Abstract
Sensing of dietary triacylglycerol in the proximal small intestine results in physiological, hormonal and behavioural responses. However, the exact physiological pathways linking intestinal fat sensing to food intake and the activation of brain circuits remain to be identified. In this study we examined the role of triacylglycerol digestion for intestinal fat sensing, and compared the effects of the triacylglycerol digestion products, fatty acids and 2-monoacylglycerol, on behavioural, hormonal and dopaminergic responses in behaving mice. Using an operant task in which mice are trained to self-administer lipid emulsions directly into the stomach, we show that inhibiting triacylglycerol digestion disrupts normal behaviour of self-administration in mice, indicating that fat sensing is conditional to digestion. When administered separately, both digestion products, 2-monoacylglycerol and fatty acids, were sensed by the mice, and self-administration patterns of fatty acids were affected by the fatty acid chain length. Peripheral plasma concentrations of the gut hormones GLP-1, GIP, PYY, CCK and insulin did not offer an explanation of the differing behavioural effects produced by 2-monoacylglycerol and fatty acids. However, combined with behavioural responses, striatal dopamine effluxes induced by gut infusions of oleic acid were significantly greater than those produced by equivalent infusions of 2-oleoylglycerol. Our data demonstrate recruitment of different signalling pathways by fatty acids and 2-monoacylglycerol, and suggest that the structural properties of fat rather than total caloric value determine intestinal sensing and the assignment of reward value to lipids.
Key points
Digestion is required for intestinal sensing of triacylglycerol in this behavioural model.
The hydrolysis products of triacylglycerol, fatty acids and 2-monoacylglycerol, regulate feeding via separate mechanisms.
Sensing of long-chain fatty acids, but not of 2-monoacylglycerol, stimulated central dopaminergic signalling.
Fatty acid chain length regulates behavioural responses to fatty acids.
Introduction
Eating is essential to sustain metabolism and growth. Accordingly, regulation of appetite involves the brain reward circuitry, and nutrient intake is linked to the activation of satiation as well as reward-related dopaminergic pathways, even when food is placed directly into the gut, bypassing the oral cavity (Ferreira et al. 2012). A thorough understanding of the mechanisms underlying this nutrient-mediated intestinal regulation of appetite is highly relevant to disorders such as obesity, where appetite regulation may be dysfunctional (Stewart et al. 2011;Vucetic & Reyes, 2010). However, the physiological pathways linking gut nutrient sensing to central control of food intake remain incompletely understood.
Triacylglycerol may be sensed after digestion by gut intestinal cells via a number of different pathways, including β-oxidation of absorbed fatty acids (Langhans et al. 2011), fatty acid/protein kinase C interactions (Rasmussen et al. 2012) or binding of fatty acid metabolites to PPARα (Guerre-Millo et al. 2000; Hostetler et al. 2005; Piomelli, 2013; Hansen, 2014). Additionally, the apical surface of enteroendocrine cells express receptors (Engelstoft et al. 2008), that have digested nutrients as their natural ligands, such as the amino-acid/oligopeptide-activated G-protein coupled receptor 93 (GPR93) (Cordier-Bussat et al. 1998), the monosaccharide-activated sweet GPR T1R2/T1R3 (Gerspach et al. 2011), and the fatty-acid sensitive GPR40, GPR41, GPR43 and GPR120 (Offermanns, 2014). Finally, GPR119 is activated by the other digestion product of triacylglycerol, namely 2-monoacylglycerols (Hansen et al. 2011).
These receptors are believed to function as first-hand reporters on the composition and density of ingested foods (Wellendorph et al. 2010; Liou et al. 2011; Hansen et al. 2012; Ichimura et al. 2012; Tolhurst et al. 2012; Duca et al. 2013) via activation of several downstream pathways, which include the release of gut hormones and/or stimulation of local nerve endings (Hansen et al. 2011; Little and Feinle-Bisset, 2011; Blad et al. 2012). Therefore, these membrane receptors have been proposed to function as intestinal nutrient sensors, potentially regulating food intake via a combination of paracrine, endocrine and neuronal actions (Schwartz & Zeltser, 2013).
Mice have been shown to achieve precise regulation of caloric intake when self-administering emulsified triacylglycerol directly into the stomach (Ferreira et al. 2012). Specifically, mice proportionally increased the number of gut infusions upon dilutions of the emulsion, implying the existence of a gastrointestinal mechanism enabling animals to sense the caloric density and/or molecular composition of ingested triacylglycerol. Gut infusions of triacylglycerol emulsions induced robust release of dopamine in dorsal striatum (Ferreira et al. 2012), consistent with a critical role for this region in the regulation of food intake (Szczypka et al. 2001; Small et al. 2003). More precisely, dopamine release reflected the amount of triacylglycerol administered to the gut (Ferreira et al. 2012); conversely, the ability to sense the density of the emulsion was disrupted by administration of the dopamine receptor antagonist haloperidol, implying that normal triacylglycerol sensing involves central dopamine signalling (Ferreira et al. 2012).
Because, to date, no triacylglycerol receptors have been described in either intestinal or brain tissue, we hypothesized that the behavioural and dopaminergic effects of intragastric triacylglycerol infusions are mediated by either moiety of digested triacylglycerols, i.e. fatty acids and the 2-monoacylglycerol. Additionally, 2-monoacylglycerol and fatty acids bind to different apical G-protein coupled receptors (GPCRs) on enteroendocrine cells, and differ in terms of partitioning to β-oxidation, re-esterification and nuclear receptor binding; we further hypothesized that lipid sensing depends on the molecular structure of the infused emulsions. Thus, the aim of our study was to determine whether triacylglycerol digestion is required for taste-independent self-administration of triacylglycerol, i.e. whether the triacylglycerol metabolites, fatty acid and 2-monoacylglycerol, could be individually sensed, and whether they are individually sufficient to stimulate dopamine release in dorsal striatum. More specifically, because the lipid messenger, oleoylethanolamide, has been proposed to link gut lipid sensing to the activation of the reward circuitry (Tellez et al. 2013), and because acute infusion of oleic acid, but not of 2-monoacylglycerol, enhances intestinal levels of oleoylethanolamide, we anticipated that the gut sensing of the oleic acid moiety in triacylglycerol, but not of 2-monoacylglycerol, would be sufficient to activate the brain reward circuitry.
Methods
Animals and housing
Seventy C57Bl6/J mice (male, 10–24 weeks of age) were used in the experiments. Mice were obtained from the Jackson Laboratory (Bar Harbour, ME, USA) and from Harlan Laboratories (Boxmeer, The Netherlands). Upon arrival, the mice were allowed to acclimatize for 1 week in a temperature- and humidity-controlled facility with a 12/12 h light cycle before undergoing any surgical or behavioural procedures. They had ad libitum access to standard chow (3.02 kcal g−1, 13.5%E fat in the US, and 2.99 kcal g−1, 14.8%E fat in Denmark) and water, unless otherwise described in the protocols below. Experiments conducted at The J. B. Pierce Laboratory were approved by the J. B. Pierce Laboratory Institutional Care and Use Committee (IACUC) and were in accordance with J. B. Pierce Laboratory and Yale University regulations on usage of animals in research. Experiments conducted at The University of Copenhagen were approved by the Danish Committee for Animal Research (Licence 2013-15-2934-00766 C1) and fully compliant with nationally and internationally accepted principles for the care and use of laboratory animals.
