Abstract
Rationale
Platelets are anuclear cell fragments derived from bone marrow megakaryocytes (MKs) that safeguard vascular integrity but may also cause pathological vessel occlusion. One major pathway of platelet activation is triggered by 2 receptors that signal through an (hem)immunoreceptor tyrosine-based activation motif (ITAM), the activating collagen receptor glycoprotein (GP) VI and the C-type lectin-like receptor 2 (CLEC-2). Growth factor receptor–bound protein 2 (Grb2) is a ubiquitously expressed adapter molecule involved in signaling processes of numerous receptors in different cell types, but its function in platelets and MKs is unknown.
Objective
We tested the hypothesis that Grb2 is a crucial adapter protein in (hem)immunoreceptor tyrosine-based activation motif signaling in platelets.
Methods and Results
Here, we show that genetic ablation of Grb2 in MKs and platelets did not interfere with MK differentiation or platelet production. However, Grb2-deficiency severely impaired glycoprotein VI–mediated platelet activation because of defective stabilization of the linker of activated T-cell (LAT) signalosome and activation of downstream signaling proteins that resulted in reduced adhesion, aggregation, and coagulant activity on collagen in vitro. Similarly, CLEC-2–mediated signaling was impaired in Grb2-deficient platelets, whereas the cells responded normally to stimulation of G protein–coupled receptors. In vivo, this selective (hem) immunoreceptor tyrosine-based activation motif signaling defect resulted in prolonged bleeding times but affected arterial thrombus formation only after concomitant treatment with acetylsalicylic acid, indicating that defective glycoprotein VI signaling in the absence of Grb2 can be compensated through thromboxane A2–induced G protein–coupled receptor signaling pathways.
Conclusions
These results reveal an important contribution of Grb2 in (hem)immunoreceptor tyrosine-based activation motif signaling in platelets in hemostasis and thrombosis by stabilizing the LAT signalosome.
Keywords: blood platelets, hemostasis, mice, signal transduction, thrombosis
At sites of vascular injury, platelets adhere and aggregate on the exposed subendothelial extracellular matrix and thereby form a plug that seals the wound. This process is crucial for normal hemostasis, but in diseased vessels it may lead to pathological thrombus formation and infarction of vital organs.1 Among the multiple macromolecular constituents of the extracellular matrix, collagens are the most thrombogenic because they directly induce powerful cellular activation. This activation is mediated by glycoprotein VI (GPVI), a receptor of the Ig superfamily, which is closely related to the Fcα receptor (FcαR) and the natural killer cell receptors.2 GPVI is noncovalently associated with an FcR γ-chain homodimer, which contains a classical immunoreceptor tyrosine-based activation motif (ITAM) and initiates a signaling pathway similar to that of immune receptors, such as the T cell receptor (TCR), the B cell receptor (BCR), or the FcγRs. Ligand-induced cross-linking of GPVI triggers tyrosine phosphorylation of the FcR γ-chain on its ITAMs by the Src kinases Fyn and Lyn.3 This induces the recruitment and subsequent activation of the tandem Src homology (SH) 2 domain–containing tyrosine kinase Syk, which in turn initiates a downstream signaling cascade involving the adapters linker of activated T cells (LAT) and SH2 domain–containing leukocyte protein of 76 kDa (SLP-76) and culminates in the activation of different effector molecules, including phospholipase C (PLC) γ2 and phosphoinositide-3 kinase. GPVI has emerged as a promising antithrombotic target because its absence or functional inhibition provides protection from pathological thrombus formation and stroke in mice without causing major bleeding complications.3
A similar but not identical signaling pathway is used by the more recently discovered activated platelet surface glycoprotein, C-type lectin-like receptor 2 (CLEC-2), which initiates signaling on tyrosine phosphorylation of only a single YXXL motif (hemITAM) in its cytoplasmic tail.1 Compared with GPVI, CLEC-2 might also become a target for antithrombotic agents4 because CLEC-2 deficiency has only a minor effect on hemostasis but prevents formation of stable vessel occluding thrombi.5 Importantly, however, CLEC-2 is also required for the separation of the lymphatic from the blood vasculature, most likely because of its interaction with its only known physiological ligand, podoplanin.6,7 Although several proteins and their functions within the (hem)ITAM signaling pathway have been elucidated, this complex machinery is still not fully understood.
Growth factor receptor–bound protein 2 (Grb2) is an adapter protein comprising a central SH2 domain flanked by 2 SH3 domains.8 It has been identified as a major mediator in Ras-mitogen-activated protein kinase (MAPK) activation, which is induced by many growth factor receptors because of its association with son of sevenless homolog (SOS1), a GDP–GTP exchange factor for Ras.9,10 Grb2 is ubiquitously expressed and has essential functions during embryo development and malignant transformation.11,12 On TCR stimulation, Grb2 associates with LAT or the CD3 complex via SH2 and collagen homology domain–containing protein (Shc).13 Analysis of mice with a T cell-specific Grb2-deficiency revealed that the adapter plays a pivotal role in early T cell development and that it amplifies TCR signaling at the proximal end of the tyrosine phosphorylation cascade.14 In addition, Houtman et al15 have demonstrated a critical role for LAT in amplifying T cell signaling through the generation of an LAT signalosome in association with SOS1. Recently, 2 independent studies on mice with a B cell-specific Grb2-deficiency identified, contrary to T cells, enhanced BCR-induced Ca2+ signaling in the absence of Grb2,16,17 whereas conflicting results on the role of Grb2 in MAPK activation in these cells were reported. Grb2 is also expressed in platelets and MKs, and based on in vitro studies, it has been proposed that the Grb2/SOS1/mitogen-activated protein kinase/ERK kinase pathway is of central importance for MK differentiation.18,19 Furthermore, the LAT signalosome is key to (hem)ITAM-mediated platelet activation. In platelets, Grb2 is part of the LAT signalosome20 and becomes tyrosine phosphorylated after GPVI-mediated platelet activation,21 indicating a possible role of the adapter in GPVI signaling. Interestingly, in human platelets Grb2 associates strongly with LAT after GPVI stimulation, whereas the Grb2-related adapter protein downstream of Shc (Gads) shows only a weak association, and studies in Gads-deficient mice demonstrate only a minor role of Gads in supporting GPVI signaling.22 In addition, an increased interaction of Grb2 with the adapter protein downstream of tyrosine kinase (Dok) 3 on activation of integrin αIIbβ3 has been reported, implicating a possible role in integrin outside-in signaling.23 However, because of the lack of an appropriate animal model, the exact role of Grb2 in platelet production and function has remained elusive.
Here, we show that an MK/platelet-specific Grb2-deficiency has no effect on MK differentiation and platelet production but severely affects (hem)ITAM signaling in platelets because of the defective stability of the LAT signalosome.
Methods
Detailed methods are provided in the online-only Data Supplement.
Results
Megakaryocyte-Specific Grb2-Deficiency Has No Effect on Platelet Production
To study the role of Grb2 in platelet physiology in vivo, we crossed mice carrying 2 loxP sites flanking exon 2 of the Grb2 gene (Grb2fl/fl)16 with mice carrying Cre recombinase under the control of the platelet/MK-specific platelet factor 4 promoter.24 Western blot analysis confirmed the absence of Grb2 in platelets from Grb2fl/fl,PF4-Cre+/− mice (hereon referred to as Grb2−/− mice), whereas the expression of the protein in splenocytes was unaltered (Online Figure IA). Grb2−/− mice were born in Mendelian ratios, appeared healthy, and did not show any signs of spontaneous bleeding or blood-lymph vessel separation defects. Platelet count and size, as well as surface expression levels of prominent glycoprotein receptors, were unaltered compared with the wild-type control (Online Figure IB and IC, Online Table I), indicating that megakaryopoiesis and platelet formation can occur independent of Grb2. This was corroborated by normal MK numbers in the bone marrow of the mutant animals (Grb2+/+: 7.8±0.3 MK/visual field, Grb2−/−: 8.8±0.7 MK/visual field; Online Figure ID).
