Abstract
Purpose
Polymeric quick dissolving films were developed as a solid dosage topical microbicide formulation for the vaginal delivery of the highly potent and non-toxic, dual-acting HIV nonnucleoside reverse transcriptase inhibitor (NNRTI) pyrimidinedione, IQP-0528.
Methods
Formulated from approved excipients, a polyvinyl alcohol (PVA) based film was manufactured via solvent casting methods. The film formulations were evaluated based upon quantitative physicochemical evaluations defined by a Target Product Profile (TPP)
Results
Films dosed with 0.1 % (w/w) of IQP-0528 disintegrated within 10 minutes with over 50% of drug released and near 100% total drug released after 30 minutes. The IQP-0528 films were found to be non-toxic in in vitro CEM-SS and PBMC cell-based assays and biologically active with sub-nanomolar efficacy against HIV-1 infection. In a 12 month stability protocol, the IQP-0528 films demonstrated no significant degradation at International Conference on Harmonization (ICH) recommended standard (25°C / 65% relative humidity (R.H.)) and accelerated (40°C / 75% R.H.) environmental conditions.
Conclusions
Based on the above evaluations, a vaginal film formulation has been identified as a potential solid dosage form for the vaginal delivery of the topical microbicide candidate IQP-0528.
Keywords: microbicides, HIV, formulation, films, pyrimidinedione
INTRODUCTION
Sexually transmitted infections (STIs), such as human immunodeficiency virus (HIV), herpes simplex virus type II (HSV-2), chlamydia, and gonorrhea are spread predominantly through unprotected heterosexual vaginal intercourse. Of the 2.6 million new HIV infections reported worldwide in 2009, 1.8 million of them occurred in Sub-Saharan Africa, with nearly 66% of these infections occurring in women [1]. The rate of male to female transmission is higher due to greater susceptibility of women which is attributed to biological vulnerability, absence of widely accepted female-oriented preventative methods, socio-economic status, and the inability to negotiate safe sex practices. Current strategies in HIV prevention have been limited to condom use and behavioral changes; however, in areas of the world with high HIV prevalence, condom use is low due to social stigmas [2]. As such, sexual transmission is one of the primary sources of HIV infection [3]. With over 33 million people worldwide that are HIV-positive, the need for female-controlled methods of STI prevention is of great importance.
While there are defined therapies for the treatment of viral infections such as HIV, HSV-2, and HPV, there exists no cure for the diseases. Therefore, a viable strategy currently in development to prevent the spread of infection is to employ preventative treatments to halt viral entry and transmission at the site of infection. One of the most promising of these strategies against HIV infection is the use of microbicides: agents applied topically to the vagina or rectum prior to sexual intercourse to prophylactically inhibit transmission of STIs, including HIV [4, 5]. Several microbicides that showed promising anti-HIV activity have been considered for clinical development such as PRO2000 [6] and BufferGel [7]. The completed CAPRISA 004 trial showed that a pericoitally dosed 1% vaginal gel formulation of tenofovir, a nucleotide reverse transcriptase inhibitor, had potential to be formulated as a safe, effective, and acceptable product [8]. However, the recently discontinued VOICE study indicated that a once-daily regimen of tenofovir gel did not show effectiveness in preventing HIV in women enrolled in the trial. The differences in dosing regimens is suggested to be the contributing factor in the differences between the two trials, and the currently running FACTS trial in South Africa is set to address the reproducibility of pericoital dosing.
To increase the portfolio of potential drugs that prevent HIV-infection, ImQuest BioSciences is currently developing the pyrimidinedione (PYD) class of novel nonnucleoside reverse transcriptase inhibitors (NNRTIs) as a microbicide candidate. The current lead molecule, IQP-0528 [1-(3-Cyclopropyl)methyl-6-(3,5-dimethylbenzoyl)-5-isopropyl-2,4(1H,3H)-pyrimidinedione], is a highly potent small molecule inhibitor that has a dual mechanism of action against HIV infection: viral entry and reverse transcriptase inhibition [9, 10]. In cell-based in vitro assays, IQP-0528 has shown subnanomolar levels of activity as a NNRTI and nanomolar levels of activity as inhibitors of a step in virus entry occurring prior to chemokine receptor binding and fusion [11]. Mechanistic studies have conclusively shown that the pyrimidinediones act as NNRTIs of HIV-1 and as entry inhibitors of both HIV-1 and HIV-2, and thus are the first NNRTIs with substantial inhibitory potential against HIV-2 [12]. While not as prevalent as HIV-1, HIV-2 infections are predominantly found in African nations. Therefore a microbicide that targets both HIV-1 and HIV-2 would prove advantageous in preventing the spread of HIV in the highest risk regions of the world.
Currently several dosage forms have been investigated for use as drug delivery systems for microbicide products ranging from liquid to semi-solid to solid dosage forms [13, 14]. To date, gel products have been widely studied in clinical trials as vaginal drug delivery systems for the prevention of HIV with the most success. Despite its very low solubility, IQP-0528 does not possess any stability issues that would impact formulation into a gel product [15]. However, regional economic and social conditions, as well as individual preferences may interfere with gel acceptability and reduce overall effectiveness. Ultimately, it may be necessary to develop multiple dosage platforms for a single active agent to provide users with options they can use within the constraints of their social environment, personal choice, and environmental conditions.
As an alternative formulation and a direct response to very low solubility microbicides, quick-dissolving polymeric films are being developed as solid dosage forms. Vaginal films are thin solid sheets of water soluble polymers that deliver drug incorporated within the polymer matrix locally via the ambient fluid available in the vagina. Films may provide some potential economic advantages over other dosage forms. The small size of the film and the lack of the need for applicators may result in a less expensive product that is easier to store and transport. The solid formulation may increase product stability by reducing drug degradation through oxidation or hydrolysis and by eliminating precipitation. As such, polymeric films have been gaining increased acceptance as an oral delivery method of vitamins, minerals, herbal remedies, supplements, cold remedies, pain medications, and gastric disturbance medications which provide rapid drug release and bioadhesive properties that may increase retention time at the target [16, 17]. In light of this, films have been under development as a means for topical anti-HIV drug delivery. The commercially available contraceptive vaginal film (VCF), which contains Nonoxynol-9, has been evaluated as a potential dosage form for vaginal delivery of microbicide drug candidates [18–20]. Additionally, other microbicide candidates have begun formulation into topical film delivery [21].
As part of the development of IQP-0528 as a microbicide, a vaginal film formulation was developed and identified as an effective method for rapidly delivering the drug to the vaginal tissue and was evaluated for its physicochemical properties, safety evaluation in organotypic in vitro tissue models and anti-HIV activity. As a result, this study allowed the quantitative assessment of the vaginal film formulation for the delivery of IQP-0528 as an effective microbicide product for further development.
MATERIALS AND METHODS
IQP-0528
The pyrimidinedione IQP-0528 was synthesized by Samjin Pharmaceutical Co. LTD (Seoul, Korea) and is licensed to ImQuest BioSciences Inc. (Frederick, MD).