Insertion of gastric catheters
Mice were anaesthetized with an i.p. dose of either hypnorm/dormicum (fentanyl 0.8 mg kg−1 /fluanisone 25 mg kg−1/midazolam 12.5 mg kg−1) or ketamine/xylazine (100 mg kg−1/15 mg kg−1). Eye ointment containing paraffin oil and Vaseline (Ophta A/S, Gentofte, Denmark) were applied to both eyes to avoid dehydration. The operative procedure was as described previously (Ferreira et al. 2012). Briefly, a small incision was made between the shoulder plates to allow for catheter exteriorization before a midline incision was made in the abdomen. Approximately 10 cm of catheter tubing (Micro-Renathane tubing, MRE-033, 0.33 × 0.014 inches, Braintree Scientific, Braintree, MA, USA) was then tunnelled under the skin, leaving approximately 2 cm on the outside between the shoulders. A 1 cm incision was cut in the abdominal muscle and the catheter was guided into the abdominal cavity through a hole in the abdominal muscle pricked with an 18 G needle. The stomach was exteriorized and a purse string suture (Silk 5-0, Oasis, SouthPoint Surgical Supply Inc., Coral Springs, FL, USA) was put in the greater curvature just above the antrum. The tip of the tubing was inserted and the purse string was tightened around the tubing, the stomach was re-interiorized, and both the abdominal and the dorsal incisions were sutured and disinfected. All mice were allowed to recover for at least 5 days before they entered experimental protocols.
Insertion of microdialysis guide cannula
For mice used in microdialysis studies, upon concluding the above procedure, animals were positioned in a stereotactic apparatus (David Kopff, Tujunga, CA, USA), which was connected to an isoflurane source (1%, 1.5 l min−1). The scalp was gently opened, and the skull cleaned with 30% hydrogen peroxide to expose cranial sutures. A circular craniotomy was drilled (AP = 1.4 mm, ML = ±1.5 mm) and a guide cannula (CMA 7; CMA Microdialysis, Kista, Sweden) was inserted (DV = –0.8 mm, final tip position = –2.5 mm). Dental cement was applied to keep the guide cannula in place and the skin was sutured around the implant.
Post-operative care
After surgery, mice were housed singly to prevent rupture of sutures, and were given ad libitum access to standard chow and water. They were treated with Rimadyl (Carprofen 5 mg kg−1) for 3 days and allowed a minimum of 5 days of recovery. During this period as well as during experiments, the mice were weighed daily and monitored for any signs of distress or morbidity. Whenever such signs were detected, the animal was killed.
Emulsion preparation
Lipid emulsion preparation used as basis a 30% (w/v) Intralipid (Baxter, Deerfield, IL, USA or Fresenius Kabi A/S, Copenhagen, Denmark) containing 1.2% (w/v) phospholipid and 1.7% (w/v) glycerol as emulsifiers. To ensure homogenous emulsification of all lipid species, the glycerol content was increased to 5% (w/v) and all emulsions were supplemented with sodium taurocholate (Sigma-Aldrich, Schnelldorf, Germany) to a final concentration of 5 mm. Emulsions were prepared so as to contain equimolar amounts of triacylglycerol to intact Intralipid, or to contain the corresponding molar amounts of digestion products from fully hydrolysed Intralipid, which yields 1 mol of 2-monoacylglycerol and 2 mol of fatty acids per mole of triacylglycerol. The compositions of the applied lipid emulsions are presented in Table1.
Table 1.
Composition of the emulsions applied in this study
| TAG, % (mm) | FFAs, % (mm) | 2-MAG, % (mm) | Lecithin (%) | Glycerol (%) | NaTCh (mm) | kcal ml–1 | |
|---|---|---|---|---|---|---|---|
| IL30 | 30 (350) | — | — | 1.2 | 5 | 5 | 3 |
| IL5 | 5 (58) | — | — | 1.2 | 5 | 5 | 0.8 |
| VEH | — | — | — | 1.2 | 5 | 5 | 0.3 |
| 2-OG | — | — | 12 (350) | 1.2 | 5 | 5 | 1.3 |
| 2-OG ether | — | — | 12 (350) | 1.2 | 5 | 5 | 0.3 |
| OA30 | — | 19 (700) | — | 1.2 | 5 | 5 | 2 |
| LA30 | — | 19 (700) | — | 1.2 | 5 | 5 | 2 |
| LA7.5 | — | 4.75 (175) | — | 1.2 | 5 | 5 | 0.7 |
| CA30 | — | 9.8 (700) | — | 1.2 | 5 | 5 | 1.1 |
| CA7.5 | — | 2.45 (175) | — | 1.2 | 5 | 5 | 0.5 |
| BA30 | — | 6 (700) | — | 1.2 | 5 | 5 | 0.6 |
| BA7.5 | — | 1.5 (175) | — | 1.2 | 5 | 5 | 0.4 |
TAG, triacylglycerol; FFA, free fatty acids; 2-MAG, 2-monoacylglycerol; NaTCh, sodium taurocholate; IL, intralipid; VEH, vehicle; 2-OG, 2-oleoylglycerol; 2-OG ether, 2-oleylglyceryl ether; OA, oleic acid; LA, linoleic acid; CA, caprylic acid; BA, butyric acid. All emulsions with the suffix 30 are equimolar with respect to fatty acids after digestion of IL30. IL5 is obtained by a sixfold dilution of IL30 with VEH. All emulsions with the suffix 7.5 are equimolar with respect to fatty acids and obtained by a fourfold dilution of the corresponding 30 emulsion with VEH.
Ten millilitres of 30% Intralipid was supplemented with 330 mg of glycerol and 27 mg of sodium taurocholate. This emulsion was labelled ‘IL30’. To prepare the other emulsions, lecithin (Lipoid E PC S, Lipoid AG, Steinhausen, Switzerland) and glycerol (≥99%, Sigma-Aldrich) were weighed off, heated gently to just below 50°C, and thoroughly mixed before the lipid species were added. To this mixture, 5 ml 10 mm sodium taurocholate was added dropwise under agitation by either ultraturax or vortexing. Finally, distilled water was added for a final volume of 10 ml.
Behavioural apparatus
Behavioural studies employed operant test chambers enclosed in a sound attenuating cubicle (MedAssociates, St. Albans, VT, USA). Each chamber was equipped with two slots for sipper placement at symmetrical positions on the left wall of the chamber. The sippers were connected to a contact-based relay for lick detection (‘contact lickometer’), such that lick detection was programmed to trigger the activation of a connected infusion pump via TTL pulses.
Behavioural training protocol
Once habituated to the behavioural boxes, mice were trained to associate licking on a dry sipper with obtaining intra-gastric infusions of IL30. Unless otherwise stated, mice were presented with only one out of two sippers, always kept in the same position. Mice were calorie-restricted to 75% of normal chow intake during the duration of the behavioural studies, receiving 2.8 g of chow each day after the 1 h sessions in the box. This ensured a stable day-to-day food intake and increased the motivation for the mice to obtain infusions. During the first 3–4 days of training a small amount of chow was placed behind the sipper allowing for smell but not taste cue to enhance the mice's interest in the sipper. Training continued for approximately 10 days until the mice displayed stable numbers of daily infusions. A mouse was considered trained when the variation in infusion numbers across five consecutive days did not exceed 20%. The training was further confirmed by exchanging the IL30 with a diluted emulsion (5%, ‘IL5’) to verify compensation for the dilution by increased numbers of infusions. Trained mice were sometimes used in more than one behavioural protocol, provided that prior to each experiment a stable baseline period had been achieved.
