Abstract
AMP-activated protein kinase (AMPK) is a regulator of energy homeostasis during exercise. Studies suggest muscle fibre type-specific AMPK expression. However, fibre type-specific regulation of AMPK and downstream targets during exercise has not been demonstrated. We hypothesized that AMPK subunits are expressed in a fibre type-dependent manner and that fibre type-specific activation of AMPK and downstream targets is dependent on exercise intensity. Pools of type I and II fibres were prepared from biopsies of vastus lateralis muscle from healthy men before and after two exercise trials: (1) continuous cycling (CON) for 30 min at 69 ± 1% peak rate of O2 consumption (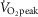 ) or (2) interval cycling (INT) for 30 min with 6 × 1.5 min high-intensity bouts peaking at 95 ± 2%
) or (2) interval cycling (INT) for 30 min with 6 × 1.5 min high-intensity bouts peaking at 95 ± 2% 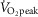 . In type I vs. II fibres a higher β1 AMPK (+215%) and lower γ3 AMPK expression (−71%) was found. α1, α2, β2 and γ1 AMPK expression was similar between fibre types. In type I vs. II fibres phosphoregulation after CON was similar (AMPKThr172, ACCSer221, TBC1D1Ser231 and GS2+2a) or lower (TBC1D4Ser704). Following INT, phosphoregulation in type I vs. II fibres was lower (AMPKThr172, TBC1D1Ser231, TBC1D4Ser704 and ACCSer221) or higher (GS2+2a). Exercise-induced glycogen degradation in type I vs. II fibres was similar (CON) or lower (INT). In conclusion, a differentiated response to exercise of metabolic signalling/effector proteins in human type I and II fibres was evident during interval exercise. This could be important for exercise type-specific adaptations, i.e. insulin sensitivity and mitochondrial density, and highlights the potential for new discoveries when investigating fibre type-specific signalling.
. In type I vs. II fibres a higher β1 AMPK (+215%) and lower γ3 AMPK expression (−71%) was found. α1, α2, β2 and γ1 AMPK expression was similar between fibre types. In type I vs. II fibres phosphoregulation after CON was similar (AMPKThr172, ACCSer221, TBC1D1Ser231 and GS2+2a) or lower (TBC1D4Ser704). Following INT, phosphoregulation in type I vs. II fibres was lower (AMPKThr172, TBC1D1Ser231, TBC1D4Ser704 and ACCSer221) or higher (GS2+2a). Exercise-induced glycogen degradation in type I vs. II fibres was similar (CON) or lower (INT). In conclusion, a differentiated response to exercise of metabolic signalling/effector proteins in human type I and II fibres was evident during interval exercise. This could be important for exercise type-specific adaptations, i.e. insulin sensitivity and mitochondrial density, and highlights the potential for new discoveries when investigating fibre type-specific signalling.
Keypoints
AMP-activated protein kinase (AMPK) is an important regulator of cellular energy status during exercise.
Most human studies investigating skeletal muscle protein signalling have been performed in whole muscle biopsy samples, yet recent studies suggest muscle fibre type-specific AMPK expression with potential fibre type-specific regulation of AMPK during exercise.
This study provides novel and comprehensive data on human muscle fibre type-specific expression levels of AMPK subunits and downstream targets of AMPK.
We show a differentiated response to exercise of key metabolic signalling proteins in human type I and type II muscle fibres during interval exercise, not evident during continuous exercise. These differences between exercise types were not present in whole muscle biopsy samples.
Our findings highlight the importance of performing fibre type-specific measurements and the increased activation of AMPK in interval vs. continuous exercise could be important for exercise type-specific adaptations, i.e. metabolism, insulin sensitivity and mitochondrial density in human skeletal muscle.
Introduction
AMP-activated protein kinase (AMPK) is an αβγ heterotrimer protein which has been proposed as a regulator of cellular energy homeostasis during exercise (for reviews see Hardie et al. 2012; O'Neill, 2013; Richter & Hargreaves, 2013). Since the first observations of AMPK activation during exercise in skeletal muscle from rats (Winder & Hardie, 1996) and humans (Chen et al. 2000; Fujii et al. 2000; Wojtaszewski et al. 2000;), the functional role of AMPK has been studied intensively. Most measurements have been performed in whole muscle biopsy samples without consideration of the influence of muscle fibre types. In fact, several rodent studies have found differences in AMPK subunit and AMPK complex expression between muscles mainly composed of slow twitch (type I) or fast twitch (type II) muscle fibres (Chen et al. 1999; Mahlapuu et al. 2004; Yu et al. 2004; Putman et al. 2007; Treebak et al. 2009a; Murphy, 2011). These studies indirectly suggest that rodent type I fibres have a higher expression of α1, α2 and β1 AMPK (Putman et al. 2007; Murphy, 2011) while type II fibres have a higher expression of β2, γ2 and γ3 AMPK as well as a higher α2β2γ3 AMPK content (Chen et al. 1999; Mahlapuu et al. 2004; Yu et al. 2004; Treebak et al. 2009a). However, in human muscle, these differences were not evident when comparing biopsies from soleus muscle (∼70% type I fibres), gastrocnemius muscle (∼50% type I fibres) and vastus lateralis muscle (∼50% type I fibres) (Jensen et al. 2012), possibly due to the small muscle–muscle variation in fibre type composition (Jensen et al. 2012). In contrast, the expression of different AMPK isoforms in human muscle has been reported to be influenced by denervation, exercise training, fitness level, sex and age (Nielsen et al. 2003; Frøsig et al. 2004; Wojtaszewski et al. 2005; Mortensen et al. 2009; Kostovski et al. 2013). All of these conditions are also associated with variations in fibre type composition, and the studies reveal that in particular the expression of γ3 AMPK is tightly and positively correlated to muscle fibre type myosin heavy chain (MHC) II isoform expression. However, one cannot distinguish between the direct effect of these conditions per se or the shift/difference in MHC composition in these subjects. Thus, in order to evaluate the consequence of different fibre types on AMPK expression and regulation in humans, muscle fibre type-specific analyses must be performed. When using immunohistochemistry, Lee-Young et al. (2009) found different γ3 AMPK expression among fibre types in the order IIx > IIa > I, whereas α1 and α2 AMPK content was similar between fibre types. In contrast, Murphy reported a higher α1 and β1 AMPK expression in type I compared to type II fibres when performing Western blotting on single human muscle fibres (Murphy, 2011). Although incongruent findings, these studies suggest that the expression of at least some AMPK subunits are fibre type dependent, yet the expression of other AMPK subunits in human muscle fibres remains to be determined. Furthermore, exercise-induced phosphorylation of AMPK at Thr172 (AMPKThr172) is higher in type IIx compared to type I and IIa fibres measured by immunohistochemistry (Lee-Young et al. 2009). This observation suggests a fibre type-specific regulation of AMPK activity during exercise. However, assay linearity is difficult to control when using immunohistochemistry and the findings need to be confirmed by other methods. In fact, lack of assay linearity in immunohistochemistry could explain the different findings regarding fibre type-specific α1 AMPK expression when using immunohistochemistry (α1 AMPK expression similar in type I vs. II) (Lee-Young et al. 2009) compared to Western blotting (higher α1 AMPK expression in type I vs. II) (Murphy, 2011).
In skeletal muscle AMPK is an upstream kinase for various targets, i.e. the acetyl-CoA carboxylase (ACC) involved in fatty acid oxidation (Winder et al. 1997), the GTPase-activating proteins TBC1 domain family members (TBC1D) 1 and 4 regulating cellular transport of GLUT4-containing vesicles (Kramer et al. 2006; Treebak et al. 2007, 2014; Pehmøller et al. 2009; Frøsig et al. 2010; Vichaiwong et al. 2010; Jessen et al. 2011), and the glycogen synthase (GS), a key regulator of glycogen synthesis (Carling & Hardie, 1989; Jørgensen et al. 2004). It is well known that phosphorylation and hence the activity of these proteins are altered with exercise (Wojtaszewski et al. 2002; Taylor et al. 2008; Pehmøller et al. 2009; Rose et al. 2009; Frøsig et al. 2010; Treebak et al. 2010; Vichaiwong et al. 2010; Jessen et al. 2011), but whether changes are fibre type specific during exercise remains elusive.
Several studies have reported that activation of AMPK is dependent on exercise intensity (Fujii et al. 2000; Birk & Wojtaszewski, 2006; Treebak et al. 2007; Rose et al. 2009; Bartlett et al. 2012). These observations possibly relate in part to changes in energy homeostasis in the individual fibre and in part to an alteration in fibre type recruitment pattern as exercise intensity increases. The latter is referred to as the motor unit size principle, in which small motor units containing type I fibres are recruited at low levels of force production (Henneman et al. 1965). As force production increases larger motor units containing type II fibres are activated (Henneman et al. 1965). Measures of fibre type-specific glycogen content have often been used as an indirect measure of the fibre type recruitment pattern during exercise (Gollnick et al. 1974; Vøllestad et al. 1984; Vøllestad & Blom, 1985; Krustrup et al. 2004; Altenburg et al. 2007). However, no studies have combined measurements of fibre type-specific regulation of AMPK and glycogen content in response to acute exercise at different intensities.
Thus the aims of the present study were to investigate the fibre type-specific expression and regulation of AMPK and its downstream targets following exercise, and in addition to study whether continuous vs. interval exercise elicits differences in the fibre type recruitment and hence signalling response.
Methods
Ethical approval
The study was approved by The Regional Ethics Commit-tee for Copenhagen (H-2-2012-085) and complied with the ethical guidelines of the Declaration of Helsinki II. Written informed consent from all participants prior to entering the study.
