Abstract
NADPH oxidases are a family of oxidases that utilize molecular oxygen to generate hydrogen peroxide and superoxide, thus indicating physiological functions of these highly reactive and short-lived species. The regulation of these NADPH oxidases (nox) enzymes is complex, with many members of this family exhibiting complexity in terms of subunit composition, cellular location, and tissue-specific expression. While the complexity of the nox family (Nox1–5, Duox1, 2) is daunting, the complexity also allows for targeting of NADPH oxidases in disease states. In this review, we discuss which inflammatory and malignant disorders can be targeted by nox inhibitors, as well as clinical experience in the use of such inhibitors.
Keywords: NADPH oxidase, Lymphoma, Melanoma, Hemangioma, Wilms tumor 1, Triphenylmethanes, Fulvenes
Introduction
NADPH oxidases are a diverse family of enzymes that give rise to superoxide and hydrogen peroxide, cellular second messengers that play critical roles in human pathology and pathophysiology. While the role of human NADPH oxidases was first elucidated in a deficiency syndrome, namely, chronic granulomatous disease, current information implicates excessive generation of reactive oxygen as a more important mechanism for inflammation and carcinogenesis. Firstly, the failure to downregulate reactive oxygen generation leads to persistent inflammation in virtually all organ systems. Secondly, persistent reactive oxygen generation is also carcinogenic, leading to a distinct tumor phenotype known as the reactive oxygen-driven tumor. Recognition of this phenotype is important because (1) it occurs in all organ systems and can be recognized epidemiologically and histologically and (2) tumors caused by reactive oxygen survive based upon reactive oxygen-driven signaling systems. The implications of these findings are that these tumors can be both prevented and treated by reactive oxygen inhibitors. Inflammatory disorders can similarly be treated by reactive oxygen inhibitors, and this may lead to prevention of neoplasms secondary to persistent inflammation. Thus, both common inflammatory and tumor systems adapt to inflammatory stress by adopting the signaling pathways involved in chronic inflammation, especially reactive oxygen-driven nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB) activation.
Identification of the reactive oxygen-driven phenotype
Initial identification of the role of reactive oxygen in neoplasia came from the pioneering studies of Warburg, who determined that advanced tumors used glycolysis as a method of generating ATP, even in the presence of adequate oxygen, and the studies of Carl Nathan, who observed high levels of reactive oxygen in melanoma cells [48, 54]. The Lambeth group identified additional members of the nox family and also demonstrated that overexpression of NADPH oxidase 1 (nox1) could contribute to angiogenesis and tumorigenesis [4]. Our interest in reactive oxygen stemmed from the different behavior of the three forms of endothelial neoplasms observed in humans. Hemangioma, which is the most common, is characterized by rapid growth, followed by regression [49]. Given the unique ability of hemangiomas to regress, we hypothesized that hemangiomas could be driven by reactive oxygen and that the sudden loss of reactive oxygen could mediate regression. Thus, hemangiomas could be an example of a reactive oxygen-driven tumor. Vascular malformations are characterized by this lack of regression and show instead continued growth with the growth of the patient [49]. Angiosarcoma, a malignant endothelial tumor, is characterized by defects in p53, especially mutant p53, and death due to invasion and distant metastasis [58]. We hypothesized that the distinct clinical behavior of these similarly appearing lesions was due to differences in in vivo signaling and subsequently created a model of angiosarcoma through the sequential introduction of a temperature-sensitive SV40 large T antigen and oncogenic H-ras [3]. Using this model, we demonstrated that Ras can upregulate vascular endothelial growth factor (VEGF) through a phosphatidylinositol-3 kinase-dependent pathway and that blockade of PI3 kinase leads to decreased tumor burden. Upon introducing a dominant negative MAP kinase kinase (MEKK) into these cells, soft agar growth was abolished, but the production of matrix metalloproteinases was paradoxically elevated. In vivo tumor growth was also enhanced, with increased lung metastasis [30]. This finding contradicted the dogma of MAP kinase activation as an oncogenic event. In order to reconcile our data with the data showing oncogenesis due to MAP kinase activation, we compared our system to the system in which MAP kinase was initially found to be oncogenic [15, 43]. MAP kinase activation is oncogenic in NIH3T3 fibroblasts, which are characterized by the loss of tumor suppressor p16ink4a. Our system, like human angiosarcoma, has defects in p53 signaling. We thus hypothesized that the loss of a tumor suppressor gene dictates the signaling pathways that occur in the tumors which develop as a consequence.
Support for a link between MAP kinase loss and p16 inactivation came from a carcinogenesis experiment in which nickel sulfide, a well-known reactive oxygen-generating carcinogen, was implanted into mouse muscle. Sarcomas grow out as a result of reactive oxygen-induced carcinogenesis, and in an analysis of these sarcomas, we observed hypermethylation of p16ink4a and MAP kinase activation, even in tumors arising in p53 heterozygous mice [24]. The conclusions we drew from this observation is that reactive oxygen-induced carcinogens cause hypermethylation of p16ink4a and MAP kinase activation.
While a deficiency in reactive oxygen is associated with inflammation, excessive reactive oxygen is a more common cause of inflammation [18]. Inflammation caused by excessive neutrophil infiltration, such as psoriasis, inflammatory bowel disease, and disorders of the inflammasome, are associated with elevated reactive oxygen [11, 27, 34, 35]. Similarly, disorders of lymphocytic inflammation, such as atopic dermatitis, persistent inflammation due to hepatitis B and C, schistosomiasis, lupus, pneumonitis, and forms of multiple sclerosis, can also be a source of reactive oxygen excess [5, 36, 44, 47].
