2013), a conclusion made by Pezet et al. (2008) after comparing young Eln+/– mice with aged wild-type mice. However, we found no effects of Eln heterozygous deletion on non-arterial tissue, including no differences in glucose tolerance between groups, although previous studies have noted mild cardiac hypertrophy with this model (Faury et al. 2003). Thus, the large artery properties seen with elastin heterozygous deletion reasonably replicate the ageing large artery phenotype and consequent effects, without other age-associated co-morbidities unrelated to changes in large artery properties.
In contrast, measurement of elastin in histological sections indicates that one Eln gene was sufficient to maintain elastin content in cerebral and skeletal muscle feed arteries, potentially due to the lower basal elastin content in resistance arteries compared with large arteries. Furthermore, we found no differences between groups for cerebral artery stiffness. In contrast, in the GFAs there was a slightly greater stiffness in Eln+/– compared with Eln+/+ mice. Therefore, Eln+/– mice represent a good model of large artery stiffening without changes in cerebral artery mechanical properties, although there may be effects in skeletal muscle feed arteries.
Cerebral artery function
In the present study, we found that greater large artery stiffness led to impaired EDD in the MCA, without changes in EID. In addition, we found that the impairment in EDD was a result of reduced NO-mediated dilatation in Eln+/– cerebral arteries. Consistent with previous studies in cerebral arteries, the dilatation in response to ACh was almost entirely mediated by NO in the MCAs from both groups (Mayhan, 1990; Walker et al. 2014), while prostaglandins appear to have an inhibitory effect on ACh-mediated dilatation, possibly through ACh-mediated release of vasoconstrictor prostaglandins. The mechanism for the lower NO-mediated dilatation in Eln+/– cerebral arteries is greater oxidative stress, as indicated by improvements in EDD with scavenging of superoxide in Eln+/– MCAs. We find greater superoxide, as indicated by oxidized DHE, in Eln+/– MCAs, and the reaction of this superoxide with NO is likely to be the formation of peroxynitrite, as indicated by greater nitrotyrosine content of a similar magnitude. Our results suggest that there is no compensatory upregulation in antioxidant SOD isoform gene expression in response to the greater superoxide present in Eln+/– MCAs. However, we did not measure SOD activity, and therefore do not know if alterations in this enzyme activity contribute to the greater superoxide content in Eln+/– MCAs. Thus, future studies are needed to determine the exact mechanism for the greater oxidative stress in the MCA of Eln+/– mice.
Skeletal muscle feed artery function
Although we found no differences in the maximal EDD response between GFAs from Eln+/– and Eln+/+ mice, there was a lower sensitivity to ACh in Eln+/– GFAs. This may be a result of a reduced sensitivity of the smooth muscle to NO, as Eln+/– GFAs also demonstrated lower sensitivity to SNP. However, these differences in SNP sensitivity, although statistically significant, are minor and may not be physiologically relevant. In addition, there was a difference between groups in the contribution of NO and prostaglandins to ACh-mediated dilatation in GFAs, with prostaglandins contributing to dilatation in Eln+/+, but not Eln+/–, GFAs. It is unclear if these differences in dilator reactivity are related to the differences in mechanical properties of the GFA or are a consequence of differences in large artery stiffness between groups. Given the lack of differences in maximal EDD between groups in the GFA, we did not determine the contribution of oxidative stress in this artery as we expected no group differences for these outcomes. Note that the lack of an effect of large artery stiffening on GFA EDD is consistent with the lack of an effect of ageing on EDD in this artery (Muller-Delp et al. 2002; Woodman et al. 2002). However, with ageing there are impairments in soleus feed artery EDD (Muller-Delp et al. 2002) and it is unknown if increased large artery stiffness may contribute to this effect.