Experimental protocol
Nine lean, healthy, non-smoking young men (age: 24 ± 1 years, BMI: 23.1 ± 0.6) were included in the study. Maximal oxygen uptake was determined 2 weeks (range: 1–4 weeks) prior to the first experimental day by an incremental cycle test to exhaustion on a Monark ergometer cycle (Ergomedic 839E, Vansbro, Sweden) using breath by breath measurements of 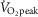 (MasterScreen CPX, IntraMedic, Gentofte, Denmark) (
(MasterScreen CPX, IntraMedic, Gentofte, Denmark) (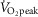 : 49.7 ± 0.9 ml kg−1 min−1). The experimental protocol consisted of 2 experimental days in a randomized order separated by 1–2 weeks. Before the first experimental day, subjects were instructed to record food intake for 3 days and abstain from alcohol and strenuous physical activity 36–48 h prior to the experiment. On the first experimental day, subjects arrived at the laboratory after an overnight fast using minimal physical activity. Each experimental day consisted of a 30 min rest period followed by 30 min of cycling exercise. During the first 30 min of rest, a catheter was inserted to the antecubital vein and the first blood sample was taken. After 30 min of rest, a needle biopsy from vastus lateralis muscle was obtained under local anaesthesia (3–5 ml Xylocaine, 20 mg ml−1) using the Bergström needle technique with suction (Bergström, 1962). The subjects were then randomly assigned to perform 30 min of either continuous cycling exercise (CON) at ∼70% (69 ± 1%) of
: 49.7 ± 0.9 ml kg−1 min−1). The experimental protocol consisted of 2 experimental days in a randomized order separated by 1–2 weeks. Before the first experimental day, subjects were instructed to record food intake for 3 days and abstain from alcohol and strenuous physical activity 36–48 h prior to the experiment. On the first experimental day, subjects arrived at the laboratory after an overnight fast using minimal physical activity. Each experimental day consisted of a 30 min rest period followed by 30 min of cycling exercise. During the first 30 min of rest, a catheter was inserted to the antecubital vein and the first blood sample was taken. After 30 min of rest, a needle biopsy from vastus lateralis muscle was obtained under local anaesthesia (3–5 ml Xylocaine, 20 mg ml−1) using the Bergström needle technique with suction (Bergström, 1962). The subjects were then randomly assigned to perform 30 min of either continuous cycling exercise (CON) at ∼70% (69 ± 1%) of 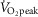 or interval cycling exercise (INT). INT consisted of six high intensity bouts of 1.5 min at a workload eliciting ∼100% (95 ± 2%)
or interval cycling exercise (INT). INT consisted of six high intensity bouts of 1.5 min at a workload eliciting ∼100% (95 ± 2%) 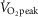 interspersed with 2.5 min active recovery at a workload corresponding to 40%
interspersed with 2.5 min active recovery at a workload corresponding to 40% 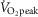 . In both protocols, the first 6 min were used as a warm-up period (INT/CON) and to adjust the load to obtain a steady state
. In both protocols, the first 6 min were used as a warm-up period (INT/CON) and to adjust the load to obtain a steady state 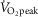 of ∼70%
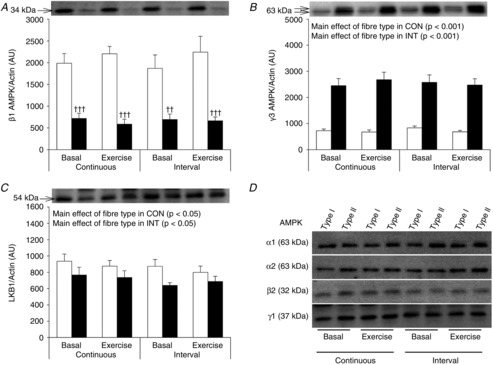
of ∼70% 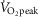 (CON). During exercise, venous antecubital blood samples were taken at 10, 18 and 29 min and expired air was measured breath by breath. Immediately after exercise, another biopsy was taken from the vastus lateralis muscle of the contralateral leg. One to two weeks after the first experimental day the subjects arrived at the laboratory to a similar experimental day except that they performed the exercise protocol they had not performed on the first trial (CON or INT) after having repeated the 3 day diet regime as in the first trial.
(CON). During exercise, venous antecubital blood samples were taken at 10, 18 and 29 min and expired air was measured breath by breath. Immediately after exercise, another biopsy was taken from the vastus lateralis muscle of the contralateral leg. One to two weeks after the first experimental day the subjects arrived at the laboratory to a similar experimental day except that they performed the exercise protocol they had not performed on the first trial (CON or INT) after having repeated the 3 day diet regime as in the first trial.
Analysis of blood and plasma metabolites, substrates and hormones
The blood content of glucose and lactate was measured by a blood gas analyser (ABL800 FLEX, Radiometer, Copenhagen, Denmark). Plasma noradrenaline and adrenaline were measured using a 2-CAT Plasma ELISAHigh Sensitive kit (Labor Diagnostika Nord GmbH & Co, Nordhorn, Germany). Plasma insulin was measured using an Insulin ELISA kit (DakoCytomation, Glostrup, Denmark) and plasma fatty acids were measured using the NEFA C ACS-ACOD method (Wako Chemicals GmbH, Neuss, Germany).
Dissection of individual muscle fibres
Muscle fibres were prepared as described previously (Murphy, 2011) but with a few modifications (Albers et al. 2014). Muscle tissue, 20–180 mg, was freeze-dried for 48 h before dissecting individual muscle fibres using a light microscope and fine forceps in a temperature- and humidity-controlled room (n = 2128 fibres in total). A minimum of 56 fibres were dissected from each biopsy (length: 1.9 ± 0.2 mm, mean ± SD). Each fibre was dissolved in 5 μl ice-cooled Laemmli sample buffer (125 mm Tris-HCl, pH 6.8, 10% glycerol, 125 mm SDS, 200 mm DTT, 0.004% Bromophenol Blue).
Preparation of pooled muscle fibre samples
A small fraction (1/5) of each solubilized fibre sample was used to identify MHC expression of the fibre using Western blotting and specific antibodies against MHC I or MHC II (see section on Immunoblotting). The remnant sample of each individual fibre was pooled according to MHC expression to form a sample of pooled type I and type II fibres from each biopsy. Hybrid fibres (<1%) expressing two MHC isoforms were excluded. The mean number of type I and type II fibres per biopsy included in each pool was 23 (range 9–52) and 27 (range 12–53), respectively.
Protein content of fibre pools
The protein concentration of the fibre pools was estimated using 4–20% Mini-PROTEAN TGX stain-free gels (BioRad, Hercules, CA, USA), which allowed for gel-protein imaging following UV activation on a ChemiDoc MP imaging system (BioRad). The intensity of the protein bands (40–260 kDa) was compared to a standard curve of mixed muscle homogenates with a known protein concentration (correlation coefficient squared (r)2 = 0.98, correlation not shown).
Glycogen content in muscle fibre pools
Muscle fibre type-specific glycogen content was measured as previously described using dot-blotting (Albers et al. 2014). A quantity of 150 ng of protein from each fibre pool was spotted onto a polyvinylidene difluoride (PVDF) membrane and incubated with an antibody against glycogen (Baba, 1993; Nakamura-Tsuruta et al. 2012) (see section on Immunoblotting). The intensity of each dot was compared to a standard curve (r2 = 0.99, correlation not shown) of muscle homogenate with a known glycogen concentration determined biochemically (see section on Muscle glycogen).
Muscle homogenate and lysate preparation
Whole muscle homogenates and lysates were prepared from 5 mg freeze-dried muscle which was dissected free of visible fat, blood and connective tissue. The muscle samples were homogenized in a buffer containing 10% glycerol, 20 mm sodium pyrophosphate, 150 mm NaCl, 50 mm Hepes (pH 7.5), 1% NP-40, 20 mm β-glycerophosphate, 10 mm NaF, 2 mm phenylmethylsulfonyl fluoride (PMSF), 1 mm EDTA (pH 8.0), 1 mm EGTA (pH 8.0), 10 μg μl−1 aprotinin, 10 μg μl−1 leupeptin, 2 mm Na3VO4, 3 mm benzamidine, 1 mm sodium butyrate, 1 mm nicotinamide and double-distilled H2O (ddH2O) during 2 × 60 s in a TissueLyser (Retsch Qiagen, Hilden, Germany) followed by 2 × 30 min rotation end-over-end at 4°C interspersed by 60 s in the TissueLyser. Lysates were recovered by centrifuging the homogenates (20 min, 18,320 g, 4°C). Homogenate and lysate protein content were determined by the bicinchoninic acid method (Pierce Biotechnology, Inc., Rockford, IL, USA).
Muscle glycogen
Glycogen content in whole muscle biopsies was measured in homogenates (150 μg protein) as glycosyl units after acid hydrolysis determined by a fluorometric method (Lowry & Passonneau, 1972).
Muscle lactate, creatine (Cr) and phosphocreatine (PCr)
Freeze-dried muscle samples were extracted with perchloric acid (1.0 mm), neutralized with potassium hydroxide (2.0 mm) and analysed for the content of lactate, Cr and PCr as previously described (Lowry & Passonneau, 1972).