Pyrimidinedione / Excipient Compatibility
The compatibility of the pyrimidinedione IQP-0528 with the film excipients was evaluated over 30 days at two environmental conditions: 25°C / 65% relative humidity (R.H.) and 40°C / 75% R.H. Measured amounts of IQP-0528 were solubilized in propylene glycol (Sigma-Aldrich) and aliquotted into 200 µL volume samples. Two milliliter (2 mL) solutions each containing a single excipient: polyvinyl alcohol (PVA-403) (Kuraray), hydroxypropyl methylcellulose (HPMC) (Fluka), polyethylene glycol 400 (PEG400) (Sigma-Aldrich), glycerin (Sigma-Aldrich), and a solution containing all the excipients were added to the drug samples and mixed together through vortexing. Sealed with parafilm and foil to protect against evaporation and light, the samples were placed under the specified conditions. At regular intervals, samples were removed and the IQP-0528 drug content was determined via HPLC analysis.
Film Formulation
The films were formulated via a solvent casting and evaporation method. Using a specific order of addition, PVA-403 was first added to DI water and mixed until the polymer was completely dissolved. Next, glycerin and PEG400 were then added to the solution. HPMC was added last to the solution and mixed under low heating until completely dissolved. Multiple film formulations were developed by varying the concentration of the excipients. The specific composition of each film formulation as a total wet weight percentage is detailed in Table I. Finally, 0.1% (film w/w) of IQP-0528 was dissolved in a solution of propylene glycol and added to the polymer solution and mixed for 1 hour at ambient room temperature. The polymer solution was distributed into film molds of (8.0 cm2) containing 3.6 mL of solution per well. The molds were then placed into an IsoTemp vacuum oven (Fisher, Pittsburgh, PA) and dried for 24 hours at 50°C and −20 in Hg. After the drying process, the films were removed from the molds and packaged into air-tight heat-sealed foil packaging and stored in a light controlled chamber at 25°C with no humidity control. Acceptable film formulation was decided upon a defined Target Product Profile (TPP) based upon physical values generally accepted within the microbicide field. In addition, the amount of IQP-0528 was varied from 0.05% to 0.1% (film w/w) to investigate the effect of loading dose on drug release.
Table I.
Vaginal Film Formulation
| Formulation | PVA | Glycerin | PEG | HPMC | Propylene Glycol |
IQP-5028 |
|---|---|---|---|---|---|---|
| P1 | 1.0 | 0.4 | 0.9 | 1.0 | 8.7 | 0.1 |
| P2 | 1.5 | 0.4 | 0.9 | 1.0 | 8.7 | 0.1 |
| P3 | 2.0 | 0.4 | 0.9 | 1.0 | 8.7 | 0.1 |
| P4 | 2.5 | 0.4 | 0.9 | 1.0 | 8.7 | 0.1 |
| H1 | 1.5 | 0.4 | 0.9 | 0.5 | 8.7 | 0.1 |
| H2 | 1.5 | 0.4 | 0.9 | 1.0 | 8.7 | 0.1 |
| H3 | 1.5 | 0.4 | 0.9 | 1.5 | 8.7 | 0.1 |
| H4 | 1.5 | 0.4 | 0.9 | 2.0 | 8.7 | 0.1 |
| G1 | 1.5 | 0.2 | 0.9 | 1.0 | 8.7 | 0.1 |
| G2 | 1.5 | 0.3 | 0.9 | 1.0 | 8.7 | 0.1 |
| G3 | 1.5 | 0.4 | 0.9 | 1.0 | 8.7 | 0.1 |
| G4 | 1.5 | 0.5 | 0.9 | 1.0 | 8.7 | 0.1 |
| PG1 | 1.5 | 0.4 | 0.7 | 1.0 | 8.7 | 0.1 |
| PG2 | 1.5 | 0.4 | 0.8 | 1.0 | 8.7 | 0.1 |
| PG3 | 1.5 | 0.4 | 0.9 | 1.0 | 8.7 | 0.1 |
| PG4 | 1.5 | 0.4 | 1.0 | 1.0 | 8.7 | 0.1 |
(% wet w/w)
In all formulations, water was used to fill to 100%
Film physical characterization
Polymeric vaginal films were evaluated for various aesthetic, physical, and mechanical parameters including thickness, dimensions, weight, moisture content, and visual inspections for appearance, color, and transparency. Physical dimension evaluation was performed through caliper measurements. The moisture content of the films was performed through the Arizona Instruments VaporPro system (Chandler, AZ) at a temperature of 120°C. To identify an acceptable film formulation, qualitative inspection was performed in mano to identify film pliability. In mano pliability was graded on a qualitative scale from “high” to “low” defined by the film’s ability to be rolled and folded. “High” pliability was defined as the film’s ability to maintain a 1 cm diameter roll and be folded in half without creating a permanent crease. “Low” pliability was defined as the film being unable to maintain a 1 cm diameter roll and producing sharp permanent crease once folded. All measurements were performed in triplicate.
Vaginal film disintegration
For gross qualitative evaluation of vaginal film disintegration, the films were placed into 3 mL of DI water in a 50 × 35mm crystallization dish. The crystallization dish was rotated at a slow pace on an orbital shaker and disintegration was measured visually. Film disintegration was defined as the time for the intact film structure to completely disappear into solution.
IQP-0528 loading of the vaginal film
Product extraction and homogeneity of IQP-0528 in the films were determined by HPLC analysis. The films were divided into 4 sections. Each section was dissolved in 2 mL of DI water followed by 1 mL of Acetonitrile. The entire solution was then filtered through a 0.22 µm filter. The filtered solution was run through the Shimadzu HPLC system (Columbia, MD). Based upon a previously developed stability indicating method [15], the separation of IQP-0528 was achieved using a HPLC ODS C18 (150 × 4.6 mm, 5 µm) column at 25°C with a mobile phase A (DI water with 0.1% TFA) and a mobile phase B (acetonitrile (ACN) for 15 minutes at a flow rate of 1.0 mL/min. The analyte peak was monitored at 267 nm and the retention time of IQP-0528 was determined to be 6.3 minutes.
In vitro release of IQP-0528 release from vaginal film
The in vitro release of IQP-0528 from the vaginal films was performed via a USP Apparatus 4 – flow through dissolution system. Dissolution conducted as per USP guidance’s for semisolid products using a Sotax CE7 apparatus was performed (Hopkinton, MA). In a dissolution volume of 60 mL containing 60% water and 40% acetonitrile, the dissolution was run through 12 mm cells at 10 mL/min and 37°C for 60 minutes. The rate of release of IQP-0528 from the films was continuously sampled and run through a UV/Vis spectrometer and was detected at 267 nm.
Vaginal film acute toxicity to the normal vaginal flora Lactobacillus
In a 15 mL conical tube 10 mL of MRS (de Man, Rogosa and Sharpe) media was inoculated with a frozen glycerol stock of Lactobacillus crispatus (ATCC 33820), jensenii (ATCC 25258), or acidophilus (ATCC 11975) and was incubated for 24 hours at 37°C in an anaerobic chamber. The overnight culture was diluted in MRS media until an absorbance of 0.06 at 670 nM was obtained. Six serial ½ log dilutions of the compound were performed and were added in a volume of 100 µL to the plate followed by the addition of 100 µL of the diluted bacteria. The plates were placed in an anaerobic chamber and were incubated at 37°C for 24 hours. Following the incubation the plates were read spectrophotometrically at 490 nm. TC50 concentrations were determined by linear regression analysis. Penicillin/Streptomycin solution was evaluated in parallel as an internal assay control.