Differences between cerebral and skeletal muscle feed arteries
We found that large artery stiffness does not affect all peripheral arteries similarly, as evidenced by effects on maximal dilatation in cerebral arteries, but not skeletal muscle feed arteries. Potential explanations for these tissue-specific effects may include (1) the difference in the distance from the heart, with the greater distance to the GFA allowing for more dampening of the pulse compared with the MCA; (2) the continuous perfusion of the brain provided by high-volume flow throughout systole and diastole that results in more pulsatile flow in the cerebral arteries (O'Rourke & Safar, 2005); (3) the difference in elasticity between arteries, with the MCA in healthy animals having less elasticity and a lower elastin content compared with other resistance arteries (Gonzalez et al. 2005), possibly leading to a greater propensity to be affected by increases in pulsatility; or (4) the greater reliance of MCAs on NO as a dilator, which may increase the susceptibility to dysfunction as a result of increased pulsatility and consequent oxidative stress.
Previous studies have found impaired endothelial function in Eln+/– mice in other resistance arteries, including a recent study demonstrating impaired mesenteric artery EDD in Eln+/– mice (Osei-Owusu et al. 2014). However, the mesenteric arteries appear to be more structurally affected by elastin insufficiency, as Eln+/– mesenteric arteries exhibit an additional elastic lamina, which we did not see in the MCAs or GFAs in this study. Previous studies also indicate that Eln+/– mice have impaired EDD in the renal arteries, but no EDD impairments in the aorta or carotids (Faury et al. 2003). As large artery stiffening is tightly linked to cerebral and renal end organ damage (O'Rourke & Safar, 2005; Mitchell, 2008), these findings support the hypothesis that resistance artery endothelial function may contribute to these associations.
Pulsatility and arterial function
A likely mechanism by which greater large artery stiffness leads to peripheral artery dysfunction is by greater pulsatility. A stiffer aorta and carotid arteries can result in less pulse dampening, leading to greater pulsatility in peripheral resistance arteries and greater pulsatile circumferential stretch of the arterial wall (O'Rourke & Safar, 2005; Mitchell, 2008). Greater pulsatile stretch of endothelial cells in culture is known to increase reactive oxygen species production from NADPH oxidase and angiotensin II release (Hishikawa & Luscher, 1997; Cheng et al. 1998; Delli Gatti et al. 2008), as well as to increase oxidative stress and p38 mitogen-activated protein kinase (MAPK) signalling in vascular smooth muscle cells (Zhu et al. 2011). Studies have examined the effect in isolated arteries of static increases in pressure on EDD and conflicting results have been found (Ungvari et al. 2003; Woodman et al. 2007), although the effect of elevated pulsatility on EDD has not been studied. Thus, future studies of increased pulsatility in isolated arteries are needed to establish impaired function and determine mechanistic causes.
Clinical significance
Advancing age and other disease conditions such as diabetes are characterized by increased large artery stiffness. In addition, animal models demonstrate impaired cerebral artery EDD associated with reduced NO bioavailability and greater oxidative stress is present in old mice (Modrick et al. 2009; Walker et al. 2014). Our findings in Eln+/– mice establish that increased large artery stiffness likely causes impaired cerebral artery function; thus, this mechanistic link may also occur with advancing age. Furthermore, we speculate that the effects of large artery stiffness on cerebral artery function could explain the relationship found in human studies between cerebral impairments (e.g. leukoaraiosis, stroke risk, memory score) and large artery stiffness (Mattace-Raso et al. 2006; Mitchell et al. 2011; Webb et al. 2012). In addition, we find Eln+/– MCAs have reduced NO bioavailability and, reduced NO production by endothelial cells can contribute to features of Alzheimer's disease (Austin et al. 2013). However, future studies are needed to investigate if the cerebral artery dysfunction in Eln+/– mice is associated with impairments in cognitive function or greater stroke risk.
