Abstract
We compared the oxygen cost of breathing (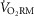 ) in healthy men and women over a wide range of exercise ventilations (
) in healthy men and women over a wide range of exercise ventilations ( ). Eighteen subjects (nine women) completed 4 days of testing. First, a step-wise maximal cycle exercise test was completed for the assessment of spontaneous breathing patterns. Next, subjects were familiarized with the voluntary hyperpnoea protocol used to estimate
). Eighteen subjects (nine women) completed 4 days of testing. First, a step-wise maximal cycle exercise test was completed for the assessment of spontaneous breathing patterns. Next, subjects were familiarized with the voluntary hyperpnoea protocol used to estimate  . During the final two visits, subjects mimicked multiple times (four to six) the breathing patterns associated with five or six different exercise stages. Each trial lasted 5 min, and on-line pressure–volume and flow–volume loops were superimposed on target loops obtained during exercise to replicate the work of breathing accurately. At ∼55 l min−1
. During the final two visits, subjects mimicked multiple times (four to six) the breathing patterns associated with five or six different exercise stages. Each trial lasted 5 min, and on-line pressure–volume and flow–volume loops were superimposed on target loops obtained during exercise to replicate the work of breathing accurately. At ∼55 l min−1
 ,
,  was significantly greater in women. At maximal ventilation, the absolute
was significantly greater in women. At maximal ventilation, the absolute  was not different (P > 0.05) between the sexes, but represented a significantly greater fraction of whole-body
was not different (P > 0.05) between the sexes, but represented a significantly greater fraction of whole-body  in women (13.8 ± 1.5 vs. 9.4 ± 1.1%
in women (13.8 ± 1.5 vs. 9.4 ± 1.1%  ). During heavy exercise at 92 and 100%
). During heavy exercise at 92 and 100%  , the unit cost of
, the unit cost of  was +0.7 and +1.1 ml O2 l−1 greater in women (P < 0.05). At
was +0.7 and +1.1 ml O2 l−1 greater in women (P < 0.05). At  , men and women who developed expiratory flow limitation had a significantly greater
, men and women who developed expiratory flow limitation had a significantly greater  than those who did not (435 ± 44 vs. 331 ± 30 ml O2 min−1). In conclusion, women have a greater
than those who did not (435 ± 44 vs. 331 ± 30 ml O2 min−1). In conclusion, women have a greater  for a given
for a given  , and this represents a greater fraction of whole-body
, and this represents a greater fraction of whole-body  . The greater
. The greater  in women may have implications for the integrated physiological response to exercise.
in women may have implications for the integrated physiological response to exercise.
Key points
The oxygen cost of breathing represents a significant fraction of total oxygen uptake during intense exercise.
At a given ventilation, women have a greater work of breathing compared with men, and because work is linearly related to oxygen uptake we hypothesized that their oxygen cost of breathing would also be greater.
For a given ventilation, women had a greater absolute oxygen cost of breathing, and this represented a greater fraction of total oxygen uptake.
Regardless of sex, those who developed expiratory flow limitation had a greater oxygen cost of breathing at maximal exercise.
The greater oxygen cost of breathing in women indicates that a greater fraction of total oxygen uptake (and possibly cardiac output) is directed to the respiratory muscles, which may influence blood flow distribution during exercise.
Introduction
During dynamic exercise, ventilation increases in proportion to the metabolic demands of the locomotor muscles. This exercise-induced increase in minute ventilation ( ) results in an increased mechanical work of breathing (WOB; Otis, 1964). Consequently, the metabolic and circulatory costs of exercise hyperpnoea are substantial. In healthy untrained humans, the oxygen uptake of the respiratory muscles (
) results in an increased mechanical work of breathing (WOB; Otis, 1964). Consequently, the metabolic and circulatory costs of exercise hyperpnoea are substantial. In healthy untrained humans, the oxygen uptake of the respiratory muscles ( ) during maximal exercise represents ∼10% of whole-body maximal oxygen uptake (
) during maximal exercise represents ∼10% of whole-body maximal oxygen uptake ( ; Nielsen, 1936; Shephard, 1966; Aaron et al. 1992b). In endurance-trained men, who possess a high
; Nielsen, 1936; Shephard, 1966; Aaron et al. 1992b). In endurance-trained men, who possess a high 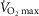 and sustain high rates of ventilation, the
and sustain high rates of ventilation, the  can represent upwards of 15% of
can represent upwards of 15% of  (Aaron et al. 1992b). Akin to other skeletal muscles, the contracting respiratory musculature requires sufficient blood flow to meet oxygen demand (Andersen & Saltin, 1985). Whilst direct blood flow measurements are not available in humans, at maximal exercise in ponies ∼15% of cardiac output is directed towards the respiratory muscles (Manohar, 1990); this value is commensurate with estimates of blood flow in humans (Coast & Krause, 1993; Harms et al. 1998b). Quantifying the metabolic demands of the respiratory muscles is necessary for understanding cardiorespiratory control during exercise. Specifically, high respiratory muscle work has been shown to alter blood flow distribution (Harms et al. 1997) and reduce cardiac output by altering preload and afterload (Harms et al. 1998b). Similar alterations in cardiac output have also been demonstrated with externally imposed expiratory loading (Stark-Leyva et al. 2004; Miller et al. 2006).
(Aaron et al. 1992b). Akin to other skeletal muscles, the contracting respiratory musculature requires sufficient blood flow to meet oxygen demand (Andersen & Saltin, 1985). Whilst direct blood flow measurements are not available in humans, at maximal exercise in ponies ∼15% of cardiac output is directed towards the respiratory muscles (Manohar, 1990); this value is commensurate with estimates of blood flow in humans (Coast & Krause, 1993; Harms et al. 1998b). Quantifying the metabolic demands of the respiratory muscles is necessary for understanding cardiorespiratory control during exercise. Specifically, high respiratory muscle work has been shown to alter blood flow distribution (Harms et al. 1997) and reduce cardiac output by altering preload and afterload (Harms et al. 1998b). Similar alterations in cardiac output have also been demonstrated with externally imposed expiratory loading (Stark-Leyva et al. 2004; Miller et al. 2006).
There are well-documented sex differences with respect to airway anatomy and the respiratory mechanics associated with exercise hyperpnoea. When compared with height-matched men, women have smaller lungs (Tan et al. 2011) and conducting airways (Mead, 1980; Sheel et al. 2009). During dynamic whole-body exercise, women develop expiratory flow limitation (EFL) more often (McClaran et al. 1998; Guenette et al. 2007) and have a greater mechanical WOB for a given  above ∼65 l min−1 (Wanke et al. 1991; Guenette et al. 2009). It has been hypothesized that mechanical ventilatory constraints, such as EFL, are associated with a higher
above ∼65 l min−1 (Wanke et al. 1991; Guenette et al. 2009). It has been hypothesized that mechanical ventilatory constraints, such as EFL, are associated with a higher  (Aaron et al. 1992b), although to our knowledge this has not been investigated systematically. Therefore, it is important to consider the potential independent effect of EFL on
(Aaron et al. 1992b), although to our knowledge this has not been investigated systematically. Therefore, it is important to consider the potential independent effect of EFL on  . There have been limited attempts to compare the
. There have been limited attempts to compare the 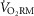 between men and women (Eckermann & Millahn, 1962; Topin et al. 2003; Lorenzo & Babb, 2012). Unfortunately, methodological inadequacies and conflicting results render the findings difficult to interpret. For example, most of the studies assessed the
between men and women (Eckermann & Millahn, 1962; Topin et al. 2003; Lorenzo & Babb, 2012). Unfortunately, methodological inadequacies and conflicting results render the findings difficult to interpret. For example, most of the studies assessed the  in men and women at ventilations where sex differences may not necessarily be present (<60 l min−1). The importance of determining sex-based differences in
in men and women at ventilations where sex differences may not necessarily be present (<60 l min−1). The importance of determining sex-based differences in  is because cardiac output is finite during maximal exercise. As such, if women have a greater
is because cardiac output is finite during maximal exercise. As such, if women have a greater  and must therefore dedicate a greater fraction of total blood flow towards their respiratory muscles during maximal exercise, performance may be impaired due to reduced locomotor muscle blood flow (Harms et al. 2000).
and must therefore dedicate a greater fraction of total blood flow towards their respiratory muscles during maximal exercise, performance may be impaired due to reduced locomotor muscle blood flow (Harms et al. 2000).
Accordingly, we sought to compare  in men vs. women to address whether, for a given ventilation, women have a greater absolute
in men vs. women to address whether, for a given ventilation, women have a greater absolute  and whether this represents a larger percentage of whole-body oxygen uptake (
and whether this represents a larger percentage of whole-body oxygen uptake ( ) compared with men. A secondary aim was to determine the effect of EFL on
) compared with men. A secondary aim was to determine the effect of EFL on  . We hypothesized that at submaximal and maximal ventilations where the WOB is greater compared with men, women have a greater
. We hypothesized that at submaximal and maximal ventilations where the WOB is greater compared with men, women have a greater  and this constitutes a larger proportion of whole-body
and this constitutes a larger proportion of whole-body  . We further hypothesized that at maximal exercise, those who develop EFL will have a higher
. We further hypothesized that at maximal exercise, those who develop EFL will have a higher  .
.
Some of the data in the current article have been presented as an abstract entitled ‘Oxygen cost of exercise hyperpnea is greater in women compared to men’, published in The FASEB Journal, and were presented at the Experimental Biology 2014 Meeting in San Diego, California.
Methods
Subjects
After providing written informed consent, eighteen (nine male, nine female) healthy subjects participated in the study. Some subjects (13 of 18) had previously participated in a study designed to determine the reproducibility of  (Dominelli et al. 2014). The primary outcome measures in the present study did not overlap with any of the previous analyses. All procedures adhered to the Declaration of Helsinki and were approved by the Clinical Research Ethics Board at the University of British Columbia. Subjects had a wide range of exercise participation (recreational to national calibre athletics), did not report any current or previous cardiorespiratory ailments and had spirometry parameters within normal limits (Tan et al. 2011; Table1). Although not universally established, studies in which conjugates of oestrogen and progesterone have been measured have demonstrated significant inter- and intrasubject variability with respect to hormone levels throughout the menstrual cycle, but with no effect on submaximal exercise ventilation (Beidleman et al. 1999; MacNutt et al. 2012). Therefore, we tested female subjects at random points throughout their menstrual cycle, and oral contraceptive use was not an exclusion criterion.
(Dominelli et al. 2014). The primary outcome measures in the present study did not overlap with any of the previous analyses. All procedures adhered to the Declaration of Helsinki and were approved by the Clinical Research Ethics Board at the University of British Columbia. Subjects had a wide range of exercise participation (recreational to national calibre athletics), did not report any current or previous cardiorespiratory ailments and had spirometry parameters within normal limits (Tan et al. 2011; Table1). Although not universally established, studies in which conjugates of oestrogen and progesterone have been measured have demonstrated significant inter- and intrasubject variability with respect to hormone levels throughout the menstrual cycle, but with no effect on submaximal exercise ventilation (Beidleman et al. 1999; MacNutt et al. 2012). Therefore, we tested female subjects at random points throughout their menstrual cycle, and oral contraceptive use was not an exclusion criterion.
Table 1.
Anthropometric and spirometric values
| Characteristic | Men (n = 9) | Women (n = 9) |
|---|---|---|
| Age (years) | 29 ± 3 | 23 ± 1* |
| Height (cm) | 183 ± 2 | 167 ± 2* |
| Mass (kg) | 75 ± 3 | 58 ± 2* |
| FVC (l) | 5.8 ± 0.2 | 4.0 ± 0.2* |
| FVC (% predicted) | 99 ± 4 | 95 ± 2 |
| FEV1 (l) | 4.7 ± 0.2 | 3.4 ± 0.1* |
| FEV1 (% predicted) | 100 ± 4 | 94 ± 2 |
| FEV1/FVC | 82 ± 2 | 85 ± 1 |
| FEV1/FVC (% predicted) | 100 ± 2 | 99 ± 2 |
Abbreviations: FEV1, forced expired volume in 1 s; and FVC, forced vital capacity. *Significantly different from men (P < 0.05). Values in this and subsequent tables are means ± SEM.