Anti-HIV efficacy of IQP-0528 vaginal films
Cytopathic Effect Inhibition (CPE) Assay
The CPE assay was performed as previously described [22]. Briefly, serially diluted compound was added to a 96-well round bottom microtiter plate in triplicate. CEM-SS cells at a concentration of 2.5 × 103 cells per well and HIV-1IIIB at the appropriate pre-determined titer to achieve 90% cell killing at day 6 were sequentially added to the microtiter plate. The cultures were incubated at 5% CO2/37°C for six days. Following the incubation, the microtiter plates were stained with XTT tetrazolium dye to evaluate the efficacy and toxicity of the test compound(s). AZT was evaluated in parallel as an assay control compound.
Anti-HIV Assay in Fresh Human Peripheral Blood Mononuclear Cells
PBMC based anti-HIV assays were performed as previously described [23]. Briefly, PHA-stimulated PBMCs cultured in the presence of IL-2 were suspended at 1 × 106 cells/mL and were added to a 96-well round-bottom plate. Serially diluted test materials were added to the plate in triplicate followed by the appropriate pre-titered strain of HIV (Clade B 92/HT/599). The culture was incubated for 7 days at 37°C/5% CO2. Following the incubation, supernatants were collected for analysis of virus replication by supernatant RT activity and cells analyzed for viability by XTT dye reduction. AZT was used as an internal assay control.
Film stability
A stability evaluation program of the IQP-0528 films was performed for 12 months under ICH conditions. Films produced were packaged in light protective air-tight foil packaging and stored under standard conditions (25°C / 65% R.H) and accelerated conditions (40°C / 75% R.H.). At specified time points (initial, 3 days, 1 week, 2 weeks, 3 weeks, 1 month, 3 months, 6 months, 12 months), the films were removed from storage and allowed to equilibrate to ambient temperature. The films were then removed from the packaging and assessed for their stability through the film evaluation assays including physical characteristics, IQP-0528 stability and content uniformity, in vitro release, toxicity, and anti-HIV efficacy. All tests were performed in triplicate. Stability in the films was defined as < 10% deviation in the initial values of the film characteristics.
Statistics
All statistics were evaluated by analysis of variation (ANOVA). P < 0.05 was considered statistically significant. All error bars represent standard deviations.
RESULTS
IQP-0528
IQP-0528 is a dual acting pyrimidinedione nonnucleoside RT inhibitor that also targets virus entry. It has nanomolar activity against laboratory strains of virus and nanomolar to subnanomolar activity against representative clinical strains of HIV [11, 24].
IQP-0528 / film excipient compatibility
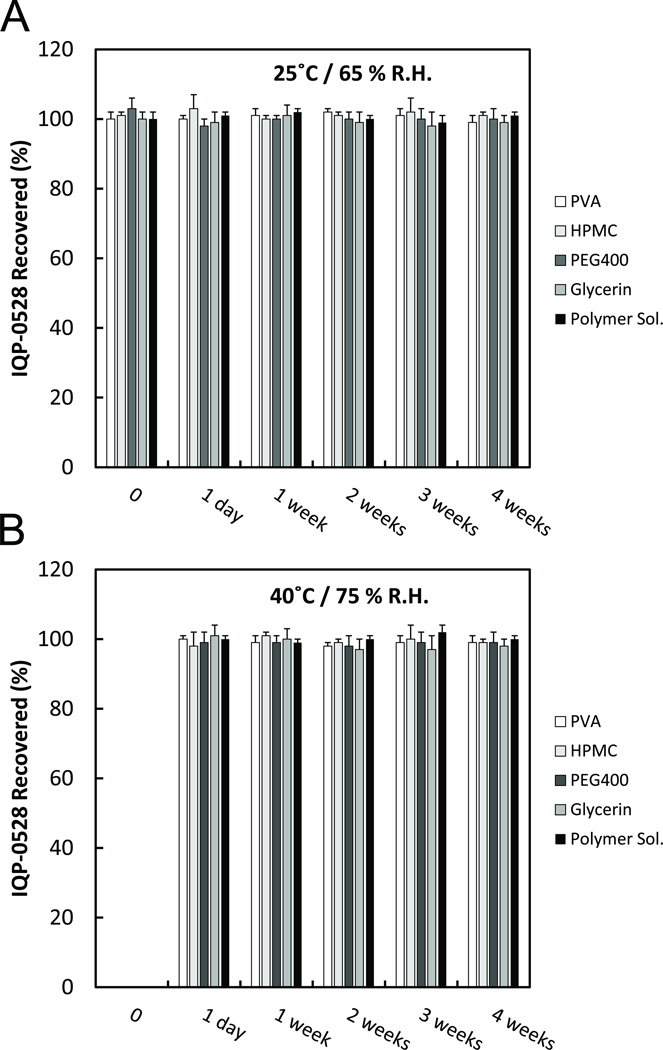
The compatibility of IQP-0528 in the planned film excipients was determined under various conditions to ensure safe effective delivery of drug from the film. For 30 days within two temperature and humidity conditions, IQP-0528 mixed with the individual film excipients PVA, HPMC, glycerin, and PEG and the combined excipients did not result in significant drug loss (p < 0.05) over this time period (Figure 1).
Figure 1.
(A) The recovery of IQP-0528 from the film excipients over 4 weeks at 25°C and 65% R.H. IQP-0528 solubilized in propylene glycol was mixed with PVA (white), HPMC (left diagonals), PEG400 (grey), glycerin (right diagonals), and the sum of all the excipients (polymer solution) (black). Each mixture was stored at 25°C and 65% R.H. and tested via HPLC every 7 days. (B) The recovery of IQP-0528 from the film excipients over 4 weeks at 40°C and 75% R.H. IQP-0528 solubilized in propylene glycol was mixed with PVA (white), HPMC (left diagonals), PEG400 (grey), glycerin (right diagonals), and the sum of all the excipients (polymer solution) (black). Each mixture was stored at 40°C and 75% R.H. and tested via HPLC every 7 days. Percent recovery was compared to IQP-0528 in propylene glycol. ± standard deviation; n = 3
Formulation of IQP-0528 into films
The various films were manufactured through solvent casting methods and the physical results of the different films are shown in Table II. From a developed TPP, the films were defined as having an area of 1200 mm2, a weight of less than 300 mg, a semi-transparent appearance with a smooth texture, a disintegration time of less than 10 minutes, a product moisture content of less than 5%, and a high in mano pliability. The formulations that did not meet these criteria were removed from further development.
Film physical characteristics
The films formulated were manufactured into molds resulting in films 30 mm × 40 mm in size. The thickness of the films varied between 0.085 and 0.108 mm suggesting that the formulation differences used in this study did not produce any significant effects (p < 0.05) to the thickness of the films (Table II). With these physical dimensions, the average weight of a film was 178 ± 14 mg. The water content of the films was 4.551 ± 0.09 % on average. The films were generally smooth, pliable, and semi-transparent in appearance. The major contributing factor that altered film appearance was the amount of HPMC in the formulation (Table II). The films became more textured, rigid, and opaque as the ratio of HPMC was increased. With a target of disintegration in less than 10 minutes, a designed characteristic of the films is the ability to rapidly dissolve upon exposure to aqueous environment.
Table II.
