Abstract
Clenbuterol is a β2-adrenergic receptor agonist known to induce skeletal muscle hypertrophy and a slow-to-fast phenotypic shift. The aim of the present study was to test the effects of chronic clenbuterol treatment on contractile efficiency and explore the underlying mechanisms, i.e. the muscle contractile machinery and calcium-handling ability. Forty-three 6-week-old male Wistar rats were randomly allocated to one of six groups that were treated with either subcutaneous equimolar doses of clenbuterol (4 mg kg−1 day−1) or saline solution for 9, 14 or 21 days. In addition to the muscle hypertrophy, although an 89% increase in absolute maximal tetanic force (Po) was noted, specific maximal tetanic force (sPo) was unchanged or even depressed in the slow twitch muscle of the clenbuterol-treated rats (P < 0.05). The fit of muscle contraction and relaxation force kinetics indicated that clenbuterol treatment significantly reduced the rate constant of force development and the slow and fast rate constants of relaxation in extensor digitorum longus muscle (P < 0.05), and only the fast rate constant of relaxation in soleus muscle (P < 0.05). Myofibrillar ATPase activity increased in both relaxed and activated conditions in soleus (P < 0.001), suggesting that the depressed specific tension was not due to the myosin head alteration itself. Moreover, action potential-elicited Ca2+ transients in flexor digitorum brevis fibres (fast twitch fibres) from clenbuterol-treated animals demonstrated decreased amplitude after 14 days (−19%, P < 0.01) and 21 days (−25%, P < 0.01). In conclusion, we showed that chronic clenbuterol treatment reduces contractile efficiency, with altered contraction and relaxation kinetics, but without directly altering the contractile machinery. Lower Ca2+ release during contraction could partially explain these deleterious effects.
Key points
Clenbuterol is an adrenergic receptor agonist known to induce skeletal muscle hypertrophy and a shift towards faster muscle fibres, when administered chronically at high doses.
However, when normalized to the muscle surface area, the increase in muscle force is no longer increased and even depressed.
We show that muscle contraction and relaxation force kinetics were significantly reduced particularly in fast contracting muscles.
We show that action potential-elicited Ca2+ transients were depressed in the fast contracting muscle.
Our data show that chronic clenbuterol treatment reduces contractile efficiency, with altered contraction and relaxation kinetics, but without directly altering the contractile machinery. Lower Ca2+ release during contraction could partially explain these deleterious effects.
Introduction
β2-Adrenergic agonists, the most effective bronchodilators used to prevent asthma in humans, act by inhibiting smooth muscle contraction and increasing epithelial mucus clearance. In addition to their anti-asthma effect, chronic administration of these agonists causes a dramatic increase in skeletal muscle growth in various mammalian species (Kim et al. 1992; Mersmann, 1998; Kissel et al. 2001) and enhances human sports performance (Collomp et al. 2005; Sanchez et al. 2012; Hostrup et al. 2014a,b2014b).
Synthetic β2-agonists such as cimaterol, clenbuterol, fenoterol, formoterol, salbutamol and salmeterol are based on the chemical structure of adrenaline and promote muscle growth through the stimulation of β2-adrenoceptors and subsequent activation of downstream signalling pathways. Clenbuterol, one of the selective β2-adrenergic agonists, is known to induce a specific protein anabolic effect in skeletal muscle (Mersmann, 1998; Kissel et al. 2001; Bricout et al. 2004; Lynch & Ryall, 2008). Because of this effect, clenbuterol has been extensively studied, especially for its therapeutic potential in various diseases affecting muscle mass or weakness. Effects of β2-adrenergic agonist treatment on muscle mass have been demonstrated in th contexts of muscular dystrophy, disuse (Ricart-Firinga et al. 2000), ageing, denervation and amyotrophic lateral sclerosis in both animals and humans (Lynch & Ryall, 2008). In addition to the effect on muscle mass, chronic clenbuterol treatment induces a fibre-type transition from slow to fast twitch fibres in animal models (Zeman et al. 1988; Polla et al. 2001; Mounier et al. 2007; Douillard et al. 2011). The phenotypic and metabolic changes induced by clenbuterol have raised questions about the impact of this treatment on muscle contractile function and ATPase activity. Previous studies have shown greater force-producing capacity in clenbuterol-treated muscle in both fast and slow twitch muscles (Dodd et al. 1996). Nevertheless, when maximal tetanic contraction was corrected for muscle cross-sectional area, muscle force was unchanged and even altered in soleus muscle (McCormick et al. 2010). These results suggest a potentially deleterious effect of chronic clenbuterol treatment on the skeletal muscle contractile machinery. A preliminary study suggested that chronic clenbuterol treatment induces significant changes in the calcium (Ca2+) signals associated with excitation–contraction coupling in fast twitch skeletal muscles (Sirvent et al. 2014). However, the mechanisms responsible for the changes in clenbuterol-induced contractility are not well understood.
Ca2+ handling is the primary regulator of force generation by cross-bridges in striated muscle. A reduction in Ca2+ release from the sarcoplasmic reticulum at each contraction would explain, at least in part, the reduced contractile function. However, muscle contractile function depends on contraction and relaxation processes. Relaxation involves at least three processes: termination of Ca2+ release, the kinetics of calcium dissociation from troponin and cross-bridge detachment, and Ca2+ uptake by the sarcoplasmic reticulum and myoplasmic Ca2+ buffering (Poggesi et al. 2005). Strong evidence indicates that cross-bridge kinetics is the major determinant of the time course of striated muscle relaxation and is responsible for ‘load-dependent’ changes in relaxation kinetics. Last, the kinetics of myosin head detachment from the thin filament is related to ATP binding. Thus, beyond muscle hypertrophy, an effect of clenbuterol on contraction and relaxation kinetics is expected.
The aim of the present study was to test the effect of chronic clenbuterol treatment on muscle force generation and relaxation kinetics in slow and fast twitch muscles and on myofibrillar ATPase activity in both relaxed and activated conditions. In addition, Ca2+ signalling was evaluated as it is the main regulator of contraction and relaxation kinetics.
Methods
All experiments complied with the Guide for the Care and Use of Laboratory Animals, published by the National Institutes of Health (NIH Publications No. 85-23, Revised 1996). All experiments were approved by the Research Ethics Committee of Languedoc-Roussillon region [‘Ethique sur l'Expérimentation Animale’ approved the experimental protocol (# 36)].
Animals
Forty-three 6-week-old male Wistar rats (body mass: 236.3 ± 3.8 g; Charles River, Wilmington, MA, USA) were randomly allocated to one of six groups and housed in standard cages with a 12:12 h light–dark cycle and food and water available ad libitum. Treated rats received subcutaneous equimolar doses of clenbuterol (4 mg kg−1 day−1) for 9 (CB-G9, n = 7), 14 (CB-G14, n = 7) or 21 days (CB-G21, n = 8). Body weights were measured daily and injection doses adjusted accordingly. To control for maturation effects due to the treatment duration, control rats were recruited and received a daily subcutaneous injection of an identical volume of saline solution for 9 (CTL-G9, n = 7), 14 (CTL-G14, n = 7) or 21 days (CTL-G21, n = 7). All measurements were made 24 h after the last treatment. While animals were anaesthetized with pentobarbital sodium, extensor digitorum longus (EDL), soleus (SOL) and flexor digitorum brevis (FDB) muscles of both left and right hindlimbs were rapidly dissected out. At the end of the surgical procedure, the animals were killed with a lethal dose of pentobarbital.
Muscle phenotype
SOL and EDL muscle phenotypes were determined by the Kaiser and Brooke myosin-ATPase coloration method (Brooke & Kaiser, 1970). All twitch fibres from each muscle were selected and identified as being type I fibres, type IIa fibres or type IIx-IIb fibres.