Experimental design
Subjects completed 4 days of testing. Day 1 consisted of maximal incremental cycle exercise in order to obtain spontaneous ventilatory parameters during exercise. Day 2 served to familiarize subjects with the voluntary hyperpnoea protocol used to estimate  . Days 3 and 4 were experimental days, during which subjects mimicked their exercise breathing patterns at rest while
. Days 3 and 4 were experimental days, during which subjects mimicked their exercise breathing patterns at rest while  was assessed. Days 1 and 2 were separated by at least 48 h, whereas the experimental days were separated by at least 24 h. Subjects were instrumented with oesophageal and gastric catheters on days 1, 3 and 4 for the assessment of respiratory pressures.
was assessed. Days 1 and 2 were separated by at least 48 h, whereas the experimental days were separated by at least 24 h. Subjects were instrumented with oesophageal and gastric catheters on days 1, 3 and 4 for the assessment of respiratory pressures.
Maximal exercise (day 1)
To obtain spontaneous breathing patterns, a step-wise incremental test on a cycle ergometer (Excalibur Sport; Lode, Groningen, The Netherlands) was performed to the limit of tolerance after insertion and placement of oesophageal and gastric balloon-tipped catheters. To ensure that subjects exercised for similar durations, men began at 120 W and women began at 80 W, with a 20 W increase every 2 min for both groups. Testing was terminated when subjects could not maintain >60 r.p.m. despite encouragement. Cardiorespiratory variables, including EFL, were assessed using customized hardware and software as described elsewhere (Dominelli et al. 2011, 2014).
Voluntary hyperpnoea (days 3 and 4)
To estimate  , subjects rested on the cycle ergometer and mimicked tidal volume, frequency,
, subjects rested on the cycle ergometer and mimicked tidal volume, frequency,  , duty cycle and respiratory pressures associated with their exercise hyperpnoea (Dominelli et al. 2014). End-tidal CO2 tension was set to a level similar to each respective exercise stage. Briefly, each subject mimicked the breathing pattern associated with four or five submaximal exercise stages and maximal exercise. The experimental trials were performed in random order over 2 days. Each stage was mimicked four to six times sequentially and each trial was 5 min in duration. The first 4 min was used to provide feedback and ensure sufficient time for mixed expired gas fractions to reach a steady state. The final minute of each stage was used for subsequent analysis. During each of the voluntary hyperpnoea trials, the subjects maintained the same body position as during cycle exercise. Ample rest was allowed between trials, ranging from 5 min for lower intensity
, duty cycle and respiratory pressures associated with their exercise hyperpnoea (Dominelli et al. 2014). End-tidal CO2 tension was set to a level similar to each respective exercise stage. Briefly, each subject mimicked the breathing pattern associated with four or five submaximal exercise stages and maximal exercise. The experimental trials were performed in random order over 2 days. Each stage was mimicked four to six times sequentially and each trial was 5 min in duration. The first 4 min was used to provide feedback and ensure sufficient time for mixed expired gas fractions to reach a steady state. The final minute of each stage was used for subsequent analysis. During each of the voluntary hyperpnoea trials, the subjects maintained the same body position as during cycle exercise. Ample rest was allowed between trials, ranging from 5 min for lower intensity  [40% of maximum (
[40% of maximum ( )] to >30 min for higher levels of
)] to >30 min for higher levels of  (>75%
(>75% 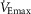 ). Heart rate and blood pressure were measured during the rest periods to ensure complete recovery before the subsequent hyperpnoea trial. Similar clothing was worn during exercise and voluntary hyperpnoea to avoid any clothing-induced changes in pulmonary mechanics, and the ergometer configuration was identical for both conditions. We included a familiarization day because it significantly improves the accuracy of tidal volume and
). Heart rate and blood pressure were measured during the rest periods to ensure complete recovery before the subsequent hyperpnoea trial. Similar clothing was worn during exercise and voluntary hyperpnoea to avoid any clothing-induced changes in pulmonary mechanics, and the ergometer configuration was identical for both conditions. We included a familiarization day because it significantly improves the accuracy of tidal volume and  and the matching of expiratory flow during voluntary hyperpnoea (Dominelli et al. 2014). During the familiarization day, each hyperpnoea trial was mimicked several times, and similar feedback (except oesophageal and gastric pressure) was provided.
and the matching of expiratory flow during voluntary hyperpnoea (Dominelli et al. 2014). During the familiarization day, each hyperpnoea trial was mimicked several times, and similar feedback (except oesophageal and gastric pressure) was provided.
Several procedures were used to ensure that the ventilatory responses to exercise were replicated during the voluntary hyperpnoea trials (Dominelli et al. 2014). Numerical tidal volume was displayed to the subjects on a breath-by-breath basis, and verbal feedback was provided throughout. Breathing frequency and duty cycle were maintained by breathing in time to a metronome. Directly in front of the subjects was a screen displaying online flow–volume and pressure–volume loops that were overlaid on loops obtained during exercise. Representative traces of a subject's oesophageal pressure during exercise and voluntary hyperpnoea are shown in Fig. 1. Subjects were coached by the same investigator to match their online flow–volume and pressure–volume loops with the target loop. End-expiratory lung volume was monitored using the surrogate measure of end-expiratory oesophageal pressure. During each trial, the inspired gas was modified to ensure that end-tidal CO2 tension was similar to the respective exercise stage. In this regard, the inspired percentage of CO2 ranged from 2 to 6%, whereas inspired O2 was always ∼21%.
Figure 1. Oesophageal pressure–volume loops for a representative male subject during exercise (A–D) and voluntary hyperpnoea (E–H).

Loops representing exercise are an average composite from 30 s of breathing. Loops representing voluntary hyperpnoea are average composites of the final minute of several trials. Ventilation and work of breathing are presented in tables for each respective stage. Abbreviations: Ex, exercise; Hyp; hyperpnoea;  , expired minute ventilation;
, expired minute ventilation;  , maximal minute ventilation; and WOB, work of breathing.
, maximal minute ventilation; and WOB, work of breathing.
In total, 442 experimental trials were completed, with equal distribution between the sexes. Each subject performed 20–30 trials, equally distributed across the experimental days. To determine the between-day reproducibly of  , one subject repeated the entire procedure 6 months after initial testing; all estimates were within 5–7% of the original measures over their full range of
, one subject repeated the entire procedure 6 months after initial testing; all estimates were within 5–7% of the original measures over their full range of  (40–150 l min−1).
(40–150 l min−1).
Data analysis
Data were collected using a 16-channel analog-to-digital data acquisition system (PowerLab/16Sp model ML 795; ADInstruments, Colorado Springs, CO, USA), sampled at 200 Hz, and stored on a computer for subsequent analysis using bespoke software (GNARx, developed in LabView 2013, National Instruments Austin, TX, USA). The final 30 s of each exercise stage was used for subsequent analysis. During voluntary hyperpnoea, the final minute was used for analysis. A longer time was used for the voluntary hyperpnoea protocols to ensure steady-state  . Flow–volume and pressure–volume loops for each trial were constructed from ensemble-averaged breaths. Tidal flow–volume loops were placed within the maximal expiratory flow–volume (MEFV) envelope by determining end-expiratory lung volume from an inspiratory capacity manoeuvre (Guenette et al. 2010). The WOB, extent of EFL and ventilatory capacity were determined using previously described methods (Dominelli et al. 2013). The efficiency of the respiratory muscles was estimated by dividing the measured
. Flow–volume and pressure–volume loops for each trial were constructed from ensemble-averaged breaths. Tidal flow–volume loops were placed within the maximal expiratory flow–volume (MEFV) envelope by determining end-expiratory lung volume from an inspiratory capacity manoeuvre (Guenette et al. 2010). The WOB, extent of EFL and ventilatory capacity were determined using previously described methods (Dominelli et al. 2013). The efficiency of the respiratory muscles was estimated by dividing the measured  by the ideal oxygen uptake needed to perform the WOB (Aaron et al. 1992a). To calculate the ideal oxygen uptake, the measured WOB was converted into units of oxygen with changes in respiratory exchange ratio taken into account. The maximal effective ventilation was defined as when the change in
by the ideal oxygen uptake needed to perform the WOB (Aaron et al. 1992a). To calculate the ideal oxygen uptake, the measured WOB was converted into units of oxygen with changes in respiratory exchange ratio taken into account. The maximal effective ventilation was defined as when the change in  per unit of
per unit of  equalled the change in whole-body
equalled the change in whole-body  per unit
per unit  (Otis, 1954; Shephard, 1966). Once the maximal effective ventilation was determined, maximal exercise gas exchange parameters and the alveolar gas equation were used to predict the maximal alveolar ventilation and the corresponding
(Otis, 1954; Shephard, 1966). Once the maximal effective ventilation was determined, maximal exercise gas exchange parameters and the alveolar gas equation were used to predict the maximal alveolar ventilation and the corresponding  .
.
Statistics
Anthropometric and maximal exercise variables were compared between men and women using Student's unpaired t tests. An equation was fitted to each subject's relationship for WOB vs. 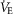 ,
,  vs. WOB and
vs. WOB and  vs.
vs.  . The
. The  vs. WOB relationship was fitted with a linear equation, whereas the WOB vs.
vs. WOB relationship was fitted with a linear equation, whereas the WOB vs.  and
and  vs.
vs.  were fitted with an exponential equation. The respective constants for each equation were pooled, and the sexes were compared using Student's unpaired t tests. To determine the specific
were fitted with an exponential equation. The respective constants for each equation were pooled, and the sexes were compared using Student's unpaired t tests. To determine the specific  or WOB for which the groups were different, each subject's equation was solved for successive independent variables, with the resultants compared with t tests and Bonferroni correction. To determine sex-independent differences, subjects were grouped into those with and without EFL during exercise, and similar comparisons were completed. Expiratory flow limitation was defined as >5% overlap of the tidal flow–volume loop with the MEFV curve. The effect of sex and EFL was also compared at different percentages of
or WOB for which the groups were different, each subject's equation was solved for successive independent variables, with the resultants compared with t tests and Bonferroni correction. To determine sex-independent differences, subjects were grouped into those with and without EFL during exercise, and similar comparisons were completed. Expiratory flow limitation was defined as >5% overlap of the tidal flow–volume loop with the MEFV curve. The effect of sex and EFL was also compared at different percentages of  (45, 60, 75 and 100%) using a two-factor (sex and percentage
(45, 60, 75 and 100%) using a two-factor (sex and percentage  ) repeated-measures ANOVA. When significant F ratios were detected, Tukey's post hoc test was conducted. The percentages of
) repeated-measures ANOVA. When significant F ratios were detected, Tukey's post hoc test was conducted. The percentages of  were selected for the following reasons: (i) they spanned a wide range of ventilation; (ii) they represented ∼10% increments in
were selected for the following reasons: (i) they spanned a wide range of ventilation; (ii) they represented ∼10% increments in  (see Table3); and (iii) all subjects had mimicked an exercise stage within this range. Statistical significance was set at P < 0.05. All values are presented as means ± SEM unless otherwise noted.
(see Table3); and (iii) all subjects had mimicked an exercise stage within this range. Statistical significance was set at P < 0.05. All values are presented as means ± SEM unless otherwise noted.
Table 3.