Grb2−/− Platelets Show Diminished Responses to GPVI and CLEC-2 Stimulation But Normal Integrin Outside-In Signaling
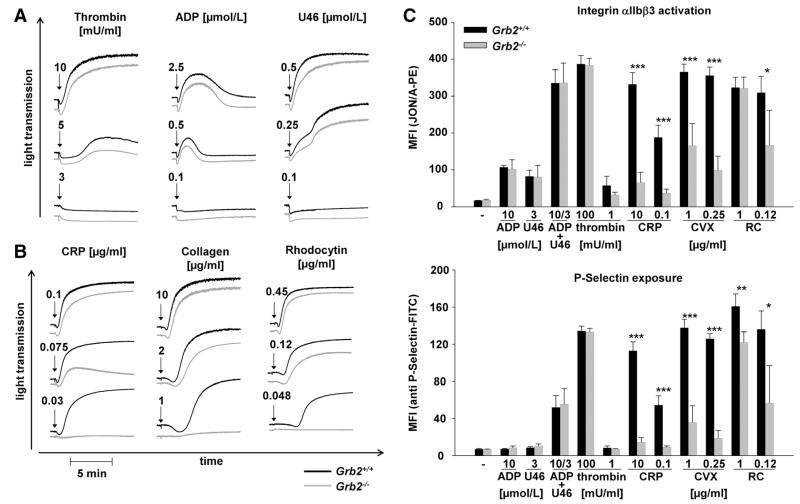
To investigate the consequences of Grb2-deficiency on platelet function, we performed ex vivo aggregation studies. Grb2−/− platelets aggregated normally in response to the G protein–coupled receptor agonists thrombin, ADP, and the thromboxane A2 (TxA2) analog U46619 (Figure 1A). In contrast, the mutant platelets displayed a marked aggregation defect in response to intermediate and low doses of the GPVI agonists collagen and collagen-related peptide (CRP). These defects could, however, be overcome by high concentrations of these agonists in which Grb2-deficient platelets showed a slightly delayed aggregation onset but a full aggregation response (Figure 1B). Similar observations were made with the snake venom toxin rhodocytin that induces aggregation via the (hem)ITAM receptor CLEC-2 (Figure 1B). This selective (hem)ITAM receptor signaling defect was confirmed by flow cytometric analysis of integrin αIIbβ3 activation and of degranulation-dependent P-selectin surface exposure (Figure 1C). These results demonstrated that Grb2 is required for (hem)ITAM receptor–induced platelet activation, whereas it is dispensable for platelet activation downstream of G protein–coupled receptors.
Figure 1. Impaired aggregation responses, αIIbβ3 activation, and granule release in Grb2−/− platelets in response to glycoprotein VI and C-type lectin-like receptor 2 stimulation.
Washed platelets from Grb2+/+ (black line) and Grb2−/− (gray line) mice were activated with the indicated concentrations of (A) ADP, U46619, and thrombin and (B) collagen, collagen-related peptide (CRP), or rhodocytin, and light transmission was recorded on a Fibrintimer 4-channel aggregometer. ADP measurements were performed in platelet-rich plasma. Representative aggregation traces of ≥3 individual experiments are depicted. C, Flow cytometric analysis of integrin αIIbβ3 activation (top) and degranulation-dependent P-selectin exposure (bottom) in response to the indicated agonists in wild-type and Grb2−/− platelets. Results are mean fluorescence intensities (MFI)±SD of 4 mice per group and representative of 4 individual experiments (*P<0.05, **P<0.01, ***P<0.001). CVX indicates convulxin; FITC, fluorescein isothiocyanate; PE, phycoerythrin; and RC, rhodocytin.
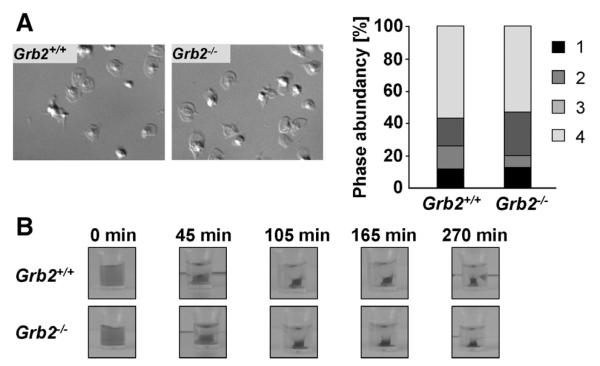
Ligand-occupied integrin αIIbβ3 mediates outside-in signaling, leading to cytoskeletal reorganization and platelet spreading.25 Grb2 has been proposed to play a role in this process,23 as well as in the formation of actin-rich protrusions.26 To test this directly, Grb2−/− and wild-type platelets were allowed to spread on a fibrinogen-coated surface in the presence of low concentrations of thrombin (Figure 2A). Grb2−/− and wild-type platelets formed filopodia and lamellipodia with similar kinetics, and after 30 minutes, the number of fully spread platelets was comparable between the 2 groups. We confirmed these findings by performing the spreading experiments also in the presence of apyrase and indomethacin to inhibit stimulation by released secondary mediators in the absence (Online Figure IIA) or presence of thrombin (Online Figure IIB). Integrin αIIbβ3 outside-in signaling also regulates clot retraction.27 Therefore, we induced clot formation in platelet-rich plasma by the addition of a high dose of thrombin (5 U/mL) and 20 mmol/L CaCl2 and monitored retraction over time. No differences between wild-type and Grb2−/− platelets were observed (Figure 2B), demonstrating that the adapter is not required for this integrin αIIbβ3-controlled process.
Figure 2. Normal integrin outside-in signaling in Grb2−/− platelets.
A, Washed platelets of Grb2+/+ and Grb2−/− mice were allowed to spread on fibrinogen (100 μg/mL) for 30 minutes after stimulation with 0.01 U/mL thrombin. Representative differential interference contrast images of 2 individual experiments (left) and statistical evaluation of the percentage of spread platelets at different spreading stages (right). 1 indicates roundish; 2, only filopodia; 3, filopodia and lamellipodia; and 4, fully spread. B, Clot retraction of platelet-rich plasma on activation with 5 U/mL thrombin in the presence of 20 mmol/L CaCl2 at the indicated time points (n=3 for Grb2+/+ and n=7 for Grb2−/−).
Defective GPVI-Induced Ca2+ Mobilization in Grb2−/− Platelets
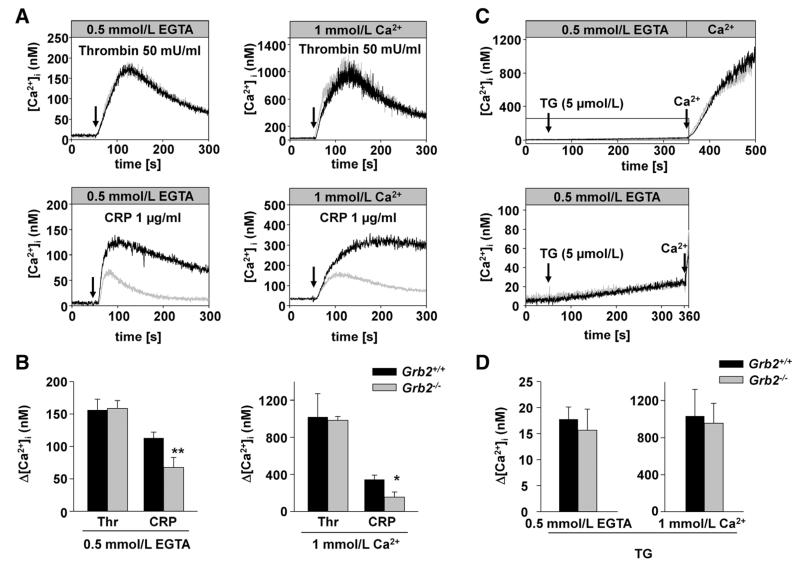
Agonist-induced platelet activation leads to an increase in cytosolic calcium concentrations ([Ca]2+i) through release of Ca2+ from intracellular stores and Ca2+ entry across plasma membrane Ca2+ channels.28 To test whether the observed GPVI signaling defect in Grb2−/− platelets was based on impaired Ca2+ signaling, we studied agonist-induced changes in [Ca]2+i fluorimetrically. Grb2−/− platelets displayed markedly reduced Ca2+ store release and entry in response to CRP but showed normal responses to thrombin (Figure 3A and 3B). Notably, passive store depletion with thapsigargin did not lead to any differences in the kinetics of store release or store-operated Ca2+ entry between wild-type and Grb2−/− platelets (Figure 3C and 3D).
Figure 3. Defective glycoprotein VI–induced Ca2+ mobilization.
A, Time course of intracellular Ca2+ mobilization in Grb2+/+ (black line) and Grb2−/− platelets (gray line) in response to thrombin and collagen-related peptide (CRP; addition indicated by an arrow). The experiment was performed in the absence (left) and presence (right) of extracellular Ca2+. The results shown are representative of ≥3 individual experiments. B, Maximal increase in cytosolic Ca2+ concentration ([Ca]2+i) of Grb2+/+ (black bars) and Grb2−/− platelets (gray bars) after activation with the indicated agonists (thrombin, 0.1 U/mL; CRP, 5 μg/mL). C, Time course of intracellular Ca2+ mobilization after treatment with thapsigargin (TG, top). The boxed area comprises the time frame before addition of extracellular Ca2+ and is shown magnified in the bottom. D, Maximal increase in cytosolic Ca2+ concentration after treatment with TG. Results are given as mean [Ca]2+i (nmol/L)±SD, n=3 to 4 per group (*P<0.05, **P<0.01, ***P<0.001). The experiment was performed in the presence of 1 mmol/L Ca2+ or 0.5 mmol/L EGTA.