Vaginal Film Physical Properties
| Formulation | Dimension (mm × mm) |
Thickness (µm) |
Weight (mg) |
Water Content (%) | Film Disintegration (minutes) |
Appearance | Pliability |
|---|---|---|---|---|---|---|---|
| P1 | 30 × 40 | 90 ± 4 | 228 ± 3 | 4.555 ± 0.034 | 5.5 ± 0.8 | Semi-transparent | Fragile |
| P2 | 30 × 40 | 101 ± 5 | 230 ± 9 | 4.663 ± 0.063 | 7.6 ± 2.1 | Semi-transparent | High |
| P3 | 30 × 40 | 105 ± 2 | 233 ± 1 | 4.899 ± 0.045 | 10.4 ± 1.1 | Semi-transparent | Moderate |
| P4 | 30 × 40 | 108 ± 6 | 236 ± 4 | 4.331 ± 0.021 | 25.3 ± 5.6 | Semi-transparent | Low |
| H1 | 30 × 40 | 85 ± 8 | 228 ± 9 | 4.322 ± 0.045 | 6.3 ± 1.9 | Transparent | Fragile |
| H2 | 30 × 40 | 94 ± 2 | 230 ± 4 | 4.665 ± 0.100 | 10.4 ± 1.1 | Semi-transparent | High |
| H3 | 30 × 40 | 101 ± 2 | 234 ± 5 | 4.100 ± 0.094 | 20.1 ± 3.4 | Opaque | Low |
| H4 | 30 × 40 | 109 ± 5 | 238 ± 2 | 5.000 ± 0.012 | 35.2 ± 2.5 | Opaque | Very Low |
| G1 | 30 × 40 | 100 ± 2 | 231 ± 5 | 4.555 ± 0.032 | 6.9 ± 2.5 | Semi-transparent | High |
| G2 | 30 × 40 | 99 ± 3 | 230 ± 4 | 4.655 ± 0.014 | 7.7 ± 1.8 | Semi-transparent | High |
| G3 | 30 × 40 | 98 ± 6 | 228 ± 9 | 4.338 ± 0.064 | 7.6 ± 2.1 | Semi-transparent | High |
| G4 | 30 × 40 | 101 ± 2 | 230 ± 1 | 4.298 ± 0.098 | 8.0 ± 1.4 | Semi-transparent | Moderate |
| PG1 | 30 × 40 | 102 ± 1 | 232 ± 8 | 4.587 ± 0.059 | 7.6 ± 1.4 | Transparent | High |
| PG2 | 30 × 40 | 100 ± 2 | 232 ± 4 | 4.627 ± 0.068 | 7.1 ± 2.1 | Transparent | High |
| PG3 | 30 × 40 | 101 ± 6 | 232 ± 2 | 4.583 ± 0.058 | 7.6 ± 2.1 | Semi-transparent | High |
| PG4 | 30 × 40 | 102 ± 3 | 230 ± 7 | 4.713 ± 0.085 | 7.8 ± 1.7 | Semi-transparent | Low |
0.1 % (w/w) IQP-0528
± standard deviation; n = 3;
The effect of changing formulation on disintegration time was measured in formulations P1 – P4 and H1 – H4 (Table II). The disintegration time of the film is dependent upon the PVA and HPMC amounts in the formulations with P1 = 5.5 ± 0.8 minutes, P2 = 7.6 ± 2.1 minutes, P3 = 10.4 ± 1.1 minutes, P4 = 25.3 ± 5.6 minutes, H1 = 6.3 ± 1.9 minutes, H2 = 10.4 ± 1.1 minutes, H3 = 20.1 ± 3.4 minutes, and H4 = 35.2 ± 2.5 minutes. As both PVA and HPMC is increased, the time of film disintegration increases as well. Changes in propylene glycol, PEG400, and glycerin did not have any effect upon film disintegration (Table II). Finally, none of the dosing levels of IQP-0528 tested in this study had any effect on film appearance, texture, disintegration, pliability, or moisture content (data not shown).
Under the definitions of the TPP, various film formulations met the requirements for an acceptable film. However, several undefined qualitative factors, such as the film adhering to itself (G1 – G2) or completely transparent appearance (PG1 – PG2), eliminated these potential film formulations. Therefore, the formulation that best met the overall defined physical TPP criteria was formulation P2, containing 13.77% PVA-403, 3.44% glycerin, 6.89% PEG400, 6.89% HPMC, and 68.85% propylene glycol (dry w/w). P2 was highly pliable, semi-transparent, had a moisture content of 4.663 ± 0.063%, and a disintegration time of 7.6 ± 2.1 minutes. Based upon dosing levels of IQP-0528 in microbicide gel products currently under development, a target dose of 0.1% (w/w) IQP-528 per film was chosen (0.8 mg per film). To determine the effect of dosing levels on drug delivery and film properties, films also containing 0.05% (w/w) IQP-0528 (0.4 mg per film) and 0.085% (w/w) IQP-0528 (0.68 mg per film) were evaluated.
IQP-0528 release from film
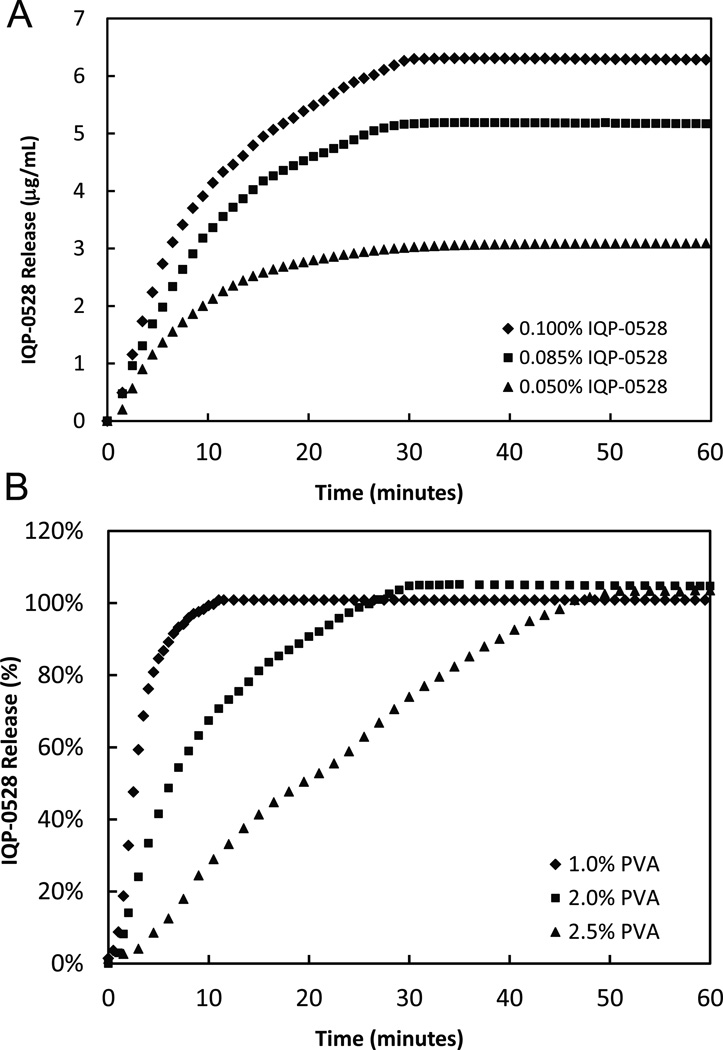
The release of IQP-0528 from the films was measured via a class IV USP dissolution apparatus at 37°C with a flow rate of 5 mL/min. From the film formulation P2, the effect of dosing levels of IQP-0528 on drug release rates was measured (Figure 2a). With the IQP-0528 dosing levels of 0.05%, 0.085%, and 0.10%, a loading dependent release was measured where increased dosage yielded increased drug release. In all cases, over 50% of IQP-0528 was released into the system by 10 minutes and near 100% total drug release was measured by 60 minutes. The effect that PVA amounts in the formulation have upon the release of IQP-0528 from the film was investigated (Figure 2b). Similar to disintegration time, the release of IQP-0528 was dependent upon PVA amounts where increased amounts of PVA in the formulation resulted in a slower release of drug.