Behavioural responses to Orlistat
The effect of the lipase inhibitor Orlistat on intragastric self-administration was tested by administering the agent through the gastric catheters immediately before the trained mice (n = 6) were place in the test chambers. Orlistat was dosed (1 g kg−1 in vehicle, ‘VEH’, total volume 300 μl) immediately before the 1 h session. During two consecutive days mice received either VEH or Orlistat in randomized order.
Behavioural responses to fatty acids and 2-monoacylglycerol
Trained mice self-infused IL30, in daily 1 h sessions, 24 h apart, for three consecutive days for baseline data collection. On the fourth day IL30 was replaced with one of the emulsions described in Table1, containing oleic acid (Sigma-Aldrich), 2-oleoylglycerol (SiChem GmbH, Bremen, Germany), 2-oleylglyceryl ether (Larodan Fine Chemicals, Malmö, Sweden), linoleic acid (Sigma-Aldrich), caprylic acid (Sigma-Aldrich) or butyric acid (Sigma-Aldrich) (n = 6–8 per group). In experiments where two concentrations of lipid species were tested, the mice received the test emulsions on days 4 and 5 in a cross-over design to rule out effects of exposure to one emulsion affecting the response to the other. After exposure to new emulsions, IL30 was reintroduced to verify returning to baseline levels.
OA30 vs. 2-OG preference protocol
Mice were divided into two groups (n = 6), testing their preference towards either the oleic acid emulsion described in Table1, ‘OA30’, or the 2-oleoylglycerol emulsion, described in Table1, ‘2-OG’, against IL30. In the self-administration chamber, the mice were trained to associate the position (left or right) with the infusion of either IL30 or one of the two test emulsions. For all mice the side and emulsion associations were balanced to accommodate for any innate side preferences the mice might have. For 12 days the mice were trained, receiving IL30 through licks on one spout on one day and the other emulsion through licks on the other spout the next day during 1 h sessions. During the actual test, the mice were presented with both spouts, and their preference in terms of the number of licks on each spout was recorded during a 10 min session where licks on any spout elicited infusions of vehicle only.
Hormonal response protocol
Naïve mice were calorie restricted to 75% of normal chow intake overnight and divided into five groups (n = 6). The gastric catheter was connected to a syringe pump and after 1 h of habituation to the experimental set-up, the mice received passive infusions of lipid emulsion, (6 min, 0.6 ml). Thirty minutes after infusion, the mice were killed by cervical dislocation and blood was collected by decapitation and subsequent bleeding into EDTA-coated vials and supplemented with 10 μl dipeptidyl peptidase IV (DPPIV) inhibitor (Merck-Millipore, Billerica, MA, USA) and 500 kIU aprotinin (Sigma-Aldrich). Plasma was obtained by centrifugation at 10 000 g for 10 min at 4°C and immediately frozen on dry ice after separation. The samples were stored at −20°C until further analysis.
Hormone analyses
All hormone analysis (n = 4–6) was performed on snap-frozen plasma and according to the manufacturer's protocol. Peptide YY (PYY)total (PYY1–36 and PYY3–36) was measured using the rat/mouse-specific PYY radioimmunoassay (Cat. No. RMPYY-68HK; Millipore) and radioactivity was counted in a Packard Cobra II Gamma Counter (GMI Inc., Ramsey, MN, USA). Data were analysed using Packard RiaSmart software (GMI).
Glucose-dependent insulinotropic polypeptide (GIP)total (GIP1–42 and GIP3–42) was measured using the rat/mouse-specific GIP ELISA (Cat. No. EZRMGIP-55 K; Millipore) and colorimetric fluorescence was measured using a Biotek EL800 Absorbance Microplate Reader (BioTek Instruments, Winokoske, VT, USA). Data were analysed using Gen5 data analysis software (BioTek Instruments).
Glucagon-like peptide 1 (GLP-1)total (GLP-17-36/37 and GLP-19-36/37) and insulin were measured using a custom made multiarray ELISA from Meso Scale Discovery (Rockville, MD, USA) combining measurements of total GLP-1 (vers.2) (Cat. No. K150JVC-1) and mouse/rat insulin (Cat. No. K152BZC-1). Electrochemiluminescence was measured using a SECTOR Imager 2400 instrument (Meso Scale Discovery) and data were analysed using Discovery Workbench (Meso Scale Discovery).
Cholecystokinin (CCK) was measured using a radioimmunoassay that reacts fully with mouse CCK peptides without cross-reactivity with any gastrins (Rehfeld, 1998). Plasma was mixed with barbital buffer added albumin (BBA), tracer (10 μCi 125I-CCK8/20 ml BBA and antibody Ab92128), and incubated at 4°C for 3–7 days. Separation of bound and un-bound CCK was performed by adding barbital buffer, 20% human plasma and 6 g active charcoal/100 ml (Sigma) for 15 min and subsequent centrifugation. Bound 125I-CCK8 was counted in a Wallac Wizard 1470 gamma counter (GMI).
Microdialysis protocol
Mice were calorie-restricted to 75% of normal chow intake during microdialysis experiments. A microdialysis membrane (CMA7; CMA Microdialysis) was inserted in the dorsal striatum through the surgically implanted guide cannula in naïve (non-trained) mice. After insertion and connection to a syringe pump the membrane was perfused with artificial cerebrospinal fluid (Harvard Apparatus, Holliston, MA, USA) at 1.2 μl min–1 and microdialysate was collected and analysed for dopamine content every sixth minute. For at least 30 min or until five consecutive dopamine measurements lay within 3% (97–103%) of each other, baseline dopamine levels were recorded. The mice then received passive intragastric infusions (6 min, 0.6 ml) of lipid emulsion (IL30, OA30, 2-OG or VEH) and the dopamine was recorded for another 60 min. The mice (n = 11) received all stimuli on separate days (one stimulus per day) in a randomized order. Microdialysate samples were manually injected into a HTEC-500 HPLC unit (Eicom, Kyoto, Japan), separated on a PP-ODS affinity column (Eicom) and electrochemically detected. Resulting chromatograms were analysed using the adjuvant EPC-300 software and sample concentration was calculated with reference to 0.5 pg μl−1 dopamine standards.
Results
Intestinal triacylglycerol sensing is affected by luminal lipase inhibition
To elucidate if triacylglycerol hydrolysis is required for post-ingestive sensing, the lipase inhibitor Orlistat was administered to trained mice prior to the behavioural task. Administering Orlistat immediately prior to placing the animals in the behavioural chambers resulted in a moderate yet significant increase in the number of self-administered IL infusions during the first 15 and 30 min of the session (Fig.1). This confirms that normal triacylglycerol post-ingestive sensing depends on the digestion and hydrolysis of triacylglycerol to fatty acids and 2-monoacylglycerol.
Figure 1. Lipase inhibition increases the self-administration of IL30.
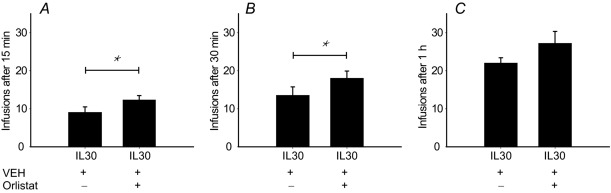
Mice (n = 6) trained to self-administer IL30 were receiving either 300 μl VEH or 300 μl Orlistat solubilized in VEH (1 mg kg−1) on two consecutive days in a randomized manner. Lipase inhibition increased the number of self-administered IL30 infusions during the first 15 min (t5 = –2.911, P = 0.033) and 30 min (t5 = –2.730, P = 0.041). The effect was not significant after 1 h at the end of the session (t5 = -2.419, P = 0.060). *P < 0.05.