Defective Adhesion, Aggregate Formation, and Coagulant Activity of Grb2−/− Platelets on Collagen Under Flow
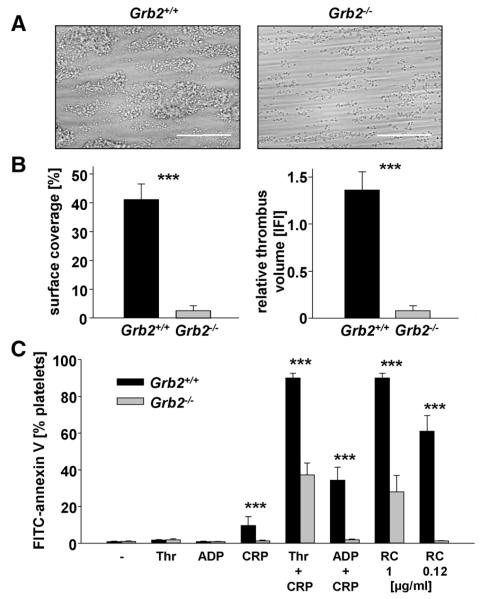
Thrombus formation at the site of vascular injury requires stable shear-resistant platelet adhesion on the extracellular matrix and auto- and paracrine platelet activation by locally released secondary mediators.3 To address the effect of Grb2-deficiency on these processes, platelet adhesion to collagen was studied in an ex vivo whole blood perfusion system. When blood was perfused over immobilized collagen at a shear rate of 1000 s−1, wild-type platelets rapidly adhered to the collagen surface and recruited additional platelets from the blood stream, resulting in the formation of stable 3-dimensional thrombi (Figure 4A, left). In sharp contrast, adhesion of Grb2−/− platelets was markedly impaired, and aggregate formation was virtually abrogated. As a result, both the surface area covered by platelets and thrombus volume at the end of the perfusion period were reduced by ≈94% for Grb2−/− platelets compared with the wild type (Figure 4B). These results demonstrated that Grb2 is essential for platelet adhesion and aggregate formation on collagen under flow.
Figure 4. Impaired adhesion and defective aggregate formation of Grb2−/− platelets on collagen under flow and defective procoagulant activity.
A, Whole blood from Grb2+/+ or Grb2−/− mice was perfused over a collagen-coated surface (0.2 mg/mL) at a shear rate of 1000 s−1. Representative phase contrast images of aggregate formation on collagen after 4 minutes of perfusion time. Scale bar, 50 μm. B, Mean surface coverage (left) and relative thrombus volume expressed as integrated fluorescence intensity (IFI; right)±SD of 4 Grb2+/+ and 5 Grb2−/− mice (***P<0.001). C, Flow cytometric analysis of phosphatidylserine (PS) exposure in response to the indicated agonists in Grb2+/+ and Grb2−/− platelets. Washed platelets were stained with annexin V-DyLight-488 in the presence of Tyrode’s buffer containing 2 mmol/L Ca2+ agonist concentrations: Thr, 0.1 U/mL; ADP, 10 μmol/L; collagen-related peptide (CRP), 20 μg/mL. Results are mean percentage of annexin V–positive platelets±SD of 4 Grb2+/+ and 5 Grb2−/− mice and representative of 2 individual experiments (***P<0.001). RC indicates rhodocytin; and Thr, thrombin.
GPVI-stimulated platelets facilitate coagulation by the exposure of negatively charged phosphatidylserine (PS) on their outer surface, thereby providing high-affinity binding sites for key coagulation factors.29 To test a possible role of Grb2 in the induction of coagulant activity, washed platelets were stimulated with different agonists, and PS exposure was analyzed. After stimulation with the combination of CRP/thrombin or CRP/ADP, as well as high concentrations of rhodocytin, the majority of wild-type platelets exposed PS on their surface. In marked contrast, only partial or absent responses were seen in Grb2−/− platelets under these conditions, and they displayed virtually no PS exposure in response to CRP, which still produced a small increase in PS exposure in wild-type platelets (Figure 4C). These results revealed a prominent role for Grb2 in facilitating (hem)ITAM-induced coagulant activity in platelets.
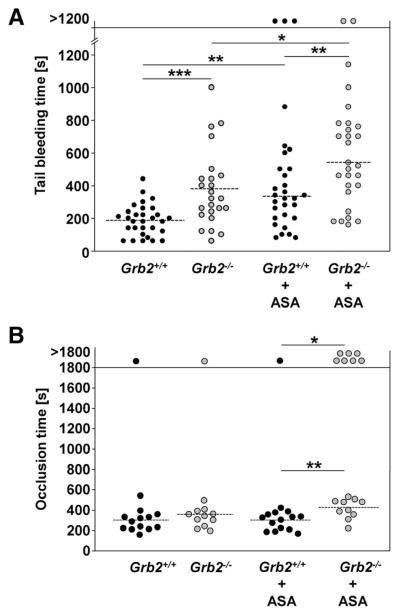
Impaired Hemostasis and Partially Defective Arterial Thrombus Formation in Grb2−/− Mice
To study the effect of Grb2-deficiency on hemostasis, tail bleeding times were determined (Figure 5A). Grb2−/− mice exhibited prolonged (mean bleeding time: Grb2+/+: 197±107 seconds versus Grb2−/−: 381±237 seconds; P<0.0005) and highly variable bleeding times, suggesting thrombus instability as recently also described for mice lacking the adapter LAT.30,31
Figure 5. Impaired hemostasis and partially defective thrombus formation in Grb2−/− mice.
A, Tail bleeding times of Grb2+/+ and Grb2−/− mice without or with acetylsalicylic acid (ASA) treatment (1 mg/kg i.v.) 15 minutes before the start of the experiment. Each symbol represents 1 animal. B, Time to stable vessel occlusion of Grb2+/+ and Grb2−/− mice without or with ASA treatment (1 mg/kg i.v.) 15 minutes before the start of the experiment. The abdominal aorta was injured by firm compression with a forceps, and blood flow was monitored for 30 minutes. Each symbol represents 1 animal (*P<0.05, **P<0.01, ***P<0.001).
To examine to which extent the in vitro observed defects of Grb2−/− platelets influenced thrombotic events in vivo, we studied occlusive thrombus formation in a model where the abdominal aorta is mechanically injured, and blood flow is monitored with an ultrasonic perivascular Doppler flowmeter. Unexpectedly, wild-type and mutant mice formed occlusive thrombi with similar kinetics (mean occlusion time: Grb2+/+: 303±101 seconds versus Grb2−/−: 351±98 seconds; Figure 5B), whereas the vessels of GPVI-deficient mice did not occlude.32 We have previously shown that defective GPVI signaling can be compensated through TxA2-induced G protein–coupled receptor signaling pathways.33 Therefore, we inhibited TxA2 synthesis in control and Grb2−/− mice by administration of a low dose of acetylsalicylic acid (ASA) intravenously (1 mg/kg) and assessed thrombus formation in the injured aorta after 15 minutes. Strikingly, although ASA treatment had no significant effect on thrombus formation in control mice (14 of 15 occluded, mean occlusion time: 296±84 seconds), a strong effect was seen in the mutant mice where 7 of 17 vessels did not occlude (P<0.05), and occlusion times for the remaining vessels were increased compared with the wild-type (419±99 seconds; P<0.005). Similar effects were observed in a model of FeCl3-induced injury of mesenteric arterioles where vessel occlusion times of Grb2-deficient mice were prolonged only in the presence of ASA compared with ASA-treated wild-type mice (17.89±4.13 versus 25.73±5.20 minutes; P<0.005; Online Figure III), confirming that the GPVI signaling defect in these animals is at least, in part, compensated by TxA2-dependent activation pathways in vivo. In contrast, ASA treatment prolonged tail bleeding times of wild-type and Grb2−/− mice to a similar extent (Figure 5A).
