Abstract
These studies have examined the effect of hypoxia inducible factor 1α (HIF-1α) on nucleotide metabolism in the ischemic heart using a genetic mouse model with heart-specific and regulated expression of a stable form of HIF-1α. We find that AMP deaminase (AMPD), the entry point of the purine nucleotide cycle (PNC), is induced by HIF-1α at the level of mRNA, protein, and activity. AMP that accumulates during ischemia can be metabolized to adenosine by 5′-nucleotidase or to IMP by AMPD. Consistent with the finding of AMPD induction, adenosine accumulation during ischemia was much attenuated in HIF-1α-expressing hearts. Further investigation of nucleotide salvage enzymes found that hypoxanthine phosphoribosyl transferase (HPRT) is also upregulated in HIF-1α-expressing hearts. Treatment of hearts with an inhibitor of the PNC, hadacidin, hastens the fall of the adenylate energy charge during ischemia and the accumulation of AMP. The results provide new insight into the role of the PNC in heart, especially as it relates to ischemia, and indicate that HIF-1α regulates nucleotide metabolism as a compensatory response to hypoxia.
INTRODUCTION
These studies have used a mouse model as described by Bekeredjian et al [1] containing a cardiac-specific, oxygen-stabilized, doxycycline (Dox)-off regulated HIF-1α transgene (HIF-1α-PPN) to probe the role of HIF-1α in purine metabolism. Hypoxia inducible factor 1α (HIF-1α) is a master regulatory transcription factor that directs the transcription of a multitude of genes that provide adaptive responses when O2 levels decrease. The cardio-specific role of HIF-1α is less understood, however recent evidence indicates that HIF-1α plays a central role in the protection of myocardium against hypoxic stress. Several studies indicate that HIF-1α is necessary for preconditioning protection [2,3] and our previous work showed that the overexpression of HIF-1α in cardiomyocytes confers robust protection in adult hearts subjected to ex vivo ischemia [4]. We attributed the protection by HIF-1α to the cardiomyocyte’s ability to maintain mitochondrial polarization during anoxia or when cytochrome c oxidase was inhibited with cyanide [4]. We also demonstrated preservation of electron transport chain (ETC) activity by utilization of fumarate as a terminal electron acceptor in the absence of O2 [5]. Fumarate is reduced to succinate at complex 2 and allows for the continued pumping of H+ by complex 1 in the absence of cytochrome c oxidase activity. This reduction of fumarate with the concomitant production of succinate has been shown to operate in kidney tubule and heart tissue previously; albeit at low levels [6,7]. Thus, the capacity to employ fumarate reduction is inducible through HIF-1α stabilization [5,8].
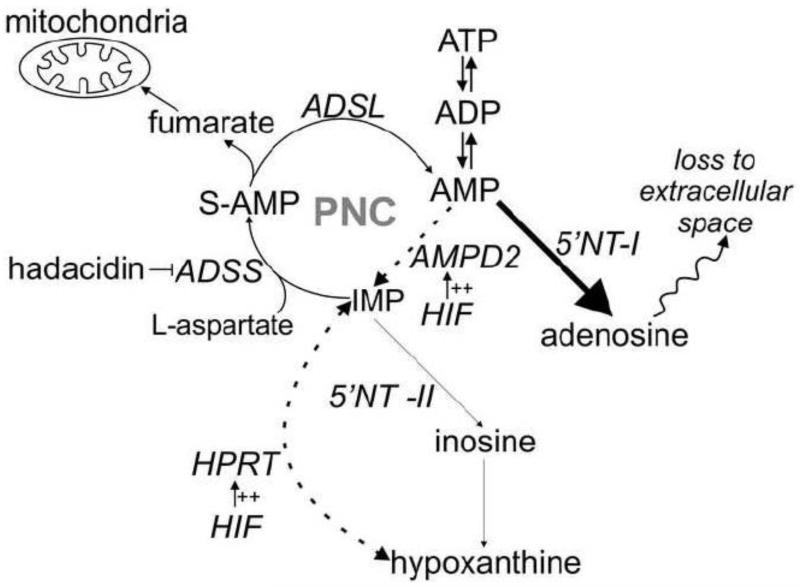
Further studies identified the source of fumarate used for anaerobic ETC activity as the purine nucleotide cycle (PNC) and incidentally suggested that PNC activity might be higher in cardiomyocytes treated with prolyl hydroxylase inhibitors to induce HIF-1α levels [5]. The previous suggestion that the PNC metabolic pathway is upregulated by HIF-1α largely motivates the present work; a systematic examination of the enzymes of the PNC and the catabolism of adenonucleotides during ischemia in HIF-1α-over-expressing hearts. The entry reaction of the PNC is catalyzed by AMP deaminase and converts AMP into IMP (Figure 1). In a series of two reactions, catalyzed by adenylosuccinate synthetase and adenylosuccinate lyase, aspartate and GTP are consumed and fumarate and AMP are produced to complete the cycle. The PNC has been mostly studied in skeletal muscle where it has been suggested to help maintain the energy charge (EC); where EC=([ATP]+0.5[ADP])/([ATP]+[ADP]+[AMP]) by preventing the rise of AMP and ADP during strenuous exercise [9]. The energy charge ranges from 0 to 1 as proposed by Atkinson and is an index of the cellular energy state [10]. A high energy charge is indicative of favorable conditions for ATP utilization to carry out cellular work. The PNC may also act as an anaplerotic pathway that generates fumarate for the citric acid cycle [11]. Direct confirmation of the function of the PNC in skeletal muscle is lacking, whereas a significant metabolic role for the PNC in heart has been discounted entirely by some investigators [12].
Figure 1. Nucleotide degradation pathways.
During ischemia, the rate of ATP consumption exceeds its synthesis from glycolysis. As a result, there is a net degradation of ATP and the heart’s nucleotide pool while nucleosides and nucleobases accumulate. PNC = purine nucleotide cycle. AMPD2 = AMP deaminase isoform 2. ADSS = adenylosuccinate synthetase. ADSL = adenylosuccinate lyase. 5′NT-I = 5′-nucleotidase isoform 1 (AMP specific). 5′NT-II = 5′-nucleotidase isoform 2 (IMP specific). HPRT = hypoxanthine phosphoribosyl transferase. Major pathways are indicated by bold arrows. HIF-1α induced pathways are indicated by dashed arrows.