The sensing of 2-oleoylglycerol is not correlated to sensing of calories
The mice's response to replacing IL30 with emulsions of either one of the digestion products (OA30 and 2-OG) was investigated to establish if the mice were able to sense both moieties (Fig.2A–D). When IL30 was replaced with IL5, the mice compensated for the dilution and administered the same total amount of calories. When IL30 was replaced with OA30 the mice self-administered an amount of calories equal to those associated with IL30 infusions, although only the fatty acid moiety was present. Thus, mice were able to sense the fatty acid and adjusted the infusions to a level where calories matched what they would have obtained from the triacylglycerol IL30 (Fig.2A, B).
Figure 2. Mice do not compensate for lower caloric content when IL30 is changed to 2-OG in a self-administration set-up.
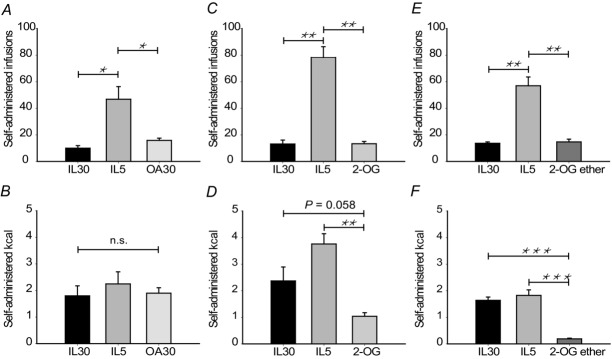
Mice were trained to self-administer IL30 in sessions of 1 h duration. When administrating a stable number of calories over three consecutive days the mice were presented with IL5 as a positive control for compensative behaviour. The next day they were presented with OA30, 2-OG or 2-OG ether (n = 6 in each group). A, C and E, mice compensated for and administered more infusions when IL30 is diluted. When the emulsion was changed to OA30, 2-OG or 2-OG ether the mice did not increase infusions compared to IL30, indicating that they sense the components of the administered emulsions. B, D and F, when the mice administer OA30, they attain the same caloric level compared as with IL30. When they administer 2-OG ether the number of infused calories is reduced compared to IL30 (t5 = 12.030, P = 0.000). 2-OG infusions also reduce administered calories although not significantly (t5 = 2.450, P = 0.058). Data are expressed as mean±SEM. *P < 0.05, **P < 0.01, ***P < 0.001 in paired sample t-tests.
Interestingly, when IL30 was replaced with 2-OG (Fig.2C, D) the mice did not adjust the amount of infused calories to the level of IL30 but infused only about half the calories, although the difference was not significant (P = 0.058, Fig.2D). However, mice did not significantly increase the number of infusions as they did in response to IL5, suggesting a different sensing mechanism for 2-monoacylglycerol compared to fatty acids. To rule out luminal hydrolysis of 2-OG after admistration, the trial was performed in an additional group of mice self-infusing an emulsion containing a structural analogue to 2-oleoylglycerol, 2-oleylglyceryl ether (‘2-OG ether’), which is not susceptible to degradation by luminal lipases. The results obtained with 2-OG ether (Fig.2E, F) were very similar to those obtained with 2-OG (Fig.2C, D). Thus, in this behavioural set up both triacylglycerol metabolites can be sensed, and the sensing of 2-OG may not involve β-oxidation or fatty acid receptors.
The sensing of fatty acids is affected by fatty acid chain length
To investigate whether structural differences among fatty acids also influenced sensing, we prepared emulsions of linoleic acid (C18:2), caprylic acid (C8:0) and butyric acid (C4:0), having the same molarity as OA30 and consistent with digested IL30 with respect to fatty acid molarity. We also prepared four-fold diluted emulsions to monitor compensation. The results are presented in Fig.3. The self-administration of LA30 was very similar to both OA30 and IL30, illustrating that when administering structurally related long-chain fatty acids (LCFAs), the mice adjusted the self-administered infusions to similar levels of calories (approximately 2 kcal h−1, Figs 2B and 3B). Additionally, mice comparably increased the number of infusions of the diluted emulsion (Fig.3B).
Figure 3. Sensing of free fatty acids depends on chain length.
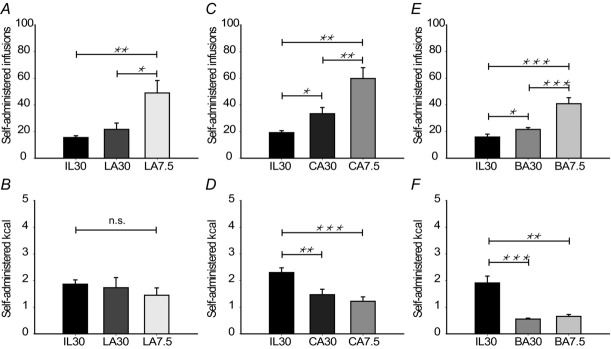
Male mice (n = 6–8 per group) were trained to self-administer intragastric infusions of IL30. After 3 days on IL30, the emulsion was changed to emulsions of either linoleic acid (LA), caprylic acid (CA) or butyric acid (BA) in one of two concentrations. The next day the mice received the other concentration of the corresponding fatty acid. The molarity of all 7.5-emulsions are equal. The 30-emulsions have a fatty acid concentration equal to digested IL30 (see Table1 for details). A, C and E, number of administered infusions. Compared to IL30 the mice increased the number of infusions when administering CA30 and BA30 and further increased the number of infusions when LA, CA and BA were diluted. B, D and F, administered calories. Mice infusing LA ended up administering the same amount of calories as IL30. However, mice administering CA and BA ended up administrating a lower amount of calories as IL30. Data are expressed as mean±SEM. *P < 0.05, **P < 0.01, ***P < 0.001 in paired sample t-tests.
Interesting effects were observed in experiments involving fatty acids of lower chain lengths: caloric compensation was not fully achieved when mice self-administered caprylic acid (approximately 1.5 kcal), an effect that was even more pronounced when they administered butyric acid (approximately 0.7 kcal). This indicates that the sensing of fatty acids is affected by chain length and might be due to the differences in intestinal processing. Due to the spread in time among these behavioural tests, we compared the obtained results relative to their preceeding baseline levels. This comparison (Fig.4) revealed that although the number of infusions was not significantly different among the stimuli, the amount of administered calories was affected by the chain length of the fatty acid, and apparently the mice sense short- and medium-chain fatty acids (SCFAs and MCFAs) more pontently per calorie than LCFAs.
Figure 4. Fatty acid chain length affects the number of administered calories.
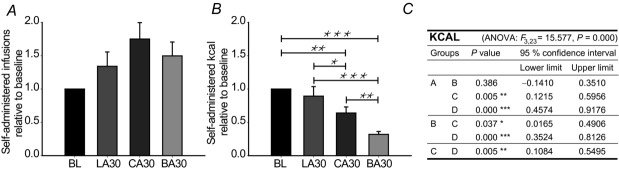
Mice (n = 6–8) trained to self-administer IL30 were presented with either LA, CA or BA in concentrations corresponding to the fatty acid content in digested IL30. A, the number of infusions was not significantly different in a one-way ANOVA with Fischer's least significant difference (LSD) post hoc correction (F3,23 = 2.778, P = 0.064). B, the number of administered calories was significantly affected by the chain length of the administered fatty acid. C, detailed statistics. A, IL30; B, LA30; C, CA30; D, BA30. Data are expressed as mean±SEM. *P < 0.05, **P < 0.01, ***P < 0.001 in Ficher's LSD post hoc corrected ANOVA.