Limitations
There are a few limitations of the present study that should be noted. (1) As increased systolic blood pressure is likely a consequence of large artery stiffening (Franklin et al. 1997), we are unable to isolate the effects of large artery stiffness from those of blood pressure. As increases in systolic blood pressure with primary ageing are mostly a result of the increases in large artery stiffness, we believe that Eln+/– is a more accurate model of the arterial ageing process than a model without increased blood pressure. (2) Due to the small size of cerebral and skeletal muscle resistance arteries we were only able to measure elastin in histological sections and not by a more robust technique (e.g. Western blot). Furthermore, we cannot dismiss the possibility that altered elastin genotype, even despite similar elastin content, can affect the cellular environment in small arteries. For example, reduced elastin is known to promote increased smooth muscle cell proliferation in the aorta of mice (Li et al. 1998), as well as in cultured cells from humans with genetic reductions in elastin (Urban et al. 2002). However, we found that for the MCA, wall thickness and artery stiffness were similar between Eln+/– and Eln+/+ mice, suggesting that one copy of the elastin gene is sufficient to maintain the mechanical properties of this small artery. In addition, the aorta, which is most affected by elastin deletion due to its highly elastic properties, has not been found to have impaired EDD in Eln+/– mice (Faury et al. 2003; Pezet et al. 2008). Thus, we believe the impairments in MCA EDD in Eln+/– mice relate directly to the increases in large artery stiffness. (3) We used a range of phenylephrine doses (2–6 μm) to achieve the desired preconstriction prior to EDD measurement. Therefore, we cannot comment on any group/condition differences in artery constrictor reactivity. However, as preconstriction did not differ between groups for each condition, we do not believe the amount of preconstriction affected our EDD results. (4) We do not currently have the methodology to measure pulsatility in the resistance arteries and therefore cannot prove the presence of a greater pulsatility in the MCA of Eln+/– mice. However, human studies have demonstrated that large artery stiffness is strongly related to cerebral artery pulsatility (Mitchell et al. 2011; Webb et al. 2012). Measuring MCA pressure pulsatility, as well as blood flow, in future studies will help us better understand the mechanisms by which large artery stiffness affects resistance artery function. (5) Measurements of PWV are done under anaesthesia, which may affect the outcome, although as heart rate during testing was similar between groups, we believe that this effect was similar for both genotypes. (6) We did not measure carotid stiffness in these mice and thus cannot comment of the properties of the major conduit artery leading to the MCA. However, in vivo carotid compliance measures have been shown to relate to in vivo measures of aortic stiffness by PWV in humans (Nagai et al. 1999).
Conclusions
In this study, we used mice with altered elastin content to model greater large artery stiffness without other co-morbidities unrelated to the large artery properties. We found that mice with greater large artery stiffness have cerebral artery endothelial dysfunction as a result of reduced NO and increased oxidative stress. In contrast, greater large artery stiffness was only associated with minimal effects on endothelial function in skeletal muscle feed arteries. These results confirm that large artery stiffness probably leads to peripheral artery dysfunction, a concept that was previously hypothesized but never empirically tested. Thus, interventions that target large artery stiffness may be important to reduce disease risk associated with cerebral artery dysfunction.
Acknowledgments
We thank Siavash Ghaffari for his technical assistance.
Glossary
- ACh
acetylcholine
- Eln
elastin gene
- EDD
endothelium-dependent dilatation
- EID
endothelial-independent dilatation
- DHE
dihydroethidium
- GFA
gastrocnemius feed artery
- GTT
glucose tolerance test
- l-NAME
Nω-nitro-l-arginine methyl ester
- MCA
middle cerebral artery
- NO
nitric oxide
- PSS
physiological salt solution
- PWV
pulse wave velocity
- SNP
sodium nitroprusside
- SOD
superoxide dismutase
Additional information
Competing interests
None.
Author contributions
A.E.W., L.A.L. and A.J.D. contributed to all aspects of the studies and manuscript preparation. D.Y.L. contributed to conception of experiments and manuscript revision. G.D.H., K.D.R., R.G.M., P.S.D., E.I.N., J.L. and R.P.M. contributed to data collection and analysis and manuscript revision.