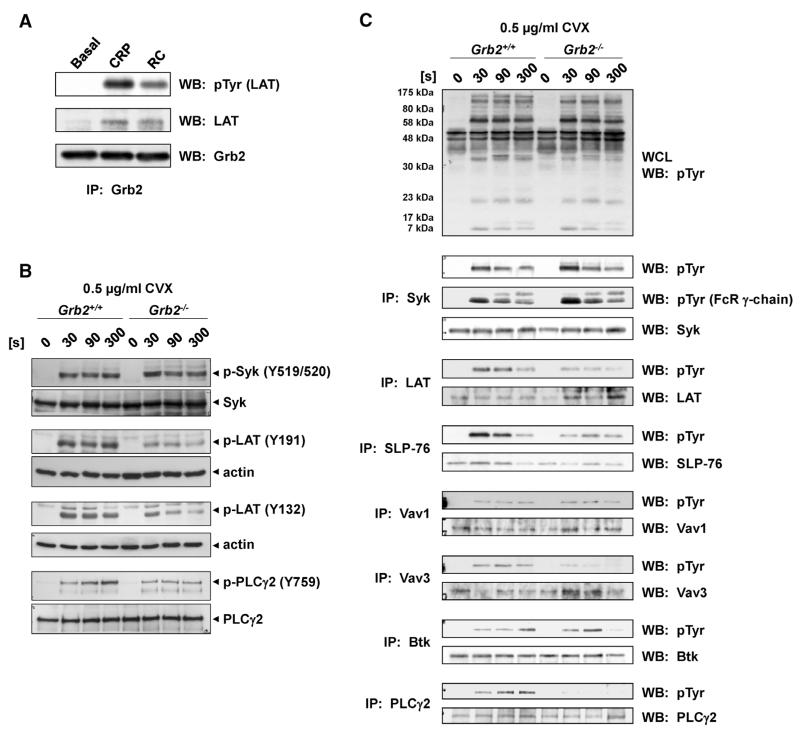
Grb2 Stabilizes the LAT Signalosome to Mediate (hem)ITAM Signaling
To investigate the molecular basis of Grb2 in transmitting (hem)ITAM-mediated signaling, Grb2 was immunoprecipitated from resting and GPVI-, as well as CLEC-2–, stimulated wild-type platelets, and samples were analyzed for protein tyrosine phosphorylation (Figure 6A). In line with our previous study,22 LAT immunoprecipitated with Grb2 in (hem) ITAM-stimulated platelets, demonstrating that Grb2 forms part of the LAT signalosome (Figure 6A). Significantly, analysis of protein tyrosine phosphorylation of key components of the (hem)ITAM signaling cascade by Western blot analysis revealed that in Grb2−/− platelets phosphorylation of proteins located upstream of the LAT signalosome, most importantly the FcR γ-chain, and CLEC-2, respectively, and the tyrosine kinase Syk is maintained (Figure 6B and 6C; Online Figure IV). In contrast, phosphorylation of LAT at Y195, which is bound by Grb2 and contributes to PLCγ2 activation, and Y136, the high-affinity binding site for PLCγ,34 was strongly reduced in Grb2-deficient compared with wild-type platelets. This resulted in reduced phosphorylation of proteins that lie downstream of the LAT signalosome, including not only key effector proteins such as PLCγ2, but also the adapter SLP-76 and the guanine nucleotide exchange factor Vav3 (Figure 6B and 6C; Online Figure IV). These results position Grb2 as a central adapter molecule of the LAT signalosome in platelets, stabilizing the LAT complex by direct association, thereby enabling downstream signaling and platelet activation.
Figure 6. Defective immunoreceptor tyrosine-based activation motif–induced signal transduction in Grb2−/− platelets.
A, Washed platelets (5×108/mL) were stimulated with 10 μg/mL collagen-related peptide (CRP) or 300 nmol/L rhodocytin (RC) for 90 seconds and subsequently lysed with nonidet P-40 (NP-40) detergent. Growth factor receptor–bound protein 2 (Grb2) was immunoprecipitated, and proteins were separated by reducing SDS-PAGE (10%) and transferred to a polyvinylidene fluoride (PVDF) membrane. The membrane was probed with an anti-pTyr mAb (4G10) and reprobed with an anti–linker of activated T-cell (LAT) and anti-Grb2 antibody. B, Washed platelets (7×108/mL) from Grb2+/+ and Grb2−/− mice were stimulated with 0.5 μg/mL convulxin (CVX) under stirring conditions at 37°C. Aliquots were taken at the indicated time points and subsequently lysed with NP-40 detergent. Proteins were separated by reducing SDS-PAGE (10%), blotted on a PVDF membrane, and stained using the indicated phospho-specific antibodies. Staining of the respective nonphosphorylated proteins or actin served as loading controls. The result shown is representative of 3 individual experiments. C, Washed platelets (5×108/mL) were stimulated with 0.5 μg/mL CVX for the indicated time points and subsequently lysed with NP-40 detergent. Syk, LAT, SLP-76, Vav1, Vav3, and PLCγ2 were immunoprecipitated, and proteins were separated by reducing SDS-PAGE (10%) and transferred to a PVDF membrane. The membrane was probed with an anti-pTyr mAb (4G10) and reprobed with Syk, LAT, SLP-76, Vav1, Vav3, and PLCγ2 antibodies.
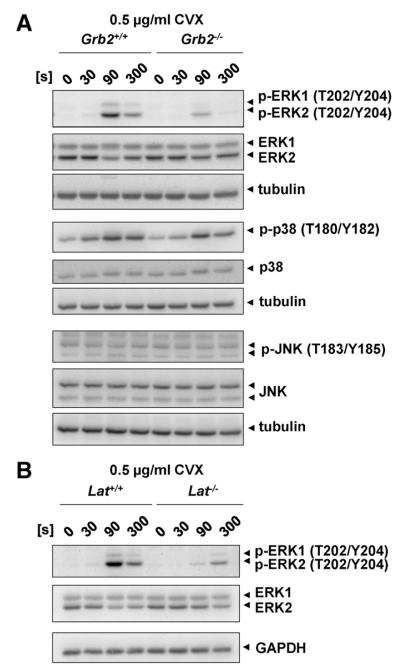
Furthermore, we analyzed Ras-MAPK signaling because Grb2 has initially been identified as a major mediator of this signaling pathway induced by numerous receptors,8 but the exact function of MAPK signaling in platelets is still not fully understood.35 Interestingly, phosphorylation of extracellular signal–regulated protein kinase 1/2 (ERK1/2) in response to convulxin was severely reduced in Grb2−/− platelets compared with wild-type platelets, whereas phosphorylation of p38 was unaltered (Figure 7A). To reveal how Grb2 regulates ERK1/2 phosphorylation, we analyzed MAPK signaling in LAT-deficient platelets, demonstrating a comparable reduction in ERK1/2 phosphorylation after GPVI stimulation (Figure 7B), indicating that Grb2-dependent recruitment of SOS1 to the LAT signalosome seems to be required for full ERK1/2 activation.15 Alternatively, loss of ERK1/2 phosphorylation might be a result of impaired activation by protein kinase C, which lies downstream of PLCγ2 and is critical for the activation of MAPK signaling in platelets.36
Figure 7. Defective extracellular signal–regulated protein kinase 1/2 (ERK1/2) signaling in Grb2−/− and Lat−/− platelets.
Determination of mitogen-activated protein kinase (MAPK) phosphorylation. Washed platelets (5×108/mL) from Grb2+/+ and Grb2−/− mice (A) or Lat+/+ and Lat−/− mice (B) were stimulated with 0.5 μg/mL convulxin (CVX) under stirring conditions at 37°C. Aliquots were taken at the indicated time points and subsequently lysed with NP-40 detergent. Proteins were separated by reducing SDS-PAGE (10%), blotted on a PVDF membrane, and stained using the indicated phospho-specific antibodies. Staining of the respective nonphosphorylated proteins, GAPDH or tubulin served as loading controls. The result shown is representative of 3 individual experiments.
Discussion
In this study, we have shown that Grb2-deficient platelets display severely impaired (hem)ITAM signaling but unaltered G protein–coupled receptor function and integrin outside-in signaling. This highly specific defect resulted in impaired hemostasis and partially defective arterial thrombus formation, indicating that Grb2-dependent signaling in platelets may contribute to the pathogenesis of acute ischemic disease states.
Grb2 is widely expressed throughout the body and associates with a large variety of receptors and signaling pathways in different cell types.9 Its physiological importance is best highlighted by the early embryonic lethality of Grb2−/− mice because of defective differentiation of endodermal cells and formation of the epiblast.11 Grb2 is also strongly expressed in the hematopoietic system, and a previous study suggested a role of the Grb2/SOS1/mitogen-activated protein kinase/ERK kinase complex in thrombopoietin receptor (c-Mpl) signaling during MK maturation.37 We found, however, normal platelet counts and bone marrow MK numbers in mice with an MK-specific Grb2-deficiency, demonstrating that Grb2 is dispensable for megakaryopoiesis and platelet production in vivo. This might be explained by a compensatory alternative pathway downstream of c-Mpl involving the small GTPase Rap1,18 but further studies will be required to address this in detail.