Immunoblotting
To determine the MHC isoform in single muscle fibres, an equal volume of sample was loaded onto pre-cast 7.5% gels (BioRad). To measure total and phosphorylated levels of relevant proteins in muscle fibre pools, homogenates and lysates, 6 μg of protein was separated using self-cast 9% gels. An internal standard of muscle lysate (6 μg) was loaded in both sides on each gel to be able to adjust for gel to gel variation. On one gel, a standard curve of muscle homogenate or lysate was loaded, as appropriate, validating that the quantification of each protein probed for was within the linear range of detection. Following SDS-PAGE, proteins were transferred (semidry) from multiple gels (representing all samples, standards and standard curve) onto one single PVDF membrane. The membranes were blocked in 2% skimmed milk in TBS containing 0.05% Tween 20 for 60 min at room temperature (RT), followed by incubation in primary antibody overnight at 4°C. The following primary antibodies were used: anti-ACC (streptavidin, Dako, Glostrup, Denmark); anti-phospho-ACC Ser221, anti-phospho-AMPK Thr172 and anti-LKB1 (serine/threonine kinase 11 (STK11)) (Cell Signaling Technology, Danvers, MA, USA). Anti-β-actin (Sigma-Aldrich, St Louis, MO, USA); anti-α1 and -γ1 AMPK (Abcam, Cambridge, UK); anti-α2 AMPK (Santa Cruz Biotechnology, Dallas, TX, USA); anti-β1 AMPK (Arexis, Mölndal, Sweden); anti-β2 AMPK, anti-phospho-GS Ser7 + Ser10 (GS2+2a) and Ser640 + Ser644 (GS3a+3b) (kindly provided by Professor D. Grahame Hardie, University of Dundee, UK); anti-γ3 AMPK (Zymed Laboratories Inc., South San Francisco, CA, USA and also kindly provided by Professor James Hastie, University of Dundee, UK); anti-phospho-TBC1 domain family member 1 (TBC1D1) Ser231 (Millipore, Billerica, MA, USA); anti-phospho-TBC1D4 Ser704 (kindly provided by Professor Laurie Goodyear, Joslin Diabetes Centre and Harvard Medical School, USA); anti-TBC1D1 (kindly provided by Professor D. Grahame Hardie and Professor James Hastie, University of Dundee, UK); anti-TBC1D4 (Upstate, now Millipore, MA, USA); anti-MHC I and anti-MHC II (Developmental Studies Hybridoma Bank, University of Iowa, Iowa City, IA, USA). Membranes were incubated for 1 h at RT with secondary antibodies (Jackson ImmunoResearch, West Grove, PA, USA) which were conjugated with either horseradish peroxidase (HRP) or biotin. Membranes probed with biotin-conjugated antibody were further incubated in HRP-conjugated streptavidin (Jackson ImmunoResearch) for 45 min at RT. Protein bands were visualized using enhanced chemiluminescence (SuperSignal West Femto, Rockford, Pierce, IL, USA) and ChemiDoc MP imaging system (BioRad). Band quantification was performed using BioRad Image Lab. Membranes were re-probed with an alternative antibody after removal of the first antibody by incubation in stripping buffer (62.3 mm Tris-HCl, 69.4 mm SDS, ddH2O and 0.08% β-mercaptoethanol pH 6.7) for 60 min at 58°C. The membranes were checked for successful removal of the primary antibody before re-probing.
AMPK activity
Isoform-specific AMPK activity was measured on 300 μg muscle lysate by sequential immunoprecipitation (IP) of the γ3 subunit (isolating the α2β2γ3 complex) followed by IP of the α2 subunit (isolating α2β2γ1) and lastly the α1 subunit (isolating α1β2γ1) as described previously (Birk & Wojtaszewski, 2006). AMPK activity was measured in the presence of 200 μm AMP and 100 μm AMARA-peptide (Schafer-N, Copenhagen, Denmark) as substrate, as described previously (Birk & Wojtaszewski, 2006).
Statistics
Data are presented as means ± SEM. Data obtained in whole muscle homogenates/lysates and in blood/plasma were evaluated by a two-way repeated measures (RM) ANOVA (factors: ‘exercise type’ (CON vs. INT) and ‘time’ (basal vs. exercise)). To evaluate changes in muscle fibre pools, four two-way RM ANOVAs were used. Two tests were used to test for the factors ‘fibre type’ and ‘intervention’ (basal vs. exercise) in the CON and INT trial separately, and two tests were used to test for the factors ‘intervention’ and ‘exercise type’ in type I and type II fibres, separately. Significant interactions were evaluated by Tukey's post hoc test. Pearson correlation was used for correlated data. All statistical analyses were performed in Sigma Plot (version 11, Systat Software, Chicago, IL, USA). The level of significance was P < 0.05.
Results
AMPK subunit and LKB1 expression
AMPK subunit and LKB1 protein expression were unaffected by exercise intervention and trial. The expression of α1, α2, β2 and γ1 AMPK was similar between fibre types (Fig. 1D). In contrast, type I fibres had a higher expression of the β1 subunit (+215%) (Fig. 1A) and a lower expression of the γ3 subunit (−71%) (Fig. 1B) compared to type II fibres. Furthermore, type I fibres had a higher protein expression of LKB1 (+24%) compared to type II fibres (Fig. 1C). Actin was used as a reference protein with no difference in expression between fibre types, exercise intervention and trial (data not shown).
Figure 1. AMPK subunit and LKB1 protein expression in type I and type II muscle fibre pools AMPK subunit and LKB1 protein expression in type I and type II muscle fibre pools.
Muscle fibre type-specific protein expression of β1 AMPK (A), γ3 AMPK (B), LKB1 (C) and α1, α2, β2 and γ1AMPK (D) was evaluated by Western blotting. Two bands are apparent for γ3 AMPK both being γ3 AMPK. This was verified by immunoprecipitation of γ3 AMPK followed by Western blotting (data not shown). Quantified values of each protein are expressed relative to actin content. Open bars represent type I fibres and filled bars represent type II fibres. Representative blots are shown above bars for each protein loaded in the same order as the graph below. D shows the representative blots of the AMPK subunits with no difference between fibre types. Data are expressed as means ± SEM. AU, arbitrary units. ††P < 0.01 vs. type I muscle fibres, †††P < 0.001 vs. type I muscle fibres.
Metabolic characteristics during CON and INT
Despite the fact that 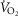 peaked between 90 and 99%
peaked between 90 and 99% 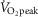 during the six high intensity exercise bouts of 1.5 min, the mean intensity for the last 24 min of the exercise session was not significantly different between CON and INT (69 ± 1 and 71 ± 2%
during the six high intensity exercise bouts of 1.5 min, the mean intensity for the last 24 min of the exercise session was not significantly different between CON and INT (69 ± 1 and 71 ± 2%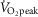 , respectively) (Table1). The glycogen breakdown in response to CON and INT was similar between the two trials when measured in whole muscle homogenate (Table2). In addition, similar increases in venous plasma adrenaline and noradrenaline concentrations in CON and INT (Table1) were evident. The mean respiratory exchange ratio during the last 24 min of the exercise session was significantly higher during INT compared to CON (Table1). Blood lactate was significantly increased in both trials but especially after INT compared to CON (Table1). In line with this, muscle lactate concentration was significantly increased after INT only (Table2). Furthermore, the muscle PCr/(Cr + PCr) ratio decreased in both trials but to a greater extent after INT (−52%) compared to CON (−23%) (Table2). Glucose and insulin concentrations in venous plasma remained unchanged during CON whereas they both increased after INT by 49% and 90%, respectively (Table1).
, respectively) (Table1). The glycogen breakdown in response to CON and INT was similar between the two trials when measured in whole muscle homogenate (Table2). In addition, similar increases in venous plasma adrenaline and noradrenaline concentrations in CON and INT (Table1) were evident. The mean respiratory exchange ratio during the last 24 min of the exercise session was significantly higher during INT compared to CON (Table1). Blood lactate was significantly increased in both trials but especially after INT compared to CON (Table1). In line with this, muscle lactate concentration was significantly increased after INT only (Table2). Furthermore, the muscle PCr/(Cr + PCr) ratio decreased in both trials but to a greater extent after INT (−52%) compared to CON (−23%) (Table2). Glucose and insulin concentrations in venous plasma remained unchanged during CON whereas they both increased after INT by 49% and 90%, respectively (Table1).
Table 1.
Metabolic characteristics during exercise interventions
| Continuous | Interval | |||
|---|---|---|---|---|
| Basal | Exercise | Basal | Exercise | |
| Blood glucose (mmol l−1) | 5.3 ± 0.1 | 5.5 ± 0.2 | 5.1 ± 0.4 | 7.6 ± 0.4***### |
| Plasma insulin (μIU ml−1) | 3.81 ± 0.43 | 2.31 ± 0.33 | 4.43 ± 0.42 | 8.44 ± 1.11***### |
| Blood lactate (mmol l−1) | 0.7 ± 0.1 | 4.6 ± 0.6*** | 0.7 ± 0.1 | 14.0 ± 0.8***# |
| Plasma fatty acids (μmol l−1) | 435 ± 61 | 329 ± 39† | 351 ± 65‡ | 248 ± 28†‡ |
| Adrenaline (nmol l−1) | 0.20 ± 0.07 | 2.84 ± 0.94††† | 0.31 ± 0.12 | 2.71 ± 0.62††† |
| Noradrenaline (nmol l−1) | 1.58 ± 0.40 | 21.48 ± 3.55††† | 1.71 ± 0.38 | 29.86 ± 5.41††† |
%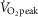 (mean) (mean) |
— | 69.2 ± 0.9 | — | 71.1 ± 1.8 |
| RER (mean) | — | 0.91 ± 0.01 | — | 1.01 ± 0.01### |
Values are means ± SEM. ***P < 0.001 vs. basal, ###P < 0.001 vs. CON, †P < 0.05 main effect of exercise, †††P < 0.001 main effect of exercise, ‡P < 0.05 main effect of exercise type. RER, respiratory exchange ratio.
Table 2.