Kinetics of muscle force activation and relaxation kinetics
EDL and SOL muscles from the right hindlimb were surgically exposed. The excised muscles were immediately placed ib a custom-built Plexiglas chamber filled with Krebs-Ringer solution [composition (in mm): NaCl 11.9, KCl 0.5, CaCl2 0.125, MgSO4 0.1, KH2PO4 0.1, glucose 1, NaHCO3 2.5, mannitol 0.11] equilibrated with 95% O2/5% CO2 gas and maintained at 25°C, pH 7.4.
Contractile properties of the right EDL and SOL were assessed in vitro according to the methods described in detail previously (Lynch et al. 2001; Ryall et al. 2002). In vitro contractile measurements began with the determination of the muscle optimal length (Lo) for isometric tension development. After a 15 min equilibration in the bath, the muscle was connected to an isotonic force transducer (model 305B; Cambridge Instruments, Aurora Scientific, Inc., Ontario, Canada) and stimulated along its entire length with platinum wire electrodes. After the determination of Lo from the maximum isometric twitch force, all subsequent contractile properties were measured at Lo. Optimum twitch fibre length (Lf) was determined by multiplying Lo by previously determined ratios of fibre length to muscle length: 0.44 for EDL and 0.71 for SOL (Brooks & Faulkner, 1988; Ryall et al. 2002). Isometric tetanic tension was determined at different frequencies of stimulation (701B Stimulator; Aurora Scientific), ranging from 1 to 100 Hz for SOL and 150 Hz for EDL (pulse duration 0.5 ms, train duration 500 ms). A rest period of 1 min was applied between each tetanic stimulation. Maximal isometric tetanic tension (Po) was determined from the plateau of the frequency–force curve. After all measurements, the muscle was removed from the bath, trimmed of connective tissue, blotted dry and weighed on an analytical balance. Specific force (sPo; N cm−2) was calculated for each muscle according to the well-accepted procedure that accounts for muscle cross-sectional area, determined after dividing muscle mass by the product of Lf and 1.06 mg mm−3, according to Lynch et al. (2001).
Fitting of muscle mechanical kinetics
A non-linear regression was used to fit the data obtained in the CTL-G21 and CB-G21 groups only to a model that defines the kinetics of muscle force production and relaxation in EDL and SOL muscles during a single 500 ms twitch (pulse duration of 0.5 ms at 100 Hz for SOL and 150 Hz for EDL). The model used to fit the force development and relaxation kinetics for EDL and SOL and determine the parameter values is presented in Fig.1. Data were fitted using GraphPad Prism 4 (GraphPad Prism Software, San Diego, CA, USA). During the force production phase, the rate constant of the force development (kACT, s−1) was determined. The time course of full force relaxation was biphasic, starting with a slow, seemingly linear, phase (rate constant, Slow kREL, s−1) followed, after a ‘shoulder’, by a fast, approximately monoexponential, relaxation phase (rate constant, Fast kREL, s−1).
Figure 1. Fitting of force activation and relaxation in EDL (A) and SOL (B) muscles.
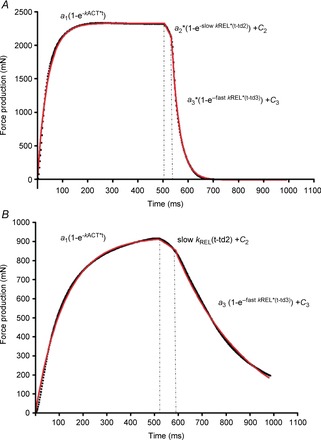
Recording shows the fitting curve (red line) obtained from the data curve (black line) recorded at 150 and 100 Hz for EDL and SOL, respectively. The kinetics of force development was described by one exponential increase: a1(1–e−kACT*t), where a1 is the value of force at the maximal curve asymptote (mN); e is the exponential function; kACT (s−1) is the rate constant of the force development; and x is the time (ms). The kinetics of force relaxation was described using two exponential slow and fast decays: a2(1–e−slow kREL*(t-td2)) + c2 and a3(1–e− fast kREL*(t-td3)) +c3 where c2 and c3 are the force levels at the beginning of each decay; slow kREL and fast kREL are the rate constants of the slow and fast phases of tension decline, respectively; a2 and a3 are the basal relaxation force level c2 and c3, respectively; and td2 and td3 are the time delays at the beginning of each decay.
Myofibril preparation
SOL (slow twitch) muscle from CTL-G21, CB-G14 and CB-G21 groups as well as EDL (fast twitch) muscle from CTL-G21, CB-G9, CB-G14 and CB-G21 groups was used for these experiments. Briefly, muscles were immersed in ice-cooled Ringer buffer [composition (in mm): Tris-HCl 50, pH 7.0, NaCl 100, KCl 2, MgCl2 2, EGTA 1, dithiothreitol 1, phenylmethanesulfonyl fluoride 0.2, leupeptin 0.01, pepstatin 0.005 and NaN3 0.5] at rest length. From these latter SOL and EDL muscles, myofibrils were prepared as previously described (Candau et al. 2003). The total myosin head concentration in the myofibrillar suspension was measured by absorbance at 280 nm of a 1:10 dilution of the suspension in 2% SDS (Houadjeto et al. 1992), based on the assumption that the molar extinction coefficient and percentage of myosin in SOL and EDL myofibrils are the same as in psoas myofibrils (Herrmann et al. 1994; Iorga et al. 2004).
Myofibrillar ATPase activity
ATPase measurements were carried out in a home-built, thermostatically controlled, Rapid Flow Quench apparatus (Barman & Travers, 1985). Experiments were performed at 4°C to slow down the unloaded shortening velocity (V0), and thus to increase the duration of the unloaded shortening phase (tB) and improve the time resolution. The chosen temperature of 4°C, although it slows the shortening velocity, still allows the myofibrils to behave and contract normally (Candau et al. 2003). In the Rapid Flow Quench apparatus, myofibrils were mixed with [γ-32P]ATP (2.5 μm myosin heads + 25 μm [γ-32P]ATP in the reaction mixture at 4°C). Reaction mixtures were quenched in acid (22% trichloroacetic acid and 1 mm KH2PO4) at different times and the [32P]Pi was assayed. Reaction mixtures with incubation times from 200 ms to 10 s were obtained with this apparatus. For longer incubation times, myofibrils and [γ-32P]ATP were mixed in a beaker at 4°C, where one sample of the reaction mixture was taken and quenched every 10–15 s for 3–10 min. The amounts of total Pi (i.e. free Pi plus myosin head-bound Pi) were determined in the quenched reaction mixtures by the filter paper method of Reimann and Umfleet (1978). Relaxing and activating buffers were used to obtain relaxed and activated conditions, respectively, i.e. 50 mm Tris-acetate, pH 7.4, 100 mm potassium acetate, 5 mm KCl and either 0.1 mm CaCl2 and 2 mm magnesium acetate for the activating buffer (with Ca2+), or 2 mm EGTA and 5 mm magnesium acetate for the relaxing buffer (without Ca2+).