Grb2 has been implicated in antigen receptor signaling in lymphocytes, with recent reports showing prominent roles of the adapter during B cell maturation by negatively regulating proximal BCR signaling16,17 and a crucial function in T cell selection by amplifying TCR signaling.14,38 Similar to the BCR and TCR complexes, the GPVI/FcR γ-chain complex and CLEC-2 in platelets use a (hem)ITAM signaling module to transduce extracellular signals, thereby mediating platelet activation, aggregation, and thrombus formation. Previous studies in human platelets demonstrated that Grb2 binds to the membrane-bound adapter molecule LAT20 and that it is phosphorylated after GPVI stimulation,21 indicating a role of Grb2 in ITAM signaling in platelets. However, given the opposing effects of Grb2-deficiency in B and T cells, it was not clear what the exact function of the adapter in platelets would be. We found markedly impaired integrin αIIbβ3 activation, degranulation, and aggregation of Grb2−/− platelets in response to GPVI or CLEC-2 stimulation, which phenocopies the defects seen in LAT-deficient mice,22 although to a somewhat lesser extent (Figure 1). Therefore, like in T cells, Grb2 acts as a positive regulator of (hem)ITAM receptor–induced cellular activation.
Compared with immune receptor stimulation, agonist-induced platelet activation requires an increase in [Ca]2+i that is mainly triggered by PLC-mediated inositol 1,4,5-triphosphate production, resulting in Ca2+ store release and subsequent Ca2+ entry.28 The tyrosine phosphorylation cascade leading to PLCγ2 activation shows striking similarities among the GPVI, the BCR, and the TCR, involving phosphorylation of accessory receptor chains, activity of Src and Syk family kinases, and involvement of various adapter proteins, such as SLP-65/SLP-76 and LAT.3,13 Our data clearly show that Grb2 function in platelets resembles its function in TCR signaling in thymocytes, in that in both cell types deletion of Grb2 results in impaired Ca2+ signaling (Figure 3),14 in contrast to B cells in which Ca2+ influx is enhanced in the absence of the adapter.16,17 However, although in thymocytes Grb2-deficiency resulted in defects at the proximal end and at the level of the LAT signalosome of the TCR signaling cascade,14,15 in platelets Grb2 seems to only act downstream of platelet GPVI at the level of the LAT signalosome. Our data demonstrate that Grb2 interacts with LAT after (hem)ITAM stimulation and that in the absence of Grb2 tyrosine phosphorylation of LAT at Y136 and Y195 (corresponding to LAT Y132 and Y191 in humans, respectively) is reduced (Figures 6 and 7). Grb2 strongly associates via its SH2 domains with 3 phosphotyrosines of LAT (Y171, Y191, Y226),39,40 whereas PLCγ preferentially binds at Y132.39,41 Previous studies in T-cell lines have shown that mutations of the Gads/Grb2-binding residues of LAT (Y171, Y191, Y226) result in reduced binding of PLCγ1 to LAT and downstream signaling events, including tyrosine phosphorylation of the phospholipase.39,41,42 Therefore, a concept of cooperative binding among LAT-associated proteins that stabilize the signaling complex has been proposed.15,34 In line with this, Grb2-deficient platelets display reduced tyrosine phosphorylation of the key downstream signaling molecules PLCγ2, SLP-76, and Vav3 after GPVI and CLEC-2 stimulation, whereas tyrosine phosphorylation of the FcR γ-chain, CLEC-2, and Syk is maintained (Figure 6; Online Figure IV). Taken together, our present observations emphasize that Grb2 is an important adapter protein of the LAT signalosome, which stabilizes this signaling complex by its direct association after (hem)ITAM-induced stimulation, thereby enabling downstream signaling and platelet activation.
The residual phosphorylation of LAT and PLCγ2 in the absence of Grb2 implicates that other proteins may partially compensate its loss. One possible candidate is the adapter protein Gads, a second member of the Grb2 family, which has been shown to play a supportive but not essential role in GPVI- and CLEC-2–mediated platelet activation.22 Based on our data, we speculate that Grb2 is the major adapter protein in platelets stabilizing the LAT signalosome, thereby activating PLCγ2, whereas Gads stabilizes SLP-76 binding to support platelet activation in response to weak (hem)ITAM stimuli. This might also explain the less pronounced hem(ITAM) signaling defect in Grb2−/− mice compared with Lat−/− mice.
Besides LAT, further interaction partners of Grb2 are known (http://plateletweb.bioapps.biozentrum.uni-wuerzburg.de/plateletweb.php).43 Thus, it is feasible that Grb2 also exerts functions beyond LAT-dependent signaling. Subtle effects on other signaling pathways may have been masked by the pronounced hem(ITAM) signaling defect that arose from the deletion of the protein. With regard to human platelets, it is conceivable that Grb2 may contribute to the signaling pathway of the ITAM-bearing platelet Fc receptor, FcγRIIa.44,45 However, FcγRIIa is not expressed in murine platelets, demonstrating a principal limitation of mouse studies. Certainly, further investigations involving mouse models with distinct mutations of Grb2 are necessary to explicitly address this point.
The GPVI signaling defect in Grb2−/− platelets translated into severely impaired platelet adhesion, aggregate formation, and procoagulant activity on collagen under flow in vitro. A similar phenotype has been reported in mouse lines lacking LAT22 and Rac1,31 indicating a prominent role for Grb2 in thrombus formation by linking signaling molecules within the LAT signalosome. However, we found unaltered platelet spreading on fibrinogen and normal clot retraction in the absence of Grb2 (Figure 2), excluding a major function of the adapter in integrin outside-in signaling. This stands in contrast to a previous report suggesting a role of Grb2 in this process in human platelets because of its induced association with the adapter proteins Dok-1 and Dok-323 and might be explained by the fact that Dok-3 is not expressed in mouse platelets.
Platelets express 3 different members of MAPK family: p38, c-Jun N-terminal kinase, and ERK1/2.35 In our studies, we found that Grb2 is a positive regulator of ERK1/2 signaling and that p38 was not affected by the loss of Grb2 (we did not see activation of c-Jun N-terminal kinase in wild-type platelets). Furthermore, our data demonstrate that Grb2 mediates ERK1/2 activation in an LAT-dependent manner (Figure 7). This can be explained by recruitment of SOS1 to the LAT–Grb2 complex,15 which in turn activates the Ras-ERK1/2 signaling pathway. The presence of SOS1 in platelets has been described.46 ERK1/2, however, is also regulated downstream of protein kinase C in platelets,36 and this would also be disrupted in Grb2-deficient platelets through loss of PLCγ2 activation. Interestingly, activation of ERK1/2 is delayed relative to protein kinase C,36 suggesting that this alone is not sufficient to activate the MAPK pathway, leading us to speculate that this delay is as a result of assembly of the LAT-Grb2-SOS1 signaling complex. Regardless of the mechanism, the exact function of MAPKs in platelets is controversially discussed, possibly because of off-target effects of MAPK inhibitors, but increasing evidence suggests a role in promoting thrombus formation.35 Interestingly, Mazharian et al47 have previously shown that treatment of platelets with an ERK1/2 inhibitor resulted in decreased platelet adhesion to collagen under high shear flow. Based on this observation, it seems possible that the impaired adhesion and thrombus formation of Grb2−/− platelets may, in part, be a consequence of impaired ERK1/2 signaling.
GPVI-deficient mice are protected from pathological thrombus formation but display only mildly prolonged bleeding times.32 In contrast, although LAT deficiency is also protective in experimental thrombosis models, it additionally causes a significant hemostatic defect.30 We found that Grb2−/− mice, similar to Lat−/− mice, display highly variable and overall prolonged bleeding times, indicating that Grb2 has an important functional role downstream of GPVI and CLEC-2 in normal hemostasis. Surprisingly, however, we found that Grb2-deficiency alone was not protective in models of arterial thrombosis, suggesting that other signaling pathways can fully compensate the partial loss of hem(ITAM) signaling in this setting. We have previously shown that defective GPVI signaling can be compensated by TxA2-mediated activation of integrin α2β1 via Gq/G13-induced signaling pathways, thereby enabling platelets to arrest on collagen and to reinforce activation through outside-in signals.33,48 In line with this report, we found that ASA treatment (1 mg/kg) markedly reduced or delayed occlusive thrombus formation in Grb2−/− mice, whereas it had no significant effect in wild-type mice. These results indicate that the residual hem(ITAM) signaling capacity in Grb2−/− platelets is sufficient to trigger arterial thrombus formation by TxA2-mediated reinforcement of platelet activation, whereas hemostasis is affected in the absence of Grb2, independent of ASA treatment.