Parameters of cellular energy status and whole muscle AMPK activities
| Continuous | Interval | |||
|---|---|---|---|---|
| Basal | Exercise | Basal | Exercise | |
| Glycogen (nmol (mg protein)−1) | 607 ± 31 | 328 ± 22*** | 664 ± 54 | 278 ± 31*** |
| Lactate (mmol (kg dry wt)−1) | 75.5 ± 3.2 | 88.9 ± 5.7 | 69.8 ± 5.9 | 153.6 ± 7.8***### |
| Cr (mmol (kg dry wt)−1) | 29.2 ± 4.8 | 45.5 ± 4.9(*) | 25.9 ± 2.6 | 61.8 ± 4.3***## |
| PCr (mmol (kg dry wt)−1) | 58.8 ± 3.9 | 49.3 ± 3.6†† | 60.1 ± 3.5 | 31.0 ± 3.9†† |
| PCr/(Cr + PCr) (× 100) | 67.8 ± 3.5 | 52.4 ± 3.0** | 69.9 ± 2.6 | 33.4 ± 4.2***# |
| α1β2γ1 AMPK activity | 1.66 ± 0.17 | 1.42 ± 0.15†† | 1.54 ± 0.18 | 1.25 ± 0.17†† |
| α2β2γ1 AMPK activity | 0.95 ± 0.16 | 1.10 ± 0.17 | 1.00 ± 0.09 | 1.20 ± 0.22 |
| α2β2γ3 AMPK activity | 0.20 ± 0.02 | 2.59 ± 0.48** | 0.20 ± 0.04 | 5.70 ± 0.89***### |
Values are means ± SEM. **P < 0.01 vs. basal, ***P < 0.001 vs. basal, #P < 0.05 vs. CON, ##P < 0.01 vs. CON, ###P < 0.001 vs. CON, ††P < 0.01 main effect of exercise, (*)P = 0.06 vs. basal. Units of AMPK activity: pmol min−1 mg−1.
Whole muscle AMPK activity
AMPK activity was measured in muscle lysates on the three main heterotrimeric complexes found in human skeletal muscle. The increase in α2β2γ3 AMPK activity with exercise was significantly higher after INT compared to CON (27- and 12-fold, respectively) (Table2). Independently of exercise trial, α1β2γ1 AMPK activity decreased with exercise whereas α2β2γ1 AMPK activity was unchanged (Table2).
Protein phosphorylation in whole muscle homogenate
When measured in whole muscle homogenates phospho-rylation of AMPKThr172, ACCSer221, TBC1D1Ser231, TBC1D4Ser704 and GS2+2a increased during exercise to a similar extent in CON and INT (Table3, Fig. 2). Phosphorylation of GS3a+3b decreased with exercise in both trials but to a greater extent after INT (Table3, Fig. 2).
Table 3.
Protein phosphorylation before and after CON and INT in whole muscle homogenate (values are arbitrary units)
| Continuous | Interval | |||
|---|---|---|---|---|
| Basal | Exercise | Basal | Exercise | |
| pAMPKThr172 | 89 ± 9 | 184 ± 30††† | 87 ± 12 | 216 ± 32††† |
| pACCSer221 | 49 ± 6 | 315 ± 29††† | 45 ± 6 | 392 ± 40††† |
| pTBC1D4Ser704 | 231 ± 16 | 422 ± 89†† | 222 ± 18 | 604 ± 109†† |
| pTBC1D1Ser231 | 216 ± 20 | 308 ± 24†† | 214 ± 26 | 401 ± 46†† |
| pGS2+2a | 108 ± 16 | 367 ± 58††† | 129 ± 16 | 288 ± 42††† |
| pGS3a+3b | 750 ± 62 | 439 ± 46*** | 777 ± 61 | 326 ± 32***# |
Values are means ± SEM. ***P < 0.001 vs. basal, #P < 0.05 vs. CON, ††P < 0.01 main effect of exercise, †††P < 0.001 main effect of exercise. Representative Western blots are shown in Fig. 2.
Figure 2. Representative Western blots of protein phosphorylation before and after CON and INT in whole muscle homogenate.
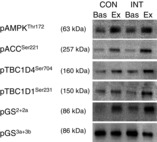
Phosphorylation of AMPKThr172, ACCSer221, TBC1D4Ser704, TBC1D1Ser231, GS2+2a and GS3a+3b in whole muscle homogenate was evaluated by Western blotting. Quantified data are given in Table3.
Glycogen content in fibre pools
Basal glycogen content was lower in type I fibres compared to type II fibres (−25%) (Fig. 3A). This was only significant in INT, whereas a tendency towards a main effect of fibre type was found in CON (P = 0.1). Glycogen decreased to a similar extent in both fibre types after CON. During INT glycogen degradation was significantly smaller in type I fibres (−40%) compared to type II fibres (−73%), resulting in higher glycogen content in type I vs. II fibres after INT.
Figure 3. Glycogen content and AMPKThr172 phosphorylation in type I and type II muscle fibre pools before and after CON and INT.
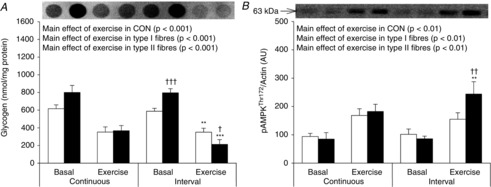
A, muscle fibre type-specific glycogen content measured by dot-blotting. B, muscle fibre type-specific phosphorylation of AMPKThr172 evaluated by Western blotting. Quantified values of AMPKThr172 phosphorylation are expressed relative to actin content. Open bars represent type I fibres and filled bars represent type II fibres. Representative blots are shown above bars in the same order as the graph below. Data are expressed as means ± SEM. AU, arbitrary units. **P < 0.01 vs. basal conditions, ***P < 0.001 vs. basal conditions, †P < 0.05 vs. type I muscle fibres, ††P < 0.01 vs. type I muscle fibres, †††P < 0.001 vs. type I muscle fibres. A borderline main effect of fibre type in glycogen content was observed in CON (P = 0.1). Furthermore, a borderline interaction between exercise and exercise type was observed in glycogen content in type II fibres (P = 0.08) (A).
AMPK phosphorylation in fibre pools
In the basal state, the phosphorylation of AMPKThr172 was similar between fibre types in both trials (Fig. 3B). After CON there was a main effect of exercise to increase phosphorylation of AMPKThr172 with no difference between fibre types. In contrast, after INT the phosphorylation of AMPKThr172 increased by 52% in type I fibres (not significant) and by 184% in type II fibres (P < 0.01).
ACC in fibre pools
In response to both CON and INT there was a main effect of exercise to increase ACCSer221 phosphorylation. Yet, a tendency towards a different fibre type response to exercise was observed after INT (P = 0.08) (Fig. 4A). When ACCSer221 phosphorylation was related to total ACC protein (Fig. 4B) this difference became significant as phosphorylation of ACCSer221/ACC increased less in type I fibres compared with type II fibres. In both fibre types, phosphorylation of ACCSer221/ACC was increased to a higher level after INT compared to CON. ACC protein expression was not different between fibre types. A minor increase in ACC protein expression with exercise was observed after CON and in type I fibres (Fig. 4C). This was unexpected; however, in whole muscle homogenate no effect of exercise could be detected (data not shown).
Figure 4. ACCSer221 phosphorylation and ACC expression in type I and type II muscle fibre pools before and after CON and INT.
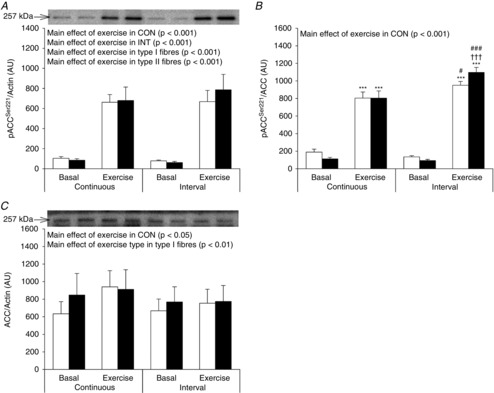
Muscle fibre type-specific phosphorylation of ACCSer221 (A) and protein expression of ACC (C) was evaluated by Western blotting. Quantified values are expressed relative to actin content. Phosphorylation of ACCSer221 was also expressed relative to ACC content (B). Open bars represent type I fibres and filled bars represent type II fibres. Representative blots are shown above bars for each protein loaded in the same order as the graph below. Data are expressed as means ± SEM. AU, arbitrary units. ***P < 0.001 vs. basal conditions, †††P < 0.001 vs. type I muscle fibres, #P < 0.05 vs. CON and ###P < 0.001 vs. CON. A borderline interaction between exercise and fibre type was observed for ACCSer221 phosphorylation after INT (P = 0.08) (A).
TBC1D1 in fibre pools
TBC1D1 expression was 52% lower in type I fibres compared to type II fibres (Fig. 5A) independent of exercise per se and exercise trial. In response to CON, phosphorylation of TBC1D1Ser231/TBC1D1 increased with no difference between fibre types. In contrast, in response to INT TBC1D1Ser231/TBC1D1 phosphorylation increased in both fibre types but to a lesser extent in type I fibres (+63%) compared to type II fibres (+97%) (Fig. 5E). Furthermore, in type II fibres, the increase in TBC1D1Ser231/TBC1D1 phosphorylation with exercise was higher after INT compared to CON (P = 0.05).
Figure 5. TBC1D1 and TBC1D4 in type I and type II muscle fibre pools before and after CON and INT.
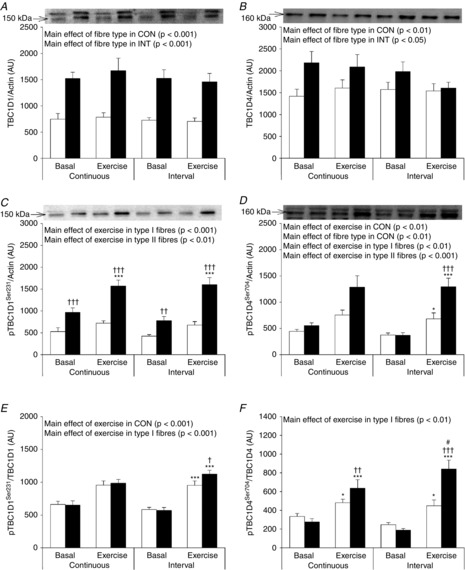
Muscle fibre type-specific protein expression of TBC1D1 (A) and TBC1D4 (B) and phosphorylation of TBC1D1Ser231 (C), TBC1D4 Ser704 (D) were evaluated by Western blotting. Quantified values are expressed relative to actin content. Phosphorylation of TBC1D1Ser231 was expressed relative to TBC1D1 content (E) and phosphorylation of TBC1D4Ser704 was expressed relative to TBC1D4 content (F). Open bars represent type I fibres and filled bars represent type II fibres. Representative blots are shown above bars in the same order as the graph below. Data are expressed as means ± SEM. AU, arbitrary units. *P < 0.05 vs. basal conditions, ***P < 0.001 vs. basal conditions, †P < 0.05 vs. type I muscle fibres, ††P < 0.01 vs. type I muscle fibres, †††P < 0.001 vs. type I muscle fibres, #P < 0.05 vs. CON. Borderline interaction between exercise and fibre type in CON was observed for TBC1D4Ser704 phosphorylation (P = 0.05) (D) and between exercise and exercise type in type II fibres for TBC1D1Ser231 expressed relative to TBC1D1 (P = 0.05) (E).