Occurrence of the reactive oxygen-driven tumor in humans
Burkitts lymphoma is a high-grade lymphoma that is associated with Epstein–Barr virus (EBV) in Africa and in patients with immune disorders. It is associated with a characteristic immunoglobulin c-myc translocation and is classified as either epidemic (African) or sporadic. Of interest, virtually all patients with sporadic Burkitt’s lymphoma have mutant p53, while those with epidemic (EBV associated) have wild-type p53 and hypermethylation of p16ink4a [28]. In our study of Burkitt’s lymphoma, we found greatly elevated levels of reactive oxygen in EBV-associated lymphoma compared with Burkitt’s sporadic lymphoma [12], as well as increased activation of MAP kinase in the former compared with the latter. EBV infection induced reactive oxygen species production in lymphocytes, and reactive oxygen generation was dependent on autocrine interleukin (IL)-10 production, EBNA2 and LMP1 [12]. Consistent with our findings, in another study, reactive oxygen was found to induce DNA methyltransferase 1 (DNMT1), the enzyme primarily responsible for hypermethylation of p16ink4a [38].
Melanoma is a common solid tumor characterized by the loss of p16ink4a and MAP kinase activation. In fact, germline loss of p16ink4a is the most common cause of familial melanoma [55]. We found that MAP kinase is activated in 90 % of all melanomas, and not in atypical nevi [14]. The most common and second-most common oncogenic mutations in melanoma are Braf V600E and Nras, respectively, both of which activate MAP kinase [16]. We demonstrated that MAP kinase is transformed in melanocytes but that this transformation is not sufficient to fully recapitulate the fully malignant phenotype of melanoma, implying additional pathways [23]. In order to elucidate additional pathways, we transfected a constitutively activated Akt into an early-stage melanoma cell line, WM35. Introduction of active Akt into these cells led to a dramatic increase in reactive oxygen levels, especially superoxide, and an upregulation of VEGF. Blockade of Akt with a small molecule led to the downregulation of VEGF, rictor, an mTORC2 subunit responsible for rapamycin resistance, and SIRT1, which contributes to the immortalization of tumor cells [26]. Most importantly, introduction of Akt led to malignant transformation in vivo. Other research groups have also observed Akt amplification in melanoma and the expression of high levels of Akt, especially in brain metastasis [17]. One of the genes upregulated as a result of Akt expression is nox4, which other groups have described as also being expressed in melanoma.
The signaling pathway of hemangiomas was elucidated in part by studies of verruga peruana, an endothelial lesion induced by the bacterium Bartonella bacilliformis [13]. Histologically, verruga peruana is nearly histologically identical to hemangiomas and can therefore be confused with hemangiomas except for the presence of intracellular bacteria. Like hemangiomas, infection of primary endothelial cells with Bartonella leads to upregulation of angiopoietin-2 (Ang-2), accompanied by upregulation of the Rac signaling pathway [52]. Rac1 and 2 are components of NADPH oxidases, implying that hemangiomas are reactive oxygen-driven tumors. We tested the functionality of nox4 in a murine model of hemangiomas. Using an Ang trap, we first established that, like human hemangiomas, this model is Ang-2 dependent [41], and then we demonstrated that nox4 small interfering RNA (siRNA) resulted in a potent inhibition of tumor growth in vivo. Third, we synthesized a novel inhibitor of nox4, fulvene-5, that had potent inhibitory activity against bend3 tumors in mice. Fulvene 5 downregulated Notch-related ankyrin-related protein, indicating that the notch signaling pathway is downstream of reactive oxygen signaling [7].
Proof of principle of reactive oxygen inhibition in humans
In order to test the efficacy of NADPH oxidase inhibition in humans, we examined Federal Drug Administration-approved drugs that are structurally similar to diphenyleneiodonium, a well-established—albeit not entirely specific—NADPH oxidase inhibitor. We hypothesized that gentian violet and brilliant green would have similar activity to diphenyleneiodonium, in that these compounds have a central carbon with a cationic charge that can be delocalized [41]. Diphenyleneiodonium also has a central atom, in this case an iodine rather than a carbon. These cationic triphenylmethanes inhibit the production of reactive oxygen by both nox2 and nox4 and also inhibit the production of Ang-2 by bend3 hemangioma cells, causing the regression of experimental hemangiomas in mice. Given these findings, we were able to assess the effects of nox inhibitors in humans.
In a trial of infants with ulcerated hemangiomas, another triphenylmethane, eosin, was applied daily under occlusion for 1 month. This treatment resulted in the regression of these otherwise painful lesions, thus providing a safe and effective tumor treatment [31]. A second mode of treatment has also gained favor in the treatment of large infantile hemangiomas, namely propranolol, a beta adrenergic blocker. Its effectiveness was discovered by observing an infant with large hemangioma who received propranolol for the treatment of cardiac disease [33]. Surprisingly, the hemangioma of the infant regressed, leading to a clinical trial in which propranolol was demonstrated to cause the rapid regression of hemangiomas. Propranolol treatment usually requires that infants be hospitalized because of the side effects of the beta blockade, such as hypoglycemia, as well as the need for blood pressure monitoring. Interestingly, both the beta blocking active isoform of propranolol and the inactive isoform of propranolol have nox inhibitory activity, indicating that beta blockade may not be required for the activity of propranolol [42].