Modelling ATPase activity kinetics
Although the first phase of steady-state ATP hydrolysis was used as a measure of the ATPase activity during unloaded shortening of the calcium-activated myofibrils, the entire kinetics of ATP hydrolysis was modelled with a three-component model to determine the kinetic parameters:
| 1 |
| 2 |
where the third component eqn (2) starts when t>tB, [Total Pi]/[myosin heads] is the amount of total phosphate determined per myosin head (mol of ATP hydrolysed per mol of myosin heads), A is the amplitude (mol/mol) of the Pi burst, kF is the rate constant (s−1) of the fast steady state during the unloaded shortening phase in activated conditions, kS is the rate constant (s−1) of the steady state during ‘over-contraction’ when the thin filaments of the two halves of the sarcomeres start to overlap each other (a non-physiological state), and tB is the time break (s) corresponding to the unloaded shortening phase duration (Lionne et al. 1996). The model parameters were determined using an iterative process that minimizes the sum of the mean squares between the total phosphate measured and that predicted by the model. The ATP cost of contraction during the unloaded shortening phase was determined based on the number of ATPs consumed per myosin head (ATPtB) until tB.
The titration of the myofibril preparation ([active site]), i.e. the number of fully active ATPase sites per total sites, was estimated from the amplitude (A) of the Pi burst as proposed by Iorga et al. (2004) for Pi burst experiments on SOL myofibrils. Titration experiments were performed in relaxed conditions because (i) A is similar in relaxed and Ca2+-activated conditions, (ii) the cleavage step that controls the Pi burst amplitude occurs in the detached state whatever the condition and (iii) A is determined more accurately in relaxed than in activated conditions, due a slower reaction compared with activated conditions.
The limiting step of the cross-bridge cycle, the isomerization step that occurs just before Pi release (k4), was evaluated from the catalytic activity (kcat) and the equilibrium constant of the ATP cleavage step (K3) (Iorga et al. 2004):
| 3 |
where kcat = ks/[active site], and K3>10.
Stopped-flow experiments
The low temperature retained for the enzyme kinetic experiments allowed measurement of the kinetics of the ATP-induced myosin head detachment from the thin filament and the ATP cleavage (K3) (Stehle & Brenner, 2000). The experiment was carried out in a Hi-Tech Scientific stopped-flow apparatus (model SF-61 DX2; Hi-Tech Ltd, Salisbury, UK). The excitation wavelength was 295 nm and emission was >320 nm using a cut-off filter (WG320; Hi-Tech) to measure tryptophan fluorescence. For each experimental condition, a series of 12 shots was carried out and averaged. The fluorescence of the myofibrillar solution, without ATP, was set as 100% just before the start of the experiment. Fluorescence time courses were fitted with a double exponential model of rate constants kTrp fast and kTrp slow. The fast phase is related to the ATP-induced detachment of the myosin head from the actin filaments. Its kinetics is hyperbolically dependent on ATP concentration, and the initial slope of the dependence gives information on the rate of detachment. The slow phase kinetics is governed by the ATP cleavage step and is independent of ATP concentration. For further details, see Stehle and Brenner (2000).
Modelling ATPase activity as a function of twitch fibre type
To identify the changes in ATPase activity due to changes in the twitch fibre type in response to clenbuterol treatment, a multilinear regression analysis including specific ATPases for each twitch fibre type was used:
| 4 |
where F-I, F-IIa and F-IIx/b were the percentages of twitch fibre types I, IIa and IIx/b, respectively, and kss was the relaxed ATPase steady state, assumed to be equal to 0.125kf.
Ca2+-transient measurement
Due to its relatively small size and the technical constraints associated with Ca2+-transient measurement, the FDB muscle (fast twitch) was selected, dissected and enzymatically dissociated at 37°C for 1 h with 3 mg ml−1 collagenase (type 1; Sigma, St Louis, MO, USA) dissolved in an external medium (in mm: NaCl 145, KCl 4, CaCl2 1.8, MgSO4 1, Hepes 10 and glucose 10, pH 7.4). Bundles of fibres were then transferred to an external medium without collagenase, and the fibres were mechanically separated by gentle trituration. Intact single fibres were plated on extracellular matrix (Sigma)-coated coverslips attached across a 12 mm hole in the bottom of 35 mm Petri dishes. FDB fibres bathed in an external medium were loaded for 30 min at room temperature with Fluo-3 AM (5 μm; TefLabs, Austin, TX, USA). Action potential (AP)-induced Ca2+ transients were triggered by field stimulation with a single 2 ms pulse just above the stimulation threshold. Fluorescence images were acquired in line-scan mode (spatial [x] vs. temporal [t], 1.5 ms per line) with a confocal system (Zeiss LSM 510 Meta, 63× objective, NA = 1.2, H2O immersion; Oberkochen, Germany). Fluo-3 was excited with an argon/krypton laser at 488 nm, and the emitted fluorescence was recorded at 525 nm. Single-fibre Ca2+ transients were identified on raw fluorescence images. Image strips of the manually identified area of a single fibre were extracted. After subtracting the photomultiplier offset current, strip images were converted to ΔF/F, where F is the mean resting fluorescence of the fibre calculated from the image area preceding the AP stimulation and subsequent Ca2+ transient. The temporal fluorescence time courses of each fibre were generated by spatial compression of the ΔF/F images, resulting in a mean spatial F value for each temporal coordinate.
Statistics
Variables were compared between groups using ANOVA and the Newman–Keuls post hoc multiple comparison procedure when significance was detected. The significance of the model parameters retained in the kinetic analysis was tested by an F-test. Significance was set at P < 0.05. All values are expressed as mean ± SEM.
Results
Morphometric properties, shift in muscle phenotype
Clenbuterol treatment for 21 days resulted in significant increases in muscle mass, more pronounced in EDL (+22%; 220.9 ± 5.4 mg CB-G21 vs. 176.3 ± 8.3 CTL-G21; P < 0.001) than SOL (+9%; 202.0 ± 5.8 mg CB-G21 vs. 176.7 ± 6.1 mg CTL-G21; P < 0.01). The muscle mass/body mass ratio was not different compared with control groups. In the clenbuterol-treated rats, the ratio was unchanged in SOL (0.53 ± 0.01 CB-G21 vs. 0.54 ± 0.01 CTL-G21) but significantly increased in EDL (0.59 ± 0.01 CB-G21 vs. 0.52 ± 0.02 CTL-G21; P < 0.001).
Non-statistically significant differences were found between CTL-G9, CTL-G14 and CTL-G21 groups for both fibre type distribution or cross-sectional area (CSA) (data not shown for CTL-G9 and CTL-G14 groups) and thus we decided to present results of the CTL-G21 group only. EDL muscle underwent a phenotypic shift toward faster muscle twitch fibres, as determined by the myosin ATP (Table1). This shift was noticeable from day 9 onward. The increased percentage of type IIx-IIb twitch fibres at day 9 was accompanied by a decreased percentage of type IIa twitch fibres. At day 21, a 17% (P < 0.05) increase and a 15% (P < 0.05) decrease in IIx-IIb and IIa twitch fibre types, respectively, were observed compared with CTL-G21. Similar results in the SOL muscle were found (Table1). At day 14, the proportion of type I muscle fibres was 11% (P < 0.05) lower in the treated group compared with CTL-G14 group. The percentage of type IIx-IIb twitch fibres was significantly higher in the treated group compared with CTL-G9 at day 9 (+6%, P < 0.01). Later (i.e. between day 9 and day 21), no further increase in the percentage of type IIx-IIb twitch fibres was observed. After 21 days of treatment, the percentage of type IIa fibres was significantly increased from 14 to 29% (Table1).
Table 1.