Funding
This work was supported by National Institutes of Health grants: AG040297, AG043952, AG046326, HL007576, HL53325 and HL105314.
References
- Anwar MA, Shalhoub J, Lim CS, Gohel MS. Davies AH. The effect of pressure-induced mechanical stretch on vascular wall differential gene expression. J Vasc Res. 2012;49:463–478. doi: 10.1159/000339151. [DOI] [PubMed] [Google Scholar]
- Austin SA, Santhanam AV, Hinton DJ, Choi DS. Katusic ZS. Endothelial nitric oxide deficiency promotes Alzheimer's disease pathology. J Neurochem. 2013;127:691–700. doi: 10.1111/jnc.12334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumbach GL. Hajdu MA. Mechanics and composition of cerebral arterioles in renal and spontaneously hypertensive rats. Hypertension. 1993;21:816–826. doi: 10.1161/01.hyp.21.6.816. [DOI] [PubMed] [Google Scholar]
- Briones AM, Gonzalez JM, Somoza B, Giraldo J, Daly CJ, Vila E, Gonzalez MC, McGrath JC. Arribas SM. Role of elastin in spontaneously hypertensive rat small mesenteric artery remodelling. J Physiol. 2003;552:185–195. doi: 10.1113/jphysiol.2003.046904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng JJ, Wung BS, Chao YJ. Wang DL. Cyclic strain-induced reactive oxygen species involved in ICAM-1 gene induction in endothelial cells. Hypertension. 1998;31:125–130. doi: 10.1161/01.hyp.31.1.125. [DOI] [PubMed] [Google Scholar]
- De Vriese AS, Verbeuren TJ, Van de Voorde J, Lameire NH. Vanhoutte PM. Endothelial dysfunction in diabetes. Br J Pharmacol. 2000;130:963–974. doi: 10.1038/sj.bjp.0703393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delli Gatti C, Osto E, Kouroedov A, Eto M, Shaw S, Volpe M, Luscher TF. Cosentino F. Pulsatile stretch induces release of angiotensin II and oxidative stress in human endothelial cells: effects of ACE inhibition and AT1 receptor antagonism. Clin Exp Hypertens. 2008;30:616–627. doi: 10.1080/10641960802443183. [DOI] [PubMed] [Google Scholar]
- Didion SP, Kinzenbaw DA, Schrader LI. Faraci FM. Heterozygous CuZn superoxide dismutase deficiency produces a vascular phenotype with aging. Hypertension. 2006;48:1072–1079. doi: 10.1161/01.HYP.0000247302.20559.3a. [DOI] [PubMed] [Google Scholar]
- Donato AJ, Gano LB, Eskurza I, Silver AE, Gates PE, Jablonski K. Seals DR. Vascular endothelial dysfunction with aging: endothelin-1 and endothelial nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2009;297:H425–432. doi: 10.1152/ajpheart.00689.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Henson GD, Morgan RG, Enz RA, Walker AE. Lesniewski LA. TNF-α impairs endothelial function in adipose tissue resistance arteries of mice with diet-induced obesity. Am J Physiol Heart Circ Physiol. 2012;303:H672–679. doi: 10.1152/ajpheart.00271.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Magerko KA, Lawson BR, Durrant JR, Lesniewski LA. Seals DR. SIRT-1 and vascular endothelial dysfunction with ageing in mice and humans. J Physiol. 2011;589:4545–4554. doi: 10.1113/jphysiol.2011.211219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donato AJ, Walker AE, Magerko KA, Bramwell RC, Black AD, Henson GD, Lawson BR, Lesniewski LA. Seals DR. Life-long caloric restriction reduces oxidative stress and preserves nitric oxide bioavailability and function in arteries of old mice. Aging Cell. 2013;12:772–783. doi: 10.1111/acel.12103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn WR. Gardiner SM. Differential alteration in vascular structure of resistance arteries isolated from the cerebral and mesenteric vascular beds of transgenic [(mRen-2)27], hypertensive rats. Hypertension. 1997;29:1140–1147. doi: 10.1161/01.hyp.29.5.1140. [DOI] [PubMed] [Google Scholar]