Preference for IL30 vs. 2-OG is greater than IL30 vs oleic acid
Tofurther elucidate the observed differences in sensing between fatty acids and 2-oleoylglycerol, the rewarding potential of OA30 and 2-OG compared to IL30 was investigated in a preference test. The mice were allocated to one of two groups, investigating in one group the relative preference towards OA30 compared to IL30, and in the other the relative preference towards 2-OG compared to IL30. The results are shown in Fig.5. In both cases the mice exhibited a preference towards IL30, although this preference was stronger in the 2-OG group. The preference for IL30 was 0.68 over OA30 and 0.86 over 2-OG, a significant difference in preference ratios (Fig.5), indicating that 2-OG is perceived as less rewarding by mice.
Figure 5. IL30 is preferred over OA and 2-OG.
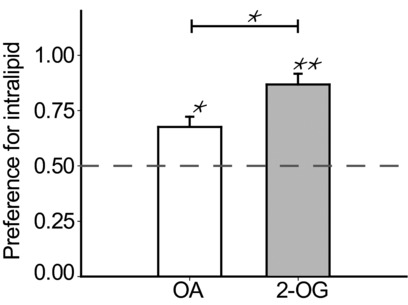
In a preference test mice preferred IL30 when given the choice between either IL30 and OA or IL30 and 2-OG (t5 = 3.727, P = 0.014 and t5 = 7.447, P = 0.001, respectively). The mice (n = 6 per group) were trained for 12 days to associate the location of the spout (right or left) with the tested emulsions. Each mouse always received the same emulsion from the same side and on the actual test day the mice were presented with both sippers, but received only vehicle through the catheter. The preference for IL30 was higher in mice where the alternative was 2-OG than in mice where the alternative emulsion was OA (t10 = –2.817, P = 0.018). The dashed line indicates a 50–50 preference for the tested emulsions. *P < 0.05, **P < 0.01.
Differences in sensing may not be explained by intestinal hormone secretion
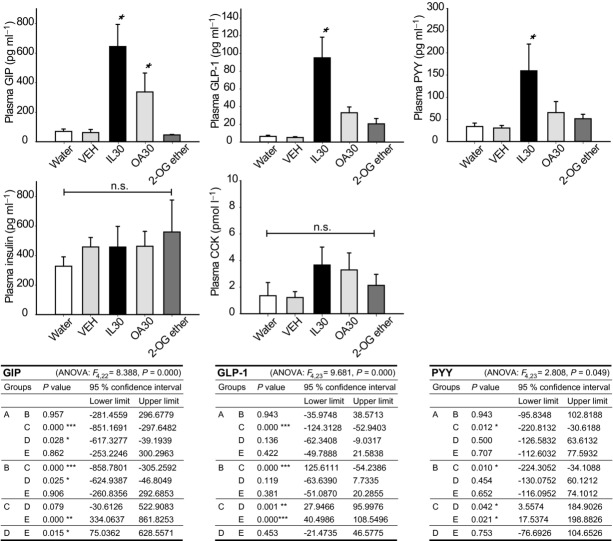
To evaluate whether the different sensing mechanisms revealed by the behavioural task were reflected in gut hormone concentrations, we measured in peripheral plasma GIP, PYY, GLP-1 insulin and CCK concentrations in response to passive infusions of IL30, OA30 and 2-OG ether at one time point. 2-OG ether was used to control for post-infusion degradation of 2-OG, and VEH and water were used as negative controls. Naïve mice were used in this experiment and received passive infusions of either of the emulsions. Plasma was isolated after 30 min, leaving sufficient time for the administered emulsions to leave the stomach and stimulate intestinal hormone secretion. The results are presented in Fig.6. IL30 produced the highest concentration of GIP, with OA30 producing an intermediate response that was not significantly different from that of IL30. For GLP-1 and PYY, only IL30 caused an increased plasma concentration compared to water and VEH, and there were no differences between the stimuli for CCK and insulin. Based on these limited data, the hormone responses appear not to be explanatory for the observed differences in sensing between fatty acids (OA30) and 2-monoacylglycerol (2-OG ether), except perhaps in the case of GIP, where responses to OA30 and IL30 were similar, as also observed in the self-operant task.
Figure 6. Plasma concentrations of gut hormones do not explain the differences in sensing of fatty acids and 2-monoacyl glycerol.
Mice with surgically inserted gastric catheters received passive infusions of either stimuli (water, VEH, IL30, OA or 2-OG ether, n = 4–6 per group). The passive infusions were 600 μl over 6 min as in the microdialysis. Thirty minutes after infusion plasma was isolated and analysed for GIP, GLP-1, PYY, insulin and CCK. IL30 was the strongest stimulator of GIP, GLP-1 and PYY, with a tendency for OA to give an intermediate response, although only significantly so for GIP. There was no difference between the stimuli on insulin and CCK levels (F4,23 = 0.345, P = 0.845 and F4,22 = 1.007, P = 0.425, respectively). In A–F, an asterisk indicates a significant difference from water and VEH in a one-way ANOVA with Fischer's LSD correction. The tables present detailed statistics. A, water; B, VEH; C, IL30; D, OA30; E, 2-OG ether. *P < 0.05, **P < 0.01, ***P < 0.001.
Fatty acids are responsible for the triacylglycerol-mediated increase in dorsal striatal dopamine
To study the involvement of reward in the observed behavioural effects, we measured the concentration of extracellular dopamine in dorsal striatum in response to passive infusions of IL30, OA30 and 2-OG. Only infusions of IL30 and OA30 induced significant release of dopamine in dorsal striatum (Fig.7A) and the increase did not reflect the caloric density of the emulsions, but rather the content of fatty acids in the intestine, as the increases in dopamine overlapped between these two emulsions. Neither 2-OG (Fig.7) nor VEH (data not shown) were able to provoke any increases in dopamine. These results indicate that the fatty acid moiety generated from digestion of IL30 accounts for the increase in dopamine observed after IL30 infusions and further corroborates that the sensing of fatty acids and 2-monoacylglycerol activates different pathways. There was no difference in the baseline dopamine levels across days or across the different stimuli (Fig. 7B and C).
Figure 7. Fatty acids account for the triacylglycerol-mediated increase in dorsal striatal dopamine.
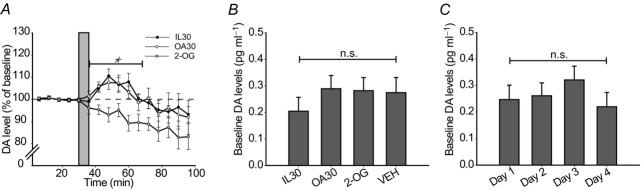
Mice with surgically inserted gastric catheters and implanted probes in the dorsal striatum received 0.6 ml intragastric infusions of IL30, OA30, 2-OG or VEH as presented in Table1. All mice (n = 11) were subjected to all stimuli on separate days in a randomized cross-over design and the dopamine levels of the dorsal striatum was recorded. A, relative dopamine (DA) levels in the dorsal striatum, obtained from microdialysis sampling at 6 min intervals. VEH (data not shown) was not significantly different from 2-OG at any time. Grey bar indicates the 6 min infusion period. A two-way (stimulus × time) repeated measures ANOVA revealed a significant effect of stimuli over the indicated (*) time points (F2,9 = 5.905, P = 0.023). Likewise, there was an effect of time (F5,6 = 7.378, P = 0.015), but no interaction (F10,1 = 17.303, P = 0.185). B, absolute levels of dopamine compared between stimuli groups (F3,8 = 0.487, P = 0.701). C, absolute levels of dopamine compared between days (F3,8 = 0.659, P = 0.600).