Time course of clenbuterol-induced phenotype shift in EDL and SOL muscles
| EDL | SOL | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| CTL-G21 | CB-G9 | CB-G14 | CB-G21 | CTL-G21 | CB-G9 | CB-G14 | CB-G21 | ||
| Fibre types | I | 7.5 ± 2.2 | 7.3 ± 2.6 | 7.6 ± 2.8 | 5.4 ± 1.4 | 80.0 ± 7.4 | 72.0 ± 8.3 | 69.4 ± 9.5* | 60.7 ± 8.5*** |
| (%) | IIa | 25.6 ± 1.9 | 14.7 ± 4.3* | 9.9 ± 2.4** | 11.0 ± 3.7** | 14.8 ± 8.5 | 16.9 ± 8.4 | 20.4 ± 9.7 | 29.1 ± 10** |
| IIx-IIb | 66.9 ± 2.7 | 78.0 ± 4.3* | 82.4 ± 3.1** | 83.6 ± 4.7** | 5.3 ± 3.6 | 11.1 ± 1.8* | 10.2 ± 2.8* | 10.2 ± 6.8* |
Values are mean percentages (± SD, n = 8) of the different fibre types in EDL and SOL. *Different from CTL-G21; **different from CTL-G21 and CB-G9; ***different from CTL-G21, CB-G9 and CB-G14. P < 0.05. Because non-statistically differences exist between CTL-G9, CTL-G14 and CTL-G21 groups for both fibre type distribution and CSA, results of the CTL-G21 group only are given.
Kinetics of muscle force activation and relaxation
Muscle force development
In EDL muscles, maximal isometric tension (Po) exhibited an 89% increase in CB-G21 compared with CTL-G21 (2232 ± 192 vs. 1177 ± 206 mN, respectively; P < 0.05, Fig.2A). However, when Po was corrected to account for muscle CSA, sPo was not different in EDL among groups (12.3 ± 1.0 vs. 12.0 ± 1.8 N cm−2 in CB-G21 and CTL-G21, respectively, Fig.2A).
Figure 2. Effect of clenbuterol treatment on maximal isometric tetanic power (Po), specific isometric tetanic power (sPo), and force development and force relaxation kinetics in EDL (A, C) and SOL (B, D) muscle.
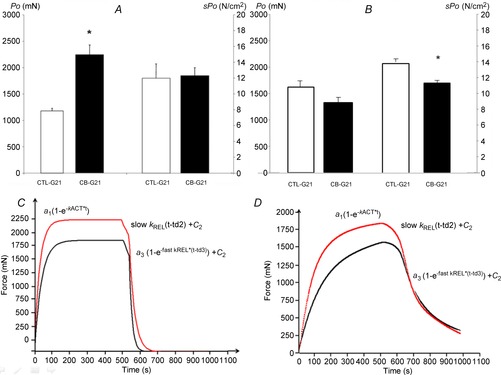
Mean (± SEM) EDL (A) and SOL (B) maximal isometric tetanic power (Po, left vertical axis) and specific maximal isometric tetanic power (sPo, right vertical axis) recorded from CTL-G21 (white bar) and CB-G21 animals (dark bar). C and D, recording shows representative fitting curves in a CB-treated rat (red line) and a CTL-treated rat (black line) in EDL muscle (C) and SOL muscle (D). The kinetics of force development was described by one exponential increase: a1(1–e−kACT*t), where a1 is the value of force at the maximal curve asymptote (mN); e is the exponential function; kACT (s−1) is the rate constant of the force development; and x is the time (ms). The kinetics of force relaxation was described using two exponential slow and fast decays: a2(1–e−slow kREL*(t-td2)) + c2 and a3(1–e− fast kREL*(t-td3)) +c3 where c2 and c3 are the force levels at the beginning of each decay; slow kREL and fast kREL are the rate constants of slow and fast phases of tension decline, respectively; a2 and a3 are the basal relaxation forces level c2 and c3, respectively; and td2 and td3 are the time delasy at the beginning of each decay. *P < 0.05 CTL-G21 vs. CB-G21.
In SOL muscle, no significant difference in Po was observed between CB-G21 and CTL-G21 (1648 ± 133 vs. 1365 ± 50 mN, respectively) (Fig.2B). However, clenbuterol treatment significantly reduced SOL sPo in CB-G21 compared with CTL-G21 (11.9 ± 0.7 vs. 14.0 ± 0.6 N cm−2; P < 0.05, Fig.2B).
Kinetics of force development and relaxation
Fitting of thekinetics of force activation and relaxation for EDL and SOL gave high determination coefficients, with a mean r2 value greater than 0.997 and 95% confidence intervals inferior to 1% for EDL and SOL. Figure 2C and D illustrates representative recordings from fitting curves obtained from G21-CB and CTL-G21 for both EDL and SOL. The results obtained from fitting the muscle mechanical kinetics showing the clenbuterol effect after 21 days of treatment are summarized in Table2. In EDL, the main clenbuterol effect was observed for kACT with a significant decrease compared with the untreated condition (Table2; P < 0.01). In addition, clenbuterol treatment significantly decreased Slow kREL and Fast kREL (Table2; P < 0.01 and P < 0.001, respectively). In SOL muscle, a significant decrease in Fast kREL was observed in CB-21 compared with CTL-G21 (Table2; P < 0.05).
Table 2.
Clenbuterol alters contraction and relaxation kinetics in fast muscle
| EDL | SOL | |||
|---|---|---|---|---|
| CTL-G21 (n = 7) | CB-G21 (n = 8) | CTL-G21 (n = 7) | CB-G21 (n = 8) | |
| kACT (s−1) | 23.1 ± 0.8 | 19.3 ± 1.2** | 8.5 ± 0.8 | 7.6 ± 0.0 |
| Slow kREL (s−1) | 3.4 ± 0.1 | 1.8 ± 0.0** | 1.8 ± 0.2 | 1.4 ± 0.1 |
| Fast kREL (s−1) | 38.7 ± 2.2 | 28.7 ± 1.6*** | 10.4 ± 1.7 | 6.3 ± 0.5* |
Kinetic parameters for force development and relaxation during maximal tetanic eletrostimuation following clenbuterol treatment in EDL and SOL muscles. Values are expressed as means ± SEM. kACT, rate constant of force development; Slow kREL, rate constant of the slow relaxation phase; Fast kREL, rate constant of the fast relaxation phase; CTL-G21, untreated control group; CB-G21, clenbuterol-treated group. *P < 0.05; **P < 0.01; ***P < 0.001.
Chemical kinetics
Slow-type muscle
Relaxed condition. A significant increase in myosin ATPase activity was observed during clenbuterol treatment compared with CTL (Figs 3A and 4A, P < 0.001) in myofibrils prepared from SOL muscle. This increase was explained by the twitch fibre shift from slow to fast type (P < 0.001). The lack of significant change (P > 0.05) in the titration index suggests that the number of myosin heads fully competent in the myofibril suspension, from an enzymatic point of view, was not affected by the clenbuterol treatment. The fast rate constant of the tryptophan fluorescence signal increased and the steeper initial slope of the dependence with ATP concentration (P < 0.01; Fig.4B) indicated an increase in the rate constant of ATP binding-induced myosin detachment with clenbuterol treatment compared with the control group. In contrast, the rate constant of the tryptophan fluorescence slow phase was not altered by clenbuterol treatment (P > 0.05; Fig.4C). As this slow phase was attributed to the ATP cleavage step (Stehle & Brenner, 2000), the present result suggests a lack of clenbuterol effect on this ATP cleavage step. The increase in the rate constant of Pi release under clenbuterol treatment (P < 0.001; Fig.4D) suggests that the enhancement in relaxed ATPase activity was due to an increase in the Pi release step, a rate-limiting step in the steady state of ATPase activity.
Figure 3. Time courses of SOL myosin ATPase in relaxed (A) or Ca2+-activated (B) myofibrils in control group (filled circles), and after 21 days of clenbuterol treatment (open circles).