Cardiorespiratory variables during voluntary hyperpnoea at different percentages of maximal exercise ventilation
45% 
|
60% 
|
75% 
|
100% 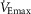
|
|||||
|---|---|---|---|---|---|---|---|---|
| Parameter | Men | Women | Men | Women | Men | Women | Men | Women |
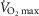 (% max) (% max) |
70 ± 3 | 72 ± 3 | 83 ± 1 | 83 ± 2 | 92 ± 1 | 92 ± 2 | 100 | 100 |
| VT (l) | 3.1 ± 0.2 | 1.7 ± 0.1* | 3.3 ± 0.2 | 1.8 ± 0.1* | 3.3 ± 0.2 | 1.9 ± 0.1* | 3.1 ± 0.2 | 1.8 ± 0.1* |
| fR (breaths min−1) | 27 ± 2 | 34 ± 2* | 33 ± 2 | 39 ± 2 | 41 ± 3 | 46 ± 2 | 53 ± 2 | 60 ± 2* |
| ΔHR (beats min−1) | 0 ± 3 | −2 ± 3 | 7 ± 2 | 6 ± 4 | 7 ± 1 | 11 ± 3 | 20 ± 3 | 25 ± 3 |
| tE/ttot | 0.55 ± 0.01 | 0.55 ± 0.01 | 0.54 ± 0.01 | 0.55 ± 0.01 | 0.53 ± 0.01 | 0.55 ± 0.01 | 0.51 ± 0.01 | 0.53 ± 0.01 |
 (mmHg) (mmHg) |
40 ± 1 | 37 ± 1 | 40 ± 1 | 37 ± 1 | 35 ± 1 | 36 ± 1 | 31 ± 2 | 35 ± 2 |
 (l min−1) (l min−1) |
81 ± 7 | 55 ± 2* | 106 ± 5 | 71 ± 3* | 133 ± 7 | 88 ± 3* | 165 ± 4 | 109 ± 4* |
 / /  (ml O2 l−1) (ml O2 l−1) |
1.4 ± 0.1 | 1.5 ± 0.1 | 1.7 ± 0.1 | 1.9 ± 0.1 | 1.7 ± 0.1 | 2.4 ± 0.2* | 2.4 ± 0.2 | 3.5 ± 0.3* |
 /WOB (ml O2 J−1) /WOB (ml O2 J−1) |
0.8 ± 0.1 | 1.1 ± 0.1* | 0.8 ± 0.05 | 1.2 ± 0.1* | 0.7 ± 0.04 | 1.1 ± 0.1* | 0.7 ± 0.0 | 1.1 ± 0.1* |
| EfficiencyRM (%) | 6.7 ± 0.5 | 5.1 ± 0.6* | 6.4 ± 0.4 | 4.4 ± 0.3* | 7.7 ± 0.94 | 4.8 ± 0.3* | 7.2 ± 0.2 | 4.6 ± 0.4* |
| ΔPoe (cmH2O) | 24 ± 1 | 19 ± 2 | 31 ± 2 | 24 ± 2 | 39 ± 3 | 32 ± 2 | 55 ± 4 | 47 ± 3* |
| WOB (J min−1) | 142 ± 21 | 75 ± 8 | 232 ± 23 | 122 ± 12* | 347 ± 38 | 199 ± 15* | 593 ± 60 | 339 ± 27* |
| WOBres (% total) | 40 ± 3 | 51 ± 5* | 57 ± 3 | 54 ± 5 | 65 ± 3 | 65 ± 4 | 76 ± 2 | 76 ± 3 |
| WOBel (% total) | 60 ± 3 | 49 ± 6* | 43 ± 3 | 46 ± 5 | 35 ± 3 | 35 ± 4 | 24 ± 2 | 24 ± 3 |
| EELV (% FVC) | 35 ± 2 | 44 ± 3* | 33 ± 1 | 44 ± 3* | 32 ± 1 | 43 ± 2* | 38 ± 2 | 46 ± 3* |
| EILV (% FVC) | 83 ± 2 | 83 ± 3 | 85 ± 2 | 86 ± 3 | 85 ± 2 | 88 ± 2 | 87 ± 1 | 88 ± 1 |
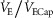 (%) (%) |
38 ± 3 | 33 ± 3 | 52 ± 3 | 40 ± 3* | 67 ± 3 | 51 ± 3* | 74 ± 4 | 67 ± 4 |
Abbreviations: EELV, end-expiratory lung volume; EfficiencyRM, efficiency of the respiratory muscles determined by dividing the measured  by the calculated ideal oxygen uptake needed to perform the work; EILV, end-inspiratory lung volume; fR, breathing frequency; ΔHR, change in heart rate from rest to mimic;
by the calculated ideal oxygen uptake needed to perform the work; EILV, end-inspiratory lung volume; fR, breathing frequency; ΔHR, change in heart rate from rest to mimic;  , end-tidal carbon dioxide tension; ΔPoe, oesophageal pressure swing; tE, expiratory time; ttot, total breath time;
, end-tidal carbon dioxide tension; ΔPoe, oesophageal pressure swing; tE, expiratory time; ttot, total breath time;  , minute ventilation;
, minute ventilation;  , ventilatory capacity;
, ventilatory capacity;  , maximal oxygen uptake; VT, tidal volume;
, maximal oxygen uptake; VT, tidal volume;  , respiratory muscle oxygen uptake; WOB, work of breathing; WOBel, elastic work of breathing; and WOBres, resistive work of breathing. *Significantly different from men (P < 0.05).
, respiratory muscle oxygen uptake; WOB, work of breathing; WOBel, elastic work of breathing; and WOBres, resistive work of breathing. *Significantly different from men (P < 0.05).
Results
Subject characteristics and cardiorespiratory responses
Subject characteristics are presented in Table1. Maximal cardiorespiratory and respiratory mechanics values are presented in Table2. At peak exercise, men had significantly greater absolute and relative oxygen uptake, carbon dioxide output, tidal volume and  (P < 0.05), but there were no differences in respiratory exchange ratio, heart rate, end-tidal CO2, ventilatory equivalents or operational lung volumes (P > 0.05). At maximal exercise, men had significantly greater WOB owing to a greater
(P < 0.05), but there were no differences in respiratory exchange ratio, heart rate, end-tidal CO2, ventilatory equivalents or operational lung volumes (P > 0.05). At maximal exercise, men had significantly greater WOB owing to a greater  (P < 0.05). For a given
(P < 0.05). For a given  , however, women had a greater WOB due to a significantly greater resistive component (P < 0.05; Figs 2 and 3).
, however, women had a greater WOB due to a significantly greater resistive component (P < 0.05; Figs 2 and 3).
Table 2.
Cardiorespiratory values at maximal exercise
| Parameter | Men (n = 9) | Women (n = 9) |
|---|---|---|
 (l min−1) (l min−1) |
4.4 ± 0.2 | 2.8 ± 0.2* |
 (ml kg−1 min−1) (ml kg−1 min−1) |
58.7 ± 1.9 | 48.1 ± 2.1* |
| Range | 50.3–68.5 | 41.4–60.4 |
 (l min−1) (l min−1) |
4.8 ± 0.2 | 3.0 ± 0.1* |
| VT (l) | 3.1 ± 0.1 | 1.9 ± 0.1* |
| fR (breaths min−1) | 56 ± 3 | 61 ± 3 |
 (l min−1) (l min−1) |
173 ± 10 | 114 ± 4* |
| RER | 1.11 ± 0.02 | 1.10 ± 0.02 |
| HR (beats min−1) | 183 ± 2 | 189 ± 3 |
 (mmHg) (mmHg) |
28 ± 1 | 28 ± 1 |
 |
36 ± 1 | 38 ± 1 |
 |
40 ± 2 | 42 ± 2 |
| EELV (% FVC) | 40 ± 2 | 43 ± 2 |
| EILV (% FVC) | 88 ± 1 | 87 ± 1 |
| ΔPoe (cmH2O) | 54 ± 4 | 46 ± 1* |
| WOB (J min−1) | 605 ± 59 | 354 ± 19* |
| PTPoe (cmH2O s−1 min−1) | 606 ± 35 | 500 ± 30* |
| PTPdi (cmH2O s−1 min−1) | 457 ± 44 | 406 ± 75 |
| PTPoe/PTPdi | 0.77 ± 0.07 | 0.84 ± 0.11 |
 (l min−1) (l min−1) |
220 ± 15 | 164 ± 9* |
 (%) (%) |
80 ± 3 | 72 ± 5 |
| EFL (%) | 23 ± 9 | 21 ± 8 |
| EFL (n) | 5 | 5 |
Abbreviations: EELV, end-expiratory lung volume; EFL, expiratory flow limitation; EILV, end-inspiratory lung volume; fR, breathing frequency; HR, heart rate;  , end-tidal carbon dioxide tension; ΔPoe, oesophageal pressure swing; PTPoe, oesophageal pressure–time product; PTPdi, diaphragmatic pressure–time product; RER, respiratory exchange ratio;
, end-tidal carbon dioxide tension; ΔPoe, oesophageal pressure swing; PTPoe, oesophageal pressure–time product; PTPdi, diaphragmatic pressure–time product; RER, respiratory exchange ratio;  , carbon dioxide output;
, carbon dioxide output;  , expired minute ventilation;
, expired minute ventilation;  , ventilatory capacity;
, ventilatory capacity;  , oxygen uptake; VT, tidal volume; and WOB, work of breathing. *Significantly different from men (P < 0.05).
, oxygen uptake; VT, tidal volume; and WOB, work of breathing. *Significantly different from men (P < 0.05).
Figure 2. Relationship between work of breathing and minute ventilation during voluntary hyperpnoea.

The work of breathing is significantly greater in women at and above a ventilation of ∼75 l min−1. Abbreviations:  , expired minute ventilation; and WOB, work of breathing.
, expired minute ventilation; and WOB, work of breathing.
Figure 3. Relationship between elastic (A) or resistive work of breathing (B) and minute ventilation.

*Greater resistive work of breathing in women at isoventilation (P < 0.05). †Significantly greater elastic work of breathing and resistive work of breathing in men when maximal exercise ventilations are compared (P < 0.05). Abbreviation:  , expired minute ventilation.
, expired minute ventilation.
Work of breathing and 
Figure 4 shows the absolute and relative  at different absolute and relative ventilations. While there was minimal within-subject variability for
at different absolute and relative ventilations. While there was minimal within-subject variability for  , there was greater between-subject variability, which was more pronounced at higher ventilations (Fig. 4A). At a
, there was greater between-subject variability, which was more pronounced at higher ventilations (Fig. 4A). At a  of ∼95 l min−1, for example, the
of ∼95 l min−1, for example, the  in women ranged from 200 to 400 ml min−1. The variability in
in women ranged from 200 to 400 ml min−1. The variability in  is explained by differences in the WOB vs.
is explained by differences in the WOB vs. 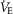 relationship, which is dependent on airway size and was presumably different in our groups. The order of trials had no effect on
relationship, which is dependent on airway size and was presumably different in our groups. The order of trials had no effect on  . Specifically, when replicating maximal exercise ventilation, the average
. Specifically, when replicating maximal exercise ventilation, the average  for all subjects was not statistically different across trials (381 ± 32, 377 ± 31, 404 ± 34 and 389 ± 31 ml O2 min−1 for the 1st–4th trials, respectively; P > 0.05). There was also no effect of order when the subjects were grouped by sex (P > 0.05). At an isoventilation of ∼55 l min−1, women had a greater absolute
for all subjects was not statistically different across trials (381 ± 32, 377 ± 31, 404 ± 34 and 389 ± 31 ml O2 min−1 for the 1st–4th trials, respectively; P > 0.05). There was also no effect of order when the subjects were grouped by sex (P > 0.05). At an isoventilation of ∼55 l min−1, women had a greater absolute  (P < 0.05; Fig. 4B). The group mean coefficient of variation for
(P < 0.05; Fig. 4B). The group mean coefficient of variation for 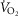 was 4.9, 4.9, 5.3 and 6.0% at 45, 60, 75 and 100%
was 4.9, 4.9, 5.3 and 6.0% at 45, 60, 75 and 100%  , respectively, with no difference between the sexes (P > 0.05). When compared at relative ventilations, men and women had a similar absolute
, respectively, with no difference between the sexes (P > 0.05). When compared at relative ventilations, men and women had a similar absolute  (P > 0.05; Fig. 4C), but this represented a greater fraction of whole-body
(P > 0.05; Fig. 4C), but this represented a greater fraction of whole-body 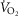 in women at 75 and 100%
in women at 75 and 100%  (Fig. 4D). At maximal exercise,
(Fig. 4D). At maximal exercise,  represented 13.8% of whole-body
represented 13.8% of whole-body  in women and 9.4% in men (P < 0.05; Fig. 5).
in women and 9.4% in men (P < 0.05; Fig. 5).
Figure 4. Respiratory muscle oxygen uptake at absolute and relative ventilations.