Figure 2.
(A) The in vitro release of various loaded doses of IQP-0528 from the vaginal films (formulation P2) was performed in a USP Apparatus 4 – flow through dissolution system. At a flow rate of 10 mL/min, films containing 0.100% (w/w) IQP-0528 (diamond), 0.085% (w/w) IQP-0528 (square), and 0.050% (w/w) IQP-0528 (triangle) were evaluated for 60 minutes. A dose dependent release of IQP-0528 was measured with each film release near 100% of the loaded drug. (B) The in vitro release of IQP-0528 from the vaginal films containing varying concentrations of PVA (formulations P1, P3, and P4) was performed in a USP Apparatus 4 – flow through dissolution system. At a flow rate of 10 mL/min, films containing 1.0% (w/w) PVA (diamond), 2.0% (w/w) PVA (square), and 2.5% (w/w) PVA (triangle) were evaluated for 60 minutes. With an IQP-0528 loading dose of 0.100% (w/w), the increased concentration of PVA resulted in a reduced rate of drug release.
IQP-0528 film toxicity
The toxicity of the IQP-0528 film formulation P2 was evaluated against three strains of the normal vaginal flora Lactobacillus(L. acidophilus, L. crispatus, and L. jensenii) upon manufacturing and throughout the 12 month stability testing (Table III). At both environmental conditions, the IQP-0528 films did not display any toxic effects to the bacteria at the highest concentrations evaluated (28.4 µM for times 0 – 180 days and 19.9 µM for time 365 days).
Table III.
Toxicity of the P2 Films to the Normal Vaginal Flora Lactobacillus
| TC50 (µg/mL) | |||
|---|---|---|---|
| L. acidophilus | L. crispatus | L. jensenii | |
| 35°C / 65 %R.H. | |||
| 0 days | >10 | >10 | >10 |
| 3 days | >10 | >10 | >10 |
| 7 days | >10 | >10 | >10 |
| 14 days | >10 | >10 | >10 |
| 21 days | >10 | >10 | >10 |
| 28 days | >10 | >10 | >10 |
| 90 days | >10 | >10 | >10 |
| 180 days | >10 | >10 | >10 |
| 365 days | >7 | >7 | >7 |
| 40°C / 75 %R.H. | |||
| 0 days | >10 | >10 | >10 |
| 3 days | >10 | >10 | >10 |
| 7 days | >10 | >10 | >10 |
| 14 days | >10 | >10 | >10 |
| 21 days | >10 | >10 | >10 |
| 28 days | >10 | >10 | >10 |
| 90 days | >10 | >10 | >10 |
| 180 days | >10 | >10 | >10 |
| 365 days | >7 | >7 | >7 |
Anti-HIV efficacy of the IQP-0528 film
The P2 film formulation, at a loading dose of 0.800 mg/film, was evaluated for anti-HIV activity in CEM-SS cells and human PBMCs against HIV-1IIIB and HIV-192/HT/599, respectively. The initial evaluation of the IQP-0528 film at both stability conditions yielded an EC50 against HIV-1IIIB of 0.3 ± 0.1 nM and an EC50 of 0.16 ±0.06 nM against HIV-192/HT/599 (Table IV). The TC50 in both assays were greater than the “high test concentrations”: 200 nM in the cytopathic effect (CPE) assay and 1 µM in the viral replication assay.
Table IV.
Activity of the P2 Films in CEM-SS Cells Against HIV-1IIIB
| Time (days) |
25°C / 65% Relative Humidity CEM-SS/HIV-1IIIB EC50 (nM) |
40°C / 75% Relative Humidity CEM-SS/HIV-1IIIB EC50 (nM) |
|---|---|---|
| 0 | 0.30 ± 0.00 | 0.30 ± 0.00 |
| 3 | 0.30 ± 0.14 | 0.35 ± 0.07 |
| 7 | 0.30 ± 0.10 | 0.30 ± 0.00 |
| 14 | 0.35 ± 0.07 | 0.30 ± 0.00 |
| 21 | 0.20 ± 0.00 | 0.25 ± 0.07 |
| 28 | 0.30 ± 0.00 | 0.30 ± 0.00 |
| 90 | 0.55 ± 0.64 | 0.40 ± 0.14 |
| 180 | 0.55 ± 0.64 | 0.60 ± 0.00 |
| 365 | 0.42 ± 0.00 | 0.25 ± 0.00 |
± standard deviation; n = 3
Stability of IQP-0528 films
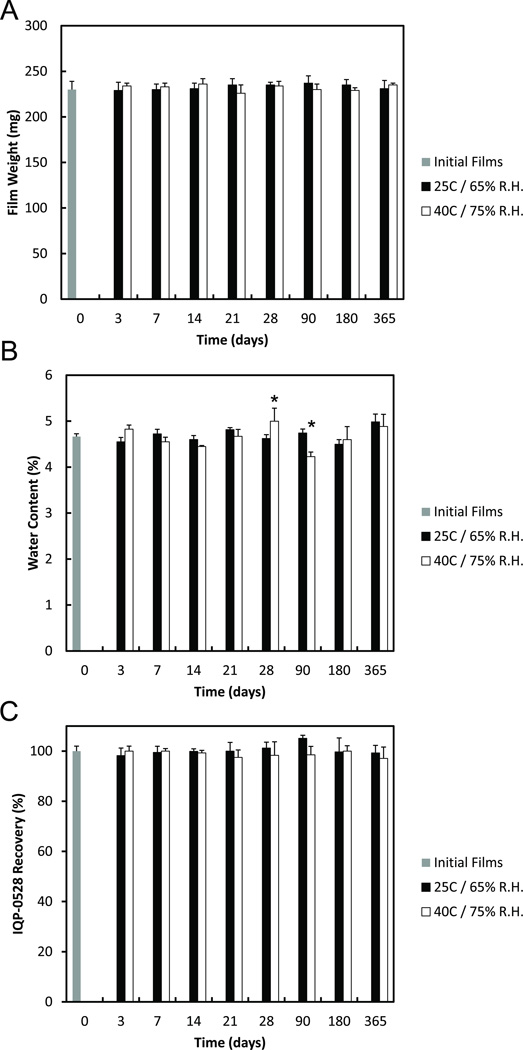
The stability of the P2 film formulation with IQP-0528 was evaluated for 12 months under ICH recommend environmental conditions. The films were packaged and stored at 25°C / 65% R.H. and at accelerated conditions 40°C / 75% R.H. for 12 months. At each time point (initial, 3 days, 1 week, 2 weeks, 3 weeks, 1 month, 3 months, 6 months, and 12 months) stability indicating assays were performed on the films: film weights (Figure 3a), water content (Figure 3b), and drug content and recovery (Figure 3c). The film’s toxicity and anti-HIV efficacy over the stability time period was measured and is presented in Tables III – V, respectively. In all physical testing, the films maintained a stable characteristic and behavior with less than 10% deviation from initial manufacturing. The only significant deviation (p < 0.05) occurred in the accelerated condition moisture content analysis at 3 months and 9 months. Additionally, the IQP-0528 films maintained their anti-HIV activity throughout the 1 year stability program at both standard ICH and accelerated ICH environmental conditions (Table V).