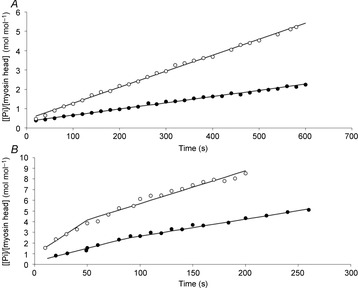
Reaction mixtures were 3 μm myofibrils (as myosin heads) plus 30 μm [γ-32P]ATP at 4°C. The reaction mixtures were quenched at the times indicated and the total [32P]Pi concentrations were determined. In the relaxed condition, the amplitude of Pi burst and ATPase activity were 0.34 ± 0.05 vs. 0.45 ± 0.07 mol mol−1, and 0.0032 ± 0.0005 vs. 0.0083 ± 0.0011 s−1 for the control and 21-day clenbuterol-treated groups, respectively. In the Ca2+-activated condition, the slope of the fast linear phase (ATPase activity in unloaded shortening), slope of the slow linear phase, duration of the unloaded shortening phase (dashed lines) and the ATP cost of shortening were 0.025 ± 0.004 vs. 0.065 ± 0.01 s−1, 0.016 ± 0.003 vs. 0.031 ± 0.006 s−1, 87 ± 9 vs. 50 ± 5 s and 2.4 ± 0.2 vs. 4.2 ± 0.4 mol mol−1 for the control (CTL-G21) and 21-day clenbuterol-treated groups (CB-G21), respectively.
Figure 4. Effect of clenburerol on SOL myofibrilar ATPase activity in relaxed (A–E) and Ca2+-activated (F–H) conditions at 4°C.
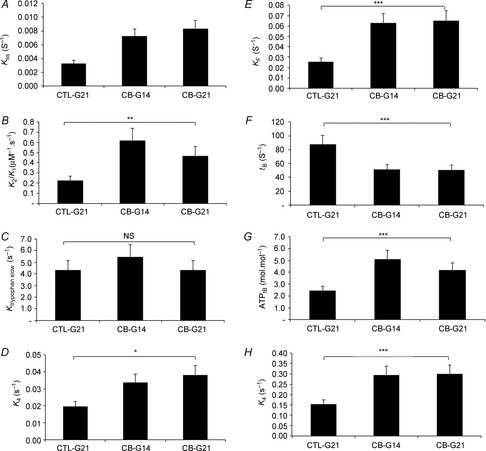
Reaction mixture concentrations were 3 and 30 μm for myosin heads and [γ-32P]ATP concentrations in Rapid Flow Quench experiments, respectively. In Stopped-Flow experiments (B and C), myosin head concentration was 1 μm reaction mixture, and ATP concentrations ranged from 30 μm to 1 mm. CTL-G21, control group; CB-G14, 14-day clenburerol-treated group; CB-G21, 21-day clenburerol-treated group; kSS, rate constant of the steady-state phase in relaxed condition; k2/K1, ATP binding-induced myosin detachment obtained from the initial slope of the regression of ktrypophan fast as a function ATP concentration; ktryprophan slow, rate constant observed for the slow phase of tryptophan increase when mixing myofibrils with ATP; k4, rate constant of Pi release (the isomerization step preceding diffusion of Pi); kF, rate constant of the fast steady-state phase in activated condition; tB, duration of the unloaded shortening phase, an index of unloaded shortening velocity; ATPtB, ATP consumed during the unloaded shortening phase. Because no significant difference was detected between CTL-G9, CTL-G14 and CTL-G21 groups, results of the CTL-G21 group are presented. NS, not significant. **P < 0.01; ***P < 0.001.
Ca2+-activated condition
In this condition and over the present time scale, the time curve was biphasic (Fig.3B). The fast steady state corresponds to the unloaded shortening phase (Lionne et al. 1996). During this phase, myofibrils were shortened at maximal velocity in the unloaded condition. The slow steady state corresponds to a pseudo-isometric phase (non-physiological phase). The time corresponding to the transition between these two phases is the duration of the unloaded shortening (tB). A significant increase in the slope of the fast steady state (i.e. myosin ATPase activity) was observed under clenbuterol treatment in activated SOL myofibrils compared with controls (P < 0.001; Fig.4E). In association with this increase, a shorter duration of the unloaded phase was noted (P < 0.001; Fig.4F), suggesting an increase in unloaded shortening velocity. Interestingly, the amount of ATP hydrolysed during the unloaded shortening phase increased under clenbuterol treatment (Fig.4G; P < 0.001). Similarly to the relaxed condition, an increase (P < 0.001) in Pi release rate was also noted in the Ca2+-activated condition, suggesting that the increased myofibrillar ATPase was due to an increase in the rate constant of Pi release. The multilinear regression analysis revealed that the increases in the Pi release rate constant and myofibrillar ATPase activity in SOL were mainly explained (P < 0.001) by the phenotype shift induced by clenbuterol.
Fast-type muscle
No significant change was seen in myofibrillar ATPase activity (Fig.5) in myofibrils prepared from EDL muscle. The rate constant of the fast and slow steady-state phases (Fig.5A and B, respectively), the duration of the unloaded shortening phase (Fig.5C) and the amount of ATP consumed during this shortening (Fig.5D) were not modified by clenbuterol treatment. The multilinear regression analysis revealed that the increase in the Pi release rate constant and myofibrillar ATPase activity in EDL were not significantly explained by the phenotype shift induced by clenbuterol.
Figure 5. Effect of clenburerol on EDL myofibrilar ATPase activity in Ca2+-activated conditions at 4°C.
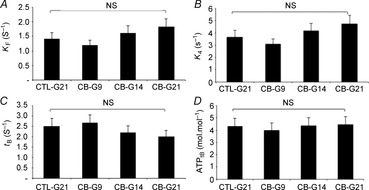
Reaction mixture concentrations were 3 and 30 μm for myosin heads and [γ-32P]ATP concentrations in Rapid Flow Quench experiments, respectively. CTL-G21, control group; CB-G9, 9-day clenbuterol-treated group; CB-G14, 14-day clenburerol-treated group; CB-G21, 21-day clenburerol-treated group; k4, rate constant of Pi release (the isomerization step preceding diffusion of Pi); kF, rate constant of the fast steady-state phase in activated condition; tB, duration of the unloaded shortening phase, an index of unloaded shortening velocity; ATPtB, ATP consumed during the unloaded shortening phase. Because no significant difference was detected between CTL-G9, CTL-G14 and CTL-G21 groups, results of the CTL-G21 group are presented. NS, not significant.
Ca2+ transients
The action potential-elicited Ca2+ transients in field-stimulated intact FDB twitch fibres from the clenbuterol-treated animals showed a progressively decreased amplitude after 14 days (−19%, P < 0.01) and 21 days (−25%, P < 0.01), but not after 9 days, when compared with the control groups (Fig.6).
Figure 6. Effect of clenbuterol treatment on FDB Ca2+ transient amplitude.
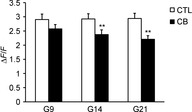
Bar graphs comparing amplitude for single-fibre AP-elicited Ca2+ transients in FDB fibres of control (CTL; n = 7) or clenbuterol-treated (CB; n = 8) rats after 9, 14 and 21 days of treatment. Amplitude was calculated from individual exponential fits and averaged. **P < 0.01.
Discussion
In this study, we showed that chronic clenbuterol administration induced a strong side effect, in contrast to the positive functional effect associated with muscle hypertrophy and absolute maximal tetanic force (Po). In agreement with the study of Head & Ha (2011) showing that clenbuterol treatment induces a loss in force production, we demonstrated that this loss is linked to (i) a diminution of the contraction and relaxation kinetics during muscle force generation, especially in fast-type muscle, and (ii) calcium handling.