A, average oxygen uptake for each stage of voluntary hyperpnoea performed by each subject. B, regression lines for men and women performing voluntary hyperpnoea trials. The asterisk, vertical line and arrow indicate that women have a significantly higher 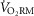 above a ventilation of ∼55 l min−1. The regression was fitted using the average of each subject's constants. C, absolute
above a ventilation of ∼55 l min−1. The regression was fitted using the average of each subject's constants. C, absolute  at different percentages of maximal ventilation. Men had significantly greater ventilations at every comparison (see also Table3). D,
at different percentages of maximal ventilation. Men had significantly greater ventilations at every comparison (see also Table3). D,  as a percentage of whole-body oxygen uptake at different percentages of maximal ventilation. All average values are means ± SEM. Abbreviations:
as a percentage of whole-body oxygen uptake at different percentages of maximal ventilation. All average values are means ± SEM. Abbreviations:  , expired minute ventilation;
, expired minute ventilation; 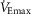 , maximal minute ventilation;
, maximal minute ventilation;  , oxygen uptake;
, oxygen uptake;  , maximal oxygen uptake; and
, maximal oxygen uptake; and 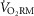 , oxygen uptake of the respiratory muscles. *Significantly greater in women (P < 0.05).
, oxygen uptake of the respiratory muscles. *Significantly greater in women (P < 0.05).
Figure 5. Box-and-whisker plot, showing individual subject data and group mean ± SEM for  as a percentage of whole-body oxygen uptake at maximal exercise in women and men.
as a percentage of whole-body oxygen uptake at maximal exercise in women and men.

Squares in the box-and-whisker plot represent 5th and 95th percentiles, and the horizontal line is the median. Abbreviations:  , oxygen uptake;
, oxygen uptake;  , maximal oxygen uptake; and
, maximal oxygen uptake; and  , oxygen uptake of the respiratory muscles. *Significantly greater compared with men (P < 0.05).
, oxygen uptake of the respiratory muscles. *Significantly greater compared with men (P < 0.05).
Without exception, every subject demonstrated a significant linear relationship between  and WOB. During maximal intensity trials, however, the
and WOB. During maximal intensity trials, however, the  rose out of proportion to the increase in WOB (Fig. 6). When WOB was related to
rose out of proportion to the increase in WOB (Fig. 6). When WOB was related to  as a percentage of total whole-body
as a percentage of total whole-body  , the average slope of the regression line was significantly greater in women (P < 0.05; Fig. 6B).
, the average slope of the regression line was significantly greater in women (P < 0.05; Fig. 6B).
Figure 6. Oxygen uptake of the respiratory muscles in absolute (A) and relative units (B) at different WOB for each subject performing each trial.

Group regression lines were developed by averaging each subject's regression and producing a composite. All subjects demonstrated a significant relationship between  and WOB (P < 0.01). For A, there was no difference in the intercepts of the regression lines, but women had a significantly greater slope (1.29 ± 0.12 ml J vs. 0.85 ± 0.05 ml J, P < 0.05). Likewise, for B, there was no difference in the intercepts of the lines, but women had a significantly greater slope (0.034 ± 0.002 %
and WOB (P < 0.01). For A, there was no difference in the intercepts of the regression lines, but women had a significantly greater slope (1.29 ± 0.12 ml J vs. 0.85 ± 0.05 ml J, P < 0.05). Likewise, for B, there was no difference in the intercepts of the lines, but women had a significantly greater slope (0.034 ± 0.002 % vs. 0.012 ± 0.001 %
vs. 0.012 ± 0.001 % , P < 0.05). Abbreviations:
, P < 0.05). Abbreviations:  , oxygen uptake;
, oxygen uptake;  , oxygen uptake of the respiratory muscles; and WOB, work of breathing.
, oxygen uptake of the respiratory muscles; and WOB, work of breathing.
Table3 displays variables for men and women during the voluntary hyperpnoea trials at different percentages of  . Men had greater absolute
. Men had greater absolute  for all comparisons (P < 0.05), primarily due to greater tidal volume. The changes from rest to hyperpnoea in heart rate, expiratory duty cycle and end-tidal carbon dioxide tension were not different between the sexes at any percentage of
for all comparisons (P < 0.05), primarily due to greater tidal volume. The changes from rest to hyperpnoea in heart rate, expiratory duty cycle and end-tidal carbon dioxide tension were not different between the sexes at any percentage of  (P > 0.05). The
(P > 0.05). The  /
/ relationship increased in both sexes as
relationship increased in both sexes as  increased, and men were statistically lower at 75 and 100%
increased, and men were statistically lower at 75 and 100%  (P < 0.05). The calculated efficiency of the respiratory muscles was greater in men at all ventilations (P < 0.05). Women performed the hyperpnoea trials at significantly higher end-expiratory lung volume (P < 0.05), with no difference in end-inspiratory lung volume (P > 0.05).
(P < 0.05). The calculated efficiency of the respiratory muscles was greater in men at all ventilations (P < 0.05). Women performed the hyperpnoea trials at significantly higher end-expiratory lung volume (P < 0.05), with no difference in end-inspiratory lung volume (P > 0.05).
Expiratory flow limitation
The effect of stratifying the subjects based on the appearance of EFL on  is shown in Fig. 7 and Table4. There were similar numbers of men and women in each group, and there was no difference between the percentage of EFL during exercise or hyperpnoea for either sex (men, 24 ± 9 vs. 23 ± 9% and women, 22 ± 8 vs. 18 ± 6%, for exercise and hyperpnoea, respectively; P > 0.05 for both). The MEFV curve was significantly larger in the group that did not develop EFL (P < 0.05; Fig. 7C). There were no differences in
is shown in Fig. 7 and Table4. There were similar numbers of men and women in each group, and there was no difference between the percentage of EFL during exercise or hyperpnoea for either sex (men, 24 ± 9 vs. 23 ± 9% and women, 22 ± 8 vs. 18 ± 6%, for exercise and hyperpnoea, respectively; P > 0.05 for both). The MEFV curve was significantly larger in the group that did not develop EFL (P < 0.05; Fig. 7C). There were no differences in 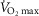 or
or  (P < 0.05); however, the
(P < 0.05); however, the  was significantly greater in the EFL group during the maximal ventilation trials (P < 0.05; Fig. 7B). At ≤75%
was significantly greater in the EFL group during the maximal ventilation trials (P < 0.05; Fig. 7B). At ≤75% 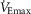 , where there was no or minimal EFL (three subjects, <20% overlap with the MEFV curve), the
, where there was no or minimal EFL (three subjects, <20% overlap with the MEFV curve), the  and
and  were similar between groups (Fig. 7B). Although the absolute WOB was not different between groups (485 ± 63 vs. 443 ± 63 J min−1, P > 0.05), the resistive work contributed a significantly greater percentage in the EFL group (79 ± 1 vs. 72 ± 2% of total WOB, P < 0.05) for the EFL and NEFL groups, respectively. At all ventilations lower than 100%, there were no differences between the EFL and NEFL groups (Table4). At 100%
were similar between groups (Fig. 7B). Although the absolute WOB was not different between groups (485 ± 63 vs. 443 ± 63 J min−1, P > 0.05), the resistive work contributed a significantly greater percentage in the EFL group (79 ± 1 vs. 72 ± 2% of total WOB, P < 0.05) for the EFL and NEFL groups, respectively. At all ventilations lower than 100%, there were no differences between the EFL and NEFL groups (Table4). At 100%  , the EFL group had a greater breathing frequency and
, the EFL group had a greater breathing frequency and  per
per  and a lower estimated respiratory muscle efficiency (P < 0.05; Table4).
and a lower estimated respiratory muscle efficiency (P < 0.05; Table4).
Figure 7. Composite average maximal expiratory flow -volume (MEFV) curves for subjects displaying expiratory flow limitation (EFL; A) and those with no expiratory flow limitation (NEFL; C) along with respiratory muscle oxygen uptake at relative ventilations.

Placed within the MEFV curves are resting (thin continuous lines) and the 100%  tidal flow–volume loops during maximal exercise and voluntary hyperpnoea. There was no difference in
tidal flow–volume loops during maximal exercise and voluntary hyperpnoea. There was no difference in  or
or  between the groups. At 100%
between the groups. At 100%  , the EFL group had a greater
, the EFL group had a greater  , whether expressed as the absolute value (B) or as a percentage of
, whether expressed as the absolute value (B) or as a percentage of  (13.5 vs. 9.2%
(13.5 vs. 9.2%  for the EFL and NEFL groups, P < 0.05). Abbreviations:
for the EFL and NEFL groups, P < 0.05). Abbreviations:  , maximal minute ventilation;
, maximal minute ventilation;  , maximal oxygen uptake; and
, maximal oxygen uptake; and  , oxygen uptake of the respiratory muscles. Values in B are means ± SEM. *Significantly higher in EFL vs. NEFL (P < 0.05).
, oxygen uptake of the respiratory muscles. Values in B are means ± SEM. *Significantly higher in EFL vs. NEFL (P < 0.05).
Table 4.
Cardiorespiratory variables during voluntary hyperpnoea at different percentages of maximal exercise ventilation for the EFL group (n = 10, 50% men) and NEFL group (n = 8, 50% men)
45% 
|
60% 
|
75% 
|
100% 
|
|||||
|---|---|---|---|---|---|---|---|---|
| Parameter | EFL | NEFL | EFL | NEFL | EFL | NEFL | EFL | NEFL |
Percentage of 
|
74 ± 2 | 69 ± 4 | 83 ± 1 | 84 ± 2 | 93 ± 1 | 92 ± 1 | 100 | 100 |
| VT (l) | 2.2 ± 0.2 | 2.5 ± 0.4 | 2.4 ± 0.2 | 2.7 ± 0.4 | 2.4 ± 0.2 | 2.8 ± 0.4 | 1.9 ± 0.3 | 2.6 ± 0.3 |
| fR (breaths min−1) | 32 ± 2 | 28 ± 3 | 37 ± 2 | 35 ± 3 | 44 ± 2 | 43 ± 3 | 60 ± 2* | 53 ± 3 |
| ΔHR (beats min−1) | −3 ± 3 | 2 ± 3 | 8 ± 2 | 4 ± 3 | 10 ± 2 | 6 ± 2 | 24 ± 3 | 19 ± 3 |
| tE/ttot | 0.56 ± 0.01 | 0.54 ± 0.01 | 0.56 ± 0.01 | 0.53 ± 0.01 | 0.54 ± 0.01 | 0.53 ± 0.01 | 0.53 ± 0.01 | 0.52 ± 0.01 |
 (mmHg) (mmHg) |
38 ± 1 | 39 ± 1 | 39 ± 1 | 38 ± 1 | 36 ± 1 | 32 ± 2 | 33 ± 2 | 34 ± 2 |
 (l min−1) (l min−1) |
69 ± 6 | 67 ± 8 | 87 ± 6 | 91 ± 9 | 108 ± 9 | 114 ± 10 | 136 ± 11 | 138 ± 12 |
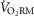 / / 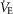 (ml O2 l−1) (ml O2 l−1) |
1.4 ± 0.1 | 1.5 ± 0.1 | 1.8 ± 0.1 | 1.8 ± 0.1 | 2.2 ± 0.3 | 2.0 ± 0.1 | 3.3 ± 0.4* | 2.4 ± 0.1 |
 /WOB (ml O2 J−1) /WOB (ml O2 J−1) |
0.9 ± 0.1 | 1.0 ± 0.2 | 1.0 ± 0.1 | 1.0 ± 0.1 | 0.9 ± 0.1 | 0.8 ± 0.1 | 1.0 ± 0.1 | 0.8 ± 0.1 |
| EfficiencyRM (%) | 4.0 ± 0.8 | 4.7 ± 0.5 | 4.7 ± 0.8 | 4.4 ± 0.9 | 4.5 ± 1.0 | 6.2 ± 0.7 | 4.6 ± 0.8* | 6.2 ± 0.5 |
| ΔPoe (cmH2O) | 23 ± 1 | 20 ± 2 | 28 ± 2 | 28 ± 3 | 35 ± 2 | 37 ± 3 | 52 ± 3 | 49 ± 3 |
| WOB (J min−1) | 114 ± 15 | 108 ± 25 | 168 ± 19 | 183 ± 33 | 265 ± 33 | 296 ± 45 | 485 ± 63 | 443 ± 67 |
| WOBres (% total) | 50 ± 5 | 43 ± 2 | 56 ± 4 | 54 ± 4 | 67 ± 3 | 64 ± 4 | 79 ± 1* | 72 ± 2 |
| WOBel (% total) | 50 ± 6 | 57 ± 5 | 44 ± 4 | 46 ± 4 | 33 ± 3 | 36 ± 4 | 21 ± 1 | 27 ± 2 |
| EELV (% FVC) | 40 ± 3 | 39 ± 3 | 40 ± 3 | 38 ± 3 | 39 ± 3 | 37 ± 2 | 43 ± 2 | 42 ± 2 |
| EILV (% FVC) | 83 ± 2 | 83 ± 3 | 86 ± 2 | 85 ± 3 | 86 ± 2 | 86 ± 2 | 86 ± 1 | 89 ± 2 |
 (%) (%) |
39 ± 3 | 31 ± 3 | 48 ± 3 | 43 ± 4 | 63 ± 4 | 54 ± 5 | 77 ± 2* | 63 ± 3 |
Abbreviations: EFL, expiratory flow limited; NEFL, non-expiratory flow limited; other abbreviations are as for Table3. *Significantly different from NEFL (P < 0.05).