Growth factors induce constitutive reactive oxygen signaling
Many tumors, including hemangiomas, are thought to arise through uncontrolled proliferative responses to growth factors. In order to demonstrate this in human cells, we overexpressed platelet-derived growth factor BB (PDGF-BB) in SV7tert human angiomyolipoma cells, a model of tuberous sclerosis. SV7tert cells express the receptor for PDGF-BB, namely PDGFRβ [2]. Overexpression of PDGF-BB resulted in upregulation of VEGF and reactive oxygen, and in vivo transformation. Transformation was accompanied by the downregulation of p16ink4a [25]. Overexpression of Id-1, a transcriptional suppressor, accelerated in vivo transformation, indicating that the loss of p16ink4a is a necessary step to reactive oxygen-mediated transformation. The high levels of reactive oxygen caused oxidative inactivation of tyrosine phosphatases, including two putative tumor suppressors, PTEN and shp2 [9]. Reactive oxygen has also been shown to inactivate the tumor suppressor gene p53 and IκB-alpha. Thus, reactive oxygen signaling leads to the activation of Akt through the inactivation of PTEN, the activation of NFκB through the inactivation of IκB-alpha, and functional inactivation of p53. These are the hallmarks of the reactive oxygen-driven tumor [20]. In addition, inflammatory processes can use the same processes of NFκB activation, Akt activation, and functional inactivation of p53, without malignant transformation, similar to what has been observed in hemangiomas. Thus, both malignant and inflammatory processes can be targeted by reactive oxygen inhibitors, such as cationic triphenylmethanes and fulvenes.
Recognition of the reactive oxygen-driven phenotype in clinical practice
In order to fully target the reactive oxygen-driven phenotype, one must be able to identify it in a paraffin section. Certain tumors, such as melanoma, glioblastoma multiforme, and pancreatic cancer, have a preponderance of p16ink4a loss. However, epidemiologic data is not sufficient to allow for clinical decisions to be made for an individual patient. One marker that might have utility in this respect is cytoplasmic Wilms tumor 1 (WT1). Our initial interest in WT1 is based on our discovery that WT1 is highly expressed in hemangiomas but not in vascular malformations [32]. This was recently confirmed in a larger study. We also observed the expression of WT1 in melanoma but not in atypical nevi [1, 51]. Cytoplasmic WT1 has been shown to bind to actin and may contribute to invadopodia [19], and the loss of WT1 has been associated with a greatly decreased ability for Ras to induce tumorigenesis [53]. Thus, WT1 may both be a valuable histological marker of reactive oxygen-driven processes, as well as a pharmacologic target, as WT1, by binding actin, may regulate the F actin to G actin transition to cause tumor invasion (manuscript in preparation).
Algorithm for clinical use
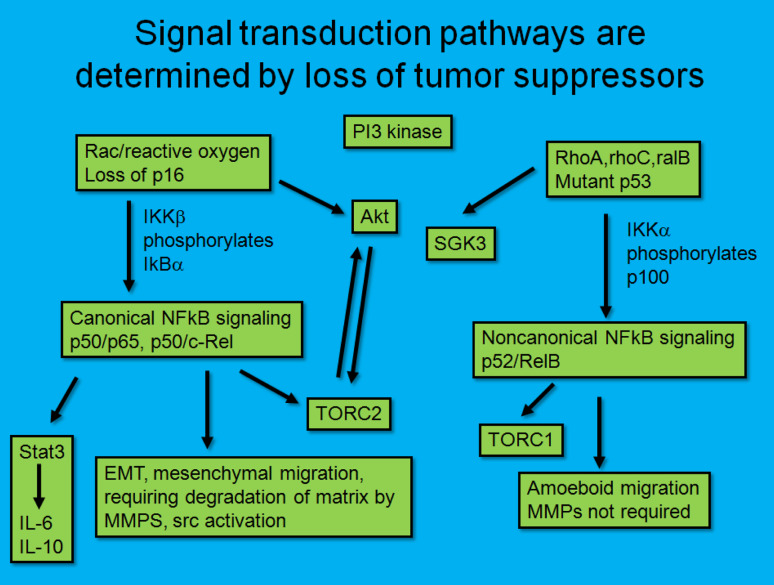
While gene arrays are highly popular, their expense currently prevents widespread use as a clinical tool. In addition, the relatively inexpensive methods of immunohistochemistry on formalin-fixed sections allows spatial examination of tumor and stromal cells for the presence of markers. Therefore, we predict that the pathologist of the future will stain tumors with a limited panel of antibodies, including p53 (to detect mutant p53), WT1 (to detect the ability to use reactive oxygen), and NFκB surrogates, such as ICAM1. Positive staining for WT1 and ICAM1, in the absence of p53 immunopositivity, will then indicate the presence of the reactive oxygen driven-tumor. P53 mutant tumors may also demonstrate NFκB activation, but these are not driven by reactive oxygen (Fig. 1). Compounds that elevate reactive oxygen should be preferentially targeted towards tumors with mutant p53, and those which inhibit NADPH oxidases should be preferentially targeted towards reactive oxygen-driven tumors.
Fig. 1.
Dependence on signaling pathways based upon tumor suppressor status. Tumors that are deficient in p16ink4a are more likely to use a reactive oxygen/Rac/NFκB signaling pathway, while tumors that have mutant p53 are more likely to use a noncanonical NFκB signaling pathway and a phosphoinositol-3 kinase-dependent but Akt-independent pathway
Treatment of the reactive oxygen-driven tumor
Two approaches have been discussed for treating tumors in terms of reactive oxygen. One method is to overwhelm the tumor with reactive oxygen, based on the hypothesis that tumors which have high levels of reactive oxygen may undergo apoptosis if subjected to a reactive oxygen-generating process. Alternatively, we would like to focus on the inhibition of reactive oxygen as a target, especially since we have provided proof of principle in human disease. As mentioned earlier, reactive oxygen is capable of reversibly inactivating proteins involved in NFκB signaling. This means that NFκB can be downregulated by pharmacologic means by downregulating the production of superoxide. This finding is of major pharmacologic importance because it means that it is possible to selectively inhibit cells that rely on superoxide for NFκB-mediated survival and, in the case of tumors, achieve NFκB-mediated immune evasion.