TBC1D4 in fibre pools
TBC1D4 expression was 21% lower in type I fibres compared to type II fibres (Fig. 5B). The phosphorylation of TBC1D4Ser704/TBC1D4 increased in response to exercise in both CON and INT. The increase was less in type I fibres (+43% and +82% after CON and INT, respectively) compared to type II fibres (+131% and +347% after CON and INT, respectively) (Fig. 5F). Furthermore, in type II fibres the increase in phosphorylation of TBC1D4Ser704/TBC1D4 was significantly smaller after CON (−25%) compared to INT.
Glycogen synthase in fibre pools
GS protein expression was 10% higher in type I fibres compared to type II fibres; however, this was only significant in the INT trial (main effect of fibre type P < 0.05 in INT, Fig. 6A). In CON, there was a main effect of exercise to increase GS2+2a phosphorylation. Although not significant, the absolute values indicate that the increment was higher in type I compared to type II fibres. In line with this, after INT the increase in GS2+2a phosphorylation was significant only in type I fibres (+76%) and not in type II fibres (+16%) (Fig. 6B). In type I but not in type II fibres, statistical main effects were evident for increased GS2+2a phosphorylation by exercise.
Figure 6. Glycogen synthase in type I and type II muscle fibre pools before and after CON and INT.
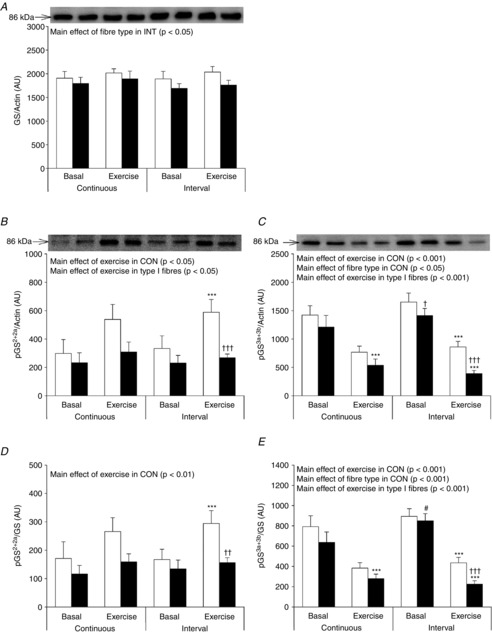
Muscle fibre type-specific protein expression of GS (A) and phosphorylation of GS2+2a (B) and 3a+3b (C) were evaluated by Western blotting. Quantified values are expressed relative to actin content. Open bars represent type I fibres and filled bars represent type II fibres. Representative blots are shown above bars for each protein loaded in the same order as the graphs below. Data are expressed as means ± SEM. AU, arbitrary units. ***P < 0.001 vs. basal conditions, †P < 0.05 vs. type I muscle fibres, ††P < 0.01 vs. type I muscle fibres, †††P < 0.001 vs. type I muscle fibres, #P < 0.05 vs. CON.
After CON phosphorylation of GS3a+3b decreased to a similar extent in type I (−52%) and type II fibres (−56%) (Fig. 6C). In contrast, after INT GS3a+3b phosphorylation decreased to a lesser extent in type I fibres (−51%) compared to type II fibres (−73%) (Fig. 6C). Similar effects of fibre type and exercise trial were observed when phosphorylation of GS2+2a and GS3a+3b was related to total GS protein (Fig. 6D and E).
Discussion
This study provides evidence that exercise-induced regulation of AMPK, ACC, TBC1D1, TBC1D4 and GS is dependent on muscle fibre type and exercise intensity. In fact, most proteins were regulated to a similar extent in type I and II muscle fibres during exercise in CON, whereas exercise during INT elicited a fibre type-specific regulation. All protein-signalling responses investigated (except GS3a+3b phosphorylation) were similar in the two exercise trials when measured traditionally in whole muscle homogenate as reported by others (Bartlett et al. 2012). These findings underscore the relevance of measuring exercise-induced protein signalling on a muscle fibre type-specific level.
The ∼70% lower γ3 AMPK protein content observed in type I vs. II muscle fibres is more pronounced than previously reported (Lee-Young et al. 2009). Immunohistochemical detection of γ3 AMPK in muscle cryosections showed that the expression level of γ3 AMPK was highest in type IIx > IIa > I muscle fibres (approximately 14% lower in type I vs. IIa muscle fibres and 33% lower in type I vs. IIx muscle fibres) (Lee-Young et al. 2009). In human skeletal muscle γ3 selectively associates with α2 and β2 (Wojtaszewski et al. 2005) and thus our findings indicate a lower expression level of the α2β2γ3 AMPK complex in human type I vs. II muscle fibres. This is also in agreement with observations in mice, in which the abundance of α2β2γ3 AMPK complexes constitutes less than 2% of total AMPK in type I/IIa-abundant soleus muscle and approximately 20% in the type II-fibre-rich extensor digitorum longus muscle (Treebak et al. 2009a). γ3 AMPK protein in muscle is decreased by exercise training and increased by detraining (muscle denervation) and associations with MHC expression have been reported in both conditions (Nielsen et al. 2003; Frøsig et al. 2004; Wojtaszewski et al. 2005; Mortensen et al. 2009; Kostovski et al. 2013). Our data may allow us to speculate that prioritization of an expression programme for MHC type I may at the same time decrease expression of γ3 AMPK protein. However, this needs to be verified.
Confirming previous observations (Birk & Wojtaszewski, 2006; Treebak et al. 2007), α2β2γ3 AMPK was the only complex activated during exercise in whole muscle biopsies, in the present study. As the glycogen degradation pattern suggests a lower activation/recruitment of type I vs. type II muscle fibres during exercise in INT, we expected a lower α2β2γ3 AMPK activity in type I compared to type II fibres during exercise in INT. Fibre type-specific AMPKThr172 phosphorylation during exercise supports this assumption. However, since the α2β2γ3 AMPK complex accounts for only ∼20% of all AMPK complexes in human skeletal muscle (Birk & Wojtaszewski, 2006), phosphorylation of AMPKThr172 is not necessarily a precise measure of AMPK complex activation. Thus, we cannot exclude the possibility of a differentiated AMPK activation in type I and II muscle fibres during exercise in CON based on this measurement alone.
Since AMPK activity is also regulated allosterically, and to further delineate AMPK activation in type I and II muscle fibres during exercise, we measured site-specific phosphorylation of several confirmed AMPK targets: ACCSer221, TBC1D1Ser231, TBC1D4Ser704 and GS2+2a. In skeletal muscle, these targets have been verified using pharmacological and exercise-induced AMPK activation in various transgenic animal models in which AMPK expression has been ablated (Carling & Hardie, 1989; Ha et al. 1994; Jørgensen et al. 2004; Pehmøller et al. 2009; Treebak et al. 2010; Frøsig et al. 2010). In line with this, phosphorylation of ACCSer221, TBC1D1Ser231 and TBC1D4Ser704 were closely associated with α2β2γ3 AMPK activity measured in whole muscle lysate in the present study (Fig. 7A–C).
Figure 7. Correlations between AMPK activity and phosphorylation of downstream targets of AMPK in whole muscle lysate.
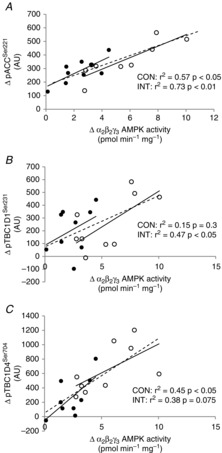
Correlations between Δα2β2γ3 AMPK activity and Δphosphorylation of ACCSer221 (A), Δphosphorylation of TBC1D1Ser231 (B) and Δphosphorylation of TBC1D4Ser704 (C) in whole muscle lysate after CON (filled circles) and INT (open circles). Phosphorylation of ACCSer221, TBC1D1Ser231 and TBC1D4Ser704 was evaluated by Western blotting. The dashed line represents both trials together (A: r2 = 0.67, P < 0.001; B: r2 = 0.29, P < 0.05; C: r2 = 0.56, P < 0.001). Δ = change with exercise (exercise − basal).
Furthermore, these three estimates of endogenous AMPK activity showed no fibre type-specific regulation during exercise in CON whereas all depicted this during exercise in INT in agreement with the phosphoregulation of AMPKThr172. Altogether, regulation of these AMPK downstream targets suggests that interval exercise elicits a fibre type-specific response and we propose that activation of α2β2γ3 AMPK complexes is a major contributor to this response. Our study allows us only to speculate on the consequences of this fibre type-specific regulation. TBC1D1 is proposed to regulate exercise-induced glucose uptake (An et al. 2010), and one interpretation of our findings is that glucose uptake by this mechanism is prioritized in type II vs. type I muscle fibres during interval exercise. Also, studies in rodents (Funai et al. 2009) and humans (Treebak et al. 2009b; Vendelbo et al. 2014) suggest that TBC1D4 phosphorylation in skeletal muscle is maintained for a prolonged period during recovery from exercise and may be important for post-exercise insulin sensitivity. If this is the case, our data would then suggest that type II muscle fibres contribute mostly to post-exercise insulin sensitivity – an adaptation facilitating glycogen re-synthesis in those fibres in which glycogen utilization seemingly has been largest. As discussed below such prioritization of glycogen synthesis may also be linked to the fibre type-specific regulation of GS.