Figure 3.
(A) The stability of IQP-0528 film weight over 12 months. After manufacturing, the weight of the films was measured to provide a baseline film weight (grey). The films were then packaged into individual air-tight light-proof pouches and stored under 25°C / 65% R.H. (black) and 40°C / 75% R.H. (white), respectively. (B) The stability of IQP-0528 film moisture content (% water) over 12 months. After manufacturing, the moisture content of the films was measured to provide a baseline film weight (grey). The films were then packaged into individual air-tight light-proof pouches and stored under 25°C / 65% R.H. (black) and 40°C / 75% R.H. (white), respectively. (C) The stability of IQP-0528 from the film over 12 months. After manufacturing, the IQP-0528 content of the film was measured via HPLC as a percent recovery of the loaded amount (grey). The films were then packaged into individual air-tight light-proof pouches and stored under 25°C / 65% R.H. (black) and 40°C / 75% R.H. (white), respectively. For each evaluation, at the specified time points, the films were removed from storage and allowed to equilibrate to ambient temperature. The films were then removed from their packaging and evaluated for their stability. ± standard deviation; n = 3; * p < 0.05
Table V.
Activity of the P2 Films Against HIV-192/HT/599 in Human PBMCs
| EC50 (nM) | TC50 (nM) | TI | |
|---|---|---|---|
| 25°C / 65 %R.H. | |||
| 0 days | 0.15 ± 0.05 | >50 | >333 |
| 3 days | 0.20 ± 0.00 | >50 | >250 |
| 7 days | 0.25 ± 0.07 | >50 | >200 |
| 14 days | 0.10 ± 0.00 | >50 | >500 |
| 21 days | 0.20 ± 0.00 | >50 | >250 |
| 28 days | 0.25 ± 0.21 | >50 | >200 |
| 90 days | 0.29 ± 0.00 | >50 | >172 |
| 180 days | 0.35 ± 0.00 | >50 | >143 |
| 365 days | 0.28 ± 0.18 | >50 | >178 |
| 40°C / 75 %R.H. | |||
| 0 days | 0.15 ± 0.05 | >50 | >333 |
| 3 days | 0.15 ± 0.07 | >50 | >333 |
| 7 days | 0.25 ± 0.07 | >50 | >200 |
| 14 days | 0.15 ± 0.07 | >50 | >333 |
| 21 days | 0.24 ± 0.00 | >50 | >208 |
| 28 days | 0.28 ± 0.11 | >50 | >182 |
| 90 days | 0.46 ± 0.26 | >50 | >108 |
| 180 days | 0.29 ± 0.10 | >50 | >172 |
| 365 days | 0.18 ± 0.00 | >50 | >278 |
± standard deviation; n = 3
DISCUSSION
Pyrimidinediones (PYDs) are highly potent small molecule inhibitors that have a dual mechanism of action against HIV infection targeting viral entry and reverse transcription. IQP-0528 was identified as the lead candidate for the topical microbicide development from a SAR series of 68 molecules. In standard in vitro assays, IQP-0528 was active against clinical strains of virus in the nanomolar to sub-nanomolar concentration range in PBMCs, monocytes-macrophages, and monocyte-derived dendritic cells [11, 24]. Although stable, IQP-0528, and PYDs in general have a low solubility in aqueous solutions making formulation into aqueous-based dosage forms, including gels and creams, challenging. Therefore, the goal of this study was to develop and evaluate a solid dosage film formulation for the vaginal delivery of IQP-0528.
In developing a vaginal film for IQP-0528 delivery, the choice of excipients for the film formulation needs to be compatible with the microbicide drug candidate. The films developed in this study were composed of polyvinyl alcohol, hydroxypropyl methylcellulose, propylene glycol, polyethylene glycol, and glycerin. All the excipients, with the exception of PVA which is listed for ophthalmic, oral topical use but not specifically vaginal, are listed in the FDA Inactive Ingredients Database as accepted excipients in vaginal products. Despite PVA not being in the FDA database for vaginal products, PVA is used in commercially available products such as Vaginal Contraceptive Film (VCF) and VCF Dissolving Vaginal Cleansing Film. Additionally, PVA is used in a wide variety of other pharmaceutical products at higher concentrations than those used in the developed film. Similarly, HPMC and PEG do not have stated total allowable amounts in vaginal products in the database; however, these ingredients are also found in pharmaceutical products in far greater concentrations than those used in the developed vaginal film. From the compatibility assays performed on the film excipients and IQP-0528, no detrimental effects were observed, either individually or as a mixture of all the excipients. IQP-0528 was recovered fully without degradation.
Having established that the potential excipients were compatible with IQP-0528, a film formulation was developed using the compatible excipients. Our study tested a wide range of film formulation compositions where each of the individual excipients was varied. The excipients of the film formulation were varied to determine the specific effect each has upon the film’s physicochemical characteristics: PVA (P1–P4), HPMC (H1–H4), glycerin (G1–G4), and PEG400 (PG1–PG4). The specific order of addition of the films was developed to produce uniform integration of the proceeding excipient to the total polymer solution. The IQP-0528 was solubilized separately in propylene glycol before lastly being adding to prevent the drug from precipitating into the aqueous polymer solution. Due to this manufacturing protocol, the films were produced in uniform molds resulting in consistent film area. Additionally, since the formulation molds were poured based upon volume and dried under identical conditions, the films all resulted in constant weight, thickness, and moisture content. Therefore, it is suggested that the physical dimensions of the films are not affected by formulation but by manufacturing process. Cosmetic appearance of the films was only affected by the amount of HPMC in the formulation. As the concentration of HPMC was increased, the films became more opaque in coloration and rigid in pliability. In all the film formulation variations evaluated, the physical dimension and cosmetic appearance were not dependent upon film composition producing no identifiable lead formulation. However, film pliability was dependent upon excipient concentrations. While glycerin and PEG400 had no effect, increased concentrations of PVA and HPMC resulted in films becoming increasingly rigid.
The defining physical characteristic of the vaginal film is the rate of disintegration. In the formulation variations evaluated in this study, it was observed that film disintegration time was dependent upon PVA and HPMC concentrations and independent upon PEG400 and glycerin concentrations. As concentrations of the PVA were increased in the formulation, the time for complete visual film disintegration was increased. Increased concentrations of the HPMC similarly resulted in increased film disintegration times. With a defined TPP disintegration time of less than 10 minutes, only film formulations P1, P2, and H1 fell within parameters. When combined with the physical evaluations of the film formulations, formulation “P2” was identified as a potential final film formulation. Film P2 resulted in a smooth pliable semi-transparent film with a surface area of 30 mm × 40mm, 0.1 mm thickness, 230 mg, 4.663 % water, and disintegration time of 7.6 minutes. While the films themselves have a physical rapid disintegration time, the release of drug from the polymer matrix is more important as a microbicide product. From USP in vitro release studies, we confirmed that under sink conditions, IQP-0528 is released rapidly from the films. At the bench mark of 10 minutes, 50% of the IQP-0528 is freely available and by 30 minutes, ~100% of the loaded IQP-0528 is available. The drug release gradient beyond the measured disintegration time suggests that while the overall films structure has visually disintegrated, there remain semi-solid portions of the film that have yet completely dissolved to release IQP-0528. The release of IQP-0528 was found to be dose level dependent. No upper limit was detected for the dosing level amounts investigated in this study. Additionally, as increased PVA concentrations in the formulation increases the time for film disintegration, the release rate of IQP-0528 is similarly PVA concentration specific.