In summary, our data demonstrate that Grb2 is dispensable for platelet formation but plays an important role in platelet activation in hemostasis and thrombosis by coordinating and stabilizing the formation of the LAT signalosome after GPVI/CLEC-2 receptor stimulation.
Supplementary Material
Online Table I. Levels of platelet glycoproteins in Grb2+/+ and Grb2−/− mice. Expression of glycoproteins on the platelet surface was determined by flow cytometry. Diluted whole blood from the indicated mice was incubated with FITC-labeled antibodies at saturating conditions for 15 minutes at RT, and platelets were analyzed directly. Data are expressed as mean fluorescence intensity ± SD (n=4) and are representative of 3 individual experiments.
Online Figure I. Specific deletion of Grb2 in platelets. (A) Analysis of Grb2 expression in Grb2+/+, Grb2+/− and Grb2−/− platelets and splenocytes by Western Blot. Expression of GPIIIa and actin were used as loading control. (B) Peripheral platelet counts and (C) platelet volume of wild-type and Grb2−/− mice measured with a blood cell counter are depicted. Results are mean ± SD of 7 mice per group. (D) Determination of MK numbers per visual field (294 × 221 μm) in hematoxylin and eosin stained BM sections. Values are mean ± SD (n ≥ 3).
Online Figure II. Normal integrin outside-in signaling in Grb2−/− platelets. Washed platelets of Grb2+/+ and Grb2−/− mice were allowed to spread on fibrinogen (100 μg/ml) for 30 minutes in the presence of high dose apyrase (2 U/mL) and indomethacin (1.4 μM) either without stimulaton (A) or after simultaneous stimulation with 0.01 U/ml thrombin (B). Representative differential interference contrast (DIC) images of 2 individual experiments (left) and statistical evaluation of the percentage of spread platelets at different spreading stages (right). 1: roundish, 2: only filopodia, 3: filopodia and lamellipodia, 4: fully spread.
Online Figure III. Thrombosis model of FeCl3-induced injury of mesenteric arterioles. Small mesenteric arterioles were injured by topical application of FeCl3, and thrombus formation of fluorescently labeled platelets was monitored using intravital microscopy. Time to occlusion in the presence or absence of ASA is depicted. Each symbol represents one arteriole. ** P < 0.01.
Online Figure IV. Defective hemITAM-induced signal transduction in Grb2−/− platelets. (A) Washed platelets (7 × 105/μL) from Grb2+/+ and Grb2−/− mice were stimulated with 2 μg/ml rhodocytin (RC) under stirring conditions at 37 °C. Aliquots were taken at the indicated time points and subsequently lysed with NP-40 detergent. Proteins were separated by reducing SDS-PAGE (10%), blotted on a PVDF membrane, and stained using the indicated phospho-specific antibodies. Staining of the respective non-phosphorylated proteins or actin served as loading controls. The result shown is representative for three individual experiments. (B) Washed platelets (5 × 105/μL) were stimulated with 2 μg/ml RC for the indicated time points and subsequently lysed with NP-40 detergent. Syk, LAT, SLP-76, Vav1, Vav3, and PLCγ2, were immunoprecipitated and proteins were separated by reducing SDS-PAGE (10%) and transferred to a PVDF membrane. The membrane was probed with an anti-pTyr mAb (4G10), and reprobed with Syk, LAT, SLP-76, Vav1, Vav3, and PLCγ2 antibodies.
Novelty and Significance.
What Is Known?
Growth factor receptor–bound protein 2 (Grb2) is a ubiquitously expressed adapter protein involved in signaling processes of several cell surface receptors, including the immunoreceptor tyrosine-based activation motif (ITAM)–bearing complexes of the T cell and B cell receptors.
The major platelet-activating collagen receptor glycoprotein VI (GPVI) and the C-type lectin-like receptor 2 (CLEC-2) trigger platelet activation via (hem)ITAM signaling.
Grb2 is expressed in platelets and suggested to be involved in (1) signaling of GPVI, (2) integrin outside-in signaling, and (3) platelet production.
What New Information Does This Article Contribute?
Loss of Grb2 impairs (hem)ITAM signaling downstream of GPVI and CLEC-2, but it is dispensable for G protein–coupled receptor signaling, integrin outside-in signaling, and platelet production.
This selective (hem)ITAM signaling defect impairs hemostasis, whereas thrombus formation in vivo can be compensated by other signaling pathways.
Grb2 stabilizes the linker of activated T cell (LAT) signalosome, a protein complex required for signal propagation after (hem)ITAM stimulation.
Stimulation of the major platelet-activating collagen receptor GPVI at sites of vascular injury induces a signaling pathway that culminates in platelet activation required for subsequent platelet aggregation and thrombus development. The formation of a signaling complex called LAT signalosome is central for proper signal transduction of (hem)ITAM-bearing receptors. This signalosome comprises several different adapter and effector proteins, including Grb2, but its exact function in platelets was entirely speculative. By using a genetic mouse model with a megakaryocyte/platelet-specific deletion of Grb2, we demonstrate that Grb2 contributes to the stabilization of this signaling complex. Consequently, platelet activation induced by GPVI and CLEC-2, a related (hem)ITAM receptor expressed in platelets, is strongly impaired by the loss Grb2, resulting in reduced adhesion and aggregation on collagen under flow in vitro. In contrast, the signaling pathways of G protein–coupled receptors that do not use the LAT signalosome were unaffected. In vivo, the impaired (hem)ITAM signaling causes prolonged bleeding times of mice, whereas thrombus formation is only affected under conditions of impaired G protein–coupled receptor signaling (eg, aspirin treatment). These results reveal an important role for the adapter protein Grb2 in propagation of GPVI- and CLEC-2–induced signals, which could be relevant during treatment with certain medications.
Acknowledgments
We thank Sylvia Hengst for excellent technical assistance.
Sources of Funding This work was supported by the Deutsche Forschungsgemeinschaft (grant Ni556/10-1 to B. Nieswandt and Sonderforschungsbereich [SFB] 688).
Nonstandard Abbreviations and Acronyms
- ASA
acetylsalicylic acid
- CLEC-2
C-type lectin-like receptor 2
- CRP
collagen-related peptide
- ERK1/2
extracellular signal–regulated protein kinase 1/2
- FcR
Fc receptor
- GPVI
glycoprotein VI
- Grb2
growth factor receptor–bound protein 2
- ITAM
immunoreceptor tyrosine-based activation motif
- LAT
linker of activated T cell
- MAPK
mitogen-activated protein kinase
- PLC
phospholipase C
- SH
Src homology
- SLP
Src homology domain–containing leukocyte protein
- SOS1
son of sevenless homolog
- TxA2
thromboxane A2
Footnotes
Disclosures None.
The online-only Data Supplement is available with this article at http://circres.ahajournals.org/lookup/suppl/doi:10.1161/CIRCRESAHA.114.302670/-/DC1.