Chronic clenbuterol treatment leads to hypertrophy but reduced specific force in slow twitch muscle
We confirmed previous studies showing that chronic clenbuterol administration increases both muscle mass and absolute isometric force, especially in fast-type muscle (Mounier et al. 2007). In the present study, the 89% increase in EDL absolute Po in clenbuterol-treated rats (4 mg kg−1 day−1) is greater than the 31% increase found by Zeman et al. (1988) for the same duration of treatment with rats receiving about 1.6 mg kg−1 day−1 or the 20% increase in maximal isometric force in the study of Ryall et al. (2002) with the commonly used dose of 2 mg kg−1 day−1. Thus, it seems that a dose–response effect exists between clenbuterol and the related increase in maximal tetanic force for treatment lasting at least 2–4 weeks (Zeman et al. 1988; Ryall et al. 2002). Moreover at the dose of 1.6 mg kg−1 day−1 it seems that no further increase in type II fibre CSA or Po occurs in EDL muscle between 2 and 12 weeks of treatment (Zeman et al. 1988). Nevertheless, when normalized to CSA, the maximal tetanic force was unchanged and even depressed in slow twitch muscle. Moreover, it is well known that high dose clenbuterol administration, as in our study, causes inflammation and apoptonecrosis in muscle early on in the treatment and that these effects seem to be greater in slow twitch fibres (Burniston et al. 2005; Douillard et al. 2011). This could be due to higher β2-adrenoreceptor and protein Gαs subunit density in slow-type as opposed to fast-type muscles (Martin et al. 1989). The main consequences of the apoptonecrotic period could be delayed muscle growth and an increased number of infiltrated cells and damaged fibres. Thus, these transient deleterious effects might explain the subsequent decrease in the maximal tetanic contraction obtained at 9, 14 and 21 days in SOL and, to a lesser extent, the decrease in the maximal tetanic contraction obtained at 14 days in EDL.
Clenbuterol treatment did not increase specific myofibrillar ATPase activity
Our results show that the myofibrillar ATPase activities and the content of type II twitch fibres increased in the same proportion in slow twitch muscle during clenbuterol treatment, indicating that the ATPase activity associated with each twitch fibre type was not altered. This suggests that the depressed specific tension was not due to the contractile machinery itself. Two myofilament proteins are possibly phosphorylated in response to β-adrenoceptor activation, i.e. troponin-I, which reduces the affinity for Ca2+ binding to troponin-C, and myosin binding protein-C (C-protein), which increases the maximal myosin ATPase rate, although neither protein seems phosphorylated in mammalian skeletal muscle under β-adrenergic agonist treatment (Manning & Stull, 1982; Gruen et al. 1999). The amino acid residues that are phosphorylated by protein kinase A in cardiac troponin-I or C-protein are absent from the skeletal muscle isoforms of these proteins (Shaffer & Gillis, 2010). Another protein, myosin light-chain kinase, is phosphorylated under β-agonist treatment in smooth muscle, but again this does not occur in skeletal muscle (Manning & Stull, 1982). β-Agonists such as clenbuterol seem to have no notable ergogenic effect on myosin head function in skeletal muscle, except for the well-known change in twitch fibre type.
Calcium handling is altered under chronic clenbuterol treatment
Another explanation is that the loss of force production induced by clenbuterol reflects different aspects of problems in calcium handling.
We found an alteration in the calcium handling ability in fast-type muscle with a reduction in Ca2+-transient amplitude that may explain, at least in part, the reduced kinetics of muscle contraction. Sarcolemma Ca2+ current and sarcoplasmic reticulum Ca2+ handling are altered differently during acute β2-agonist administration compared with chronic administration. The earlier demonstration that acute β2-agonists influence intracellular Ca2+ handling in intact skeletal muscle was achieved by administering terbutaline to isolated fast twitch FDB fibres from mice (Cairns & Dulhunty, 1993). These authors found a potentiation of myoplasmic [Ca2+] response ([Ca2+]i) during tetanic stimulation (20–100 Hz), especially at high stimulation frequencies. A series of subsequent studies further highlighted the acute β2-agonist-induced augmentation in peak twitch or tetanic [Ca2+]i on skeletal muscles for doses ranging from 1 to 50 μm (Bruton et al. 1996; Ha et al. 1999; Prakash et al. 1999). Overall, β2-agonists do not change the resting [Ca2+]i in rodent muscles (Prakash et al. 1999). A recent study by Rudolf et al. (2006) showed that isoprenaline (10 μm) facilitated emptying of Ca2+ from the lumen of the sarcoplasmic reticulum of mouse tibialis anterior muscle in vivo and that this effect was greater with tetanic stimulation. This strongly suggests that the increased amplitude of Ca2+ transients involves greater Ca2+ efflux from the sarcoplasmic reticulum. However, data on the effect of chronic β2-agonist treatment on skeletal muscle calcium homeostasis are sparse (Sirvent et al. 2014). In this study, high-dose chronic clenbuterol treatment had a deleterious effect given that Ca2+-transient amplitudes were decreased. Although we do not understand the precise mechanisms that would explain why chronic clenbuterol treatment has the opposite effect on Ca2+ handling compared with that usually reported for acute treatment, the observations by Burniston et al. (2005) and Douillard et al. (2011) suggest some directions for investigation. First, chronic high doses (4 mg kg−1 day−1) of clenbuterol induce transient apoptosis and necrosis in fast skeletal muscle. In addition, Lavoie et al. (2002) used the same dose and observed a decrease of 45 and 40% in β2-adrenoreceptor density in SOL and gastrocnemius muscle, respectively.
Chronic clenbuterol treatment alters contraction and relaxation kinetics
Beyond the specific maximal force, the original results of our study were related to the changes in muscle contraction and relaxation kinetics induced by clenbuterol. Indeed, our data demonstrated altered contraction and relaxation kinetics during muscle force generation, especially in EDL, with significant decreases in kACT, Slow kREL and Fast kREL. Regarding contraction development, kACT depends on both the cross-bridge isomerization rate constant (dependent on Pi liberation) and the Ca2+ release velocity (i.e. Ca2+ transient). In EDL, our results do not provide evidence for the first mechanism because myosin k4 and ATPase activity did not change under clenbuterol treatment. However, we showed a reduction in the muscle Ca2+ transient of FDB muscle that approximates the EDL muscle fast phenotype (Fig.6). This could contribute to the reduced kinetics of muscle contraction and strongly suggests that clenbuterol acts on some of the processes involved in excitation–contraction coupling, thereby modulating force and contractility characteristics, probably via an alteration in calcium handling. It has been well documented that muscle contraction efficiency and resistance to fatigue are altered by clenbutetol treatment (Chen & Alway, 2001; Polla et al. 2001). Interestingly, based on the fitting kinetics, we found altered muscle relaxation after treatment. Slow kREL was dependent on the myosin head detachment rate constant and the velocity of calcium uptake, confirming the role of calcium handling ability in the alteration of the relaxation properties of skeletal muscle. In our study, the mechanism responsible for the decreased relaxation rate in EDL induced by any alteration of the myosin head detachment kinetics can be ruled out because of the lack of significant change in the ATP binding rate constant. The slowed time course of skeletal muscle contraction and relaxation that clenbuterol induced during force generation counterbalanced the positive effects associated with skeletal muscle hypertrophy and may have functional relevance for physical activities. As the rate constants of both force development and relaxation control the maximal shortening velocity, such treatment could be considered as having a negative impact on the activities of daily living. On the one hand, slowing muscle relaxation might be associated with altered agonist–antagonist coordination but, on the other, a reduced myosin head detachment rate would be associated with reduced maximal shortening velocity. Another potentially negative effect for slow twitch muscle lies in the increased ATP cost of shortening, suggesting an altered efficiency in mechanochemical transduction, which would explain, at least in part, the lower resistance to fatigue observed with high doses (He et al. 2000). These last negative effects are of particular importance in sports where performance depends on endurance and muscle oxidative capacity. However, even with altered contraction and relaxation kinetics, there remains the increase in absolute tetanic force that could confer to athletes the expected ergogenic aid.