Because there are no previous data on the in vivo delivery of microbicides from films, the defined TPP for acceptable film disintegration and subsequent drug delivery was based upon the goal of formulating a film that would dissolve rapidly in the target environment similar to other microbicide films under development [21]. The volumes of dissolution fluid in the disintegration and dissolution assays were minimized to reflect projected in vivo vaginal fluid levels. For these studies DI water was primary fluid and due the insolubility of IQP-0528 in aqueous media, acetonitrile was used to maintain sink conditions.
Beyond its physical characteristics, the IQP-0528 films must be non-toxic to vaginal flora while providing efficacious anti-HIV protection. Lactobacillus is a common bacteria comprising the vaginal microflora that acts as a natural defense against infection [25]. Lactobacillus in vitro assays provide a representative determination of a product’s toxicity to the local vaginal environment. The IQP-0528 films demonstrated no toxicity to a concentration of 28.4 µM against three strains of the normal flora: L. acidophilus, L. crispatus, and L. jensenii which is a 1000-fold higher than the expected concentrations of the vaginal film. Toxicity evaluations performed on films under stability protocols resulted in similar non-toxic effects over the course of 12 months suggesting a safe formulation.
In anti-HIV efficacy evaluations, 0.100% (w/w) IQP-0528 dosing resulted in EC50 values of 0.3 ± 0.1 nM against HIV-1IIIB and 0.16 ±0.06 nM against HIV-192/HT/599. These values are comparable to preformulation EC50 values for IQP-0528 [11]. The comparable sub-nanomolar EC50 values indicate that the film formulation does not negatively affect the in vitro activity of IQP-0528 to prevent HIV-1 infection. With 50% toxicity concentration (TC50) values exceeding the highest evaluated concentrations in both the CPE assay (200 nM) and viral replication assay (1 µM) against cell-based assays, the formulated film dosage amount results in efficacious anti-HIV activity well below toxic concentrations. The resulting in vitro therapeutic index of the IQP-0528 film suggests that films are an effective method of microbicide delivery.
The stability of the IQP-0258 film was the final characteristic investigated in this study to support the development of vaginal films as a dosage formulation for IQP-0528. Over 12 months, and under two ICH recommended environmental conditions, packaged IQP-0528 films were evaluated for their stability. Setting the level of stability at less than 10% deviation from initial manufactured parameters, the packaged films showed no changes in physical characteristics, no degradation in IQP-0528 within the film, and no additional toxicity. The films maintained their anti-HIV activity within acceptable assay variation over the stability evaluation period. The temporary significant deviations in film moisture content observed at 3 and 9 months in the accelerated storage conditions can be attributed to faults in the packaging. Despite this, the manufactured films demonstrated evidence of stability holding IQP-0528 under conditions common to developed nations such as the US, and more importantly, in environmental conditions similar to regions of the world most affected by HIV infection.
In choosing a microbicide product, there are many factors other than API bioactivity that will influence product development, such as API chemical characteristics or patient acceptability/compliance. With HIV infection being a worldwide issue, various culture sensitivities need to be considered when presenting preventative measures to the populous. Therefore, it is of great importance to have numerous dosage forms available regardless of the success of any one particular dosage form. With semi-solids such as gels and creams being the predominant dosage form, the increased development of small molecule drugs may make the compatibility between hydrophobic molecules in a hydrophilic dosage form complicated. However, despite wide-spread use and numerous studies into gel formulations, the semi-solid nature of the products results in leakage or general “messiness” being a common problem encountered by the women that use them. In product acceptability studies for cellulose sulfate (CS) vaginal gel and KY gel, 20% reported product leakage where such leakage would prevent future gel use [26–28]. And while in coital acceptability studies, 40% of the women reported leakage during sex, 100% reported that the gels made the sexual activity feel “more wet” with 96% reporting favorable usage [29]. Yet despite this, the acceptability of gels may not be the highest ranked product in other areas of the world. In a marketing study conducted by the International Partnership for Microbicides (IPM), a product acceptability study (PASII) was conducted in urban areas of Burkina Faso, Mozambique, Zambia, and Tanzania comparing tablets, films, and soft gels. The outcome of this study resulted in an opinion that the films were favorably accepted by women [30].
Films as an additional dosage form for vaginal microbicide products offers several advantages over traditionally established dosage forms. The small size of the film and the lack of the need for applicators results in a less expensive product that is easier to store which, in turn, may reduce the cost and material needed for product manufacturing and shipping. While this study produced the films in small scale molds, the solvent evaporation manufacturing process can be scaled up through the process of solvent-cast film application where the polymeric solution is thinly applied to a substrate on a continuous roll that is rapidly dried and then die-cut into strips. The reduced cost is an important consideration in product development in both developing and developed nations. A high cost/dose precludes wide spread acceptance of this approach to reducing HIV transmission in the developing world. Finally, an important characteristic of female controlled HIV prevention may be the ability to conceal their usage from their partners. The small packaging footprint of the films allows for easy concealment, and the rapidly dissolving low volume nature of the films does not provide any physical sensation within the vagina after application.
CONCLUSION
In this study, we have formulated the potential microbicide agent IQP-0528 into a stable non-toxic vaginal film that quickly dissolves and releases drug with high anti-HIV activity. The film’s stability under various environmental conditions demonstrates its viability as a robust product. With varying economic and social conditions around the world, as well as specific consumer/patient preferences, the unacceptability of semi-solids to consumers, may ultimately result in reducing the effectiveness of microbicide products. Therefore, dosage forms such as films show promise and utility and warrant further development.
ACKNOWLDEGMENTS
We would like to acknowledge the International Partnership for Microbicides (IPM) for their initial work in vaginal film formulations as a microbicides which assisted in the development of this study.