References
- 1.Nieswandt B, Pleines I, Bender M. Platelet adhesion and activation mechanisms in arterial thrombosis and ischaemic stroke. J Thromb Haemost. 2011;9(Suppl 1):92–104. doi: 10.1111/j.1538-7836.2011.04361.x. [DOI] [PubMed] [Google Scholar]
- 2.Clemetson JM, Polgar J, Magnenat E, Wells TN, Clemetson KJ. The platelet collagen receptor glycoprotein VI is a member of the immunoglobulin superfamily closely related to FcalphaR and the natural killer receptors. J Biol Chem. 1999;274:29019–29024. doi: 10.1074/jbc.274.41.29019. [DOI] [PubMed] [Google Scholar]
- 3.Dütting S, Bender M, Nieswandt B. Platelet GPVI: a target for antithrombotic therapy?! Trends Pharmacol Sci. 2012;33:583–590. doi: 10.1016/j.tips.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 4.O’Callaghan CA. Thrombomodulation via CLEC-2 targeting. Curr Opin Pharmacol. 2009;9:90–95. doi: 10.1016/j.coph.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 5.May F, Hagedorn I, Pleines I, Bender M, Vögtle T, Eble J, Elvers M, Nieswandt B. CLEC-2 is an essential platelet-activating receptor in hemostasis and thrombosis. Blood. 2009;114:3464–3472. doi: 10.1182/blood-2009-05-222273. [DOI] [PubMed] [Google Scholar]
- 6.Abtahian F, Guerriero A, Sebzda E, Lu MM, Zhou R, Mocsai A, Myers EE, Huang B, Jackson DG, Ferrari VA, Tybulewicz V, Lowell CA, Lepore JJ, Koretzky GA, Kahn ML. Regulation of blood and lymphatic vascular separation by signaling proteins SLP-76 and Syk. Science. 2003;299:247–251. doi: 10.1126/science.1079477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Watson SP, Herbert JM, Pollitt AY. GPVI and CLEC-2 in hemostasis and vascular integrity. J Thromb Haemost. 2010;8:1456–1467. doi: 10.1111/j.1538-7836.2010.03875.x. [DOI] [PubMed] [Google Scholar]
- 8.Lowenstein EJ, Daly RJ, Batzer AG, Li W, Margolis B, Lammers R, Ullrich A, Skolnik EY, Bar-Sagi D, Schlessinger J. The SH2 and SH3 domain-containing protein GRB2 links receptor tyrosine kinases to ras signaling. Cell. 1992;70:431–442. doi: 10.1016/0092-8674(92)90167-b. [DOI] [PubMed] [Google Scholar]
- 9.Jang IK, Zhang J, Gu H. Grb2, a simple adapter with complex roles in lymphocyte development, function, and signaling. Immunol Rev. 2009;232:150–159. doi: 10.1111/j.1600-065X.2009.00842.x. [DOI] [PubMed] [Google Scholar]
- 10.Neumann K, Oellerich T, Urlaub H, Wienands J. The B-lymphoid Grb2 interaction code. Immunol Rev. 2009;232:135–149. doi: 10.1111/j.1600-065X.2009.00845.x. [DOI] [PubMed] [Google Scholar]
- 11.Cheng AM, Saxton TM, Sakai R, Kulkarni S, Mbamalu G, Vogel W, Tortorice CG, Cardiff RD, Cross JC, Muller WJ, Pawson T. Mammalian Grb2 regulates multiple steps in embryonic development and malignant transformation. Cell. 1998;95:793–803. doi: 10.1016/s0092-8674(00)81702-x. [DOI] [PubMed] [Google Scholar]
- 12.Rozakis-Adcock M, Fernley R, Wade J, Pawson T, Bowtell D. The SH2 and SH3 domains of mammalian Grb2 couple the EGF receptor to the Ras activator mSos1. Nature. 1993;363:83–85. doi: 10.1038/363083a0. [DOI] [PubMed] [Google Scholar]
- 13.Leo A, Wienands J, Baier G, Horejsi V, Schraven B. Adapters in lymphocyte signaling. J Clin Invest. 2002;109:301–309. doi: 10.1172/JCI14942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jang IK, Zhang J, Chiang YJ, Kole HK, Cronshaw DG, Zou Y, Gu H. Grb2 functions at the top of the T-cell antigen receptor-induced tyrosine kinase cascade to control thymic selection. Proc Natl Acad Sci U S A. 2010;107:10620–10625. doi: 10.1073/pnas.0905039107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houtman JC, Yamaguchi H, Barda-Saad M, Braiman A, Bowden B, Appella E, Schuck P, Samelson LE. Oligomerization of signaling complexes by the multipoint binding of GRB2 to both LAT and SOS1. Nat Struct Mol Biol. 2006;13:798–805. doi: 10.1038/nsmb1133. [DOI] [PubMed] [Google Scholar]
- 16.Ackermann JA, Radtke D, Maurberger A, Winkler TH, Nitschke L. Grb2 regulates B-cell maturation, B-cell memory responses and inhibits B-cell Ca2+ signalling. EMBO J. 2011;30:1621–1633. doi: 10.1038/emboj.2011.74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jang IK, Cronshaw DG, Xie LK, Fang G, Zhang J, Oh H, Fu YX, Gu H, Zou Y. Growth-factor receptor-bound protein-2 (Grb2) signaling in B cells controls lymphoid follicle organization and germinal center reaction. Proc Natl Acad Sci U S A. 2011;108:7926–7931. doi: 10.1073/pnas.1016451108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Garcia J, de Gunzburg J, Eychène A, Gisselbrecht S, Porteu F. Thrombopoietin-mediated sustained activation of extracellular signal-regulated kinase in UT7-Mpl cells requires both Ras-Raf-1- and Rap1-B-Raf-dependent pathways. Mol Cell Biol. 2001;21:2659–2670. doi: 10.1128/MCB.21.8.2659-2670.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsumura I, Nakajima K, Wakao H, Hattori S, Hashimoto K, Sugahara H, Kato T, Miyazaki H, Hirano T, Kanakura Y. Involvement of prolonged ras activation in thrombopoietin-induced megakaryocytic differentiation of a human factor-dependent hematopoietic cell line. Mol Cell Biol. 1998;18:4282–4290. doi: 10.1128/mcb.18.7.4282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Asazuma N, Wilde JI, Berlanga O, Leduc M, Leo A, Schweighoffer E, Tybulewicz V, Bon C, Liu SK, McGlade CJ, Schraven B, Watson SP. Interaction of linker for activation of T cells with multiple adapter proteins in platelets activated by the glycoprotein VI-selective ligand, convulxin. J Biol Chem. 2000;275:33427–33434. doi: 10.1074/jbc.M001439200. [DOI] [PubMed] [Google Scholar]
- 21.García A, Senis YA, Antrobus R, Hughes CE, Dwek RA, Watson SP, Zitzmann N. A global proteomics approach identifies novel phosphorylated signaling proteins in GPVI-activated platelets: involvement of G6f, a novel platelet Grb2-binding membrane adapter. Proteomics. 2006;6:5332–5343. doi: 10.1002/pmic.200600299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hughes CE, Auger JM, McGlade J, Eble JA, Pearce AC, Watson SP. Differential roles for the adapters Gads and LAT in platelet activation by GPVI and CLEC-2. J Thromb Haemost. 2008;6:2152–2159. doi: 10.1111/j.1538-7836.2008.03166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Senis YA, Antrobus R, Severin S, Parguiña AF, Rosa I, Zitzmann N, Watson SP, García A. Proteomic analysis of integrin alphaIIbbeta3 outside-in signaling reveals Src-kinase-independent phosphorylation of Dok-1 and Dok-3 leading to SHIP-1 interactions. J Thromb Haemost. 2009;7:1718–1726. doi: 10.1111/j.1538-7836.2009.03565.x. [DOI] [PubMed] [Google Scholar]
- 24.Tiedt R, Schomber T, Hao-Shen H, Skoda RC. Pf4-Cre transgenic mice allow the generation of lineage-restricted gene knockouts for studying megakaryocyte and platelet function in vivo. Blood. 2007;109:1503–1506. doi: 10.1182/blood-2006-04-020362. [DOI] [PubMed] [Google Scholar]
- 25.Shattil SJ, Newman PJ. Integrins: dynamic scaffolds for adhesion and signaling in platelets. Blood. 2004;104:1606–1615. doi: 10.1182/blood-2004-04-1257. [DOI] [PubMed] [Google Scholar]
- 26.Mahankali M, Peng HJ, Cox D, Gomez-Cambronero J. The mechanism of cell membrane ruffling relies on a phospholipase D2 (PLD2), Grb2 and Rac2 association. Cell Signal. 2011;23:1291–1298. doi: 10.1016/j.cellsig.2011.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Morgenstern E, Ruf A, Patscheke H. Ultrastructure of the interaction between human platelets and polymerizing fibrin within the first minutes of clot formation. Blood Coagul Fibrinolysis. 1990;1:543–546. doi: 10.1097/00001721-199010000-00035. [DOI] [PubMed] [Google Scholar]
- 28.Varga-Szabo D, Braun A, Nieswandt B. STIM and Orai in platelet function. Cell Calcium. 2011;50:270–278. doi: 10.1016/j.ceca.2011.04.002. [DOI] [PubMed] [Google Scholar]
- 29.Renné T. The procoagulant and proinflammatory plasma contact system. Semin Immunopathol. 2012;34:31–41. doi: 10.1007/s00281-011-0288-2. [DOI] [PubMed] [Google Scholar]
- 30.Kalia N, Auger JM, Atkinson B, Watson SP. Critical role of FcR gamma-chain, LAT, PLCgamma2 and thrombin in arteriolar thrombus formation upon mild, laser-induced endothelial injury in vivo. Microcirculation. 2008;15:325–335. doi: 10.1080/10739680701728822. [DOI] [PubMed] [Google Scholar]