Discussion
Major findings
The major findings from this study are threefold. First, women have a greater absolute  during submaximal and maximal rates of exercise hyperpnoea. Second, during strenuous and maximal exercise, the
during submaximal and maximal rates of exercise hyperpnoea. Second, during strenuous and maximal exercise, the  represents a significantly greater fraction of whole-body
represents a significantly greater fraction of whole-body 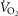 in women. Finally, regardless of sex, those who develop EFL at maximal exercise have a greater
in women. Finally, regardless of sex, those who develop EFL at maximal exercise have a greater  . Collectively, our findings indicate that the greater WOB and increased mechanical ventilatory constraints in women result in a greater absolute and relative
. Collectively, our findings indicate that the greater WOB and increased mechanical ventilatory constraints in women result in a greater absolute and relative  at submaximal and maximal exercise intensities.
at submaximal and maximal exercise intensities.
Sex differences in 
We demonstrated that women have a greater absolute and relative 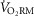 compared with men. Given that
compared with men. Given that  is linearly related to work, we hypothesized that at ventilations where women have a greater WOB than men, their
is linearly related to work, we hypothesized that at ventilations where women have a greater WOB than men, their  would also be greater. Indeed, we found that women had a greater WOB at submaximal levels of
would also be greater. Indeed, we found that women had a greater WOB at submaximal levels of  (Fig. 2), a finding consistent with work from our laboratory (Guenette et al. 2007) and others (Wanke et al. 1991). The differences in WOB were due to increased resistive work (Fig. 3). We found differences in
(Fig. 2), a finding consistent with work from our laboratory (Guenette et al. 2007) and others (Wanke et al. 1991). The differences in WOB were due to increased resistive work (Fig. 3). We found differences in  between the sexes at ∼55 l min−1, which coincides with the
between the sexes at ∼55 l min−1, which coincides with the  at which the WOB is greater in women. A ventilation of ∼55 l min−1 was achieved during submaximal exercise in both men and women, but represented a greater fraction of maximal ventilation in women. Therefore, we also used relative units to compare the sexes at similar fractions of
at which the WOB is greater in women. A ventilation of ∼55 l min−1 was achieved during submaximal exercise in both men and women, but represented a greater fraction of maximal ventilation in women. Therefore, we also used relative units to compare the sexes at similar fractions of  . As shown in Fig. 4C, when compared at similar relative
. As shown in Fig. 4C, when compared at similar relative  , there were no differences in the absolute
, there were no differences in the absolute  . However, for the comparisons presented in Fig. 4C, the men had a significantly greater
. However, for the comparisons presented in Fig. 4C, the men had a significantly greater  and their whole-body
and their whole-body 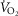 was also greater (Table3). To elucidate the comparisons fully, the units on each axis should be relative, as shown in Fig. 4D, which displays the comparison between relative
was also greater (Table3). To elucidate the comparisons fully, the units on each axis should be relative, as shown in Fig. 4D, which displays the comparison between relative  and relative
and relative  . At ≥75%
. At ≥75%  (∼90% of
(∼90% of  ), a significantly greater fraction of whole-body
), a significantly greater fraction of whole-body  was dedicated to the respiratory muscles in women. If the respiratory muscles in women command a greater percentage of cardiac output, then blood flow to locomotor muscles may become compromised (see ‘Perspectives’).
was dedicated to the respiratory muscles in women. If the respiratory muscles in women command a greater percentage of cardiac output, then blood flow to locomotor muscles may become compromised (see ‘Perspectives’).
Both sexes showed an increase in the unit rate of  per
per  and were not different at 45 and 60% of
and were not different at 45 and 60% of  . The progressive increase in
. The progressive increase in  /
/ as ventilation increases towards higher levels has been observed by others (Aaron et al. 1992b; Coast et al. 1993). At 75% of
as ventilation increases towards higher levels has been observed by others (Aaron et al. 1992b; Coast et al. 1993). At 75% of  , however, women showed a marked increase in
, however, women showed a marked increase in  /
/ , whereas men demonstrated a dramatic increase only between 75 and 100%
, whereas men demonstrated a dramatic increase only between 75 and 100%  (Table3). While the
(Table3). While the  /
/ increased with ventilation, the
increased with ventilation, the  vs. WOB relationship did not change systematically in either sex and was consistently greater in women. Despite a linear relationship for
vs. WOB relationship did not change systematically in either sex and was consistently greater in women. Despite a linear relationship for  vs. WOB in both sexes, the efficiency of the respiratory muscles was significantly lower in women, and this finding was most pronounced at maximal exercise (Table3).
vs. WOB in both sexes, the efficiency of the respiratory muscles was significantly lower in women, and this finding was most pronounced at maximal exercise (Table3).
While our study was not designed to determine the mechanism behind sex-based differences in respiratory muscle efficiency, our observations merit brief comment. Our primary concern with determining efficiency is that we are unable to account for all of the work done during breathing. For example, abdominal muscles contract to stabilize the abdominal wall during forceful expiration (De Troyer & Boriek, 2011), and work is done when the chest wall is distorted at near-maximal ventilations (>75% of  ; Grimby et al. 1968). Indeed, the work done to stabilize the abdominal wall and distort the chest wall is estimated to be upwards of 25% of total WOB (Goldman et al. 1976). A further consideration is that the velocity of muscle shortening would have been greater in the women due to a higher maximal breathing frequency (Table3), and this could also have necessitated greater work in order to stabilize the abdominal wall. Furthermore, the greater shortening velocity in women would be expected to result in an increased energy requirement (McCool et al. 1986, 1989). Accordingly, we cautiously speculate that decreased respiratory muscle efficiency in women could arise from sex differences in substrate utilization and morphology (Miller et al. 1993; Hicks et al. 2001) and/or blood vessel compression when the WOB is near maximal (Hunter, 2014).
; Grimby et al. 1968). Indeed, the work done to stabilize the abdominal wall and distort the chest wall is estimated to be upwards of 25% of total WOB (Goldman et al. 1976). A further consideration is that the velocity of muscle shortening would have been greater in the women due to a higher maximal breathing frequency (Table3), and this could also have necessitated greater work in order to stabilize the abdominal wall. Furthermore, the greater shortening velocity in women would be expected to result in an increased energy requirement (McCool et al. 1986, 1989). Accordingly, we cautiously speculate that decreased respiratory muscle efficiency in women could arise from sex differences in substrate utilization and morphology (Miller et al. 1993; Hicks et al. 2001) and/or blood vessel compression when the WOB is near maximal (Hunter, 2014).
How do our results for  in men vs. women compare with previous reports? To date, sex differences in
in men vs. women compare with previous reports? To date, sex differences in  have been found in some (Eckermann & Millahn, 1962; Topin et al. 2003) but not all studies (Lorenzo & Babb, 2012). Eckermann & Millahn (1962) assessed ventilations up to ∼120 l min−1 and concluded that women had a greater
have been found in some (Eckermann & Millahn, 1962; Topin et al. 2003) but not all studies (Lorenzo & Babb, 2012). Eckermann & Millahn (1962) assessed ventilations up to ∼120 l min−1 and concluded that women had a greater  . However, the absolute values for
. However, the absolute values for  reported in their study were excessive (>2 l min−1) and do not, therefore, represent a realistic estimation. Nonetheless, their estimation of respiratory muscle efficiency is similar to ours and others (Aaron et al. 1992a), and they noted that men may be more efficient. Although more recent estimates of
reported in their study were excessive (>2 l min−1) and do not, therefore, represent a realistic estimation. Nonetheless, their estimation of respiratory muscle efficiency is similar to ours and others (Aaron et al. 1992a), and they noted that men may be more efficient. Although more recent estimates of  appear to be reasonable, there is still no consensus on whether a sex difference is apparent (Topin et al. 2003; Lorenzo & Babb, 2012). A consideration when interpreting the two aforementioned studies is the narrow range of
appear to be reasonable, there is still no consensus on whether a sex difference is apparent (Topin et al. 2003; Lorenzo & Babb, 2012). A consideration when interpreting the two aforementioned studies is the narrow range of  investigated and the less than ideal replication of exercise breathing patterns (i.e. lack of oesophageal pressure). Specifically, both studies estimated the
investigated and the less than ideal replication of exercise breathing patterns (i.e. lack of oesophageal pressure). Specifically, both studies estimated the  in women at a
in women at a  ≤ 60 l min−1, which is approximately the threshold we found for a sex difference in
≤ 60 l min−1, which is approximately the threshold we found for a sex difference in  . Therefore, even with precise replication of exercise breathing patterns, a significant sex effect may have been masked by the low
. Therefore, even with precise replication of exercise breathing patterns, a significant sex effect may have been masked by the low  . A more suitable comparison for our data would be the work of Aaron et al (1992a,b1992). As in the present study, those authors accurately mimicked exercise breathing patterns, matched the WOB and had subjects perform multiple trials across a wide range of
. A more suitable comparison for our data would be the work of Aaron et al (1992a,b1992). As in the present study, those authors accurately mimicked exercise breathing patterns, matched the WOB and had subjects perform multiple trials across a wide range of  . When comparing our male subjects with those of Aaron et al (1992a,b1992; who tested seven men and one woman), our absolute
. When comparing our male subjects with those of Aaron et al (1992a,b1992; who tested seven men and one woman), our absolute  was similar, and we both estimated that maximal
was similar, and we both estimated that maximal  accounts for ∼10% of whole-body
accounts for ∼10% of whole-body  . The lone female subject in the previous study (Aaron et al. 1992b) had a
. The lone female subject in the previous study (Aaron et al. 1992b) had a  that represented ∼15% of whole-body
that represented ∼15% of whole-body 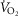 , which is commensurate with our results for women (13.8 ± 1.5% of
, which is commensurate with our results for women (13.8 ± 1.5% of  ). Notably, one woman in our study had a
). Notably, one woman in our study had a  representing 24% of total
representing 24% of total  for two trials (Fig. 5), which is remarkable considering others have shown that active muscle tissue can account for upwards of 85% of
for two trials (Fig. 5), which is remarkable considering others have shown that active muscle tissue can account for upwards of 85% of  (Poole et al. 1992). While we note that 24% is on the upper limit of expected respiratory muscle oxygen uptake, others have reported similar values (Aaron et al. 1992b). As expected, this subject developed EFL, used 85% of their ventilatory capacity and had an end-exercise
(Poole et al. 1992). While we note that 24% is on the upper limit of expected respiratory muscle oxygen uptake, others have reported similar values (Aaron et al. 1992b). As expected, this subject developed EFL, used 85% of their ventilatory capacity and had an end-exercise  and WOB that was greater than the female average. Thus, many of the predisposing factors associated with sympathetically mediated blood flow redistribution are present, and there is evidence from animal models to suggest that the diaphragm may be less sensitive to vasoconstrictor activity (Aaker & Laughlin, 2002). Given the above influencing factors and the repeatability of our measures, we are confident that our values are physiological. Overall, we conclude that women have a greater
and WOB that was greater than the female average. Thus, many of the predisposing factors associated with sympathetically mediated blood flow redistribution are present, and there is evidence from animal models to suggest that the diaphragm may be less sensitive to vasoconstrictor activity (Aaker & Laughlin, 2002). Given the above influencing factors and the repeatability of our measures, we are confident that our values are physiological. Overall, we conclude that women have a greater  than men as a result of a greater WOB and decreased efficiency.
than men as a result of a greater WOB and decreased efficiency.