AMPK is suggested to be a GS site 2 kinase (Jørgensen et al. 2004). Our data only support this in type I and not in type II fibres as we did not observe any changes in GS2+2a phosphorylation in type II fibres after INT despite increased AMPKThr172 phosphorylation in type II fibres. GS3a+3b are the other major phosphorylation sites regulating GS activity. These sites were dephosphorylated by exercise in both fibre types, and to a lesser degree in type I vs. II fibres during exercise in INT. Although we do not know the resulting effect of these phosphorylation events on GS activity, our data allow us to speculate that GS activation is considerably less in type I compared to type II muscle fibres. This could be important immediately post exercise and would suggest a lower glycogen re-synthesis rate in type I compared to II muscle fibres at the time of exercise cessation.
We used exercise-induced glycogen degradation to evaluate fibre type recruitment during exercise in CON and INT. The similar glycogen degradation in type I fibres between CON and INT suggests similar type I fibre recruitment between the two trials. Although there is no statistical difference in glycogen levels after exercise in type II fibres in CON and INT, the glycogen degradation was statistically larger in type II fibres compared to type I fibres in INT but not in CON. We interpret this to indicate a larger recruitment of type II fibres during exercise in INT compared to CON. In line with this, we found increased lactate concentration and a greater decrease in PCr/(Cr + PCr) ratio in muscle after INT compared to CON, suggesting periods of increased metabolic stress during exercise in INT. Whether protein signalling/activity measurements mainly reflect the averaged exercise intensity or a more acute regulation at the end of exercise is unknown. To support an acute regulation, Birk & Wojtaszewski (2006) showed that activity of the AMPK α2β2γ3 complex increased after 30 s of an all-out sprint. In addition, ACC phosphorylation has been shown to be acutely (<30 s) regulated during an all-out sprint (Birk & Wojtaszewski, 2006; Gibala et al. 2009). Thus, a higher increase in α2β2γ3 AMPK activity in whole muscle samples as well as ACCSer221 phosphorylation in fibre pools during exercise in INT compared to CON in the present study is probably influenced by the last interval exercise bout.
Studies in mouse muscle predominantly expressing one fibre type have revealed that TBC1D1 and TBC1D4 has a remarkable fibre type-specific expression pattern, i.e. TBC1D1 is highly expressed in muscle rich in type II fibres whereas expression in muscle rich in type I fibres is very low (Taylor et al. 2008). The expression profile of TBC1D4 is reported to be exactly the opposite (Taylor et al. 2008). However, it is unknown whether these observations were associated with the difference in MHC expression or activity pattern between the muscles. Here we provide evidence that in young healthy men both TBC1D1 and TBC1D4 expression are lower in type I vs. type II muscle fibres. This is in line with what we have previously reported in middle-aged subjects (Albers et al. 2014). However, the differences between fibre types in humans are much smaller than those reported in mice. Our findings of a substantial amount of TBC1D1 and TBC1D4 in both fibre types is an important observation as we now, to a greater extent than previously thought, can expect findings on TBC1D1 and TBC1D4 from rodent muscles also to be of importance in human muscle. A limitation to this interpretation, however, is that our data do not allow generalization to other human muscles as we do not know whether type I and type II muscle fibres from vastus lateralis muscle resemble type I and type II fibres from other muscles (Daugaard & Richter, 2004). It has been shown in untrained subjects ( 44.8 ml kg−1 min−1) that training-induced increases in GLUT4 content mainly occur in type I fibres (Daugaard et al. 2000). Thus, the training status of the subjects in the present study (
44.8 ml kg−1 min−1) that training-induced increases in GLUT4 content mainly occur in type I fibres (Daugaard et al. 2000). Thus, the training status of the subjects in the present study (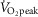 49.7 ± 0.9 ml kg−1 min−1) could potentially influence the differences in protein expression between muscle fibres. Another limitation to the present study is the lack of distinction between type IIa and IIx fibres in the pools of type II fibres. Yet in vastus lateralis muscle of young, healthy, active men the expression of type IIx fibres is relatively small compared to the proportion of type IIa and type I fibres (Hvid et al. 2011; Jensen et al. 2012; Prats et al. 2013) whereby the proportion of type IIx fibres in pools of type II fibres is thought to be limited. Thus, the differences observed between type I and type II fibres are probably influenced only to a minor degree by the differences in protein expression/regulation between type IIa and IIx fibres.
49.7 ± 0.9 ml kg−1 min−1) could potentially influence the differences in protein expression between muscle fibres. Another limitation to the present study is the lack of distinction between type IIa and IIx fibres in the pools of type II fibres. Yet in vastus lateralis muscle of young, healthy, active men the expression of type IIx fibres is relatively small compared to the proportion of type IIa and type I fibres (Hvid et al. 2011; Jensen et al. 2012; Prats et al. 2013) whereby the proportion of type IIx fibres in pools of type II fibres is thought to be limited. Thus, the differences observed between type I and type II fibres are probably influenced only to a minor degree by the differences in protein expression/regulation between type IIa and IIx fibres.
In conclusion, this study provides evidence for a differentiated response to exercise of key metabolic signalling/effector proteins in human type I and II fibres during interval exercise, emphasizing the potential for new discoveries when investigating muscle fibre type-specific signalling. Although the Western blotting procedure used is semi-quantitative, the use of a calibration curve to ensure assay linearity in fact ensures that changes/differences are detectable (Murphy & Lamb, 2013). AMPK is proposed as a mediator of at least some health benefits of physical activity (Richter & Ruderman, 2009). Increased AMPK activation during exercise in INT vs. CON could then be important for exercise type-specific adaptations, i.e. insulin sensitivity and mitochondrial density.
Acknowledgments
We acknowledge the skilled technical assistance of Betina Bolmgren, Irene B. Nielsen and Nicoline R. Andersen and clinical insight and expertise from Erik A. Richter (University of Copenhagen, Denmark). We are grateful for the kind donation of material essential for this work by the following scientists: L. J. Goodyear (Joslin Diabetes Centre and Harvard Medical School, Boston, MA, USA), O. B. Pedersen (University of Copenhagen, Denmark), J. Hastie and D. G. Hardie (University of Dundee, UK). The monoclonal antibodies against MHC I and II isoforms (A4.840 and A4.74) were developed by H. M. Blau. All MHC antibodies were obtained from the Developmental Studies Hybridoma Bank developed under the auspices of the NICHD and maintained by The University of Iowa, Department of Biology, Iowa City, IA 52242, USA.
Glossary
- ACC
acetyl-CoA carboxylase
- AMPK
AMP-activated protein kinase
- CON
continuous cycling
- Cr
creatine
- GS
glycogen synthase
- INT
interval cycling
- LKB1
serine/threonine kinase 11
- MHC
myosin heavy chain
- PCr
phosphocreatine
- RT
room temperature
- TBC1D
TBC1 domain family member
Additional information
Competing interests
None declared.
Author contributions
D.E.K., P.H.A. and J.F.P.W. contributed to the conception and design of the experiments. D.E.K., P.H.A. and J.F.P.W. performed clinical experiments.: D.E.K., P.H.A. and J.B.B. performed analyses. D.E.K., P.H.A., J.B.B. and J.F.P.W. interpreted the results. D.E.K., P.H.A. and J.F.P.W. drafted the article. All authors edited and revised the article critically for important intellectual content and approved the final version. Human experiments, as well as biochemical measurements, were performed in the laboratories of the Section of Molecular Physiology, August Krogh Centre, Department of Nutrition, Exercise and Sports, University of Copenhagen, Denmark.
Funding
This work was carried out as a part of the research programmes ‘Physical activity and nutrition for improvement of health’ funded by the University of Copenhagen (UCPH) Excellence Program for Interdisciplinary Research and the UNIK project, Food, Fitness & Pharma for Health and Disease (see www.foodfitnesspharma.ku.dk) supported by the Danish Ministry of Science, Technology and Innovation. The study was funded by the Danish Council for Independent Research Medical Sciences (FSS). P.H.A. was financed as an industrial PhD student by the Danish Agency for Science, Technology and Innovation and Novo Nordisk A/S and owns stocks in Novo Nordisk A/S. D.E.K. was partially funded by the Danish Diabetes Academy supported by the Novo Nordisk Foundation.
Translational perspective
Human skeletal muscle consists of different fibre types with molecular and functional differences. Thus, studies of skeletal muscle tissue could be confounded by the heterogeneous nature of human muscles as the proportion of the different fibre types is influenced by various factors such as training status, sex, age, health status and genetics. AMP-activated protein kinase (AMPK) is proposed as a key regulator of cellular energy homeostasis. AMPK is activated during exercise and may mediate both acute and more chronic adaptations in the skeletal muscle fibres. However, most measurements on AMPK activation in humans have been performed in whole muscle biopsy samples without consideration of the influence of muscle fibre types. In the present study, we hypothesized that the regulation of AMPK and downstream targets of AMPK occurs in a fibre type-specific manner dependent on exercise intensity. Here we show that exercise-induced regulation of AMPK and several downstream targets of AMPK is dependent on muscle fibre type and exercise intensity. Yet, when measured traditionally in whole muscle homogenate only one of several measures displayed a dependency on exercise intensity. These findings underscore the relevance of measuring exercise-induced protein signalling on a muscle fibre type-specific level. Furthermore, high intensity exercise activates AMPK predominantly in the fast twitch glycolytic fibres (type II). This might be important for the capacity of these fibres to take up and store substrates during and after exercise as AMPK is suggested to promote the ability of insulin to stimulate glucose uptake and storage.