Major mediators of both inflammation and tumor survival include transforming growth factor-beta, tumor necrosis factor-alpha (TNF-α), and CD40, all of which when activated in the tumor milieu, result in reactive oxygen generation and NFκB activation. The mechanisms include oxidative inactivation of IκB, resulting in the nuclear translocation of p50/p65 subunits of NFκB (canonical activation) [45]. Canonical activation of NFκB in a constitutive manner has been observed in melanoma, glioblastoma, hematologic malignancies, as well as in many other solid tumors [50, 57], while inducible canonical activation of NFκB has been observed in inflammatory processes, such as arthritis, inflammatory bowel disease, multiple sclerosis, scleroderma, atopic dermatitis, and psoriasis, among other disorders. We have shown the efficacy of gentian violet, a topical NADPH oxidase inhibitor, in atopic dermatitis, an extremely common disorder in which bacterial toll receptor activation combines with TNF-α to perpetuate inflammation [47]. Not all inflammatory processes are reactive oxygen mediated, a case in point being chronic granulomatous disease (CGD) in which inflammatory granulomata are a major cause of inflammation. CGD results from the loss of subunits associated with nox2, and thus it is a reactive oxygen-deficient disorder. Biomarkers are needed to determine which inflammatory processes would respond best to NADPH oxidase inhibition. Based upon currently available data, the major NADPH oxidases that merit targeting are nox1, which primarily generates superoxide, and nox4, which primarily generates hydrogen peroxide.
NOX 1 and 4
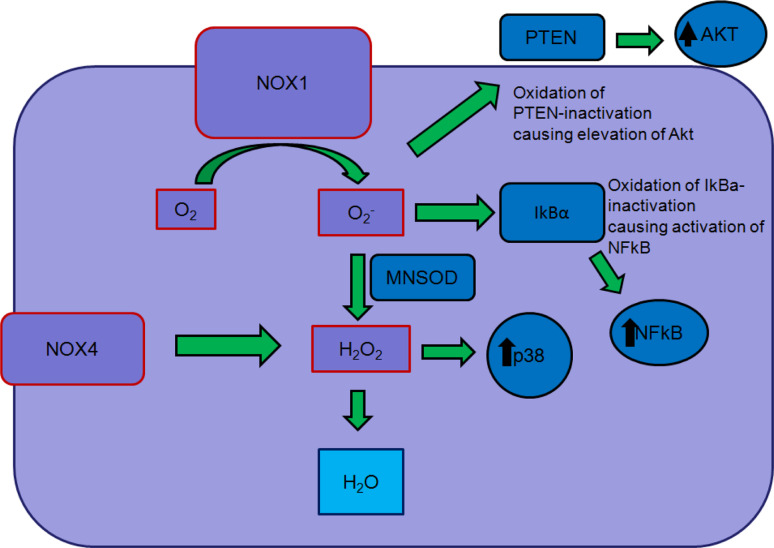
Seven proteins and several activator/organizer proteins collectively make up the NADPH oxidase or NOX family: NOX1–5, DUOX1, DUOX2, p22phox, p47phox, NOXO1/p67phox, and NOXA1/p40phox. NOX proteins (NOX1–5, DUOX1, DUOX2) are transmembrane proteins that transport electrons, reducing oxygen into superoxide or reactive oxygen species (ROS). NOX proteins (1–4), with the exception of NOX5, require p22Phox to produce ROS. NOX1–3 also depend on cytosolic subunits NOXO1 [NOX organizer 1_p47phox homolog (NOXO2)] and NOXA1 (NOX activator 1_p67phox homolog) for activation [6, 29]. In our research, we are focusing on nox1 and nox4 since these two enzymes are currently the most implicated in cancer and pathologic inflammation (Fig. 2).
Fig. 2.
Diagram of reactive oxygen-induced signaling, highlighting the role of Nox1-derived superoxide (O 2 -) and nox4-derived hydrogen peroxide (H 2 O 2) in activating signaling pathways, most notably those of Akt and NFκB
NOX1 is located on chromosome Xq22 and is well documented as being highly expressed in colon epithelium and in colon cancer cell line CaCO-2. Recent work by Shinohara et al. [46] draws a complex relationship between NOX1 and the oncogene Ras. The Shinohara group describes Ras-activated NOX1 as a mediator of cellular invasion, tumorogenesis, and angiogenesis. Experimental results drawn from K-Ras-transformed normal rat kidney cells (KNRK) and colon cancer CaCO-2 support NOX1 activation through a Ras/ERK/MEK/GATA-6 pathway. The subsequent upregulation of NOX1/ROS induces an NFκB-mediated cellular invasion through an increase production of metalloprotease-9 (MMP-9), where the upregulation of cytosolic subunits NOXO1 and NOXA1 play a significant role in NOX1/ROS-mediated MMP-9 overexpression. MMPs degrade extracellular matrix proteins, allowing for increased invasive properties. NFκB is also highly activated in adenocarcinoma cells expressing NOX1. NOX1/ROS mediate Ras-induced NFκB signaling by increasing IκB-alpha phosphorylation by IκK-alpha and degrading IκB-alpha, resulting in NFκB activation, a process reversed by NOX1 siRNA. NOX1 also mediates the downregulation of Rho via EGF and increases cellular mobility. Activation of p190RhoGAP by EGF is attributed to the downregulation or Rho activity, a process also reversed by NOX1 siRNA. This relationship is consistent with the opposing roles of rac isoforms and rhoA, with rhoA activation being associated with low levels of reactive oxygen and rac activation being associated with elevated levels of reactive oxygen [46]. Nox1 may also be the major nox isoform involved in tumor angiogenesis. Nox1 deficiency, but not nox4 deficiency, has been found to impair angiogenesis through a peroxisome proliferator-activated receptor alpha-dependent pathway [21].