HIF-1α is stabilized through inhibition of the prolyl hydroxylase domain-containing (PHD) enzymes at [O2] far above those that limit respiration, and can thus be regarded as anticipatory in nature. In this scheme HIF-1α responds to sublethal levels of hypoxia, directing compensatory changes that equip the cardiomyocyte with the ability to survive ischemic stress. For example, HIF-1α upregulates glycolytic enzymes, thereby, increasing the capacity to generate ATP anaerobically [13]. Thus, the upregulation of the PNC by HIF-1α might suggest a protective role for the PNC against ischemic stress. This is certainly in line with our finding that fumarate derived from the PNC allows for the anaerobic operation of respiratory complex 1. These considerations motivate the present studies where we have 1) evaluated the effects of HIF-1α overexpression on the enzymes that constitute the PNC, as well as other nucleotide salvage enzymes; and, 2) followed the adenylate nucleotide breakdown products during a bout of ischemia in hearts with, and without, enhanced HIF-1α expression. In heart, ischemia limits ATP production via oxidative phosphorylation and glycolysis is used to generate ATP anaerobically. However, the rate of glycolytic ATP synthesis is insufficient to meet the ATP demands of the working heart. As in skeletal muscle during intense exercise, ATP and the nucleotide pool becomes rapidly depleted while nucleosides and nucleobases accumulate (Figure 1) [14,15]. Taken together, the above considerations may suggest that the PNC plays a role in heart during ischemia similar to skeletal muscle during intense work; albeit after the pathway is induced by HIF-1α.
MATERIALS AND METHODS
Animal model
In order to examine the effect of HIF-1α on nucleotide metabolism in the ischemic heart, we utilized B6C3F1 mice that contain a doxycycline inducible HIF-1α transgene (HIF-1α-PPN) that has been previously described [1]. All mice used were males between 2 and 4 months of age. For experiments in which the HIF-1α transgene was not induced (Non-I), mice were maintained on a 625 mg/kg doxycycline-replete diet throughout (Harlan Research Laboratories, Madison, WI). In experiments requiring 2 days of HIF-1α expression (2D), mice were switched from doxycycline food to doxycycline-replete water containing 73 mM sucrose (Mallinckrodt Baker, Phillsburg, NJ) and 0.416 mM doxycycline hydrochloride (Research Product International, Mount Prospect, IL) for 2 days followed by maintenance of mice on regular food and water for two additional days. In experiments requiring 6 days of HIF-1α expression (6D), mice were maintained on doxycycline-free food and water for 5–7 days before experimentation. Results from HIF-1α-PPN mice were compared to those from B6C3F1 wildtypes obtained from Harlan Research Laboratories. Animals were handled in accordance to a protocol reviewed and approved by the East Tennessee State University Committee on Animal Care.
Realtime PCR mRNA quantification
RNA was extracted from hearts using TRIzol reagent (Life Technologies, Carlsbad, CA) and concentration as well as integrity were determined using the Agilent Bioanalyzer (Agilent Technologies, Santa Clara, CA). cDNA was synthesized using the Superscript III cDNA synthesis kit (Life Technologies). For each cDNA synthesis reaction, 2 μg of RNA was added to 1.25 μM oligo(dT)20, 50 ng random hexamers, 0.5 mM dNTP mix, 80 U RNaseOUT, 5 mM DTT, 1.25 mM MgCl2, and 400 U reverse transcriptase in a final volume of 40 μl. The cDNA synthesis reaction was allowed to proceed for 1 hour at 50°C and then terminated by heating at 70°C for 15 minutes. Quantitative PCR was performed in triplicate for all samples in a 96 well plate format on a CFX96 real-time PCR detection system (BioRad Laboratories, Richmond, CA). 40 cycles were carried out. Each reaction consisted of 50 ng cDNA and 1.5 μl of Quantitect AMPD2, HPRT, or transferrin primer from Qiagen in 1× SsoFast Evagreen supermix (BioRad Laboratories). AMPD2 and HPRT mRNA expression was normalized to that of reference gene transferrin and results were reported as a percent change in gene expression relative to WT.
Western blot – protein expression
Hearts obtained from WT, Non-I, 2D, and 6D mice were ground into a fine powder over liquid nitrogen. The frozen heart powder was then homogenized in RIPA buffer composed of 50 mM Tris·HCl, pH 7.4 (Calbiochem, Darmstadt, Germany), 1% Triton X-100 (Fisher, Pittsburgh, PA), 1% w/v sodium deoxycholate (Fisher), 0.1% w/v SDS (EMD, Billerica, MA), and 1 mM EDTA (Fisher) with 1:40 protease inhibitor cocktail mix (Sigma, St. Louis, MO). The homogenates were centrifuged at 12,000 g at 4°C for 10 minutes and the supernatant was collected. Protein concentration for mice heart homogenates was determined using the Pierce BCA protein assay kit from Thermoscientific (Rockford, IL) according to the manufacturer’s protocol. Homogenates were then subjected to SDS-PAGE using Pierce Tris-HEPES-SDS 4–20% precast polyacrylamide gels (Thermoscientific). After SDS-PAGE, proteins were transferred to PVDF membranes (BioRad Laboratories) and Ponceau S (Sigma) staining was used to ensure complete transfer and equal protein loading. Membranes were blocked in 5% nonfat dry milk in TBS with 0.1% Tween 20 (TBS-T) for 1 hour at room temperature. AMPD2 was probed using a mouse monoclonal primary antibody diluted 1:1000 in TBS-T (Abcam, Cambridge, MA). AMPD3 was probed using a rabbit polyclonal primary antibody diluted 1:100 in TBS-T (Abcam). HPRT was probed using a rabbit polyclonal primary antibody diluted 1:1000 (Novus Biologicals, Littleton, CO). The membranes were incubated in primary antibody at 4°C overnight. After incubation in primary antibody, the membranes were washed for 5 minutes in TBS-T (5×) before incubation with 1:5000 rabbit anti-mouse (Abcam) for AMPD2 or 1:2500 goat anti-rabbit (EMD Millipore) horseradish peroxidase-conjugated secondary antibodies for AMPD3 and HPRT. Protein bands were detected using the Pierce supersignal west pico chemiluminescence substrate (Thermoscientific, Rockford, IL) in the G-Box imaging system (Syngene, Frederick, MD). Densitometry of the protein bands corresponding to AMPD2, AMPD3 and HPRT was obtained using ImageJ (National Institutes of Health, Bethesda, MD) and results were normalized to that of WT.