Discussion
The mechanisms underlying the intestinal sensing of triacylglycerol remain incompletely understood. The results obtained with our behavioural model of fat self-administration strongly suggest that (i) triacylglycerol digestion is required for its sensing by gastrointestinal cells; (ii) the digestion products, fatty acids and 2-monoacylglycerol, recruit distinct signalling pathways; and (iii) fatty acids and 2-monoacylglycerol differ in reward value and in their ability to stimulate the brain reward circuitry.
Using the reversible inhibitor of pancreatic lipase, Orlistat, we demonstrated that hydrolysis is required for sensing and generation of normal behavioural responses to triacylglycerol infusions, as has been reported for other responses to intestinal triacylglycerol infusion (Feinle et al. 2003; Pilichiewicz et al. 2003; Ellrichmann et al. 2008). This confirms that the digestion products, fatty acids and 2-monoacylglycerol, are the relevant moieties being sensed by the intestine, although the effect of Orlistat was maintained only during the first 30 min of the 1 h behavioural sessions. In an aqueous environment, Orlistat is a reversible inhibitor of pancreatic lipase, which is consumed by the lipases it inhibits (Borgstrom, 1988). Thus, during the last 30 min, Orlistat was probably ousted by the triacylglycerol administered to the mice.
Many different mechanisms for fatty acid sensing have been proposed, including apical GPCRs (Offermanns, 2014), intracellular transcription factor peroxisome proliferator-activated receptor alpha (PPARα; Hostetler et al. 2005) and protein kinase C (PKC) isoenzymes (Rasmussen et al. 2012). Also, metabolic pathways such as formation of anorexic N-acylethanolamines (Piomelli, 2013; Tellez et al. 2013; Hansen, 2014) and products of β-oxidation (Langhans et al. 2011) have been implicated in intestinal sensing of fatty acids, whereas proposed mechanisms for sensing of 2-monoacylglycerol, to our knowledge, have been limited to the activation of GPR119 (Hansen et al. 2012). Whatever fatty acid sensors may be active during feeding, our results demonstrate that fatty acids and 2-monoacylglycerol are likely to be sensed by different mechanisms: the LCFA oleic acid was perceived as more rewarding and produced greater increases in dorsal striatal dopamine, compared to 2-oleoylglycerol. The magnitude of the increases in extracellular dopamine elicited by both triacylglycerol and equimolar oleic acid was similar to those previously reported from experiments with Intralipid (Ferreira et al. 2012; Tellez et al. 2013). In preference tests, the mice opted for the triacylglycerol emulsion versus the 2-OG emulsion, and for the triacylglycerol emulsion versus the oleic acid emulsion. However, the mice overwhelmingly preferred triacylglycerol over 2-OG, whereas the preference for triacylglycerol over oleic acid was less remarkable. These patterns are not directly reflected in the differences in stimulated dopamine release, as oleic acid and triacylglycerol, but not 2-OG, produced similarly robust increases in striatal dopamine levels. While this may appear as a discrepancy between the behavioural and neurochemical data, we note that the triacylglycerol emulsion, Intralipid, contains fatty acids other than oleic acid, in such a way that the reward value of a lipid solution may be enhanced by synergism involving different fatty acids (or synergy between oleic acid and 2-OG itself). The effects of such synergism on dopamine release may not be detectable by chromatographic methods. The impact on reward systems produced by the combination of different lipids in an emulsion is an interesting topic for further research.
A complete elucidation of the signalling mechanisms linking gastrointestinal fatty acid infusion to striatal dopamine release was not the objective of the present study. Our analysis of intestinally derived hormones gave little explanation about the mechanism of signalling from the intestine to the brain, perhaps because one time point measurements of hormone levels in peripheral plasma may not provide sufficient information about the paracrine function of intestinal hormones. It is important to note, however, that the present data are entirely consistent with the possibility that the lipid messenger oleoylethanolamide links gut lipid sensing to reward circuitry activation, as previously proposed (Tellez et al. 2013). Thus, acute infusion of oleic acid have been shown to increase intestinal levels of anorectic oleoylethanolamide (Schwartz et al. 2008; Piomelli 2013) and in our self-administration set up, infusion of oleoylethanolamide decreases self-administration of a 30% Intralipid emulsion (Tellez et al. 2013). However, it remains to be deter-mined exactly how endogenous oleoylethanolamide, palmitoylethanolamide or linoleoylethanolamide (Diep et al. 2011; Hansen, 2014) may be involved in mediating the signalling of dietary fat from the intestine to the brain via the vagus nerve.
We prepared the emulsions to be equimolar to digested IL30 with respect to fatty acids and 2-monoacylglycerol composition. This was based on the assumption that it is the molecular strucuture of the lipids, rather than calories, which are being sensed; consequently, the emulsions varied with respect to caloric concentration. However, it is important to keep in mind that when comparing two emulsions of the same lipid species, molecular and caloric concentrations are interchangeable. The caloric value of fatty acids is dependent on the chain length and how many ATP molecules they generate when β-oxidized. The caloric value of 2-monoacylglycerol is dependent on hydrolysis and subsequent catabolism of the fatty acid and glycerol. However, most 2-monoacylglycerol is absorbed without further hydrolysis and re-esterified to triacylglycerol intracellularly (Mu & Høy, 2004; Kleberg et al. 2014). The structural analogue to 2-oleoylglycerol, 2-oleylglyceryl ether, is resistant to luminal hydrolysis, and intracellular hydrolysis is probably considerably reduced compared to 2-oleoylglycerol. Therefore, in the short term, it can be regarded as non-caloric, although enzymatic breakdown of ether bonds in intestinal mucosa and liver has been observed (Tietz et al. 1964).
The fatty acids originating from the digestion of Intralipid (primarily linoleic acid (C18:2) and oleic acid (C18:1)) are LCFAs. Upon absorption, LCFAs are predominatly used for re-esterification inside the enterocyte and secreted as triacylglycerol in chylomicrons, while only a minor fraction is β-oxidized (Mu & Høy, 2004). The intestinal processing of SCFAs and MCFAs is different from that of LCFAs, and a much smaller fraction is directed to the lymph (Caliph et al. 2000). This might leave a larger fraction available for β-oxidation. Consequently, the real caloric value of the administered fatty acids and 2-monoacylglycerols differs depending on the intestinal processing, and might be far from the maximum caloric value, which presupposes full hydrolysis and β-oxidation. Also from this point of view, equimolarity provides a better basis for comparison than equicalority.
Additionally, in the intestinal lumen, fatty acids will be available for interaction with GPCRs on the apical membrane of enterocytes. LCFAs are ligands for GPR40 and GPR120. SCFAs are ligands for different GPCRs (GPR41 and GPR43) and MCFAs are poor ligands for GPCRs implicated in fatty acid sensning. In fact, caprylic acid is used as a negative control for fatty acid-stimulated hormone secretion (Beglinger et al. 2010; Ogawa et al. 2012).