- 31.Pleines I, Elvers M, Strehl A, Pozgajova M, Varga-Szabo D, May F, Chrostek-Grashoff A, Brakebusch C, Nieswandt B. Rac1 is essential for phospholipase C-gamma2 activation in platelets. Pflugers Arch. 2009;457:1173–1185. doi: 10.1007/s00424-008-0573-7. [DOI] [PubMed] [Google Scholar]
- 32.Bender M, Hagedorn I, Nieswandt B. Genetic and antibody-induced glycoprotein VI deficiency equally protects mice from mechanically and FeCl(3) -induced thrombosis. J Thromb Haemost. 2011;9:1423–1426. doi: 10.1111/j.1538-7836.2011.04328.x. [DOI] [PubMed] [Google Scholar]
- 33.Grüner S, Prostredna M, Aktas B, Moers A, Schulte V, Krieg T, Offermanns S, Eckes B, Nieswandt B. Anti-glycoprotein VI treatment severely compromises hemostasis in mice with reduced alpha2beta1 levels or concomitant aspirin therapy. Circulation. 2004;110:2946–2951. doi: 10.1161/01.CIR.0000146341.63677.3C. [DOI] [PubMed] [Google Scholar]
- 34.Balagopalan L, Coussens NP, Sherman E, Samelson LE, Sommers CL. The LAT story: a tale of cooperativity, coordination, and choreography. Cold Spring Harb Perspect Biol. 2010;2:a005512. doi: 10.1101/cshperspect.a005512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Adam F, Kauskot A, Rosa JP, Bryckaert M. Mitogen-activated protein kinases in hemostasis and thrombosis. J Thromb Haemost. 2008;6:2007–2016. doi: 10.1111/j.1538-7836.2008.03169.x. [DOI] [PubMed] [Google Scholar]
- 36.Börsch-Haubold AG, Kramer RM, Watson SP. Cytosolic phospholipase A2 is phosphorylated in collagen- and thrombin-stimulated human platelets independent of protein kinase C and mitogen-activated protein kinase. J Biol Chem. 1995;270:25885–25892. doi: 10.1074/jbc.270.43.25885. [DOI] [PubMed] [Google Scholar]
- 37.Séverin S, Ghevaert C, Mazharian A. The mitogen-activated protein kinase signaling pathways: role in megakaryocyte differentiation. J Thromb Haemost. 2010;8:17–26. doi: 10.1111/j.1538-7836.2009.03658.x. [DOI] [PubMed] [Google Scholar]
- 38.Gong Q, Cheng AM, Akk AM, Alberola-Ila J, Gong G, Pawson T, Chan AC. Disruption of T cell signaling networks and development by Grb2 haploid insufficiency. Nat Immunol. 2001;2:29–36. doi: 10.1038/83134. [DOI] [PubMed] [Google Scholar]
- 39.Zhang W, Trible RP, Zhu M, Liu SK, McGlade CJ, Samelson LE. Association of Grb2, Gads, and phospholipase C-gamma 1 with phosphor-ylated LAT tyrosine residues. Effect of LAT tyrosine mutations on T cell angigen receptor-mediated signaling. J Biol Chem. 2000;275:23355–23361. doi: 10.1074/jbc.M000404200. [DOI] [PubMed] [Google Scholar]
- 40.Zhu M, Janssen E, Zhang W. Minimal requirement of tyrosine residues of linker for activation of T cells in TCR signaling and thymocyte development. J Immunol. 2003;170:325–333. doi: 10.4049/jimmunol.170.1.325. [DOI] [PubMed] [Google Scholar]
- 41.Paz PE, Wang S, Clarke H, Lu X, Stokoe D, Abo A. Mapping the Zap-70 phosphorylation sites on LAT (linker for activation of T cells) required for recruitment and activation of signalling proteins in T cells. Biochem J. 2001;356:461–471. doi: 10.1042/0264-6021:3560461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Hartgroves LC, Lin J, Langen H, Zech T, Weiss A, Harder T. Synergistic assembly of linker for activation of T cells signaling protein complexes in T cell plasma membrane domains. J Biol Chem. 2003;278:20389–20394. doi: 10.1074/jbc.M301212200. [DOI] [PubMed] [Google Scholar]
- 43.Boyanova D, Nilla S, Birschmann I, Dandekar T, Dittrich M. PlateletWeb: a systems biologic analysis of signaling networks in human platelets. Blood. 2012;119:e22–e34. doi: 10.1182/blood-2011-10-387308. [DOI] [PubMed] [Google Scholar]
- 44.Boylan B, Gao C, Rathore V, Gill JC, Newman DK, Newman PJ. Identification of FcgammaRIIa as the ITAM-bearing receptor mediating alphaIIbbeta3 outside-in integrin signaling in human platelets. Blood. 2008;112:2780–2786. doi: 10.1182/blood-2008-02-142125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhi H, Rauova L, Hayes V, Gao C, Boylan B, Newman DK, McKenzie SE, Cooley BC, Poncz M, Newman PJ. Cooperative integrin/ITAM signaling in platelets enhances thrombus formation in vitro and in vivo. Blood. 2013;121:1858–1867. doi: 10.1182/blood-2012-07-443325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Robinson A, Gibbins J, Rodríguez-Liñares B, Finan PM, Wilson L, Kellie S, Findell P, Watson SP. Characterization of Grb2-binding proteins in human platelets activated by Fc gamma RIIA cross-linking. Blood. 1996;88:522–530. [PubMed] [Google Scholar]
- 47.Mazharian A, Roger S, Maurice P, Berrou E, Popoff MR, Hoylaerts MF, Fauvel-Lafeve F, Bonnefoy A, Bryckaert M. Differential Involvement of ERK2 and p38 in platelet adhesion to collagen. J Biol Chem. 2005;280:26002–26010. doi: 10.1074/jbc.M414083200. [DOI] [PubMed] [Google Scholar]
- 48.Inoue O, Suzuki-Inoue K, Dean WL, Frampton J, Watson SP. Integrin alpha-2beta1 mediates outside-in regulation of platelet spreading on collagen through activation of Src kinases and PLCgamma2. J Cell Biol. 2003;160:769–780. doi: 10.1083/jcb.200208043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Online Table I. Levels of platelet glycoproteins in Grb2+/+ and Grb2−/− mice. Expression of glycoproteins on the platelet surface was determined by flow cytometry. Diluted whole blood from the indicated mice was incubated with FITC-labeled antibodies at saturating conditions for 15 minutes at RT, and platelets were analyzed directly. Data are expressed as mean fluorescence intensity ± SD (n=4) and are representative of 3 individual experiments.
Online Figure I. Specific deletion of Grb2 in platelets. (A) Analysis of Grb2 expression in Grb2+/+, Grb2+/− and Grb2−/− platelets and splenocytes by Western Blot. Expression of GPIIIa and actin were used as loading control. (B) Peripheral platelet counts and (C) platelet volume of wild-type and Grb2−/− mice measured with a blood cell counter are depicted. Results are mean ± SD of 7 mice per group. (D) Determination of MK numbers per visual field (294 × 221 μm) in hematoxylin and eosin stained BM sections. Values are mean ± SD (n ≥ 3).
Online Figure II. Normal integrin outside-in signaling in Grb2−/− platelets. Washed platelets of Grb2+/+ and Grb2−/− mice were allowed to spread on fibrinogen (100 μg/ml) for 30 minutes in the presence of high dose apyrase (2 U/mL) and indomethacin (1.4 μM) either without stimulaton (A) or after simultaneous stimulation with 0.01 U/ml thrombin (B). Representative differential interference contrast (DIC) images of 2 individual experiments (left) and statistical evaluation of the percentage of spread platelets at different spreading stages (right). 1: roundish, 2: only filopodia, 3: filopodia and lamellipodia, 4: fully spread.
Online Figure III. Thrombosis model of FeCl3-induced injury of mesenteric arterioles. Small mesenteric arterioles were injured by topical application of FeCl3, and thrombus formation of fluorescently labeled platelets was monitored using intravital microscopy. Time to occlusion in the presence or absence of ASA is depicted. Each symbol represents one arteriole. ** P < 0.01.
Online Figure IV. Defective hemITAM-induced signal transduction in Grb2−/− platelets. (A) Washed platelets (7 × 105/μL) from Grb2+/+ and Grb2−/− mice were stimulated with 2 μg/ml rhodocytin (RC) under stirring conditions at 37 °C. Aliquots were taken at the indicated time points and subsequently lysed with NP-40 detergent. Proteins were separated by reducing SDS-PAGE (10%), blotted on a PVDF membrane, and stained using the indicated phospho-specific antibodies. Staining of the respective non-phosphorylated proteins or actin served as loading controls. The result shown is representative for three individual experiments. (B) Washed platelets (5 × 105/μL) were stimulated with 2 μg/ml RC for the indicated time points and subsequently lysed with NP-40 detergent. Syk, LAT, SLP-76, Vav1, Vav3, and PLCγ2, were immunoprecipitated and proteins were separated by reducing SDS-PAGE (10%) and transferred to a PVDF membrane. The membrane was probed with an anti-pTyr mAb (4G10), and reprobed with Syk, LAT, SLP-76, Vav1, Vav3, and PLCγ2 antibodies.