AMP deaminase activity
AMP deaminase enzyme activity was assessed using a procedure described by Raffin [16]. Hearts were homogenized in a buffer consisting of (mM): 89 KH2PO4, 180 KCl, and 0.1 DTT pH to 6.5. Protein concentration was determined using the Pierce BCA protein assay kit (Thermoscientific). The volume of heart homogenate corresponding to 1 mg protein was added to 2 ml of a reaction mixture containing (mM): 50 cacodylic acid, 150 KCl, 10 AMP with pH adjusted to 6.5 using 10 N KOH. A 250 μl aliquot of the reaction was taken upon initiation of the reaction (t0) and after 2 hours of incubation at room temperature. 125 μl of 4% perchloric acid (Alfa Aesar, Ward Hill, MA) was used to extract nucleotides from the reaction aliquots and IMP was detected at 254 nm using high performance liquid chromatography. IMP production was obtained by subtracting the IMP content at the start of the reaction (t0) from that after the 2 hour incubation period. AMP deaminase activity was expressed as nmole of IMP produced per minute per mg protein.
Nucleotide metabolism
Nucleotide metabolism during ischemia was examined in WT, Non-I, 2D, and 6D mouse hearts. All hearts were perfused with Krebs buffer containing (in mM): 118.5 NaCl, 4.7 KCl, 1.2 MgSO4, 1.2 KH2PO4, 24.8 NaHCO3, 2.5 CaCl2, and 10.6 glucose on the Langendorff apparatus for 25 minutes to allow stabilization prior to ischemic challenge. Following the stabilization period, hearts were subjected to 1) 5, 10, 20, or 30 minutes of ischemia, 2) perfusion for 2.5 minutes with glucose free Krebs buffer containing 5 mM glycolytic inhibitor iodoacetate (Acros Organics, Morris Plains, NJ) followed by 20 minutes of ischemia, or 3) perfusion for 15 minutes with 50 μg/ml PNC inhibitor hadacidin (Developmental Therapeutics Program, National Cancer Institute, Bethesda, MD) followed by 30 minutes of ischemia. After the ischemic stress protocol, nucleotides were extracted with 400 μl of 4% perchloric acid and incubated on ice for 20 minutes. Following incubation, the perchloric acid extracts were centrifuged at 15,000 g for 15 minutes at 4°C and the supernatant, which contained nucleotides was collected. The remaining pellet was lyophilized in order to obtain the dry weight (g) of tissue collected. Nucleotide content was measured with high performance liquid chromatography. Results were expressed as μmole nucleotide per gram dry tissue.
Statistical analysis
Data are expressed as means±SE. Statistical significance was detected using one-way ANOVA followed by SNK post-hoc test. Student’s t-test was used to test for significance where appropriate. Statistical analysis was performed using GraphPad Prism 5 (La Jolla, CA).
RESULTS
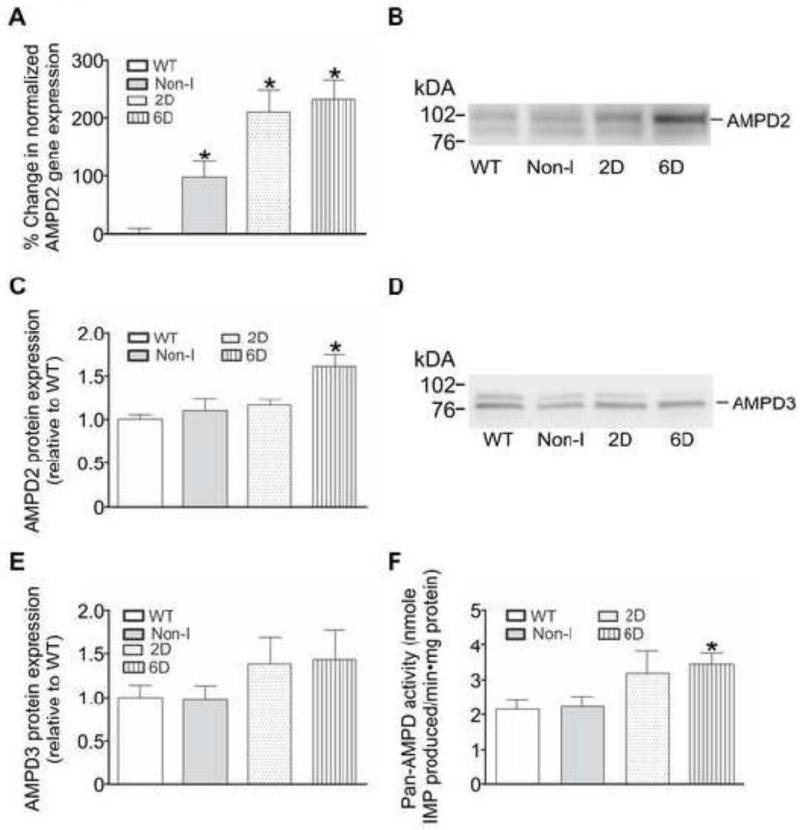
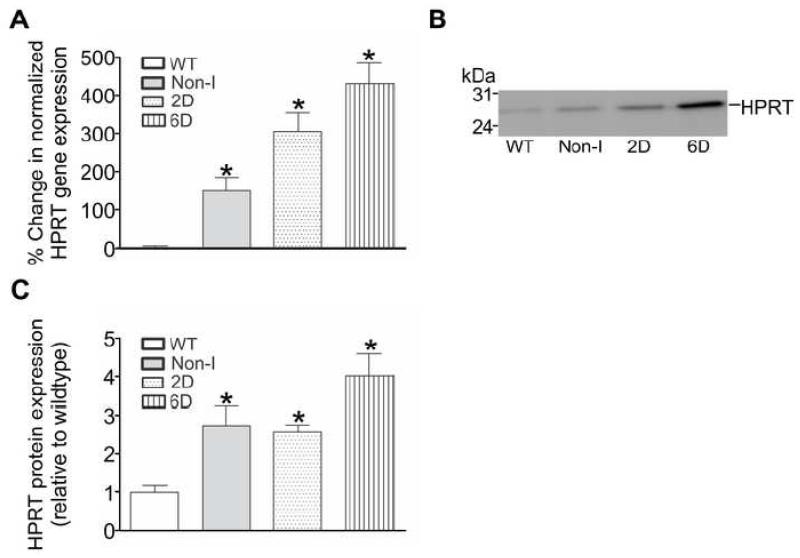
In the initial series of experiments the mRNA levels of key enzymes involved in nucleotide metabolism were examined in mouse hearts where the HIF-1α-PPN transgene was suppressed (Non-I) or expressed for 2 days (2D) or 6 days (6D). mRNA encoding the enzymes comprising the PNC, AMP deaminase (isoforms 2 and 3) and hypoxanthine phosphoribosyl transferase (HPRT) were also examined. Of the mRNA species examined, only significant elevations of AMP deaminase isoform 2 (AMPD2) and HPRT were observed in HIF-1α-PPN-expressing hearts when compared to wildtype (WT) hearts (Figures 2A and 3A). No significant differences in the expression levels of any of the other mRNA species examined were noted (data not shown). Some amount of “leak” of stabilized HIF-1α-PPN is present in Non-I hearts [4], which most likely explains the elevated level of HPRT mRNA in these hearts when compared to wildtype hearts.