We observed in the behavioural tests that fatty acids of varying chain lengths were sensed with different potency by the mice, and this might very well reflect their affinity for the different pathways described above. As the potency of the sensing increases with a presumable increasing degree of β-oxidation (SCFAs>MCFAs>LCFAs) it seems reasonable that β-oxidation is a contributing mechanism in fatty acid sensing. However, interaction with GPCRs cannot be excluded, and the rise in plasma concentrations of gut hormones support the suggestion that GPCRs are also involved. In fact, regarding sensing of 2-monoacylglycerol, interaction with GPR119 is the most likely mechanism, and this was supported by the fact that the mice's behavioural responses to 2-OG end 2-OG ether were similar despite differences in apparent caloric values.
To conclude, we have shown, for the first time, that digestion products of dietary fat are sensed differently and that LCFA sensing is coupled to central dopamine release. 2-Monoacylglycerol sensing does not involve dorsal striatal dopamine release and might occur via activation of GPR119 in the intestine. Sensing of LCFAs might thus cause both satiation and reward, whereas 2-monoacylglycerol is probaly strictly satiating via non-rewarding pathways. Both sensing mechanisms probably contribute to the behaviour of mice self-administering emulsified triacylglycerol.
Glossary
- CCK
cholecystokinin
- GIP
glucose-dependent insulinotropic polypeptide
- GLP
glucagon-like peptide
- GPCR
G-protein coupled receptor
- LCFA
long-chain fatty acid
- MCFA
medium-chain fatty acid
- PYY
peptide YY
- SCFA
short-chain fatty acid
Additional information
Competing interests
The authors have no competing interests to declare.
Author contributions
K.K. was responsible for the study design and conduction of the experiments wrote the first draft of the manuscript. A.K. and J.G. assisted with the experiments performed in Copenhagen and New Haven, respectively. J.A.W. and J.J.H. managed the GIP, GLP-1, insulin and PYY analyses. J.F.R. was in charge of the CCK analysis. I.E.A. and H.S.H. conceived the study, and contributed to the design and writing of the manuscript. All authors have read and commented on the content of the manuscript and have approved the final version.
Funding
The work has been supported by NIH grant CA180030-01 to IEA and The Novo Nordisk Foundation, Universitetsforskningens Investeringskapital (UNIK): Food, Fitness and Pharma to HSH. The UNIK project is supported by the Danish Ministry of Science, Technology and Innovation.
References
- Beglinger S, Drewe J, Schirra J, Goke B, D'Amato M. Beglinger C. Role of fat hydrolysis in regulating glucagon-like Peptide-1 secretion. J Clin Endocrinol Metab. 2010;95:879–886. doi: 10.1210/jc.2009-1062. [DOI] [PubMed] [Google Scholar]
- Blad CC, Tang C. Offermanns S. G protein-coupled receptors for energy metabolites as new therapeutic targets. Nat Rev Drug Discov. 2012;11:603–619. doi: 10.1038/nrd3777. [DOI] [PubMed] [Google Scholar]
- Borgstrom B. Mode of action of tetrahydrolipstatin: a derivative of the naturally occurring lipase inhibitor lipstatin. Biochim Biophys Acta. 1988;962:308–316. doi: 10.1016/0005-2760(88)90260-3. [DOI] [PubMed] [Google Scholar]
- Caliph SM, Charman WN. Porter CJ. Effect of short-, medium-, and long-chain fatty acid-based vehicles on the absolute oral bioavailability and intestinal lymphatic transport of halofantrine and assessment of mass balance in lymph-cannulated and non-cannulated rats. J Pharm Sci. 2000;89:1073–1084. doi: 10.1002/1520-6017(200008)89:8<1073::aid-jps12>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Cordier-Bussat M, Bernard C, Levenez F, Klages N, Laser-Ritz B, Philippe J, Chayvialle JA. Cuber JC. Peptones stimulate both the secretion of the incretin hormone glucagon-like peptide 1 and the transcription of the proglucagon gene. Diabetes. 1998;47:1038–1045. doi: 10.2337/diabetes.47.7.1038. [DOI] [PubMed] [Google Scholar]
- Diep TA, Madsen AN, Holst B, Kristiansen MM, Wellner N, Hansen SH. Hansen HS. Dietary fat decreases intestinal levels of the anorectic lipids through a fat sensor. FASEB J. 2011;25:765–774. doi: 10.1096/fj.10-166595. [DOI] [PubMed] [Google Scholar]
- Duca FA, Swartz TD, Sakar Y. Covasa M. Decreased intestinal nutrient response in diet-induced obese rats: role of gut peptides and nutrient receptors. Int J Obes (Lond) 2013;37:375–381. doi: 10.1038/ijo.2012.45. [DOI] [PubMed] [Google Scholar]
- Ellrichmann M, Kapelle M, Ritter PR, Holst JJ, Herzig KH, Schmidt WE, Schmitz F. Meier JJ. Orlistat inhibition of intestinal lipase acutely increases appetite and attenuates postprandial glucagon-like peptide-1(7–36)-amide-1, cholecystokinin, and peptide YY concentrations. J Clin Endocrinol Metab. 2008;93:3995–3998. doi: 10.1210/jc.2008-0924. [DOI] [PubMed] [Google Scholar]
- Engelstoft MS, Egerod KL, Holst B. Schwartz T. A gut feeling for obesity: 7TM sensors on enteroendocrine cells. Cell Metab. 2008;8:447–449. doi: 10.1016/j.cmet.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Feinle C, O'Donovan D, Doran S, Andrews JM, Wishart J, Chapman I. Horowitz M. Effects of fat digestion on appetite, APD motility, and gut hormones in response to duodenal fat infusion in humans. Am J Physiol Gastrointest Liver Physiol. 2003;284:G798–G807. doi: 10.1152/ajpgi.00512.2002. [DOI] [PubMed] [Google Scholar]
- Ferreira JG, Tellez LA, Ren X, Yeckel CW. de Araujo IE. Regulation of fat intake in the absence of flavour signalling. J Physiol. 2012;590:953–972. doi: 10.1113/jphysiol.2011.218289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerspach AC, Steinert RE, Schonenberger L, Graber-Maier A. Beglinger C. The role of the gut sweet taste receptor in regulating GLP-1, PYY, and CCK release in humans. Am J Physiol Endocrinol Metab. 2011;301:E317–E325. doi: 10.1152/ajpendo.00077.2011. [DOI] [PubMed] [Google Scholar]
- Guerre-Millo M, Gervois P, Raspe E, Madsen L, Poulain P, Derudas B, Herbert JM, Winegar DA, Willson TM, Fruchart JC, Berge RK. Staels B. Peroxisome proliferator-activated receptor α activators improve insulin sensitivity and reduce adiposity. J Biol Chem. 2000;275:16638–16642. doi: 10.1074/jbc.275.22.16638. [DOI] [PubMed] [Google Scholar]
- Hansen HS. Role of anorectic N-acylethanolamines in intestinal physiology and satiety control with respect to dietary fat. Pharmacol Res. 2014;86C:18–25. doi: 10.1016/j.phrs.2014.03.006. [DOI] [PubMed] [Google Scholar]