References
- 1.UNAIDS. Global Report: UNAIDS Report on the Global AIDS Epidemic 2010. 2010 [Google Scholar]
- 2.Pool R, et al. Men's attitudes to condoms and female controlled means of protection against HIV and STDs in south-western Uganda. Culture, Health, & Sexuality. 2000;2:197–211. doi: 10.1080/136910500300804. [DOI] [PubMed] [Google Scholar]
- 3.Lederman MM, Offord RE, Hartley O. Microbicides and other topical strategies to prevent vaginal transmission of HIV. Nat Rev Immunol. 2006;6(5):371. doi: 10.1038/nri1848. [DOI] [PubMed] [Google Scholar]
- 4.Turpin J. Considerations and development of topical microbicides to inhibit the sexual transmission of HIV. Expert Opin Investig. Drugs. 2002;11(8):1007–1097. doi: 10.1517/13543784.11.8.1077. [DOI] [PubMed] [Google Scholar]
- 5.Harrison P, Rosenberg Z, Bowcut J. Topical Microbicides for Disease Prevention: Status and Challenges. Clinical Infectious Diseases. 2003;36(10):1290–1294. doi: 10.1086/374834. [DOI] [PubMed] [Google Scholar]
- 6.McCormack S, et al. PRO2000 vaginal gel for prevention of HIV-1 infection (Microbicides Development Programme 301): a phase 3, randomised, double-blind, parallel-group trial. Lancet. 2010;276(9749):1329–1337. doi: 10.1016/S0140-6736(10)61086-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mayer Kenneth H, et al. Safety and Tolerability of BufferGel, a Novel Vaginal Microbicide, in Women in the United States. Clinical Infectious Diseases. 2001;32(3):476–482. doi: 10.1086/318496. [DOI] [PubMed] [Google Scholar]
- 8.Abdool Karim Q, et al. Effectiveness and Safety of Tenofovir Gel, an Antiretroviral Microbicide, for the Prevention of HIV Infection in Women. Science. 2010;329(5996):1168–1174. doi: 10.1126/science.1193748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Buckheit RW, Jr, et al. Comparative evaluation of the inhibitory activities of a series of pyrimidinedione congeners that inhibit human immunodeficiency virus types 1 and 2. Antimicrob Agents Chemother. 2008;52(1):225–236. doi: 10.1128/AAC.00972-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Buckheit RW, Jr, et al. The structure-activity relationships of 2,4(1H,3H)-pyrimidinedione derivatives as potent HIV type 1 and type 2 inhibitors. Antivir Chem Chemother. 2007;18(5):259–275. doi: 10.1177/095632020701800502. [DOI] [PubMed] [Google Scholar]
- 11.Watson KM, Yang L, Buckheit RW., Jr Development of Dual-Acting Pyrimidinediones as Novel and Highly Potent Topical Anti-HIV Microbicides. Antimicrob. Agents Chemother. 2011;55(11):5243–5254. doi: 10.1128/AAC.05237-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buckheit RW, Jr, et al. SJ-3366, a Unique and Highly Potent Nonnucleoside Reverse Transcriptase Inhibitor of Human Immunodeficiency Virus Type 1 (HIV-1) That Also Inhibits HIV-2. Antimicrobial Agents and Chemotherapy. 2001;45(2):393–400. doi: 10.1128/AAC.45.2.393-400.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Buckheit R, Jr, et al. Development of topical microbicides to prevent the sexual transmission of HIV. Antiviral Research. 2010;85:142–158. doi: 10.1016/j.antiviral.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rohan LC, Sassi AB. Vaginal Drug Delivery Systems for HIV Prevention. AAPS Journal. 2009;11(1):78–87. doi: 10.1208/s12248-009-9082-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Mahalingam A, et al. Vaginal microbicide gel for the delivery of IQP-0528, a pyrimidinedione analog with a dual mechanism of action against HIV-1. Antimicrob Agents Chemother. 2011;55(4):1650–1660. doi: 10.1128/AAC.01368-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yehia SA, El-Gazayerly ON, Basalious EB. Fluconazole mucoadhesive buccal films: in vitro/in vivo performance. Current Drug Delivery. 2009;6(1):17–27. doi: 10.2174/156720109787048195. [DOI] [PubMed] [Google Scholar]
- 17.Mizrahi B, Domb A. Mucoadhesive polymers for delivery of drugs to the oral cavity. Recent Patents in Drug Delivery Formulation. 2008;2(2):108–119. doi: 10.2174/187221108784534126. [DOI] [PubMed] [Google Scholar]
- 18.Roddy RE, et al. A controlled trial of nonoxynol 9 film to reduce male-to-female transmission of sexually transmitted diseases. New England Journal of Medicine. 1998;339:504–510. doi: 10.1056/NEJM199808203390803. [DOI] [PubMed] [Google Scholar]
- 19.Mauk CK, et al. A phase I comparative study of contraceptive vaginal films containing benzalkonium chloride and nonoxynol-9. Postcoital testing and colposcopy. Contraception. 1997;56:89–96. doi: 10.1016/s0010-7824(97)00097-8. [DOI] [PubMed] [Google Scholar]
- 20.Garg S, et al. Development and characterization of bioadhesive vaginal films of sodium polystyrene sulfonate (PSS), a novel contraceptive antimicrobial agent. Pharmaceutical Research. 2005;22:584–595. doi: 10.1007/s11095-005-2490-1. [DOI] [PubMed] [Google Scholar]
- 21.Akil A, et al. Development and characterization of a vaginal film containing dapivirine, a non-nucleoside reverse transcriptase inhibitor (NNRTI), for prevention of HIV-1 sexual transmission. Drug Delivery and Translational Research. 2011;1(3):209–222. doi: 10.1007/s13346-011-0022-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Buckheit RW, Jr, et al. Structure-activity and cross-resistance evaluations of a series of human immunodeficiency virus type-1-specific compounds related to oxathiin carboxanilide. Antimicrob Agents Chemother. 1995;39(12):2718–2727. doi: 10.1128/aac.39.12.2718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Watson KM, Buckheit CE, Buckheit RW., Jr Comparative evaluation of virus transmission inhibition by dual-acting pyrimidinedione microbicides using the microbicide transmission and sterilization assay. Antimicrob Agents Chemother. 2008;52(8):2787–2796. doi: 10.1128/AAC.01657-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahalingam A, et al. Vaginal Microbicide Gel for Delivery of IQP-0528, a Pyrimidinedione Analog with a Dual Mechanism of Action against HIV-1. Antimicrob. Agents Chemother. 2011;55(4):1650–1660. doi: 10.1128/AAC.01368-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Linhares IM, et al. Contemporary perspectives on vaginal pH and lactobacilli. American journal of obstetrics and gynecology. 2011;204(2):120.e1–120.e5. doi: 10.1016/j.ajog.2010.07.010. [DOI] [PubMed] [Google Scholar]
- 26.Holt BY, et al. Microbicide preference among young women in California. J Womens Health (Larchmt) 2006;15(3):281–294. doi: 10.1089/jwh.2006.15.281. [DOI] [PubMed] [Google Scholar]
- 27.Malonza IM, et al. Expanded Phase I safety and acceptability study of 6% cellulose sulfate vaginal gel. Aids. 2005;19(18):2157–2163. doi: 10.1097/01.aids.0000194797.59046.8f. [DOI] [PubMed] [Google Scholar]
- 28.Schwartz JL, et al. Fourteen-day safety and acceptability study of 6% cellulose sulfate gel: a randomized double-blind Phase I safety study. Contraception. 2006;74(2):133–140. doi: 10.1016/j.contraception.2006.02.008. [DOI] [PubMed] [Google Scholar]
- 29.El-Sadr WM, et al. Safety and acceptability of cellulose sulfate as a vaginal microbicide in HIV-infected women. AIDS. 2006;20(8):1109–1116. doi: 10.1097/01.aids.0000226950.72223.5f. [DOI] [PubMed] [Google Scholar]
- 30.Nel AM, et al. Acceptability of Vaginal Film, Soft-Gel Capsule, and Tablet as Potential Microbicide Delivery Methods Among African Women. Journal of Women's Health. 2011;20(8):1207–1214. doi: 10.1089/jwh.2010.2476. [DOI] [PubMed] [Google Scholar]