Figure 2. HIF-1α upregulates the mRNA, protein, and activity of AMP deaminase 2 (AMPD2) in mouse hearts.
Hearts were obtained from WT mice and those in which the HIF-1α-PPN transgene was suppressed (Non-I) or allowed to be expressed for 2 days (2D) or 6 days (6D). A: AMPD2 gene expression in mouse heart homogenates was examined using qPCR and normalized to reference gene transferrin. Results are expressed as the % change in gene expression relative to WT (n=6). B: Western blot showing AMPD2 protein expression in mouse heart homogenates. C: Quantification of AMPD2 protein levels in mouse heart homogenates (n=6-8). D: Western blot showing AMPD3 protein expression in mouse heart homogenates. E: Quantification of AMPD3 protein levels in mouse heart homogenates (n=6-10). F: AMPD activity assessed by the amount of IMP produced per minute per mg protein in a buffer system containing excess AMP (n=5). * P<0.05 versus WT.
Figure 3. HIF-1α upregulates the mRNA and protein of hypoxanthine phosphoribosyl transferase (HPRT) in mouse hearts.
Hearts were obtained from WT mice and those in which the HIF-1α-PPN transgene was suppressed (Non-I) or allowed to be expressed for 2 days (2D) or 6 days (6D). A: HPRT gene expression in mouse heart homogenates was examined using qPCR and normalized to reference gene transferrin. Results are expressed as the % change in gene expression relative to WT (n=6). B: Western blot showing HPRT protein expression in mouse heart homogenates. C: Quantification of HPRT protein levels in mouse heart homogenates (n=4-7). * P<0.05 versus WT.
Having found HIF-1α induced changes in several mRNA species encoding nucleotide salvage enzymes, the protein levels of the PNC enzymes and HPRT were evaluated using western blot analysis (Figure 2B and 3B). Similar to the results at the level of mRNA, AMPD2 and HPRT protein levels were found to be elevated in HIF-1α-PPN-expressing hearts (Figures 2C and 3C). No increase in the protein levels of AMPD3 (Figure 2D and 2E), ADSS or ADSL were observed (data not shown). In parallel experiments, enzyme activity assays for AMPD, ADSS and ADSL were performed using homogenates derived from wildtype and the HIF-1α-PPN hearts. Of these enzyme activities, only AMPD (Figure 2D) was observed to be significantly increased, with the 6D HIF-1α-PPN-expressing hearts showing more AMPD activity compared to wildtype and Non-I heart homogenates. Thus, initial experiments examining several of the nucleotide salvage enzymes, identify AMPD2 and HPRT as HIF-1α responsive genes. A retrospective analysis of the AMPD2 and HPRT genes using Unipro UGENE identified strong (>99% certainty) putative hypoxic response elements (HRE; 5′-RCGTG-3′) that occur at 323 to 326 and 1631 to 1638 base pairs upstream from the predicted AMPD2 and HPRT transcription start site, respectively. While the finding of HPRT induction may have important implications for reperfusion and recovery from ischemia, AMPD, and the resulting PNC activity, exerts its effects during ischemia [17]. Given the likely temporally separated compensatory roles of these two enzymes, we selected to focus upon the question of the potential physiological significance of AMPD in these studies.