- Hansen HS, Rosenkilde MM, Holst JJ. Schwartz TW. GPR119 as a fat sensor. Trends Pharmacol Sci. 2012;33:374–381. doi: 10.1016/j.tips.2012.03.014. [DOI] [PubMed] [Google Scholar]
- Hansen KB, Rosenkilde MM, Knop FK, Wellner N, Diep TA, Rehfeld JF, Andersen UB, Holst JJ. Hansen HS. 2-Oleoyl glycerol is a GPR119 agonist and signals GLP-1 release in humans. J Clin Endocrinol Metab. 2011;96:E1409–E1417. doi: 10.1210/jc.2011-0647. [DOI] [PubMed] [Google Scholar]
- Hostetler HA, Petrescu AD, Kier AB. Schroeder F. Peroxisome proliferator-activated receptor α interacts with high affinity and is conformationally responsive to endogenous ligands. J Biol Chem. 2005;280:18667–18682. doi: 10.1074/jbc.M412062200. [DOI] [PubMed] [Google Scholar]
- Ichimura A, Hirasawa A, Poulain-Godefroy O, Bonnefond A, Hara T, Yengo L, Kimura I, Leloire A, Liu N, Iida K, Choquet H, Besnard P, Lecoeur C, Vivequin S, Ayukawa K, Takeuchi M, Ozawa K, Tauber M, Maffeis C, Morandi A, Buzzetti R, Elliott P, Pouta A, Jarvelin MR, Korner A, Kiess W, Pigeyre M, Caiazzo R, Van HW, Van GL, Horber F, Balkau B, Levy-Marchal C, Rouskas K, Kouvatsi A, Hebebrand J, Hinney A, Scherag A, Pattou F, Meyre D, Koshimizu TA, Wolowczuk I, Tsujimoto G. Froguel P. Dysfunction of lipid sensor GPR120 leads to obesity in both mouse and human. Nature. 2012;483:350–354. doi: 10.1038/nature10798. [DOI] [PubMed] [Google Scholar]
- Kleberg K, Hassing HA. Hansen HS. Classical endocannabinoid-like compounds and their regulation by nutrients. BioFactors. 2014;40:363–372. doi: 10.1002/biof.1158. [DOI] [PubMed] [Google Scholar]
- Langhans W, Leitner C. Arnold M. Dietary fat sensing via fatty acid oxidation in enterocytes: possible role in the control of eating. Am J Physiol Regul Integr Comp Physiol. 2011;300:R554–R565. doi: 10.1152/ajpregu.00610.2010. [DOI] [PubMed] [Google Scholar]
- Liou AP, Lu X, Sei Y, Zhao X, Pechhold S, Carrero RJ, Raybould HE. Wank S. The G-protein-coupled receptor GPR40 directly mediates long-chain fatty acid-induced secretion of cholecystokinin. Gastroenterology. 2011;140:903–912. doi: 10.1053/j.gastro.2010.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Little TJ. Feinle-Bisset C. Effects of dietary fat on appetite and energy intake in health and obesity – Oral and gastrointestinal sensory contributions. Physiol Behav. 2011;104:613–620. doi: 10.1016/j.physbeh.2011.04.038. [DOI] [PubMed] [Google Scholar]
- Mu H. Høy CE. The digestion of dietary triacylglycerols. Prog Lipid Res. 2004;43:105–133. doi: 10.1016/s0163-7827(03)00050-x. [DOI] [PubMed] [Google Scholar]
- Offermanns S. Free fatty acid (FFA) and hydroxy carboxylic acid (HCA) receptors. Annu Rev Pharmacol Toxicol. 2014;54:407–434. doi: 10.1146/annurev-pharmtox-011613-135945. [DOI] [PubMed] [Google Scholar]
- Ogawa N, Ito M, Yamaguchi H, Shiuchi T, Okamoto S, Wakitani K, Minokoshi Y. Nakazato M. Intestinal fatty acid infusion modulates food preference as well as calorie intake via the vagal nerve and midbrain–hypothalamic neural pathways in rats. Metabolism. 2012;61:1312–1320. doi: 10.1016/j.metabol.2012.02.011. [DOI] [PubMed] [Google Scholar]
- Pilichiewicz A, O'Donovan D, Feinle C, Lei Y, Wishart JM, Bryant L, Meyer JH, Horowitz M. Jones KL. Effect of lipase inhibition on gastric emptying of, and the glycemic and incretin responses to, an oil/aqueous drink in type 2 diabetes mellitus. J Clin Endocrinol Metab. 2003;88:3829–3834. doi: 10.1210/jc.2003-030199. [DOI] [PubMed] [Google Scholar]
- Piomelli D. A fatty gut feeling. Trends Endocrinol Metab. 2013;24:332–341. doi: 10.1016/j.tem.2013.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen BA, Breen DM. Lam TK. Lipid sensing in the gut, brain and liver. Trends Endocrinol Metab. 2012;23:49–55. doi: 10.1016/j.tem.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Rehfeld JF. Accurate measurement of cholecystokinin in plasma. Clin Chem. 1998;44:991–1001. [PubMed] [Google Scholar]
- Schwartz GJ, Fu J, Astarita G, Li X, Gaetani S, Campolongo P, Cuomo V. Piomelli D. The lipid messenger OEA links dietary fat intake to satiety. Cell Metab. 2008;8:281–288. doi: 10.1016/j.cmet.2008.08.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz GJ. Zeltser LM. Functional organization of neuronal and humoral signals regulating feeding behavior. Annu Rev Nutr. 2013;33:1–21. doi: 10.1146/annurev-nutr-071812-161125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small DM, Jones-Gotman M. Dagher A. Feeding-induced dopamine release in dorsal striatum correlates with meal pleasantness ratings in healthy human volunteers. Neuroimage. 2003;19:1709–1715. doi: 10.1016/s1053-8119(03)00253-2. [DOI] [PubMed] [Google Scholar]
- Stewart JE, Seimon RV, Otto B, Keast RS, Clifton PM. Feinle-Bisset C. Marked differences in gustatory and gastrointestinal sensitivity to oleic acid between lean and obese men. Am J Clin Nutr. 2011;93:703–711. doi: 10.3945/ajcn.110.007583. [DOI] [PubMed] [Google Scholar]
- Szczypka MS, Kwok K, Brot MD, Marck BT, Matsumoto AM, Donahue BA. Palmiter RD. Dopamine production in the caudate putamen restores feeding in dopamine-deficient mice. Neuron. 2001;30:819–828. doi: 10.1016/s0896-6273(01)00319-1. [DOI] [PubMed] [Google Scholar]
- Tellez LA, Medina S, Han W, Ferreira JG, Licona-Limon P, Ren X, Lam TT, Schwartz GJ. de Araujo IE. A gut lipid messenger links excess dietary fat to dopamine deficiency. Science. 2013;341:800–802. doi: 10.1126/science.1239275. [DOI] [PubMed] [Google Scholar]
- Tietz A, Lindberg M. Kennedy EP. A new pteridine-requiring enzyme system for the oxidation of glycerol ethers. J Biol Chem. 1964;239:4081–4090. [PubMed] [Google Scholar]
- Tolhurst G, Reimann F. Gribble FM. Intestinal sensing of nutrients. Handb Exp Pharmacol. 2012;209:309–335. doi: 10.1007/978-3-642-24716-3_14. [DOI] [PubMed] [Google Scholar]
- Vucetic Z. Reyes TM. Central dopaminergic circuitry controlling food intake and reward: implications for the regulation of obesity. Wiley Interdiscip Rev Syst Biol Med. 2010;2:577–593. doi: 10.1002/wsbm.77. [DOI] [PubMed] [Google Scholar]
- Wellendorph P, Johansen LD. Brauner-Osborne H. The emerging role of promiscuous 7TM receptors as chemosensors for food intake. Vitam Horm. 2010;84:151–184. doi: 10.1016/B978-0-12-381517-0.00005-9. [DOI] [PubMed] [Google Scholar]