- Faury G, Pezet M, Knutsen RH, Boyle WA, Heximer SP, McLean SE, Minkes RK, Blumer KJ, Kovacs A, Kelly DP, Li DY, Starcher B. Mecham RP. Developmental adaptation of the mouse cardiovascular system to elastin haploinsufficiency. J Clin Invest. 2003;112:1419–1428. doi: 10.1172/JCI19028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng M, Whitesall S, Zhang Y, Beibel M, D'Alecy L. DiPetrillo K. Validation of volume–pressure recording tail-cuff blood pressure measurements. Am J Hypertens. 2008;21:1288–1291. doi: 10.1038/ajh.2008.301. [DOI] [PubMed] [Google Scholar]
- Franklin SS, Gustin WT, Wong ND, Larson MG, Weber MA, Kannel WB. Levy D. Hemodynamic patterns of age-related changes in blood pressure. The Framingham Heart Study. Circulation. 1997;96:308–315. doi: 10.1161/01.cir.96.1.308. [DOI] [PubMed] [Google Scholar]
- Gonzalez JM, Briones AM, Starcher B, Conde MV, Somoza B, Daly C, Vila E, McGrath I, Gonzalez MC. Arribas SM. Influence of elastin on rat small artery mechanical properties. Exp Physiol. 2005;90:463–468. doi: 10.1113/expphysiol.2005.030056. [DOI] [PubMed] [Google Scholar]
- Henson GD, Walker AE, Reihl KD, Donato AJ. Lesniewski LA. Dichotomous mechanisms of aortic stiffening in high-fat diet fed young and old B6D2F1 mice. Physiol Rep. 2014;2:e00268. doi: 10.1002/phy2.268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hishikawa K. Luscher TF. Pulsatile stretch stimulates superoxide production in human aortic endothelial cells. Circulation. 1997;96:3610–3616. doi: 10.1161/01.cir.96.10.3610. [DOI] [PubMed] [Google Scholar]
- Izzard AS, Horton S, Heerkens EH, Shaw L. Heagerty AM. Middle cerebral artery structure and distensibility during developing and established phases of hypertension in the spontaneously hypertensive rat. J Hypertens. 2006;24:875–880. doi: 10.1097/01.hjh.0000222757.54111.06. [DOI] [PubMed] [Google Scholar]
- Lakatta EG. Levy D. Arterial and cardiac aging: major shareholders in cardiovascular disease enterprises: Part I: aging arteries: a “set up” for vascular disease. Circulation. 2003;107:139–146. doi: 10.1161/01.cir.0000048892.83521.58. [DOI] [PubMed] [Google Scholar]
- Le Brocq M, Leslie SJ, Milliken P. Megson IL. Endothelial dysfunction: from molecular mechanisms to measurement, clinical implications, and therapeutic opportunities. Antioxid Redox Signal. 2008;10:1631–1674. doi: 10.1089/ars.2007.2013. [DOI] [PubMed] [Google Scholar]
- Lesniewski LA, Connell ML, Durrant JR, Folian BJ, Anderson MC, Donato AJ. Seals DR. B6D2F1 mice are a suitable model of oxidative stress-mediated impaired endothelium-dependent dilation with aging. J Gerontol A Biol Sci Med Sci. 2009;64:9–20. doi: 10.1093/gerona/gln049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li DY, Brooke B, Davis EC, Mecham RP, Sorensen LK, Boak BB, Eichwald E. Keating MT. Elastin is an essential determinant of arterial morphogenesis. Nature. 1998;393:276–280. doi: 10.1038/30522. [DOI] [PubMed] [Google Scholar]