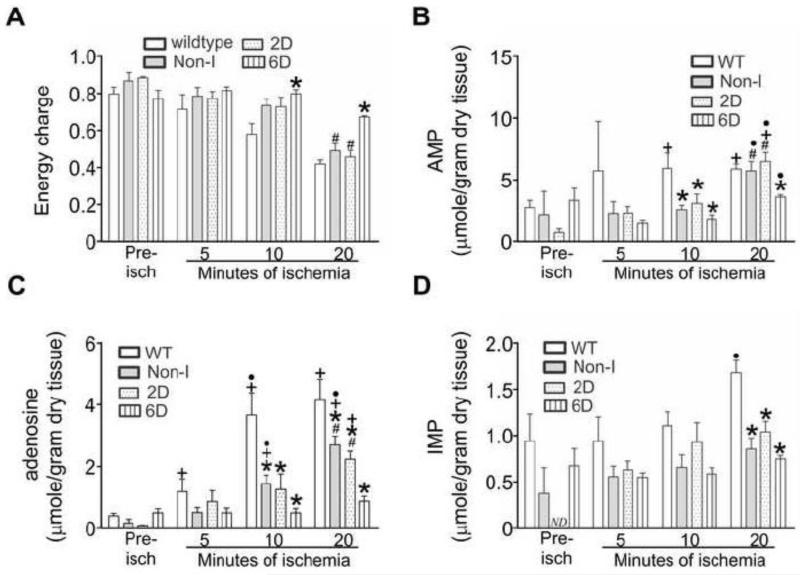
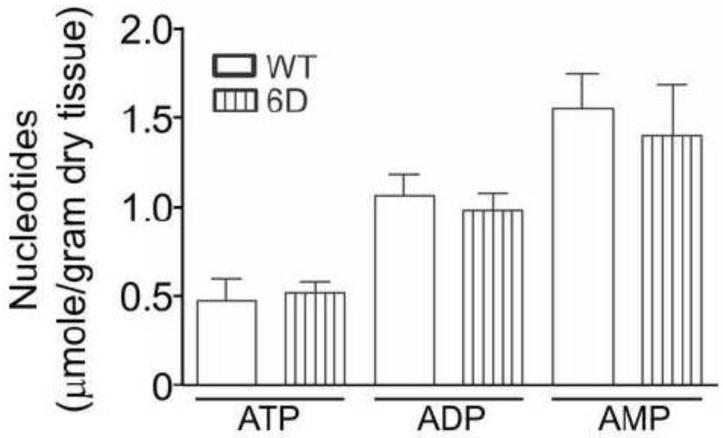
Accordingly, the next experiment compared energy charge and the accumulation of nucleotide breakdown products in wildtype, Non-I, 2D-, and 6D- HIF-1α-PPN-induced hearts during a 30 minute bout of ischemia. Figure 4A shows that HIF-1α-PPN preserved energy charge better during the initial 20 minutes of ischemia. By 30 minutes of ischemia, energy charge was equivalent across the heart groups (Table 1). Depletion of ATP leads to AMP accumulation, which has only two fates; either deamination to IMP by AMPD, or alternatively, dephosphorylation to adenosine by 5′-nucleotidase. Figure 4 shows the levels of AMP, adenosine and IMP during the initial 20 minutes of ischemia in wildtype and HIF-1α-PPN hearts, while Table 1 contains the levels of a more complete panel of ATP and its metabolites at 30 minutes of ischemia. Most prominently observed, adenosine elaboration was significantly suppressed in the HIF-1α-PPN expressing hearts starting at 5 minutes of ischemia and continuing throughout (Figure 4B and Table 1). Despite the differences in adenosine levels that accumulate, coronary flow rates, pre-ischemic and during reperfusion was not significantly different in wildtype and HIF-1α-PPN 6D hearts (data not shown). AMP accumulation was lower in the HIF-1α expressing hearts at 10 minutes but had largely normalized across the experimental groups by 20 minutes (Figure 4A). Figure 4C shows that IMP levels became elevated in wildtype hearts by 20 minutes, but interestingly, remained unchanged as compared to pre-ischemic values in the HIF-1α-PPN hearts.
Figure 4. Adenylate energy charge, AMP, adenosine, and IMP levels in ischemic adult mouse hearts.
Hearts were obtained from WT mice, mice maintained on a doxycycline diet to suppress the HIF-1α-PPN transgene (Non-I), or mice denied doxycycline to express the HIF-1α-PPN transgene for 2 days (2D) or 6 days (6D). Hearts were subjected to the indicated durations of ischemia. Nucleotides were extracted and measured using HPLC. A: Adenylate energy charge. B: AMP. C: adenosine. D: IMP. Pre-isch denotes pre-ischemia. The pre-ischemic level of IMP in 2D HIF-1α-induced hearts was not detectable (ND). * P<0.05 versus WT. # P<0.05 versus 6D. + P<0.05 versus pre-ischemic values for the corresponding experimental group. ● P<0.05 versus previous time point. n=3-9.
Table 1.
Summary of the distribution of adenine nucleotides and their breakdown products in wildtype mouse hearts as well as those in which the HIF-1α-PPN transgene has been expressed for 6 days (6D). Nucleotide levels were measured in mouse hearts prior to ischemia, after 30 minutes of ischemia or after perfusion with 50 μg/ml PNC inhibitor hadacidin followed by 30 minutes of ischemia.
| WT 30 minutes of ischemia |
6D 30 minutes of ischemia | |||||
|---|---|---|---|---|---|---|
| WT pre- ischemic |
(−) hadacidin |
(+) hadacidin |
6D pre- ischemic |
(−) hadacidin |
(+) hadacidin |
|
| ATP | 26.44±3.71 | 2.00±0.22‡ | 0.32±0.03‡§ | 21.44±2.37 | 4.44±0.55*‡ | 0.73±0.07*‡§ |
| ADP | 8.92±1.19 | 2.72±0.21‡ | 1.79±0.12‡§ | 8.84±1.32 | 6.04±0.29* | 3.23±0.29*‡§ |
| AMP | 2.76±0.57 | 5.13±0.78‡ | 8.77±0.59‡§ | 3.33±0.99 | 8.42±0.94‡ | 14.88±2.06*‡§ |
|
Energy
charge |
0.79±0.04 | 0.35±0.04‡ | 0.11±0.01‡§ | 0.77±0.05 | 0.40±0.02‡ | 0.13±0.01‡§ |
| ATP:ADP | 3.29±0.60 | 0.76±0.10‡ | 0.18±0.01‡§ | 3.05±0.63 | 0.73±0.07‡ | 0.23±0.02‡§ |
|
total adenine
nucleotide |
38.12±3.91 | 9.85±0.71‡ | 10.89±0.64‡ | 33.61±2.57 | 18.91±1.54*‡ | 18.83±2.33*‡ |
| adenosine | 0.41±0.08 | 3.19±0.92‡ | 6.82±0.77‡§ | 0.50±0.14 | 1.04±0.21*‡ | 1.00±0.20* |
| IMP | 0.94±0.29 | 3.13±0.65‡ | 3.81±0.28‡ | 0.68±0.18 | 1.26±0.29* | 1.38±0.11*‡ |
| inosine | 0.14±0.06 | 4.43±0.55‡ | 5.2±0.24‡ | 0.25±0.07 | 3.26±0.46‡ | 3.08±0.26*‡ |
| hypoxanthine | 0.14±0.06 | 1.42±0.07‡ | 1.47±0.06‡ | 0.19±0.05 | 1.38±0.09‡ | 1.29±0.05‡ |
P<0.05 versus the pre-ischemic value for the corresponding group.