NOX4 is located on chromosome 11q14.2-q21 and has been well noted to be highly expressed in kidneys and blood vessels as well as attributed to malignant melanoma and pancreatic carcinoma. Like NOX1–3, NOX4 is p22phox dependent for the generation of ROS. However, NOX4 does not require cytosolic subunits as in the case for NOX proteins 1–3. NOX4 in melanoma has been closely linked to cell survival through the activation of AKT and NκKB via ROS, as well as through the subsequently induced hypoxic factor HIF2-alpha, leading to upregulation in angiogenesis via VEGF and ANG2 [20]. The blockade of NOX4 has been associated with decreased growth of several neoplasms.
Mochizuki et al. [39] report NOX4 in pancreatic adenocarcinoma as mediating survival via the ROS/AKT/ASK1 pathway. Phosphorylation of apoptosis signal-regulating kinase 1 (ASK1) via NOX4-induced AKT provides addition insight into how NOX4/AKT may act to inhibit apoptosis and increase cell survival. Using the pancreatic cancer cell line PANC-1, the Mochizuki group found that inhibition of AKT phosphorylation of ASK1 alone was sufficient to induce apoptosis [39]. NOX4 has also been implicated in melanoma growth from two sources. First, we have demonstrated that transfection of Akt results in the induction of NOX4 in early melanoma cells. Second, the introduction of NOX4 siRNA leads to an inhibition of melanoma growth in vivo, as shown in two separate studies [10, 56]. Nox4 has been implicated in renal cell carcinoma as a direct stimulator of HIF2a, which is of physiological relevance, as tumors that express high levels of HIF2a, but not HIF1a, have a worsened prognosis [8, 22, 37]. We have found an essential role for NOX4 in hemangioma growth, and inhibitors of NOX4 result in hemangioma growth inhibition, including both fulvenes and triphenylmethanes [7, 21, 41]. Intriguingly, a recent report has shown that NOX4 mediates cell death in head and neck squamous cell carcinoma upon treatment with EGF receptor inhibitors [40]. All of the cell lines used in this study have mutant p53, highlighting the role of mutant p53 versus p16 loss in the choice of antioxidant versus prooxidant signaling pathway.
Future trends
The identification of the reactive oxygen-driven phenotype, both in inflammation and tumorigenesis, has clinical consequences. Pathologic conditions that are characterized by excessive reactive oxygen will likely be responsive to reactive oxygen inhibitors, while conditions with defective reactive oxygen may be responsive to reactive oxygen inducers. The epidemiology of reactive oxygen-driven inflammation and tumorigenesis is well-established. We have found histological tools that can be used on paraffin in humans sections to determine the presence of the reactive oxygen-driven phenotype. Finally, we and other research have developed small molecule inhibitors of nox enzymes that are active in animal models and in human patients. Wider applications of these principles may lead to improved therapies for inflammation and cancer.
Acknowledgments
Supported by NIAMS Grants RO1AR 47901 and RO1 AR 050727 to J.L.A, and Emory Skin Disease Research Core Center P30 AR 42687
References
- 1.Al DR, Powell J, McCuaig C, Kokta V. Differentiation of vascular tumors from vascular malformations by expression of Wilms tumor 1 gene: evaluation of 126 cases. J Am Acad Dermatol. 2010;63:1052–1057. doi: 10.1016/j.jaad.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 2.Arbiser JL, Govindarajan B, Bai X, Onda H, Kazlauskas A, Lim SD, Amin MB, Claesson-Welsh L. Functional tyrosine kinase inhibitor profiling: a generally applicable method points to a novel role of platelet-derived growth factor receptor-beta in tuberous sclerosis. Am J Pathol. 2002;161:781–786. doi: 10.1016/S0002-9440(10)64237-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arbiser JL, Moses MA, Fernandez CA, Ghiso N, Cao Y, Klauber N, Frank D, Brownlee M, Flynn E, Parangi S, Byers HR, Folkman J. Oncogenic H-ras stimulates tumor angiogenesis by two distinct pathways. Proc Natl Acad Sci USA. 1997;94:861–866. doi: 10.1073/pnas.94.3.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Arbiser JL, Petros J, Klafter R, Govindajaran B, McLaughlin ER, Brown LF, Cohen C, Moses M, Kilroy S, Arnold RS, Lambeth JD. Reactive oxygen generated by Nox1 triggers the angiogenic switch. Proc Natl Acad Sci USA. 2002;99:715–720. doi: 10.1073/pnas.022630199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Balasubramanian A, Ganju RK, Groopman JE. Hepatitis C virus and HIV envelope proteins collaboratively mediate interleukin-8 secretion through activation of p38 MAP kinase and SHP2 in hepatocytes. J Biol Chem. 2003;278:35755–35766. doi: 10.1074/jbc.M302889200. [DOI] [PubMed] [Google Scholar]