References
- Albers PH, Pedersen AJ, Birk JB, Kristensen DE, Vind BF, Baba O, Nøhr J, Højlund K. Wojtaszewski JF. Human muscle fiber type-specific insulin signaling: impact of obesity and type 2 diabetes. Diabetes. 2014;64:485–497. doi: 10.2337/db14-0590. [DOI] [PubMed] [Google Scholar]
- Altenburg TM, Degens H, van Mechelen W, Sargeant AJ. de Haan A. Recruitment of single muscle fibers during submaximal cycling exercise. J Appl Physiol (1985) 2007;103:1752–1756. doi: 10.1152/japplphysiol.00496.2007. [DOI] [PubMed] [Google Scholar]
- An D, Toyoda T, Taylor EB, Yu H, Fujii N, Hirshman MF. Goodyear LJ. TBC1D1 regulates insulin- and contraction-induced glucose transport in mouse skeletal muscle. Diabetes. 2010;59:1358–1365. doi: 10.2337/db09-1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba O. Production of monoclonal antibody that recognizes glycogen and its application for immunohistochemistry. Kokubyo Gakkai Zasshi. 1993;60:264–287. doi: 10.5357/koubyou.60.264. ). [ ]. [DOI] [PubMed] [Google Scholar]
- Bartlett JD, Hwa JC, Jeong TS, Louhelainen J, Cochran AJ, Gibala MJ, Gregson W, Close GL, Drust B. Morton JP. Matched work high-intensity interval and continuous running induce similar increases in PGC-1αmRNA, AMPK, p38, and p53 phosphorylation in human skeletal muscle. J Appl Physiol (1985) 2012;112:1135–1143. doi: 10.1152/japplphysiol.01040.2011. [DOI] [PubMed] [Google Scholar]
- Bergström J. Muscle electrolytes in man: determined by neutron activation analysis on needle specimens. Scand J Clin Lab Invest. 1962;14:11–13. [Google Scholar]
- Birk JB. Wojtaszewski JF. Predominant α2/β2/γ3 AMPK activation during exercise in human skeletal muscle. J Physiol. 2006;577:1021–1032. doi: 10.1113/jphysiol.2006.120972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carling D. Hardie DG. The substrate and sequence specificity of the AMP-activated protein kinase. Phosphorylation of glycogen synthase and phosphorylase kinase. Biochim Biophys Acta. 1989;1012:81–86. doi: 10.1016/0167-4889(89)90014-1. [DOI] [PubMed] [Google Scholar]
- Chen Z, Heierhorst J, Mann RJ, Mitchelhill KI, Michell BJ, Witters LA, Lynch GS, Kemp BE. Stapleton D. Expression of the AMP-activated protein kinase β1 and β2 subunits in skeletal muscle. FEBS Lett. 1999;460:343–348. doi: 10.1016/s0014-5793(99)01371-x. [DOI] [PubMed] [Google Scholar]
- Chen ZP, McConell GK, Michell BJ, Snow RJ, Canny BJ. Kemp BE. AMPK signaling in contracting human skeletal muscle: acetyl-CoA carboxylase and NO synthase phosphorylation. Am J Physiol Endocrinol Metab. 2000;279:E1202–E1206. doi: 10.1152/ajpendo.2000.279.5.E1202. [DOI] [PubMed] [Google Scholar]
- Daugaard JR, Nielsen JN, Kristiansen S, Andersen JL, Hargreaves M. Richter EA. Fiber type-specific expression of GLUT4 in human skeletal muscle: influence of exercise training. Diabetes. 2000;49:1092–1095. doi: 10.2337/diabetes.49.7.1092. [DOI] [PubMed] [Google Scholar]
- Daugaard JR. Richter EA. Muscle- and fibre type-specific expression of glucose transporter 4, glycogen synthase and glycogen phosphorylase proteins in human skeletal muscle. Pflugers Arch. 2004;447:452–456. doi: 10.1007/s00424-003-1195-8. [DOI] [PubMed] [Google Scholar]
- Frøsig C, Jørgensen SB, Hardie DG, Richter EA. Wojtaszewski JF. 5'-AMP-activated protein kinase activity and protein expression are regulated by endurance training in human skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286:E411–E417. doi: 10.1152/ajpendo.00317.2003. [DOI] [PubMed] [Google Scholar]
- Frøsig C, Pehmøller C, Birk JB, Richter EA. Wojtaszewski JF. Exercise-induced TBC1D1 Ser237 phosphorylation and 14-3-3 protein binding capacity in human skeletal muscle. J Physiol. 2010;588:4539–4548. doi: 10.1113/jphysiol.2010.194811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujii N, Hayashi T, Hirshman MF, Smith JT, Habinowski SA, Kaijser L, Mu J, Ljungqvist O, Birnbaum MJ, Witters LA, Thorell A. Goodyear LJ. Exercise induces isoform-specific increase in 5'AMP-activated protein kinase activity in human skeletal muscle. Biochem Biophys Res Commun. 2000;273:1150–1155. doi: 10.1006/bbrc.2000.3073. [DOI] [PubMed] [Google Scholar]
- Funai K, Schweitzer GG, Sharma N, Kanzaki M. Cartee GD. Increased AS160 phosphorylation, but not TBC1D1 phosphorylation, with increased postexercise insulin sensitivity in rat skeletal muscle. Am J Physiol Endocrinol Metab. 2009;297:E242–E251. doi: 10.1152/ajpendo.00194.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibala MJ, McGee SL, Garnham AP, Howlett KF, Snow RJ. Hargreaves M. Brief intense interval exercise activates AMPK and p38 MAPK signaling and increases the expression of PGC-1αin human skeletal muscle. J Appl Physiol (1985) 2009;106:929–934. doi: 10.1152/japplphysiol.90880.2008. [DOI] [PubMed] [Google Scholar]
- Gollnick PD, Piehl K. Saltin B. Selective glycogen depletion pattern in human muscle fibres after exercise of varying intensity and at varying pedalling rates. J Physiol. 1974;241:45–57. doi: 10.1113/jphysiol.1974.sp010639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ha J, Daniel S, Broyles SS. Kim KH. Critical phosphorylation sites for acetyl-CoA carboxylase activity. J Biol Chem. 1994;269:22162–22168. [PubMed] [Google Scholar]
- Hardie DG, Ross FA. Hawley SA. AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat Rev Mol Cell Biol. 2012;13:251–262. doi: 10.1038/nrm3311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henneman E, Somjen G. Carpenter DO. Functional significance of cell size in spinal motoneurons. J Neurophysiol. 1965;28:560–580. doi: 10.1152/jn.1965.28.3.560. [DOI] [PubMed] [Google Scholar]
- Hvid LG, Ortenblad N, Aagaard P, Kjaer M. Suetta C. Effects of ageing on single muscle fibre contractile function following short-term immobilisation. J Physiol. 2011;589:4745–4757. doi: 10.1113/jphysiol.2011.215434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen TE, Leutert R, Rasmussen ST, Mouatt JR, Christiansen ML, Jensen BR. Richter EA. EMG-normalised kinase activation during exercise is higher in human gastrocnemius compared to soleus muscle. PLoS One. 2012;7:e31054. doi: 10.1371/journal.pone.0031054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jessen N, An D, Lihn AS, Nygren J, Hirshman MF, Thorell A. Goodyear LJ. Exercise increases TBC1D1 phosphorylation in human skeletal muscle. Am J Physiol Endocrinol Metab. 2011;301:E164–E171. doi: 10.1152/ajpendo.00042.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jørgensen SB, Nielsen JN, Birk JB, Olsen GS, Viollet B, Andreelli F, Schjerling P, Vaulont S, Hardie DG, Hansen BF, Richter EA. Wojtaszewski JF. The α2-5'AMP-activated protein kinase is a site 2 glycogen synthase kinase in skeletal muscle and is responsive to glucose loading. Diabetes. 2004;53:3074–3081. doi: 10.2337/diabetes.53.12.3074. [DOI] [PubMed] [Google Scholar]
- Kostovski E, Boon H, Hjeltnes N, Lundell LS, Ahlsén M, Chibalin AV, Krook A, Iversen PO. Widegren U. Altered content of AMP-activated protein kinase isoforms in skeletal muscle from spinal cord injured subjects. Am J Physiol Endocrinol Metab. 2013;305:E1071–E1080. doi: 10.1152/ajpendo.00132.2013. [DOI] [PubMed] [Google Scholar]
- Kramer HF, Witczak CA, Fujii N, Jessen N, Taylor EB, Arnolds DE, Sakamoto K, Hirshman MF. Goodyear LJ. Distinct signals regulate AS160 phosphorylation in response to insulin, AICAR, and contraction in mouse skeletal muscle. Diabetes. 2006;55:2067–2076. doi: 10.2337/db06-0150. [DOI] [PubMed] [Google Scholar]
- Krustrup P, Söderlund K, Mohr M. Bangsbo J. The slow component of oxygen uptake during intense, sub-maximal exercise in man is associated with additional fibre recruitment. Pflugers Arch. 2004;447:855–866. doi: 10.1007/s00424-003-1203-z. [DOI] [PubMed] [Google Scholar]
- Lee-Young RS, Canny BJ, Myers DE. McConell GK. AMPK activation is fiber type specific in human skeletal muscle: effects of exercise and short-term exercise training. J Appl Physiol (1985) 2009;107:283–289. doi: 10.1152/japplphysiol.91208.2008. [DOI] [PubMed] [Google Scholar]
- Lowry OH. Passonneau JV. A Flexible System of Enzymatic Analysis. London: Academic Press; 1972. pp. 1–291. [Google Scholar]
- Mahlapuu M, Johansson C, Lindgren K, Hjälm G, Barnes BR, Krook A, Zierath JR, Andersson L. Marklund S. Expression profiling of the γ-subunit isoforms of AMP-activated protein kinase suggests a major role for γ3 in white skeletal muscle. Am J Physiol Endocrinol Metab. 2004;286:E194–E200. doi: 10.1152/ajpendo.00147.2003. [DOI] [PubMed] [Google Scholar]