Mechanical ventilatory constraints
To investigate the role of mechanical ventilatory constraints on  , we categorized our subjects based upon the occurrence or absence of EFL during exercise. Expiratory flow limitation occurs when maximal flow plateaus despite an increase in driving pressure (Hyatt, 1983) and can arise in young healthy subjects during intense aerobic exercise (Johnson et al. 1999; Babb, 2013). The occurrence of EFL during exercise is associated with an increase in operational lung volumes (Pellegrino et al. 1993), exacerbated exercise-induced arterial hypoxaemia (Dominelli et al. 2013) and reduced exercise performance (Iandelli et al. 2002). Others have theorized that EFL is associated with a greater
, we categorized our subjects based upon the occurrence or absence of EFL during exercise. Expiratory flow limitation occurs when maximal flow plateaus despite an increase in driving pressure (Hyatt, 1983) and can arise in young healthy subjects during intense aerobic exercise (Johnson et al. 1999; Babb, 2013). The occurrence of EFL during exercise is associated with an increase in operational lung volumes (Pellegrino et al. 1993), exacerbated exercise-induced arterial hypoxaemia (Dominelli et al. 2013) and reduced exercise performance (Iandelli et al. 2002). Others have theorized that EFL is associated with a greater  (Aaron et al. 1992b), but no specific data were presented to support this postulate. We found that those who developed EFL during maximal exercise had a greater
(Aaron et al. 1992b), but no specific data were presented to support this postulate. We found that those who developed EFL during maximal exercise had a greater  (Fig. 7). In addition, our flow-limited and non-flow-limited groups had similar
(Fig. 7). In addition, our flow-limited and non-flow-limited groups had similar  ,
,  and WOB. At submaximal exercise, minimal if any EFL was present and, as such, there were no statistical differences between the groups for any parameter (Fig. 7). Furthermore, there was an equal distribution of sexes, and both groups successfully replicated their exercise breathing patterns. Others have shown that aerobic fitness does not predict who does and does not develop EFL (Smith et al. 2014), and we have argued that women develop EFL more often than men (Guenette et al. 2007). In the present study, however, we found an equal distribution of EFL between the sexes. We intentionally recruited trained men in an attempt to ensure a similar distribution of flow-limited subjects in order to address our primary research question (regarding sex differences in
and WOB. At submaximal exercise, minimal if any EFL was present and, as such, there were no statistical differences between the groups for any parameter (Fig. 7). Furthermore, there was an equal distribution of sexes, and both groups successfully replicated their exercise breathing patterns. Others have shown that aerobic fitness does not predict who does and does not develop EFL (Smith et al. 2014), and we have argued that women develop EFL more often than men (Guenette et al. 2007). In the present study, however, we found an equal distribution of EFL between the sexes. We intentionally recruited trained men in an attempt to ensure a similar distribution of flow-limited subjects in order to address our primary research question (regarding sex differences in  ) in the most conservative fashion. If we did not recruit trained men, we anticipated that few, if any, of the male subjects would have developed EFL and we would be less able to discern sex differences in
) in the most conservative fashion. If we did not recruit trained men, we anticipated that few, if any, of the male subjects would have developed EFL and we would be less able to discern sex differences in  accurately. In all cases, subjects who developed flow limitation during exercise did so during the hyperpnoea trials, and vice versa for the non-flow-limited subjects (Fig. 7). Therefore, variation in replicating spontaneous breathing patterns was not responsible for the greater
accurately. In all cases, subjects who developed flow limitation during exercise did so during the hyperpnoea trials, and vice versa for the non-flow-limited subjects (Fig. 7). Therefore, variation in replicating spontaneous breathing patterns was not responsible for the greater  noted in the flow-limited group.
noted in the flow-limited group.
While we did not make anatomical estimations of airway size in the present study, those who develop EFL are thought to have smaller airways (McClaran et al. 1998; Dominelli et al. 2011). Smaller airways are consistent with the greater resistive WOB noted at maximal exercise in the EFL subjects, despite the total WOB being similar between groups (Table4). As the maximal effective driving pressure for flow was approached, compression of the airways may have been initiated; a phenomenon termed ‘impending flow limitation’ (Mead et al. 1967; McClaran et al. 1998). The impending flow limitation could have altered breathing patterns via compression of airways, thereby resulting in a decreased efficiency of the respiratory muscles. Other factors that could explain the greater  in the EFL groups relate to those detailed above, such as chest-wall deformation, abdominal stabilization and greater muscle shortening velocity from an increased breathing frequency. The chest-wall deformation and abdominal stabilization could lead to a greater
in the EFL groups relate to those detailed above, such as chest-wall deformation, abdominal stabilization and greater muscle shortening velocity from an increased breathing frequency. The chest-wall deformation and abdominal stabilization could lead to a greater  through additional muscular contraction, yet the work may not be accounted for, which is seen by our similar WOB values (Table4).
through additional muscular contraction, yet the work may not be accounted for, which is seen by our similar WOB values (Table4).
Technical considerations
We considered the possibility that respiratory muscle fatigue during the hyperpnoea trials could have affected our results. High-intensity exercise to exhaustion has been shown to induce respiratory muscle fatigue (Johnson et al. 1993; Taylor et al. 2006), which may persist for up to 24 h (Laghi et al. 1995). Respiratory muscle fatigue is associated with a progressive increase in muscle sympathetic nerve activity (St Croix et al. 2000; Derchak et al. 2002), alterations in resting blood flow distribution (Sheel et al. 2001) and reduced exercise capacity (Harms et al. 2000; Taylor & Romer, 2008). Voluntary hyperpnoea has also been shown to elicit respiratory muscle fatigue (Renggli et al. 2008). Thus, it is conceivable that our subjects developed respiratory muscle fatigue and the associated neurovascular effects. In the absence of heavy exercise, however, respiratory muscle fatigue develops only when the WOB is significantly greater than that achieved during maximal exercise (Babcock et al. 1995). In our study, the mechanical WOB did not exceed the maximal exercise values in any of the hyperpnoea trials. Furthermore, the trials were relatively short and the subjects were provided with substantial rest between trials. Finally, we found no effect of trial order on our estimates of  . If respiratory muscle fatigue was present, we would expect a systematic temporal change in the ability to replicate the exercise breathing pattern and/or
. If respiratory muscle fatigue was present, we would expect a systematic temporal change in the ability to replicate the exercise breathing pattern and/or  . Consequently, it is unlikely that fatigue developed, and if it did, there does not appear to be any measurable effect on the
. Consequently, it is unlikely that fatigue developed, and if it did, there does not appear to be any measurable effect on the  .
.
During the voluntary hyperpnoea trials, there was a significant increase in heart rate beyond 60%  . As such, myocardial work probably contributed in small part to the observed increase in
. As such, myocardial work probably contributed in small part to the observed increase in  . However, the increases in heart rate were similar for men and women, and we presume, therefore, that cardiac
. However, the increases in heart rate were similar for men and women, and we presume, therefore, that cardiac  was also similar between the sexes.
was also similar between the sexes.
Sex-based comparisons
A difficult and often-encountered problem when desi-gning and interpreting studies regarding sex-based differences is how best to compare men and women. Men, on average, are taller than women and will therefore have greater absolute lung volumes and flows. Due to a greater muscle mass, men will generally achieve a higher absolute  and
and  . The principal issue here is whether to compare the sexes using absolute or relative values, a concern shared in other fields (Hart & Charkoudian, 2014; Hunter, 2014). In the present study, we made several comparisons in order to address specific questions individually and provide an overall interpretation collectively.
. The principal issue here is whether to compare the sexes using absolute or relative values, a concern shared in other fields (Hart & Charkoudian, 2014; Hunter, 2014). In the present study, we made several comparisons in order to address specific questions individually and provide an overall interpretation collectively.
Another confounding variable when assessing sex-based differences is whether subjects should be matched for one or more anthropometric or functional parameter (Sheel & Guenette, 2008); for example, men and women could be matched for height, lung size, lung function, aerobic fitness or body composition. However, matching may be best justified when attempting to isolate a single mechanism rather than attempting to understand the integrative whole-body response. For example, women appear to develop exercise-induced arterial hypoxaemia to a greater degree than men (Harms et al. 1998a; Dominelli et al. 2013), but when matched for height (and consequently lung size) and aerobic fitness the gas exchange disparity is minimized (Olfert et al. 2004). Another example illustrating the effect of scaling stems from the present study. The woman with the lowest  /
/  was the tallest and had the largest lung volumes and greatest flows of all the women. Despite her high aerobic fitness (60 ml kg−1 min−1), she did not develop EFL and her WOB was similar to the men. As such, when the sexes overlap in anatomical variables, the physiological sex differences appear to be minimized. However, we emphasize that many of the variables used to match men and women are themselves extensively influenced by sex. Matching men and women for lung size may allow for certain comparisons, but it would eliminate a consistent and population-wide sex-based difference, rendering the results less generalizable.
was the tallest and had the largest lung volumes and greatest flows of all the women. Despite her high aerobic fitness (60 ml kg−1 min−1), she did not develop EFL and her WOB was similar to the men. As such, when the sexes overlap in anatomical variables, the physiological sex differences appear to be minimized. However, we emphasize that many of the variables used to match men and women are themselves extensively influenced by sex. Matching men and women for lung size may allow for certain comparisons, but it would eliminate a consistent and population-wide sex-based difference, rendering the results less generalizable.
Perspectives
What are the implications of a greater  in women on the integrative physiological response to exercise? During maximal exercise in men, the WOB influences active leg blood flow and distribution of total cardiac output through a sympathetically mediated response (Harms et al. 1997, 1998b). As shown in Fig. 6B, the slope of the
in women on the integrative physiological response to exercise? During maximal exercise in men, the WOB influences active leg blood flow and distribution of total cardiac output through a sympathetically mediated response (Harms et al. 1997, 1998b). As shown in Fig. 6B, the slope of the 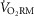 vs. WOB relationship is significantly greater in women compared with men. The greater slope of the
vs. WOB relationship is significantly greater in women compared with men. The greater slope of the  vs. WOB relationship in women indicates that for a given change in WOB, women have a greater change in the total
vs. WOB relationship in women indicates that for a given change in WOB, women have a greater change in the total  dedicated to the respiratory muscles. Therefore, it could be hypothesized that when compared with men, women may show greater changes in leg blood flow when the WOB is altered by the same amount. Further support for the above hypothesis arises from findings on anaesthetized male and female rabbits. Female rabbits dedicate a greater amount of blood towards the diaphragm in response to increases in ventilation elicited by hyperthermia (Lublin et al. 1995). A caveat to the idea that women may dedicate greater blood flow to the respiratory muscles is the greater β-adrenergic receptor activity in premenopausal women, resulting in a blunted response to sympathetically mediated vasoconstriction (Hart et al. 2011). To date, the influence of WOB on blood flow distribution during exercise has not been studied in women. To determine the potential effect of sex on WOB-related alterations in blood flow accurately, respiratory muscle work will need to be reduced experimentally while leg blood flow is measured directly.
dedicated to the respiratory muscles. Therefore, it could be hypothesized that when compared with men, women may show greater changes in leg blood flow when the WOB is altered by the same amount. Further support for the above hypothesis arises from findings on anaesthetized male and female rabbits. Female rabbits dedicate a greater amount of blood towards the diaphragm in response to increases in ventilation elicited by hyperthermia (Lublin et al. 1995). A caveat to the idea that women may dedicate greater blood flow to the respiratory muscles is the greater β-adrenergic receptor activity in premenopausal women, resulting in a blunted response to sympathetically mediated vasoconstriction (Hart et al. 2011). To date, the influence of WOB on blood flow distribution during exercise has not been studied in women. To determine the potential effect of sex on WOB-related alterations in blood flow accurately, respiratory muscle work will need to be reduced experimentally while leg blood flow is measured directly.