- Mattace-Raso FU, van der Cammen TJ, Hofman A, van Popele NM, Bos ML, Schalekamp MA, Asmar R, Reneman RS, Hoeks AP, Breteler MM. Witteman JC. Arterial stiffness and risk of coronary heart disease and stroke: the Rotterdam Study. Circulation. 2006;113:657–663. doi: 10.1161/CIRCULATIONAHA.105.555235. [DOI] [PubMed] [Google Scholar]
- Mayhan WG. Impairment of endothelium-dependent dilatation of basilar artery during chronic hypertension. Am J Physiol. 1990;259:H1455–1462. doi: 10.1152/ajpheart.1990.259.5.H1455. [DOI] [PubMed] [Google Scholar]
- Mitchell GF. Effects of central arterial aging on the structure and function of the peripheral vasculature: implications for end-organ damage. J Appl Physiol. 2008;105:1652–1660. doi: 10.1152/japplphysiol.90549.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GF, van Buchem MA, Sigurdsson S, Gotal JD, Jonsdottir MK, Kjartansson O, Garcia M, Aspelund T, Harris TB, Gudnason V. Launer LJ. Arterial stiffness, pressure and flow pulsatility and brain structure and function: the Age, Gene/Environment Susceptibility–Reykjavik study. Brain. 2011;134:3398–3407. doi: 10.1093/brain/awr253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchell GF, Vita JA, Larson MG, Parise H, Keyes MJ, Warner E, Vasan RS, Levy D. Benjamin EJ. Cross-sectional relations of peripheral microvascular function, cardiovascular disease risk factors, and aortic stiffness: the Framingham Heart Study. Circulation. 2005;112:3722–3728. doi: 10.1161/CIRCULATIONAHA.105.551168. [DOI] [PubMed] [Google Scholar]
- Modrick ML, Didion SP, Sigmund CD. Faraci FM. Role of oxidative stress and AT1 receptors in cerebral vascular dysfunction with aging. Am J Physiol Heart Circ Physiol. 2009;296:H1914–1919. doi: 10.1152/ajpheart.00300.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller-Delp JM, Spier SA, Ramsey MW. Delp MD. Aging impairs endothelium-dependent vasodilation in rat skeletal muscle arterioles. Am J Physiol Heart Circ Physiol. 2002;283:H1662–1672. doi: 10.1152/ajpheart.00004.2002. [DOI] [PubMed] [Google Scholar]
- Nagai Y, Fleg JL, Kemper MK, Rywik TM, Earley CJ. Metter EJ. Carotid arterial stiffness as a surrogate for aortic stiffness: relationship between carotid artery pressure–strain elastic modulus and aortic pulse wave velocity. Ultrasound Med Biol. 1999;25:181–188. doi: 10.1016/s0301-5629(98)00146-x. [DOI] [PubMed] [Google Scholar]
- O'Rourke MF. Safar ME. Relationship between aortic stiffening and microvascular disease in brain and kidney: cause and logic of therapy. Hypertension. 2005;46:200–204. doi: 10.1161/01.HYP.0000168052.00426.65. [DOI] [PubMed] [Google Scholar]
- Osei-Owusu P, Knutsen RH, Kozel BA, Dietrich HH, Blumer KJ. Mecham RP. Altered reactivity of resistance vasculature contributes to hypertension in elastin insufficiency. Am J Physiol Heart Circ Physiol. 2014;306:H654–666. doi: 10.1152/ajpheart.00601.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pezet M, Jacob MP, Escoubet B, Gheduzzi D, Tillet E, Perret P, Huber P, Quaglino D, Vranckx R, Li DY, Starcher B, Boyle WA, Mecham RP. Faury G. Elastin haploinsufficiency induces alternative aging processes in the aorta. Rejuvenation Res. 2008;11:97–112. doi: 10.1089/rej.2007.0587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qamirani E, Ren Y, Kuo L. Hein TW. C-reactive protein inhibits endothelium-dependent NO-mediated dilation in coronary arterioles by activating p38 kinase and NAD(P)H oxidase. Arterioscler Thromb Vasc Biol. 2005;25:995–1001. doi: 10.1161/01.ATV.0000159890.10526.1e. [DOI] [PubMed] [Google Scholar]