In conclusion, our study shows that chronic clenbuterol treatment decreases skeletal muscle contraction and relaxation kinetics, especially in fast-type muscle, and an increase in the ATP cost for contraction was noted, as well. Because we found a decreased Ca2+-transient amplitude, it seems that the reduced specific force production or the increased fatigue was dependent on depressed calcium homeostasis induced by clenbuterol. The beneficial anabolic effects of high-dose β2-agonists for treatment of sarcopenia, muscle weakness and muscular dystrophy seem counterbalanced, at least in part, by deleterious effects on muscle contractility, relaxation kinetics and Ca2+ handling.
Glossary
- AP
action potential
- CSA
cross-sectional area
- EDL
extensor digitorum longus
- FDB
flexor digitorum brevis
- SOL
soleus
Additional information
Competing interests
The authors declare that they have no conflict of interest.
Author contributions
Conception and design of the experiments: G.P. and R.C.; collection, analysis and interpretation of data: G.P., C.R., R.C., P.S., A.M.J.S., A.G.P., A.D., O.G., C.L., A.B., A.C., O.C. and A.L.; drafting the article or critically revising it for substantial intellectual content: C.R., R.C., G.P., A.M.J.S., A.G.P. and A.C.
Funding
This work was supported by the World Anti-Doping Agency, the Centre National d'Etudes Spaciales, the French Ministry of Youth and Sports, and the Fondation Française des Jeux, and it was funded in part by the University of Montpellier and the Institut National de la Recherche Agronomique (INRA). A.M.J.S., A.D. and A.G.P. received PhD scholarships from the Ministère de l'Enseignement la Recherche et de la Technologie (MENRT).
References
- Barman TE. Travers F. The rapid-flow-quench method in the study of fast reactions in biochemistry: extension to subzero conditions. Methods Biochem Anal. 1985;31:1–59. doi: 10.1002/9780470110522.ch1. [DOI] [PubMed] [Google Scholar]
- Bricout V-A, Serrurier BD. Bigard AX. Clenbuterol treatment affects myosin heavy chain isoforms and MyoD content similarly in intact and regenerated soleus muscles. Acta Physiol Scand. 2004;180:271–280. doi: 10.1046/j.0001-6772.2003.01246.x. [DOI] [PubMed] [Google Scholar]
- Brooke MH. Kaiser KK. Three human myosin ATPase systems and their importance in muscle pathology. Neurology. 1970;20:404–405. [PubMed] [Google Scholar]
- Brooks SV. Faulkner JA. Contractile properties of skeletal muscles from young, adult and aged mice. J Physiol. 1988;404:71–82. doi: 10.1113/jphysiol.1988.sp017279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruton JD, Lännergren J. Westerblad H. Effects of repetitive tetanic stimulation at long intervals on excitation–contraction coupling in frog skeletal muscle. J Physiol. 1996;495:15–22. doi: 10.1113/jphysiol.1996.sp021570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burniston JG, Chester N, Clark WA, Tan L-B. Goldspink DF. Dose-dependent apoptotic and necrotic myocyte death induced by the β2-adrenergic receptor agonist, clenbuterol. Muscle Nerve. 2005;32:767–774. doi: 10.1002/mus.20407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cairns SP. Dulhunty AF. Beta-adrenergic potentiation of E-C coupling increases force in rat skeletal muscle. Muscle Nerve. 1993;16:1317–1325. doi: 10.1002/mus.880161208. [DOI] [PubMed] [Google Scholar]
- Candau R, Iorga B, Travers F, Barman T. Lionne C. At physiological temperatures the ATPase rates of shortening soleus and psoas myofibrils are similar. Biophys J. 2003;85:3132–3141. doi: 10.1016/S0006-3495(03)74731-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen KD. Alway SE. Clenbuterol reduces soleus muscle fatigue during disuse in aged rats. Muscle Nerve. 2001;24:211–222. doi: 10.1002/1097-4598(200102)24:2<211::aid-mus60>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- Collomp K, Le Panse B, Portier H, Lecoq A-M, Jaffre C, Beaupied H, Richard O, Benhamou L, Courteix D. De Ceaurriz J. Effects of acute salbutamol intake during a Wingate test. Int J Sports Med. 2005;26:513–517. doi: 10.1055/s-2004-821223. [DOI] [PubMed] [Google Scholar]
- Dodd SL, Powers SK, Vrabas IS, Criswell D, Stetson S. Hussain R. Effects of clenbuterol on contractile and biochemical properties of skeletal muscle. Med Sci Sports Exerc. 1996;28:669–676. doi: 10.1097/00005768-199606000-00005. [DOI] [PubMed] [Google Scholar]
- Douillard A, Galbes O, Rossano B, Vernus B, Bonnieu A, Candau R. Py G. Time course in calpain activity and autolysis in slow and fast skeletal muscle during clenbuterol treatment. Can J Physiol Pharmacol. 2011;89:117–125. doi: 10.1139/y10-114. [DOI] [PubMed] [Google Scholar]
- Gruen M, Prinz H. Gautel M. cAPK-phosphorylation controls the interaction of the regulatory domain of cardiac myosin binding protein C with myosin-S2 in an on–off fashion. FEBS Lett. 1999;453:254–259. doi: 10.1016/s0014-5793(99)00727-9. [DOI] [PubMed] [Google Scholar]
- Ha TN, Posterino GS. Fryer MW. Effects of terbutaline on force and intracellular calcium in slow-twitch skeletal muscle fibres of the rat. Br J Pharmacol. 1999;126:1717–1724. doi: 10.1038/sj.bjp.0702482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He ZH, Bottinelli R, Pellegrino MA, Ferenczi MA. Reggiani C. ATP consumption and efficiency of human single muscle fibers with different myosin isoform composition. Biophys J. 2000;79:945–961. doi: 10.1016/S0006-3495(00)76349-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Head SI. Ha TNV. Acute inhibitory effects of clenbuterol on force, Ca2+ transients and action potentials in rat soleus may not involve the β2-adrenoceptor pathway. Clin Exp Pharmacol Physiol. 2011;38:638–646. doi: 10.1111/j.1440-1681.2011.05574.x. [DOI] [PubMed] [Google Scholar]
- Herrmann C, Lionne C, Travers F. Barman T. Correlation of ActoS1, myofibrillar, and muscle fiber ATPases. Biochemistry (Mosc) 1994;33:4148–4154. doi: 10.1021/bi00180a007. [DOI] [PubMed] [Google Scholar]
- Hostrup M, Kalsen A, Auchenberg M, Bangsbo J. Backer V. Effects of acute and 2-week administration of oral salbutamol on exercise performance and muscle strength in athletes. Scand J Med Sci Sports. 2014a doi: 10.1111/sms.12298. DOI: 10.1111/sms.12298. [DOI] [PubMed] [Google Scholar]
- Hostrup M, Kalsen A, Bangsbo J, Hemmersbach P, Karlsson S. Backer V. High-dose inhaled terbutaline increases muscle strength and enhances maximal sprint performance in trained men. Eur J Appl Physiol. 2014b;114:2499–2508. doi: 10.1007/s00421-014-2970-2. [DOI] [PubMed] [Google Scholar]