Previous authors have argued for the existence of a maximal effective  , defined as the ventilation beyond which further increases in external work would require the increase in
, defined as the ventilation beyond which further increases in external work would require the increase in  to be dedicated solely to the respiratory musculature (Otis et al. 1950). In men, the maximal effective ventilation is significantly above
to be dedicated solely to the respiratory musculature (Otis et al. 1950). In men, the maximal effective ventilation is significantly above  (Aaron et al. 1992b), but given the greater
(Aaron et al. 1992b), but given the greater  in women, we question whether an effective ventilation could be attained. We found that the
in women, we question whether an effective ventilation could be attained. We found that the  per
per  in women (Table3) would need to be ∼2.5 times greater to equal the change in whole-body
in women (Table3) would need to be ∼2.5 times greater to equal the change in whole-body 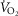 per
per 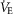 ; men would require a value ∼4 times greater. To attain the maximal effective
; men would require a value ∼4 times greater. To attain the maximal effective  , the women would have to increase their maximal ventilation by 18 l min−1, which would result in significant hypocapnia (end-tidal carbon dioxide <20 mmHg) and would not be sustainable. Using the alveolar gas equation, we estimate that the women's
, the women would have to increase their maximal ventilation by 18 l min−1, which would result in significant hypocapnia (end-tidal carbon dioxide <20 mmHg) and would not be sustainable. Using the alveolar gas equation, we estimate that the women's  would have to be 63% greater, or ∼4.5 l min−1 (∼80 ml kg−1 min−1), for the greater
would have to be 63% greater, or ∼4.5 l min−1 (∼80 ml kg−1 min−1), for the greater  to be sustainable. Likewise, for the men to achieve their maximal effective ventilation, the
to be sustainable. Likewise, for the men to achieve their maximal effective ventilation, the  would have to increase 40 l min−1 and
would have to increase 40 l min−1 and  would have to increase by 67% or to ∼98 ml kg−1 min−1. Accordingly, we conclude that women may be relatively closer to their maximal effective
would have to increase by 67% or to ∼98 ml kg−1 min−1. Accordingly, we conclude that women may be relatively closer to their maximal effective  , but the corresponding oxygen uptake is only achievable in a small percentage of highly trained athletes.
, but the corresponding oxygen uptake is only achievable in a small percentage of highly trained athletes.
Conclusion
Three primary conclusions can be drawn from our study. First, at submaximal and maximal exercise intensities,  is significantly greater in women compared with men. Second, during heavy exercise, the
is significantly greater in women compared with men. Second, during heavy exercise, the  represents a greater fraction of whole-body
represents a greater fraction of whole-body  in women. Finally, subjects who develop expiratory flow limitation during exercise have a greater maximal
in women. Finally, subjects who develop expiratory flow limitation during exercise have a greater maximal  than those who do not develop flow limitation. Overall, our findings indicate that the oxygen cost of exercise hyperpnoea is greater in healthy women than in healthy men, but neither sex readily achieves maximal effective ventilation. The greater
than those who do not develop flow limitation. Overall, our findings indicate that the oxygen cost of exercise hyperpnoea is greater in healthy women than in healthy men, but neither sex readily achieves maximal effective ventilation. The greater  in women may have implications for the integrated physiological response to exercise.
in women may have implications for the integrated physiological response to exercise.
Acknowledgments
We thank our subjects for their perseverance and enthusiastic participation.
Glossary
- EFL
expiratory flow limitation
- MEFV
maximal expiratory flow–volume

expired minute ventilation
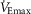
maximal expired minute ventilation

oxygen uptake

maximal oxygen uptake

oxygen uptake of the respiratory muscles
- WOB
work of breathing
Additional information
Competing interests
None declared.
Author contributions
Conception of study: P.B.D., J.N.R., G.E.F. and A.W.S. Design of experiment: P.B.D., J.N.R., G.E.F. and A.W.S. Data collection, analysis and interpretation and drafting of the article: P.B.D., J.N.R., Y.M.-S, G.E.F., L.M.R. and A.W.S. All authors approved the final version of the manuscript.
Funding
This study was supported by the Natural Science and Engineering Research Council of Canada (NSERC). P.B.D. was supported by a graduate scholarship from NSERC. J.N.R. was supported by an undergraduate student research award from NSERC. Y.M.-S. was supported by a graduate scholarship from NSERC. G.E.F. was partly supported by: (i) a focus on stroke fellowship funded by the Heart and Stroke Foundation of Canada, the Canadian Stroke Network, the Canadian Institutes of Health Research (CIHR) Institute of Circulatory and Respiratory Health, and the CIHR Institute of Aging; (ii) a research award from the Michael Smith Foundation for Health Research; and (iii) a fellowship from NSERC.
Translational perspective
We, and others, have shown that women have smaller-diameter airways than men, even when matched for lung size. Thus, a woman matched for lung size to a man will have higher airway resistance, which is important in conditions of high ventilation, such as exercise. We have also demonstrated that otherwise healthy young women have a higher mechanical work of breathing during exercise compared with men. In the present study, we found that the oxygen cost of breathing during exercise is significantly higher in women. According to the Fick equation, this suggests that women dedicate a greater fraction of cardiac output to respiratory muscles at the expense of blood flow to other exercising muscles. If our reasoning is correct, our findings become critical when considering exercise in disease. For example, pulmonary disorders are characterized by a reduced functional capacity of the respiratory system. A reduced capacity coupled with an innately accentuated respiratory muscle demand could result in sex differences in exercise responses. Exercise is widely recognized as an integral component of pulmonary rehabilitation programmes. Pulmonary rehabilitation improves symptom perceptions exercise capacity and health-related quality of life. However, rehabilitation exercise guidelines typically do not differentiate between men and women. Differences in the oxygen cost of breathing between men and women are likely to be magnified when pulmonary disease is superimposed. Additional work is required to understand how our findings may contribute to activity-related breathlessness as well as sex-specific treatment plans for the management of patients with cardiopulmonary diseases.
References
- Aaker A. Laughlin MH. Diaphragm arterioles are less responsive to α1-adrenergic constriction than gastrocnemius arterioles. J Appl Physiol. 2002;92:1808–1816. doi: 10.1152/japplphysiol.01152.2001. [DOI] [PubMed] [Google Scholar]
- Aaron EA, Johnson BD, Seow CK. Dempsey JA. Oxygen cost of exercise hyperpnea: measurement. J Appl Physiol. 1992a;72:1810–1817. doi: 10.1152/jappl.1992.72.5.1810. [DOI] [PubMed] [Google Scholar]
- Aaron EA, Seow KC, Johnson BD. Dempsey JA. Oxygen cost of exercise hyperpnea: implications for performance. J Appl Physiol. 1992b;72:1818–1825. doi: 10.1152/jappl.1992.72.5.1818. [DOI] [PubMed] [Google Scholar]
- Andersen P. Saltin B. Maximal perfusion of skeletal muscle in man. J Physiol. 1985;366:233–249. doi: 10.1113/jphysiol.1985.sp015794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babb TG. Exercise ventilatory limitation: the role of expiratory flow limitation. Exerc Sport Sci Rev. 2013;41:11–18. doi: 10.1097/JES.0b013e318267c0d2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babcock MA, Pegelow DF, McClaran SR, Suman OE. Dempsey JA. Contribution of diaphragmatic power output to exercise-induced diaphragm fatigue. J Appl Physiol. 1995;78:1710–1719. doi: 10.1152/jappl.1995.78.5.1710. [DOI] [PubMed] [Google Scholar]
-
Beidleman BA, Rock PB, Muza SR, Fulco CS, Forte VA. Cymerman A. Exercise
 and physical performance at altitude are not affected by menstrual cycle phase. J Appl Physiol. 1999;86:1519–1526. doi: 10.1152/jappl.1999.86.5.1519. [DOI] [PubMed] [Google Scholar]
and physical performance at altitude are not affected by menstrual cycle phase. J Appl Physiol. 1999;86:1519–1526. doi: 10.1152/jappl.1999.86.5.1519. [DOI] [PubMed] [Google Scholar] - Coast JR. Krause KM. Relationship of oxygen consumption and cardiac output to work of breathing. Med Sci Sports Exerc. 1993;25:335–340. [PubMed] [Google Scholar]
- Coast JR, Rasmussen SA, Krause KM, O'Kroy JA, Loy RA. Rhodes J. Ventilatory work and oxygen consumption during exercise and hyperventilation. J Appl Physiol. 1993;74:793–798. doi: 10.1152/jappl.1993.74.2.793. [DOI] [PubMed] [Google Scholar]
- Derchak PA, Sheel AW, Morgan BJ. Dempsey JA. Effects of expiratory muscle work on muscle sympathetic nerve activity. J Appl Physiol. 2002;92:1539–1552. doi: 10.1152/japplphysiol.00790.2001. [DOI] [PubMed] [Google Scholar]
- De Troyer A. Boriek AM. Mechanics of the respiratory muscles. Compr Physiol. 2011;1:1373–1300. doi: 10.1002/cphy.c100009. [DOI] [PubMed] [Google Scholar]
- Dominelli PB, Foster GE, Dominelli GS, Henderson WR, Koehle MS, McKenzie DC. Sheel AW. Exercise-induced arterial hypoxaemia and the mechanics of breathing in healthy young women. J Physiol. 2013;591:3017–3034. doi: 10.1113/jphysiol.2013.252767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominelli PB, Guenette JA, Wilkie SS, Foster GE. Sheel AW. Determinants of expiratory flow limitation in healthy women during exercise. Med Sci Sports Exerc. 2011;43:1666–1674. doi: 10.1249/MSS.0b013e318214679d. [DOI] [PubMed] [Google Scholar]
- Dominelli PB, Render JN, Molgat-Seon Y, Foster GE. Sheel A. Precise mimicking of exercise hyperpnea to investigate the oxygen cost of breathing. Respir Physiol Neurobiol. 2014;201:14–23. doi: 10.1016/j.resp.2014.06.010. [DOI] [PubMed] [Google Scholar]
- Eckermann P. Millahn HP. Der Sauerstoffverbrauch der Atmungsmuskulatur bei Frauen. Int Z Angew Physiol. 1962;19:168–172. [PubMed] [Google Scholar]
- Goldman MD, Grimby G. Mead J. Mechanical work of breathing derived from rib cage and abdominal V-P partitioning. J Appl Physiol. 1976;41:752–763. doi: 10.1152/jappl.1976.41.5.752. [DOI] [PubMed] [Google Scholar]
- Grimby G, Bunn J. Mead J. Relative contribution of rib cage and abdomen to ventilation during exercise. J Appl Physiol. 1968;24:159–166. doi: 10.1152/jappl.1968.24.2.159. [DOI] [PubMed] [Google Scholar]