P<0.05 versus hearts not treated with hadacidin.
P<0.05 versus wildtype. n=3-9.
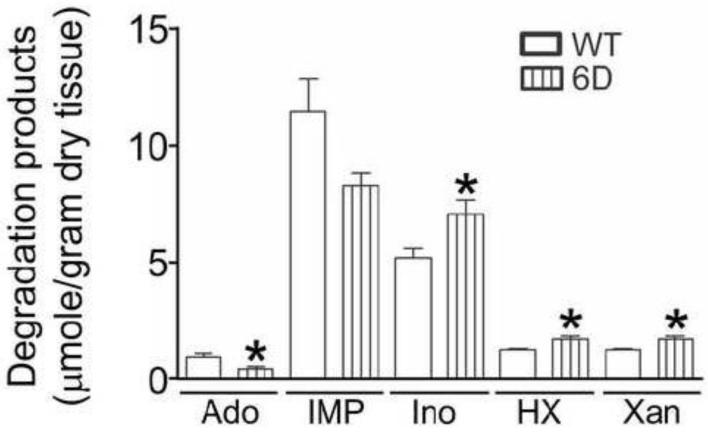
The markedly lower adenosine levels of the HIF-1α-PPN expressing hearts is highly consistent with the shunting of less AMP into the 5′-nucleotidase reaction due to higher AMPD activity. However, this interpretation is complicated by our previous findings that ATP depletion is slowed in HIF-1α hearts due to elevated glycolytic ATP production during the initial period of ischemia [4]. This raises the question of whether the lower adenosine observed in the HIF-1α-PPN hearts merely reflects the lower AMP that accumulates under these conditions. To address this question, glycolysis was blocked using treatment with iodoacetate to equalize the depletion of ATP between the wildtype and HIF-1α hearts. Previously we have used this strategy successfully[18] and here, perfusion of hearts with 5 mM iodoacetate followed by 20 minutes of ischemia reduced ATP, ADP, and AMP levels to the same extents in wildtype and 6D hearts (Figure 5). Despite excluding the effects of glycolysis, adenosine elaboration was still observed to be significantly depressed in the 6D HIF-1α-PPN hearts (Figure 6).
Figure 5. Treatment of hearts with iodoacetate followed by 20 minutes of ischemia reduced ATP, ADP, AMP to the same levels in WT and 6D hearts.
Hearts were obtained from WT mice and those in which the HIF-1α-PPN transgene was expressed for 6 days (6D). A 25 minute pre-ischemic perfusion period was performed to allow stabilization of the hearts. Then, they were perfused with glucose-free Krebs buffer containing 5 mM glycolytic inhibitor iodoacetate for 2.5 minutes prior to 20 minutes of total ischemia. After ischemic challenge, nucleotides were extracted from the hearts and measured with HPLC. n=4-5.
Figure 6. HIF-1α expression in the heart attenuates accumulation of adenosine in ischemic hearts where glycolytic activity has been blocked.
Hearts were obtained from WT mice and those in which the HIF-1α-PPN transgene was expressed for 6 days (6D). A 25 minute pre-ischemic perfusion period was performed in order to allow stabilization of the hearts. Then, they were perfused with glucose-free Krebs buffer containing 5 mM glycolytic inhibitor iodoacetate for 2.5 minutes prior to 20 minutes of total ischemia. After ischemic challenge, nucleotides were extracted from the hearts and their contents were measured using HPLC. * P<0.05 versus WT. n=4-5.
As we have shown previously, the downstream reaction steps in the PNC furnishes the fumarate that is used by cardiomyocytes to sustain anaerobic mitochondrial respiration [5]. In order to examine the PNC function downstream of AMPD at the level of adenylosuccinate synthetase, we used a stress protocol where hearts were subjected to 30 minutes of ischemia with or without prior treatment with 50 μg/ml ADSS inhibitor hadacidin. This protocol led to a reduction in ATP, energy charge as well as ATP:ADP compared to hearts subjected to ischemia alone (Table 1). Furthermore, accumulation of ADP decreased while that of AMP increased as a result of hadacidin treatment followed by ischemia (Table 1). Interestingly, the total amount of adenine nucleotides that remains after ischemia was unchanged by hadacidin.
DISCUSSION
Here we show that AMP deaminase (AMPD), the rate-limiting enzyme of the PNC [19], and the nucleotide salvage enzyme HPRT, are HIF-1α-responsive genes. The PNC has been suggested to have several key roles during metabolic stress but its precise function in metabolism, especially in heart, remains obscure. Consequently, it was of interest to explore nucleotide metabolism in ischemic wildtype mouse hearts and those that express HIF-1α and have induced AMPD.
HIF-1α has been demonstrated to confer robust hypoxic tolerance in heart [4,20]. Likewise adenosine is generally regarded as a cardioprotective molecule that contributes to preconditioning [21-23]. Thus, our finding that HIF-1α expression attenuates adenosine elaboration might be considered paradoxical. On the other hand, chronic exposure to adenosine can cause toxicity [24] and inflammation [25]. Of probably greater significance, adenosine is uncharged and freely diffuses out of the myocyte. Accordingly, the induction of AMPD can be expected to conserve the adenylate pool under conditions of chronic hypoxia.
Perhaps the most significant finding of these studies is that inhibition of the PNC with hadacidin during ischemia reduced ATP and collapsed the energy charge (Table 1). Thus, the question arises of how PNC activity might support the cellular energy charge. Lowenstein, the discoverer of the PNC, suggested that the PNC upregulates glycolysis [26]. Further, Terjung has proposed that the PNC helps preserve a high ATP:ADP ratio by limiting the accumulation of AMP and ADP during fatiguing skeletal muscle exercise [9]. Coupling of the adenylate kinase (2 ADP ↔ ATP + AMP) and the AMP deaminase reactions shifts the equilibrium of the adenylate kinase reaction towards ATP production. To our knowledge, this is the first direct evidence that the PNC supports the cellular energy charge during ischemia.