- Reddy AK, Jones AD, Martono C, Caro WA, Madala S. Hartley CJ. Pulsed Doppler signal processing for use in mice: design and evaluation. IEEE Trans Biomed Eng. 2005;52:1764–1770. doi: 10.1109/tbme.2005.855710. [DOI] [PubMed] [Google Scholar]
- Ungvari Z, Csiszar A, Huang A, Kaminski PM, Wolin MS. Koller A. High pressure induces superoxide production in isolated arteries via protein kinase C-dependent activation of NAD(P)H oxidase. Circulation. 2003;108:1253–1258. doi: 10.1161/01.CIR.0000079165.84309.4D. [DOI] [PubMed] [Google Scholar]
- Urban Z, Riazi S, Seidl TL, Katahira J, Smoot LB, Chitayat D, Boyd CD. Hinek A. Connection between elastin haploinsufficiency and increased cell proliferation in patients with supravalvular aortic stenosis and Williams-Beuren syndrome. Am J Hum Genet. 2002;71:30–44. doi: 10.1086/341035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenseil JE, Nerurkar NL, Knutsen RH, Okamoto RJ, Li DY. Mecham RP. Effects of elastin haploinsufficiency on the mechanical behavior of mouse arteries. Am J Physiol Heart Circ Physiol. 2005;289:H1209–1217. doi: 10.1152/ajpheart.00046.2005. [DOI] [PubMed] [Google Scholar]
- Walker AE, Henson GD, Reihl KD, Nielson EI, Morgan RG, Lesniewski LA. Donato AJ. Beneficial effects of lifelong caloric restriction on endothelial function are greater in conduit arteries compared to cerebral resistance arteries. Age (Dordr) 2014;36:559–569. doi: 10.1007/s11357-013-9585-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webb AJ, Simoni M, Mazzucco S, Kuker W, Schulz U. Rothwell PM. Increased cerebral arterial pulsatility in patients with leukoaraiosis: arterial stiffness enhances transmission of aortic pulsatility. Stroke. 2012;43:2631–2636. doi: 10.1161/STROKEAHA.112.655837. [DOI] [PubMed] [Google Scholar]
- Woodman CR, Price EM. Laughlin MH. Aging induces muscle-specific impairment of endothelium-dependent dilation in skeletal muscle feed arteries. J Appl Physiol (1985) 2002;93:1685–1690. doi: 10.1152/japplphysiol.00461.2002. [DOI] [PubMed] [Google Scholar]
- Woodman CR, Trott DW. Laughlin MH. Short-term increases in intraluminal pressure reverse age-related decrements in endothelium-dependent dilation in soleus muscle feed arteries. J Appl Physiol (1985) 2007;103:1172–1179. doi: 10.1152/japplphysiol.00416.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Hein TW, Wang W. Kuo L. Divergent roles of angiotensin II AT1 and AT2 receptors in modulating coronary microvascular function. Circ Res. 2003;92:322–329. doi: 10.1161/01.res.0000056759.53828.2c. [DOI] [PubMed] [Google Scholar]
- Zhu JH, Chen CL, Flavahan S, Harr J, Su B. Flavahan NA. Cyclic stretch stimulates vascular smooth muscle cell alignment by redox-dependent activation of Notch3. Am J Physiol Heart Circ Physiol. 2011;300:H1770–1780. doi: 10.1152/ajpheart.00535.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]