- Guenette JA, Dominelli PB, Reeve SS, Durkin CM, Eves ND. Sheel AW. Effect of thoracic gas compression and bronchodilation on the assessment of expiratory flow limitation during exercise in healthy humans. Respir Physiol Neurobiol. 2010;170:279–286. doi: 10.1016/j.resp.2010.01.017. [DOI] [PubMed] [Google Scholar]
- Guenette JA, Querido JS, Eves ND, Chua R. Sheel AW. Sex differences in the resistive and elastic work of breathing during exercise in endurance-trained athletes. Am J Physiol Regul Integr Comp Physiol. 2009;297:R166–R175. doi: 10.1152/ajpregu.00078.2009. [DOI] [PubMed] [Google Scholar]
- Guenette JA, Witt JD, McKenzie DC, Road JD. Sheel AW. Respiratory mechanics during exercise in endurance-trained men and women. J Physiol. 2007;581:1309–1322. doi: 10.1113/jphysiol.2006.126466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms CA, Babcock MA, McClaran SR, Pegelow DF, Nickele GA, Nelson WB. Dempsey JA. Respiratory muscle work compromises leg blood flow during maximal exercise. J Appl Physiol. 1997;82:1573–1583. doi: 10.1152/jappl.1997.82.5.1573. [DOI] [PubMed] [Google Scholar]
- Harms CA, McClaran SR, Nickele GA, Pegelow DF, Nelson WB. Dempsey JA. Exercise-induced arterial hypoxaemia in healthy young women. J Physiol. 1998a;507:619–628. doi: 10.1111/j.1469-7793.1998.619bt.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harms CA, Wetter TJ, McClaran SR, Pegelow DF, Nickele GA, Nelson WB, Hanson P. Dempsey JA. Effects of respiratory muscle work on cardiac output and its distribution during maximal exercise. J Appl Physiol. 1998b;85:609–618. doi: 10.1152/jappl.1998.85.2.609. [DOI] [PubMed] [Google Scholar]
- Harms CA, Wetter TJ, St Croix CM, Pegelow DF. Dempsey JA. Effects of respiratory muscle work on exercise performance. J Appl Physiol. 2000;89:131–138. doi: 10.1152/jappl.2000.89.1.131. [DOI] [PubMed] [Google Scholar]
- Hart EC. Charkoudian N. Sympathetic neural regulation of blood pressure: influences of sex and aging. Physiology (Bethesda) 2014;29:8–15. doi: 10.1152/physiol.00031.2013. [DOI] [PubMed] [Google Scholar]
- Hart EC, Charkoudian N, Wallin BG, Curry TB, Eisenach J. Joyner MJ. Sex and ageing differences in resting arterial pressure regulation: the role of the β-adrenergic receptors. J Physiol. 2011;589:5285–5297. doi: 10.1113/jphysiol.2011.212753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hicks AL, Kent-Braun J. Ditor DS. Sex differences in human skeletal muscle fatigue. Exerc Sport Sci Rev. 2001;29:109–112. doi: 10.1097/00003677-200107000-00004. [DOI] [PubMed] [Google Scholar]
- Hunter SK. Sex differences in human fatigability: mechanisms and insight to physiological responses. Acta Physiol (Oxf) 2014;210:768–789. doi: 10.1111/apha.12234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hyatt RE. Expiratory flow limitation. J Appl Physiol Repir Environ Exerc Physiol. 1983;55:1–7. doi: 10.1152/jappl.1983.55.1.1. [DOI] [PubMed] [Google Scholar]
- Iandelli I, Aliverti A, Kayser B, Dellaca R, Cala SJ, Duranti R, Kelly S, Scano G, Sliwinski P, Yan S, Macklem PT. Pedotti A. Determinants of exercise performance in normal men with externally imposed expiratory flow limitation. J Appl Physiol. 2002;92:1943–1952. doi: 10.1152/japplphysiol.00393.2000. [DOI] [PubMed] [Google Scholar]
- Johnson BD, Babcock MA, Suman OE. Dempsey JA. Exercise-induced diaphragmatic fatigue in healthy humans. J Physiol. 1993;460:385–405. doi: 10.1113/jphysiol.1993.sp019477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson BD, Weisman IM, Zeballos RJ. Beck KC. Emerging concepts in the evaluation of ventilatory limitation during exercise. Chest. 1999;116:488–503. doi: 10.1378/chest.116.2.488. [DOI] [PubMed] [Google Scholar]
- Laghi F, D'Alfonso N. Tobin MJ. Pattern of recovery from diaphragmatic fatigue over 24 hours. J Appl Physiol. 1995;79:539–546. doi: 10.1152/jappl.1995.79.2.539. [DOI] [PubMed] [Google Scholar]
- Lorenzo S. Babb TG. Oxygen cost of breathing and breathlessness during exercise in nonobese women and men. Med Sci Sports Exerc. 2012;44:1043–1048. doi: 10.1249/MSS.0b013e3182444c4b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lublin A, Wolfenson D. Berman A. Sex differences in blood flow distribution of normothermic and heat-stressed rabbits. Am J Physiol Regul Integr Comp Physiol. 1995;268:R66–R71. doi: 10.1152/ajpregu.1995.268.1.R66. [DOI] [PubMed] [Google Scholar]
- McClaran SR, Harms CA, Pegelow DF. Dempsey JA. Smaller lungs in women affect exercise hyperpnea. J Appl Physiol. 1998;84:1872–1881. doi: 10.1152/jappl.1998.84.6.1872. [DOI] [PubMed] [Google Scholar]
- McCool FD, McCann DR, Leith DE. Hoppin FG. Pressure-flow effects on endurance of inspiratory muscles. J Appl Physiol. 1986;60:299–303. doi: 10.1152/jappl.1986.60.1.299. [DOI] [PubMed] [Google Scholar]
- McCool FD, Tzelepis GE, Leith DE. Hoppin FG. Oxygen cost of breathing during fatiguing inspiratory resistive loads. J Appl Physiol. 1989;66:2045–2055. doi: 10.1152/jappl.1989.66.5.2045. [DOI] [PubMed] [Google Scholar]
- MacNutt MJ, De Souza MJ, Tomczak SE, Homer JL. Sheel AW. Resting and exercise ventilatory chemosensitivity across the menstrual cycle. J Appl Physiol. 2012;112:737–747. doi: 10.1152/japplphysiol.00727.2011. [DOI] [PubMed] [Google Scholar]
- Manohar M. Inspiratory and expiratory muscle perfusion in maximally exercised ponies. J Appl Physiol. 1990;68:544–548. doi: 10.1152/jappl.1990.68.2.544. [DOI] [PubMed] [Google Scholar]
- Mead J. Dysanapsis in normal lungs assessed by the relationship between maximal flow, static recoil, and vital capacity. Am Rev Respir Dis. 1980;121:339–342. doi: 10.1164/arrd.1980.121.2.339. [DOI] [PubMed] [Google Scholar]
- Mead J, Turner JM, Macklem PT. Little JB. Significance of the relationship between lung recoil and maximum expiratory flow. J Appl Physiol. 1967;22:95–108. doi: 10.1152/jappl.1967.22.1.95. [DOI] [PubMed] [Google Scholar]
- Miller AEJ, MacDougall J, Tarnopolsky M. Sale D. Gender differences in strength and muscle fiber characteristics. Eur J Appl Physiol Occup Physiol. 1993;66:254–262. doi: 10.1007/BF00235103. [DOI] [PubMed] [Google Scholar]
- Miller JD, Hemauer SJ, Smith CA, Stickland MK. Dempsey JA. Expiratory threshold loading impairs cardiovascular function in health and chronic heart failure during submaximal exercise. J Appl Physiol. 2006;101:213–227. doi: 10.1152/japplphysiol.00862.2005. [DOI] [PubMed] [Google Scholar]
- Nielsen M. Die Respirationsarbeit bei Korperruhe und bei Muskl Arbeit. Archiv Fur Physiologie. 1936;74:299–316. [Google Scholar]
- Olfert IM, Balouch J, Kleinsasser A, Knapp A, Wagner H, Wagner PD. Hopkins SR. Does gender affect human pulmonary gas exchange during exercise? J Physiol. 2004;557:529–541. doi: 10.1113/jphysiol.2003.056887. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otis AB. The work of breathing. Physiol Rev. 1954;34:449–458. doi: 10.1152/physrev.1954.34.3.449. [DOI] [PubMed] [Google Scholar]
- Otis AB. Handbook of Physiology, section 3, The Respiratory System, Washington, DC, USA: American Physiological Society; 1964. pp. 463–476. ). The work of breathing. vol. 1, chapter 17. [Google Scholar]
- Otis AB, Fenn WO. Rahn H. Mechanics of breathing in man. J Appl Physiol. 1950;2:592–607. doi: 10.1152/jappl.1950.2.11.592. [DOI] [PubMed] [Google Scholar]
- Pellegrino R, Brusasco V, Rodarte JR. Babb TG. Expiratory flow limitation and regulation of end-expiratory lung volume during exercise. J Appl Physiol. 1993;74:2552–2558. doi: 10.1152/jappl.1993.74.5.2552. [DOI] [PubMed] [Google Scholar]
- Poole DC, Gaesser GA, Hogan MC, Knight DR. Wagner PD. Pulmonary and leg VO2 during submaximal exercise: implications for muscular efficiency. J Appl Physiol. 1992;72:805–810. doi: 10.1152/jappl.1992.72.2.805. [DOI] [PubMed] [Google Scholar]
- Renggli AS, Verges S, Notter DA. Spengler CM. Development of respiratory muscle contractile fatigue in the course of hyperpnoea. Respir Physiol Neurobiol. 2008;164:366–372. doi: 10.1016/j.resp.2008.08.008. [DOI] [PubMed] [Google Scholar]
- St Croix CM, Morgan BJ, Wetter TJ. Dempsey JA. Fatiguing inspiratory muscle work causes reflex sympathetic activation in humans. J Physiol. 2000;529:493–504. doi: 10.1111/j.1469-7793.2000.00493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheel AW, Derchak PA, Morgan BJ, Pegelow DF, Jacques AJ. Dempsey JA. Fatiguing inspiratory muscle work causes reflex reduction in resting leg blood flow in humans. J Physiol. 2001;537:277–289. doi: 10.1111/j.1469-7793.2001.0277k.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheel AW. Guenette JA. Mechanics of breathing during exercise in men and women: sex versus body size differences? Exerc Sport Sci Rev. 2008;36:128–134. doi: 10.1097/JES.0b013e31817be7f0. [DOI] [PubMed] [Google Scholar]
- Sheel AW, Guenette JA, Yuan R, Holy L, Mayo JR, McWilliams AM, Lam S. Coxson HO. Evidence for dysanapsis using computed tomographic imaging of the airways in older ex-smokers. J Appl Physiol. 2009;107:1622–1628. doi: 10.1152/japplphysiol.00562.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard RJ. The oxygen cost of breathing during vigorous exercise. Q J Exp Physiol. 1966;51:336–350. doi: 10.1113/expphysiol.1966.sp001868. [DOI] [PubMed] [Google Scholar]
- Smith JR, Rosenkranz SK. Harms CA. Dysanapsis ratio as a predictor for expiratory flow limitation. Respir Physiol Neurobiol. 2014;198:25–31. doi: 10.1016/j.resp.2014.04.001. [DOI] [PubMed] [Google Scholar]
- Stark-Leyva KN, Beck KC. Johnson BD. Influence of expiratory loading and hyperinflation on cardiac output during exercise. J Appl Physiol. 2004;96:1920–1927. doi: 10.1152/japplphysiol.00756.2003. [DOI] [PubMed] [Google Scholar]
- Tan W, Bourbeau J, Hernandez P, Chapman K, Cowie R, FitzGerald M, Aaron S, Marciniuk D, Maltais F, O'Donnell D, Goldstein R, Sin D, Chan-Yeung M, Manfreda J, Anthonisen N, Tate R, Sears M, Siersted H, Becklake M, Ernst P, Bowie D, Sweet L. Til LV. Canadian prediction equations of spirometric lung function for Caucasian adults 20 to 90 years of age: results from the Canadian Obstructive Lung Disease (COLD) study and the Lung Health Canadian Environment (LHCE) study. Can Respir J. 2011;18:321–326. doi: 10.1155/2011/540396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor BJ, How SC. Romer LM. Exercise-induced abdominal muscle fatigue in healthy humans. J Appl Physiol. 2006;100:1554–1562. doi: 10.1152/japplphysiol.01389.2005. [DOI] [PubMed] [Google Scholar]
- Taylor BJ. Romer LM. Effect of expiratory muscle fatigue on exercise tolerance and locomotor muscle fatigue in healthy humans. J Appl Physiol. 2008;104:1442–1451. doi: 10.1152/japplphysiol.00428.2007. [DOI] [PubMed] [Google Scholar]
- Topin N, Mucci P, Hayot M, Prefaut C. Ramonatxo M. Gender influence on the oxygen consumption of the respiratory muscles in young and older healthy individuals. Int J Sports Med. 2003;24:559–564. doi: 10.1055/s-2003-43267. [DOI] [PubMed] [Google Scholar]
- Wanke T, Formanek D, Schenz G, Popp W, Gatol H. Zwick H. Mechanical load on the ventilatory muscles during an incremental cycle ergometer test. Eur Respir J. 1991;4:385–392. [PubMed] [Google Scholar]