Notwithstanding the above considerations, we originally examined the PNC because it is the source of fumarate that allows anaerobic complex 1 operation that maintains mitochondrial polarization during anoxia [4,5]. Here, NADH oxidation is supported by the reversal of complex 2 activity with fumarate becoming an electron acceptor resulting in succinate production. Fumarate respiration can be envisioned to support ATP levels through several mechanisms. Direct production of ATP via the ATP synthase has been argued persuasively to be unlikely on both theoretical and experimental grounds [27]. While we agree with this assessment, our previous finding clearly showed that mitochondrial polarization was maintained via fumarate reduction in the absence of glycolytic ATP input [4,5]. Thus, we favor the view that the primary contribution of NAD+ -fumarate reductase activity to the cellular energy charge is through its prevention of glycolytic ATP consumption by the reversal of the ATP synthase. Enhanced PNC activity may contribute to the higher energy charge we observe during the initial 20 minutes of ischemia in the 6D HIF-1α-PPN-expressing hearts through one, or a combination of the above-discussed mechanisms,.
We also observe that HIF-1α promotes accumulation of hypoxanthine during ischemia. This finding may have implications for the discovery that HPRT is induced by HIF-1α. In a reaction catalyzed by HPRT, the 5-phosphoribosyl group on phosphoribosyl pyrophosphate is transferred to hypoxanthine to regenerate IMP. IMP can be used by the PNC to resynthesize adenine nucleotides. The fact that HPRT is upregulated by HIF-1α may provide a significant mechanism for the re-synthesis of ATP during reperfusion. In tetanic exercised skeletal muscle, IMP accumulation is in direct proportion to ATP breakdown [28]. In contrast IMP accumulation is seldom seen in ischemic heart [14,29,30]. This led Manfredi et al to propose the existence of an unidentified factor in heart that limits IMP accumulation [30]. In this regard, the upregulation of HPRT may hold answers concerning IMP accumulation. In the HIF-1α expressing hearts, the absence of IMP accumulation is remarkable during ischemia. The HPRT reaction is known to be reversible with production of hypoxanthine from IMP. While speculative, we suggest that the induction of HPRT and its reverse activity might represent the mechanism that prevents IMP accumulation in the HIF-1α expressing hearts. The finding of significantly higher hypoxanthine in HIF-1α expressing hearts where depletion in ATP was equalized by blocking glycolysis with iodoacetate is consistent with the notion of greater reversed HPRT activity.
Another potential benefit of HPRT induction in HIF-1α expressing hearts is moderation of the ROS burst that accompanies reperfusion [31]. The burst in ROS upon reperfusion has been attributed to xanthine oxidase activity [32]. In this scenario, HPRT may reduce the hypoxanthine that is available for metabolism by xanthine oxidase, an enzyme that catalyzes the conversion of hypoxanthine to xanthine while concomitantly generating ROS. Thus, the induction of HPRT by HIF-1α may exert enhanced protection of hearts by reducing ROS induced reperfusion injury.
In summary, we show that HIF-1α upregulates the activity of the purine nucleotide cycle thru the induction of AMPD. We also show that the nucleotide salvage enzyme HPRT is induced in HIF-1α expressing hearts. The upregulation of HPRT in HIF-1α expressing hearts may help in assimilating hypoxanthine back into the nucleotide pool upon recovery from ischemic stress. These HIF mediated changes alter nucleotide metabolism in a way that helps the ischemic heart preserve the adenylate energy charge during ischemia. These studies provide evidence that in addition to glycolysis, HIF-1α upregulates the PNC as a compensatory response to hypoxia.
Highlights.
HIF-1α upregulates nucleotide salvage pathways, the PNC and HPRT in heart.
HIF-1α expression reduces the accumulation of adenosine in the ischemic heart.
The PNC helps preserve the adenylate energy charge in the ischemic heart.
ACKNOWLEDGEMENTS
We thank Collette Hunt for work to establish the transgenic animal colony at ETSU. Thanks are also extended to Megan Ewing for efforts in maintaining the mouse colony.
GRANTS
This work was supported by National Heart, Lung, and Blood Institute Grant RO1-HL-084302 to G. L. Wright.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
DISCLOSURES
No conflict of interest, financial, or otherwise, are declared by the authors.
AUTHOR CONTRIBUTIONS
J.W., R.S., and G.W. conceptualized and designed experiments. J.W., C.B., P.C., M.C., and Y.L. collected data. J.W., C.B., P.C., M.C., Y.L. analyzed data. J.W., C.B., P.C., M.C., Y.L. prepared figures. J.W. and G.W. interpreted results. J.W. and G.W. prepared and revised manuscript. J.W., C.B., P.C., M.C., Y.L., R.S. and G.W. approved the manuscript in its final form.