- 6.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 7.Bhandarkar SS, Jaconi M, Fried LE, Bonner MY, Lefkove B, Govindarajan B, Perry BN, Parhar R, Mackelfresh J, Sohn A, Stouffs M, Knaus U, Yancopoulos G, Reiss Y, Benest AV, Augustin HG, Arbiser JL. Fulvene-5 potently inhibits NADPH oxidase 4 and blocks the growth of endothelial tumors in mice. J Clin Invest. 2009;119:2359–2365. doi: 10.1172/JCI33877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Block K, Gorin Y, Hoover P, Williams P, Chelmicki T, Clark RA, Yoneda T, Abboud HE. NAD(P)H oxidases regulate HIF-2alpha protein expression. J Biol Chem. 2007;282:8019–8026. doi: 10.1074/jbc.M611569200. [DOI] [PubMed] [Google Scholar]
- 9.Boivin B, Zhang S, Arbiser JL, Zhang ZY, Tonks NK. A modified cysteinyl-labeling assay reveals reversible oxidation of protein tyrosine phosphatases in angiomyolipoma cells. Proc Natl Acad Sci USA. 2008;105:9959–9964. doi: 10.1073/pnas.0804336105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brar SS, Kennedy TP, Sturrock AB, Huecksteadt TP, Quinn MT, Whorton AR, Hoidal JR. An NAD(P)H oxidase regulates growth and transcription in melanoma cells. Am J Physiol Cell Physiol. 2002;282:C1212–C1224. doi: 10.1152/ajpcell.00496.2001. [DOI] [PubMed] [Google Scholar]
- 11.Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, Carter AB, Rothman PB, Flavell RA, Sutterwala FS. The Nalp3 inflammasome is essential for the development of silicosis. Proc Natl Acad Sci USA. 2008;105:9035–9040. doi: 10.1073/pnas.0803933105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Cerimele F, Battle T, Lynch R, Frank DA, Murad E, Cohen C, Macaron N, Sixbey J, Smith K, Watnick RS, Eliopoulos A, Shehata B, Arbiser JL. Reactive oxygen signaling and MAPK activation distinguish Epstein–Barr Virus (EBV)-positive versus EBV-negative Burkitt’s lymphoma. Proc Natl Acad Sci USA. 2005;102:175–179. doi: 10.1073/pnas.0408381102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cerimele F, Brown LF, Bravo F, Ihler GM, Kouadio P, Arbiser JL. Infectious angiogenesis: Bartonella bacilliformis infection results in endothelial production of angiopoietin-2 and epidermal production of vascular endothelial growth factor. Am J Pathol. 2003;163:1321–1327. doi: 10.1016/S0002-9440(10)63491-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cohen C, Zavala-Pompa A, Sequeira JH, Shoji M, Sexton DG, Cotsonis G, Cerimele F, Govindarajan B, Macaron N, Arbiser JL. Mitogen-activated protein kinase activation is an early event in melanoma progression. Clin Cancer Res. 2002;8:3728–3733. [PubMed] [Google Scholar]
- 15.Cowley S, Paterson H, Kemp P, Marshall CJ. Activation of MAP kinase kinase is necessary and sufficient for PC12 differentiation and for transformation of NIH 3T3 cells. Cell. 1994;77:841–852. doi: 10.1016/0092-8674(94)90133-3. [DOI] [PubMed] [Google Scholar]
- 16.Curtin JA, Fridlyand J, Kageshita T, Patel HN, Busam KJ, Kutzner H, Cho KH, Aiba S, Brocker EB, LeBoit PE, Pinkel D, Bastian BC. Distinct sets of genetic alterations in melanoma. N Engl J Med. 2005;353:2135–2147. doi: 10.1056/NEJMoa050092. [DOI] [PubMed] [Google Scholar]
- 17.Davies MA, Stemke-Hale K, Lin E, Tellez C, Deng W, Gopal YN, Woodman SE, Calderone TC, Ju Z, Lazar AJ, Prieto VG, Aldape K, Mills GB, Gershenwald JE. Integrated molecular and clinical analysis of AKT activation in metastatic melanoma. Clin Cancer Res. 2009;15:7538–7546. doi: 10.1158/1078-0432.CCR-09-1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.De SC, De SP, Di MG, Venier A, Cerimele D, Serri F. Reactive oxygen species production in circulating polymorphonuclear leukocytes in psoriasis. Acta Derm Venereol Suppl (Stockh) 1989;146:50–52. [PubMed] [Google Scholar]
- 19.Dudnakova T, Spraggon L, Slight J, Hastie N. Actin: a novel interaction partner of WT1 influencing its cell dynamic properties. Oncogene. 2010;29:1085–1092. doi: 10.1038/onc.2009.444. [DOI] [PubMed] [Google Scholar]
- 20.Fried L, Arbiser JL. The reactive oxygen-driven tumor: relevance to melanoma. Pigment Cell Melanoma Res. 2008;21:117–122. doi: 10.1111/j.1755-148X.2008.00451.x. [DOI] [PubMed] [Google Scholar]
- 21.Garrido-Urbani S, Jemelin S, Deffert C, Carnesecchi S, Basset O, Szyndralewiez C, Heitz F, Page P, Montet X, Michalik L, Arbiser J, Ruegg C, Krause KH, Imhof B. Targeting vascular NADPH oxidase 1 blocks tumor angiogenesis through a PPARalpha mediated mechanism. PLoS ONE. 2011;6:e14665. doi: 10.1371/journal.pone.0014665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gordan JD, Lal P, Dondeti VR, Letrero R, Parekh KN, Oquendo CE, Greenberg RA, Flaherty KT, Rathmell WK, Keith B, Simon MC, Nathanson KL. HIF-alpha effects on c-Myc distinguish two subtypes of sporadic VHL-deficient clear cell renal carcinoma. Cancer Cell. 2008;14:435–446. doi: 10.1016/j.ccr.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Govindarajan B, Bai X, Cohen C, Zhong H, Kilroy S, Louis G, Moses M, Arbiser JL. Malignant transformation of melanocytes to melanoma by constitutive activation of mitogen-activated protein kinase kinase (MAPKK) signaling. J Biol Chem. 2003;278:9790–9795. doi: 10.1074/jbc.M212929200. [DOI] [PubMed] [Google Scholar]