- Mortensen B, Poulsen P, Wegner L, Stender-Petersen KL, Ribel-Madsen R, Friedrichsen M, Birk JB, Vaag A. Wojtaszewski JF. Genetic and metabolic effects on skeletal muscle AMPK in young and older twins. Am J Physiol Endocrinol Metab. 2009;297:E956–E964. doi: 10.1152/ajpendo.00058.2009. [DOI] [PubMed] [Google Scholar]
- Murphy RM. Enhanced technique to measure proteins in single segments of human skeletal muscle fibers: fiber-type dependence of AMPK-α1 and -β1. J Appl Physiol (1985) 2011;110:820–825. doi: 10.1152/japplphysiol.01082.2010. [DOI] [PubMed] [Google Scholar]
- Murphy RM. Lamb GD. Important considerations for protein analyses using antibody based techniques: down-sizing Western blotting up-sizes outcomes. J Physiol. 2013;591:5823–5831. doi: 10.1113/jphysiol.2013.263251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura-Tsuruta S, Yasuda M, Nakamura T, Shinoda E, Furuyashiki T, Kakutani R, Takata H, Kato Y. Ashida H. Comparative analysis of carbohydrate-binding specificities of two anti-glycogen monoclonal antibodies using ELISA and surface plasmon resonance. Carbohydr Res. 2012;350:49–54. doi: 10.1016/j.carres.2011.12.029. [DOI] [PubMed] [Google Scholar]
- Nielsen JN, Mustard KJ, Graham DA, Yu H, MacDonald CS, Pilegaard H, Goodyear LJ, Hardie DG, Richter EA. Wojtaszewski JF. 5'-AMP-activated protein kinase activity and subunit expression in exercise-trained human skeletal muscle. J Appl Physiol (1985) 2003;94:631–641. doi: 10.1152/japplphysiol.00642.2002. [DOI] [PubMed] [Google Scholar]
- O'Neill HM. AMPK and exercise: glucose uptake and insulin sensitivity. Diabetes Metab J. 2013;37:1–21. doi: 10.4093/dmj.2013.37.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pehmøller C, Treebak JT, Birk JB, Chen S, Mackintosh C, Hardie DG, Richter EA. Wojtaszewski JF. Genetic disruption of AMPK signaling abolishes both contraction- and insulin-stimulated TBC1D1 phosphorylation and 14-3-3 binding in mouse skeletal muscle. Am J Physiol Endocrinol Metab. 2009;297:E665–E675. doi: 10.1152/ajpendo.00115.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prats C, Gomez-Cabello A, Nordby P, Andersen JL, Helge JW, Dela F, Baba O. Ploug T. An optimized histochemical method to assess skeletal muscle glycogen and lipid stores reveals two metabolically distinct populations of type I muscle fibers. PLoS One. 2013;8:e77774. doi: 10.1371/journal.pone.0077774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Putman CT, Martins KJ, Gallo ME, Lopaschuk GD, Pearcey JA, MacLean IM, Saranchuk RJ. Pette D. -Catalytic subunits of 5'AMP-activated protein kinase display fiber-specific expression and are upregulated by chronic low-frequency stimulation in rat muscle. Am J Physiol Regul Integr Comp Physiol. 2007;293:R1325–R1334. doi: 10.1152/ajpregu.00609.2006. [DOI] [PubMed] [Google Scholar]
- Richter EA. Hargreaves M. Exercise, GLUT4, and skeletal muscle glucose uptake. Physiol Rev. 2013;93:993–1017. doi: 10.1152/physrev.00038.2012. [DOI] [PubMed] [Google Scholar]
- Richter EA. Ruderman NB. AMPK and the biochemistry of exercise: implications for human health and disease. Biochem J. 2009;418:261–275. doi: 10.1042/BJ20082055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rose AJ, Bisiani B, Vistisen B, Kiens B. Richter EA. Skeletal muscle eEF2 and 4EBP1 phosphorylation during endurance exercise is dependent on intensity and muscle fiber type. Am J Physiol Regul Integr Comp Physiol. 2009;296:R326–R333. doi: 10.1152/ajpregu.90806.2008. [DOI] [PubMed] [Google Scholar]
- Taylor EB, An D, Kramer HF, Yu H, Fujii NL, Roeckl KS, Bowles N, Hirshman MF, Xie J, Feener EP. Goodyear LJ. Discovery of TBC1D1 as an insulin-, AICAR-, and contraction-stimulated signaling nexus in mouse skeletal muscle. J Biol Chem. 2008;283:9787–9796. doi: 10.1074/jbc.M708839200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treebak JT, Birk JB, Hansen BF, Olsen GS. Wojtaszewski JF. A-769662 activates AMPK β1-containing complexes but induces glucose uptake through a PI3-kinase-dependent pathway in mouse skeletal muscle. Am J Physiol Cell Physiol. 2009a;297:C1041–C1052. doi: 10.1152/ajpcell.00051.2009. [DOI] [PubMed] [Google Scholar]
- Treebak JT, Birk JB, Rose AJ, Kiens B, Richter EA. Wojtaszewski JF. AS160 phosphorylation is associated with activation of α2β2γ1- but not α2β2γ 3-AMPK trimeric complex in skeletal muscle during exercise in humans. Am J Physiol Endocrinol Metab. 2007;292:E715–E722. doi: 10.1152/ajpendo.00380.2006. [DOI] [PubMed] [Google Scholar]
- Treebak JT, Frøsig C, Pehmøller C, Chen S, Maarbjerg SJ, Brandt N, Mackintosh C, Zierath JR, Hardie DG, Kiens B, Richter EA, Pilegaard H. Wojtaszewski JF. Potential role of TBC1D4 in enhanced post-exercise insulin action in human skeletal muscle. Diabetologia. 2009b;52:891–900. doi: 10.1007/s00125-009-1294-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treebak JT, Pehmøller C, Kristensen JM, Kjøbsted R, Birk JB, Schjerling P, Richter EA, Goodyear LJ. Wojtaszewski JF. Acute exercise and physiological insulin induce distinct phosphorylation signatures on TBC1D1 and TBC1D4 proteins in human skeletal muscle. J Physiol. 2014;592:351–375. doi: 10.1113/jphysiol.2013.266338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treebak JT, Taylor EB, Witczak CA, An D, Toyoda T, Koh HJ, Xie J, Feener EP, Wojtaszewski JF, Hirshman MF. Goodyear LJ. Identification of a novel phosphorylation site on TBC1D4 regulated by AMP-activated protein kinase in skeletal muscle. Am J Physiol Cell Physiol. 2010;298:C377–C385. doi: 10.1152/ajpcell.00297.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vendelbo MH, Møller AB, Treebak JT, Gormsen LC, Goodyear LJ, Wojtaszewski JF, Jørgensen JO, Møller N. Jessen N. Sustained AS160 and TBC1D1 phosphorylations in human skeletal muscle 30 min after a single bout of exercise. J Appl Physiol (1985) 2014;117:289–296. doi: 10.1152/japplphysiol.00044.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vichaiwong K, Purohit S, An D, Toyoda T, Jessen N, Hirshman MF. Goodyear LJ. Contraction regulates site-specific phosphorylation of TBC1D1 in skeletal muscle. Biochem J. 2010;431:311–320. doi: 10.1042/BJ20101100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vøllestad NK. Blom PC. Effect of varying exercise intensity on glycogen depletion in human muscle fibres. Acta Physiol Scand. 1985;125:395–405. doi: 10.1111/j.1748-1716.1985.tb07735.x. [DOI] [PubMed] [Google Scholar]
- Vøllestad NK, Vaage O. Hermansen L. Muscle glycogen depletion patterns in type I and subgroups of type II fibres during prolonged severe exercise in man. Acta Physiol Scand. 1984;122:433–441. doi: 10.1111/j.1748-1716.1984.tb07531.x. [DOI] [PubMed] [Google Scholar]
- Winder WW. Hardie DG. Inactivation of acetyl-CoA carboxylase and activation of AMP-activated protein kinase in muscle during exercise. Am J Physiol Endocrinol Metab. 1996;270:E299–E304. doi: 10.1152/ajpendo.1996.270.2.E299. [DOI] [PubMed] [Google Scholar]
- Winder WW, Wilson HA, Hardie DG, Rasmussen BB, Hutber CA, Call GB, Clayton RD, Conley LM, Yoon S. Zhou B. Phosphorylation of rat muscle acetyl-CoA carboxylase by AMP-activated protein kinase and protein kinase A. J Appl Physiol (1985) 1997;82:219–225. doi: 10.1152/jappl.1997.82.1.219. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Birk JB, Frøsig C, Holten M, Pilegaard H. Dela F. 5'AMP activated protein kinase expression in human skeletal muscle: effects of strength training and type 2 diabetes. J Physiol. 2005;564:563–573. doi: 10.1113/jphysiol.2005.082669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wojtaszewski JF, Mourtzakis M, Hillig T, Saltin B. Pilegaard H. Dissociation of AMPK activity and ACCβphosphorylation in human muscle during prolonged exercise. Biochem Biophys Res Commun. 2002;298:309–316. doi: 10.1016/s0006-291x(02)02465-8. [DOI] [PubMed] [Google Scholar]
- Wojtaszewski JF, Nielsen P, Hansen BF, Richter EA. Kiens B. Isoform-specific and exercise intensity-dependent activation of 5'-AMP-activated protein kinase in human skeletal muscle. J Physiol. 2000;528:221–226. doi: 10.1111/j.1469-7793.2000.t01-1-00221.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu H, Fujii N, Hirshman MF, Pomerleau JM. Goodyear LJ. Cloning and characterization of mouse 5'-AMP-activated protein kinase γ3 subunit. Am J Physiol Cell Physiol. 2004;286:C283–C292. doi: 10.1152/ajpcell.00319.2003. [DOI] [PubMed] [Google Scholar]