- Houadjeto M, Travers F. Barman T. Ca2+-activated myofibrillar ATPase: transient kinetics and the titration of its active sites. Biochemistry (Mosc) 1992;31:1564–1569. doi: 10.1021/bi00120a038. [DOI] [PubMed] [Google Scholar]
- Iorga B, Candau R, Travers F, Barman T. Lionne C. Does phosphate release limit the ATPases of soleus myofibrils? Evidence that (A)M. ADP.Pi states predominate on the cross-bridge cycle. J Muscle Res Cell Motil. 2004;25:367–378. doi: 10.1007/s10974-004-0812-2. [DOI] [PubMed] [Google Scholar]
- Kim YS, Sainz RD, Summers RJ. Molenaar P. Cimaterol reduces β-adrenergic receptor density in rat skeletal muscles. J Anim Sci. 1992;70:115–122. doi: 10.2527/1992.701115x. [DOI] [PubMed] [Google Scholar]
- Kissel JT, McDermott MP, Mendell JR, King WM, Pandya S, Griggs RC, Tawil R FSH-DY Group. Randomized, double-blind, placebo-controlled trial of albuterol in facioscapulohumeral dystrophy. Neurology. 2001;57:1434–1440. doi: 10.1212/wnl.57.8.1434. [DOI] [PubMed] [Google Scholar]
- Lavoie JL, Calderone A. Béliveau L. A farnesyltransferase inhibitor attenuated β-adrenergic receptor downregulation in rat skeletal muscle. Am J Physiol Regul Integr Comp Physiol. 2002;282:R317–R322. doi: 10.1152/ajpregu.00274.2001. [DOI] [PubMed] [Google Scholar]
- Lionne C, Travers F. Barman T. Mechanochemical coupling in muscle: attempts to measure simultaneously shortening and ATPase rates in myofibrils. Biophys J. 1996;70:887–895. doi: 10.1016/S0006-3495(96)79632-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch GS, Hinkle RT, Chamberlain JS, Brooks SV. Faulkner JA. Force and power output of fast and slow skeletal muscles from mdx mice 6–28 months old. J Physiol. 2001;535:591–600. doi: 10.1111/j.1469-7793.2001.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch GS. Ryall JG. Role of β-adrenoceptor signalling in skeletal muscle: implications for muscle wasting and disease. Physiol Rev. 2008;88:729–767. doi: 10.1152/physrev.00028.2007. [DOI] [PubMed] [Google Scholar]
- Manning DR. Stull JT. Myosin light chain phosphorylation–dephosphorylation in mammalian skeletal muscle. Am J Physiol. 1982;242:C234–C241. doi: 10.1152/ajpcell.1982.242.3.C234. [DOI] [PubMed] [Google Scholar]
- Martin WH, Coggan AR, Spina RJ. Saffitz JE. Effects of fiber type and training on β-adrenoceptor density in human skeletal muscle. Am J Physiol. 1989;257:E736–E742. doi: 10.1152/ajpendo.1989.257.5.E736. [DOI] [PubMed] [Google Scholar]
- McCormick C, Alexandre L, Thompson J. Mutungi G. Clenbuterol and formoterol decrease force production in isolated intact mouse skeletal muscle fiber bundles through a β2-adrenoceptor-independent mechanism. J Appl Physiol. 2010;109:1716–1727. doi: 10.1152/japplphysiol.00592.2010. [DOI] [PubMed] [Google Scholar]
- Mersmann HJ. Overview of the effects of β-adrenergic receptor agonists on animal growth including mechanisms of action. J Anim Sci. 1998;76:160–172. doi: 10.2527/1998.761160x. [DOI] [PubMed] [Google Scholar]
- Mounier R, Cavalié H, Lac G. Clottes E. Molecular impact of clenbuterol and isometric strength training on rat EDL muscles. Pflügers Arch. 2007;453:497–507. doi: 10.1007/s00424-006-0122-1. [DOI] [PubMed] [Google Scholar]
- Poggesi C, Tesi C. Stehle R. Sarcomeric determinants of striated muscle relaxation kinetics. Pflügers Arch. 2005;449:505–517. doi: 10.1007/s00424-004-1363-5. [DOI] [PubMed] [Google Scholar]
- Polla B, Cappelli V, Morello F, Pellegrino MA, Boschi F, Pastoris O. Reggiani C. Effects of the β2-agonist clenbuterol on respiratory and limb muscles of weaning rats. Am J Physiol Regul Integr Comp Physiol. 2001;280:R862–R869. doi: 10.1152/ajpregu.2001.280.3.R862. [DOI] [PubMed] [Google Scholar]
- Prakash YS, van der Heijden HF, Gallant EM. Sieck GC. Effect of β-adrenoceptor activation on [Ca2+]i regulation in murine skeletal myotubes. Am J Physiol. 1999;276:C1038–C1045. doi: 10.1152/ajpcell.1999.276.5.C1038. [DOI] [PubMed] [Google Scholar]
- Reimann EM. Umfleet RA. Selective precipitation of 32Pi onto filter papers. Application to ATPase and cyclic AMP phosphodiesterase determination. Biochim Biophys Acta. 1978;523:516–521. doi: 10.1016/0005-2744(78)90054-2. [DOI] [PubMed] [Google Scholar]
- Ricart-Firinga C, Stevens L, Canu MH, Nemirovskaya TL. Mounier Y. Effects of β2-agonist clenbuterol on biochemical and contractile properties of unloaded soleus fibers of rat. Am J Physiol Cell Physiol. 2000;278:C582–C588. doi: 10.1152/ajpcell.2000.278.3.C582. [DOI] [PubMed] [Google Scholar]
- Rudolf R, Magalhães PJ. Pozzan T. Direct in vivo monitoring of sarcoplasmic reticulum Ca2+ and cytosolic cAMP dynamics in mouse skeletal muscle. J Cell Biol. 2006;173:187–193. doi: 10.1083/jcb.200601160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ryall JG, Gregorevic P, Plant DR, Sillence MN. Lynch GS. β2-Agonist fenoterol has greater effects on contractile function of rat skeletal muscles than clenbuterol. Am J Physiol Regul Integr Comp Physiol. 2002;283:R1386–R1394. doi: 10.1152/ajpregu.00324.2002. [DOI] [PubMed] [Google Scholar]
- Sanchez AMJ, Collomp K, Carra J, Borrani F, Coste O, Préfaut C. Candau R. Effect of acute and short-term oral salbutamol treatments on maximal power output in non-asthmatic athletes. Eur J Appl Physiol. 2012;112:3251–3258. doi: 10.1007/s00421-011-2307-3. [DOI] [PubMed] [Google Scholar]
- Shaffer JF. Gillis TE. Evolution of the regulatory control of vertebrate striated muscle: the roles of troponin I and myosin binding protein-C. Physiol Genomics. 2010;42:406–419. doi: 10.1152/physiolgenomics.00055.2010. [DOI] [PubMed] [Google Scholar]
- Sirvent P, Douillard A, Galbes O, Ramonatxo C, Py G, Candau R. Lacampagne A. Effects of chronic administration of clenbuterol on contractile properties and calcium homeostasis in rat extensor digitorum longus muscle. PloS One. 2014;9:e100281. doi: 10.1371/journal.pone.0100281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stehle R. Brenner B. Cross-bridge attachment during high-speed active shortening of skinned fibers of the rabbit psoas muscle: implications for cross-bridge action during maximum velocity of filament sliding. Biophys J. 2000;78:1458–1473. doi: 10.1016/S0006-3495(00)76699-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeman RJ, Ludemann R, Easton TG. Etlinger JD. Slow to fast alterations in skeletal muscle fibers caused by clenbuterol, a β2-receptor agonist. Am J Physiol. 1988;254:E726–E732. doi: 10.1152/ajpendo.1988.254.6.E726. [DOI] [PubMed] [Google Scholar]