- 24.Govindarajan B, Klafter R, Miller MS, Mansur C, Mizesko M, Bai X, LaMontagne K, Jr, Arbiser JL. Reactive oxygen-induced carcinogenesis causes hypermethylation of p16(Ink4a) and activation of MAP kinase. Mol Med. 2002;8:1–8. doi: 10.1007/s00894-001-0054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Govindarajan B, Shah A, Cohen C, Arnold RS, Schechner J, Chung J, Mercurio AM, Alani R, Ryu B, Fan CY, Cuezva JM, Martinez M, Arbiser JL. Malignant transformation of human cells by constitutive expression of platelet-derived growth factor-BB. J Biol Chem. 2005;280:13936–13943. doi: 10.1074/jbc.M500411200. [DOI] [PubMed] [Google Scholar]
- 26.Govindarajan B, Sligh JE, Vincent BJ, Li M, Canter JA, Nickoloff BJ, Rodenburg RJ, Smeitink JA, Oberley L, Zhang Y, Slingerland J, Arnold RS, Lambeth JD, Cohen C, Hilenski L, Griendling K, Martinez-Diez M, Cuezva JM, Arbiser JL. Overexpression of Akt converts radial growth melanoma to vertical growth melanoma. J Clin Invest. 2007;117:719–729. doi: 10.1172/JCI30102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hanselmann C, Mauch C, Werner S. Haem oxygenase-1: a novel player in cutaneous wound repair and psoriasis? Biochem J. 2001;353:459–466. doi: 10.1042/0264-6021:3530459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Klangby U, Okan I, Magnusson KP, Wendland M, Lind P, Wiman KG. p16/INK4a and p15/INK4b gene methylation and absence of p16/INK4a mRNA and protein expression in Burkitt’s lymphoma. Blood. 1998;91:1680–1687. [PubMed] [Google Scholar]
- 29.Lambeth JD, Kawahara T, Diebold B. Regulation of Nox and Duox enzymatic activity and expression. Free Radic Biol Med. 2007;43:319–331. doi: 10.1016/j.freeradbiomed.2007.03.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.LaMontagne KR, Jr, Moses MA, Wiederschain D, Mahajan S, Holden J, Ghazizadeh H, Frank DA, Arbiser JL. Inhibition of MAP kinase kinase causes morphological reversion and dissociation between soft agar growth and in vivo tumorigenesis in angiosarcoma cells. Am J Pathol. 2000;157:1937–1945. doi: 10.1016/S0002-9440(10)64832-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lapidoth M, Ben-Amitai D, Bhandarkar S, Fried L, Arbiser JL. Efficacy of topical application of eosin for ulcerated hemangiomas. J Am Acad Dermatol. 2009;60:350–351. doi: 10.1016/j.jaad.2008.10.034. [DOI] [PubMed] [Google Scholar]
- 32.Lawley LP, Cerimele F, Weiss SW, North P, Cohen C, Kozakewich HP, Mulliken JB, Arbiser JL. Expression of Wilms tumor 1 gene distinguishes vascular malformations from proliferative endothelial lesions. Arch Dermatol. 2005;141:1297–1300. doi: 10.1001/archderm.141.10.1297. [DOI] [PubMed] [Google Scholar]
- 33.Leaute-Labreze C, de la Dumas RE, Hubiche T, Boralevi F, Thambo JB, Taieb A. Propranolol for severe hemangiomas of infancy. N Engl J Med. 2008;358:2649–2651. doi: 10.1056/NEJMc0708819. [DOI] [PubMed] [Google Scholar]
- 34.Lee HM, Shin DM, Yuk JM, Shi G, Choi DK, Lee SH, Huang SM, Kim JM, Kim CD, Lee JH, Jo EK. Autophagy negatively regulates keratinocyte inflammatory responses via scaffolding protein p62/SQSTM1. J Immunol. 2011;186:1248–1258. doi: 10.4049/jimmunol.1001954. [DOI] [PubMed] [Google Scholar]
- 35.Levine SM. The role of reactive oxygen species in the pathogenesis of multiple sclerosis. Med Hypotheses. 1992;39:271–274. doi: 10.1016/0306-9877(92)90121-R. [DOI] [PubMed] [Google Scholar]
- 36.Lunec J, Herbert K, Blount S, Griffiths HR, Emery P. 8-Hydroxydeoxyguanosine. A marker of oxidative DNA damage in systemic lupus erythematosus. FEBS Lett. 1994;348:131–138. doi: 10.1016/0014-5793(94)00583-4. [DOI] [PubMed] [Google Scholar]
- 37.Maranchie JK, Zhan Y. Nox4 is critical for hypoxia-inducible factor 2-alpha transcriptional activity in von Hippel-Lindau-deficient renal cell carcinoma. Cancer Res. 2005;65:9190–9193. doi: 10.1158/0008-5472.CAN-05-2105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mishra MV, Bisht KS, Sun L, Muldoon-Jacobs K, Awwad R, Kaushal A, Nguyen P, Huang L, Pennington JD, Markovina S, Bradbury CM, Gius D. DNMT1 as a molecular target in a multimodality-resistant phenotype in tumor cells. Mol Cancer Res. 2008;6:243–249. doi: 10.1158/1541-7786.MCR-07-0373. [DOI] [PubMed] [Google Scholar]
- 39.Mochizuki T, Furuta S, Mitsushita J, Shang WH, Ito M, Yokoo Y, Yamaura M, Ishizone S, Nakayama J, Konagai A, Hirose K, Kiyosawa K, Kamata T. Inhibition of NADPH oxidase 4 activates apoptosis via the AKT/apoptosis signal-regulating kinase 1 pathway in pancreatic cancer PANC-1 cells. Oncogene. 2006;25:3699–3707. doi: 10.1038/sj.onc.1209406. [DOI] [PubMed] [Google Scholar]
- 40.Orcutt KP, Parsons AD, Sibenaller ZA, Scarbrough PM, Zhu Y, Sobhakumari A, Wilke WW, Kalen AL, Goswami P, Miller FJ, Jr, Spitz DR, Simons AL. Erlotinib-mediated inhibition of EGFR signaling induces metabolic oxidative stress through NOX4. Cancer Res. 2011;71:3932–3940. doi: 10.1158/0008-5472.CAN-10-3425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Perry BN, Govindarajan B, Bhandarkar SS, Knaus UG, Valo M, Sturk C, Carrillo CO, Sohn A, Cerimele F, Dumont D, Losken A, Williams J, Brown LF, Tan X, Ioffe E, Yancopoulos GD, Arbiser JL. Pharmacologic blockade of angiopoietin-2 is efficacious against model hemangiomas in mice. J Invest Dermatol. 2006;126:2316–2322. doi: 10.1038/sj.jid.5700413. [DOI] [PubMed] [Google Scholar]
- 42.Perry DK, Hand WL, Edmondson DE, Lambeth JD. Role of phospholipase D-derived diradylglycerol in the activation of the human neutrophil respiratory burst oxidase. Inhibition by phosphatidic acid phosphohydrolase inhibitors. J Immunol. 1992;149:2749–2758. [PubMed] [Google Scholar]
- 43.Qiu RG, Chen J, Kirn D, McCormick F, Symons M. An essential role for Rac in Ras transformation. Nature. 1995;374:457–459. doi: 10.1038/374457a0. [DOI] [PubMed] [Google Scholar]
- 44.Sanz-Cameno P, Martin-Vilchez S, Lara-Pezzi E, Borque MJ, Salmeron J, de Munoz RP, Solis JA, Lopez-Cabrera M, Moreno-Otero R. Hepatitis B virus promotes angiopoietin-2 expression in liver tissue: role of HBV X protein. Am J Pathol. 2006;169:1215–1222. doi: 10.2353/ajpath.2006.051246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Schreck R, Rieber P, Baeuerle PA. Reactive oxygen intermediates as apparently widely used messengers in the activation of the NF-kappa B transcription factor and HIV-1. EMBO J. 1991;10:2247–2258. doi: 10.1002/j.1460-2075.1991.tb07761.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Shinohara M, Shang WH, Kubodera M, Harada S, Mitsushita J, Kato M, Miyazaki H, Sumimoto H, Kamata T. Nox1 redox signaling mediates oncogenic Ras-induced disruption of stress fibers and focal adhesions by down-regulating Rho. J Biol Chem. 2007;282:17640–17648. doi: 10.1074/jbc.M609450200. [DOI] [PubMed] [Google Scholar]
- 47.Stoff B, Mackelfresh J, Fried L, Cohen C, Arbiser JL. A nonsteroidal alternative to impetiginized eczema in the emergency room. J Am Acad Dermatol. 2010;63:537–539. doi: 10.1016/j.jaad.2009.05.027. [DOI] [PubMed] [Google Scholar]
- 48.Szatrowski TP, Nathan CF. Production of large amounts of hydrogen peroxide by human tumor cells. Cancer Res. 1991;51:794–798. [PubMed] [Google Scholar]
- 49.Takahashi K, Mulliken JB, Kozakewich HP, Rogers RA, Folkman J, Ezekowitz RA. Cellular markers that distinguish the phases of hemangioma during infancy and childhood. J Clin Invest. 1994;93:2357–2364. doi: 10.1172/JCI117241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Torisu-Itakura H, Lee JH, Huynh Y, Ye X, Essner R, Morton DL. Monocyte-derived IL-10 expression predicts prognosis of stage IV melanoma patients. J Immunother. 2007;30:831–838. doi: 10.1097/CJI.0b013e318158795b. [DOI] [PubMed] [Google Scholar]
- 51.Trindade F, Tellechea O, Torrelo A, Requena L, Colmenero I. Wilms tumor 1 expression in vascular neoplasms and vascular malformations. Am J Dermatopathol. 2011;33(6):569–572. doi: 10.1097/DAD.0b013e3182092527. [DOI] [PubMed] [Google Scholar]
- 52.Verma A, Ihler GM. Activation of Rac, Cdc42 and other downstream signalling molecules by Bartonella bacilliformis during entry into human endothelial cells. Cell Microbiol. 2002;4:557–569. doi: 10.1046/j.1462-5822.2002.00217.x. [DOI] [PubMed] [Google Scholar]
- 53.Vicent S, Chen R, Sayles LC, Lin C, Walker RG, Gillespie AK, Subramanian A, Hinkle G, Yang X, Saif S, Root DE, Huff V, Hahn WC, Sweet-Cordero EA. Wilms tumor 1 (WT1) regulates KRAS-driven oncogenesis and senescence in mouse and human models. J Clin Invest. 2010;120:3940–3952. doi: 10.1172/JCI44165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Warburg O. On respiratory impairment in cancer cells. Science. 1956;124:269–270. [PubMed] [Google Scholar]
- 55.Weaver-Feldhaus J, Gruis NA, Neuhausen S, Le PD, Stockert E, Skolnick MH, Kamb A. Localization of a putative tumor suppressor gene by using homozygous deletions in melanomas. Proc Natl Acad Sci USA. 1994;91:7563–7567. doi: 10.1073/pnas.91.16.7563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yamaura M, Mitsushita J, Furuta S, Kiniwa Y, Ashida A, Goto Y, Shang WH, Kubodera M, Kato M, Takata M, Saida T, Kamata T. NADPH oxidase 4 contributes to transformation phenotype of melanoma cells by regulating G2-M cell cycle progression. Cancer Res. 2009;69:2647–2654. doi: 10.1158/0008-5472.CAN-08-3745. [DOI] [PubMed] [Google Scholar]
- 57.Yamini B, Yu X, Dolan ME, Wu MH, Kufe DW, Weichselbaum RR. Inhibition of nuclear factor-kappaB activity by temozolomide involves O6-methylguanine induced inhibition of p65 DNA binding. Cancer Res. 2007;67:6889–6898. doi: 10.1158/0008-5472.CAN-06-4496. [DOI] [PubMed] [Google Scholar]
- 58.Zietz C, Rossle M, Haas C, Sendelhofert A, Hirschmann A, Sturzl M, Lohrs U. MDM-2 oncoprotein overexpression, p53 gene mutation, and VEGF up-regulation in angiosarcomas. Am J Pathol. 1998;153:1425–1433. doi: 10.1016/S0002-9440(10)65729-X. [DOI] [PMC free article] [PubMed] [Google Scholar]