REFERENCES
- [1].Bekeredjian R, Walton CB, MacCannell KA, Ecker J, Kruse F, Outten JT, et al. Conditional HIF-1alpha expression produces a reversible cardiomyopathy. PLoS One. 2010;5:e11693. doi: 10.1371/journal.pone.0011693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [2].Eckle T, Kohler D, Lehmann R, El Kasmi K, Eltzschig HK. Hypoxia-inducible factor-1 is central to cardioprotection: a new paradigm for ischemic preconditioning. Circulation. 2008;118:166–75. doi: 10.1161/CIRCULATIONAHA.107.758516. [DOI] [PubMed] [Google Scholar]
- [3].Cai Z, Zhong H, Bosch-Marce M, Fox-Talbot K, Wang L, Wei C, et al. Complete loss of ischaemic preconditioning-induced cardioprotection in mice with partial deficiency of HIF-1 alpha. Cardiovasc Res. 2008;77:463–70. doi: 10.1093/cvr/cvm035. [DOI] [PubMed] [Google Scholar]
- [4].Wu J, Chen P, Li Y, Ardell C, Der T, Shohet R, et al. HIF-1α in heart: protective mechanisms. Am J Physiol Heart Circ Physiol. 2013;305:H821–8. doi: 10.1152/ajpheart.00140.2013. doi:10.1152/ajpheart.00140.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Sridharan V, Guichard J, Li CY, Muise-Helmericks R, Beeson CC, Wright GL. O(2)-sensing signal cascade: clamping of O(2) respiration, reduced ATP utilization, and inducible fumarate respiration. Am J Physiol Cell Physiol. 2008;295:C29–37. doi: 10.1152/ajpcell.00466.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Weinberg JM, Venkatachalam MA, Roeser NF, Nissim I. Mitochondrial dysfunction during hypoxia/reoxygenation and its correction by anaerobic metabolism of citric acid cycle intermediates. Proc Natl Acad Sci. 2000;97:2826–31. doi: 10.1073/pnas.97.6.2826. doi:10.1073/pnas.97.6.2826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Taegtmeyer H. Metabolic responses to cardiac hypoxia. Increased production of succinate by rabbit papillary muscles. Circ Res. 1978;43:808–15. doi: 10.1161/01.res.43.5.808. [DOI] [PubMed] [Google Scholar]
- [8].Sakai C, Tomitsuka E, Miyagishi M, Harada S, Kita K. Type II Fp of human mitochondrial respiratory complex II and its role in adaptation to hypoxia and nutrition-deprived conditions. Mitochondrion. 2013;13:602–9. doi: 10.1016/j.mito.2013.08.009. doi:10.1016/j.mito.2013.08.009. [DOI] [PubMed] [Google Scholar]
- [9].Hancock CR, Brault JJ, Terjung RL. Protecting the cellular energy state during contractions: role of AMP deaminase. J Physiol Pharmacol. 2006;57(Suppl 1):17–29. [PubMed] [Google Scholar]
- [10].Atkinson DE. Energy charge of the adenylate pool as a regulatory parameter. Interaction with feedback modifiers. Biochemistry. 1968;7:4030–4. doi: 10.1021/bi00851a033. doi:10.1021/bi00851a033. [DOI] [PubMed] [Google Scholar]
- [11].Ścislowski PWD, Aleksandrowicz Z, Świerczyński J. Purine nucleotide cycle as a possible anaplerotic process in rat skeletal muscle. Experientia. 1982;38:1035–7. doi: 10.1007/BF01955351. doi:10.1007/BF01955351. [DOI] [PubMed] [Google Scholar]
- [12].Taegtmeyer H. On the role of the purine nucleotide cycle in the isolated working rat heart. J Mol Cell Cardiol. 1985;17:1013–8. doi: 10.1016/s0022-2828(85)80082-1. doi:10.1016/S0022-2828(85)80082-1. [DOI] [PubMed] [Google Scholar]
- [13].Semenza GL. Hypoxia-inducible factor 1: oxygen homeostasis and disease pathophysiology. Trends Mol Med. 2001;7:345–50. doi: 10.1016/s1471-4914(01)02090-1. [DOI] [PubMed] [Google Scholar]
- [14].Jennings RB, Reimer KA, Hill ML, Mayer SE. Total ischemia in dog hearts, in vitro. 1. Comparison of high energy phosphate production, utilization, and depletion, and of adenine nucleotide catabolism in total ischemia in vitro vs. severe ischemia in vivo. Circ Res. 1981;49:892–900. doi: 10.1161/01.res.49.4.892. [DOI] [PubMed] [Google Scholar]
- [15].Swain JL, Sabina RL, McHale PA, Greenfield JC, Jr., Holmes EW. Prolonged myocardial nucleotide depletion after brief ischemia in the open-chest dog. Am J Physiol. 1982;242:H818–26. doi: 10.1152/ajpheart.1982.242.5.H818. [DOI] [PubMed] [Google Scholar]
- [16].Raffin JP, Thebault MT. A specific AMP deaminase assay and its application to tissue homogenates. Comp Biochem Physiol B. 1991;99:125–7. doi: 10.1016/0305-0491(91)90016-7. [DOI] [PubMed] [Google Scholar]
- [17].Mommsen TP, Hochachka PW. The purine nucleotide cycle as two temporally separated metabolic units: A study on trout muscle. Metabolism. 1988;37:552–6. doi: 10.1016/0026-0495(88)90170-9. doi:10.1016/0026-0495(88)90170-9. [DOI] [PubMed] [Google Scholar]
- [18].Sridharan V, Guichard J, Bailey RM, Kasiganesan H, Beeson C, Wright GL. The prolyl hydroxylase oxygen-sensing pathway is cytoprotective and allows maintenance of mitochondrial membrane potential during metabolic inhibition. Am J Physiol Cell Physiol. 2007;292:C719–28. doi: 10.1152/ajpcell.00100.2006. [DOI] [PubMed] [Google Scholar]
- [19].Sabina RL, Swain JL, Olanow CW, Bradley WG, Fishbein WN, DiMauro S, et al. Myoadenylate deaminase deficiency. Functional and metabolic abnormalities associated with disruption of the purine nucleotide cycle. J Clin Invest. 1984;73:720–30. doi: 10.1172/JCI111265. doi:10.1172/JCI111265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Ong S-G, Lee WH, Theodorou L, Kodo K, Lim SY, Shukla DH, et al. HIF-1 reduces ischaemia-reperfusion injury in the heart by targeting the mitochondrial permeability transition pore. Cardiovasc Res. 2014;104:24–36. doi: 10.1093/cvr/cvu172. doi:10.1093/cvr/cvu172. [DOI] [PubMed] [Google Scholar]
- [21].Downey JM, Cohen MV, Ytrehus K, Liu Y. Cellular mechanisms in ischemic preconditioning: the role of adenosine and protein kinase C. Ann N Y Acad Sci. 1994;723:82–98. [PubMed] [Google Scholar]
- [22].Eckle T, Krahn T, Grenz A, Köhler D, Mittelbronn M, Ledent C, et al. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation. 2007;115:1581–90. doi: 10.1161/CIRCULATIONAHA.106.669697. doi:10.1161/CIRCULATIONAHA.106.669697. [DOI] [PubMed] [Google Scholar]
- [23].Lasley R. Adenosine A1 receptor mediated protection of the globally ischemic isolated rat heart. J Mol Cell Cardiol. 1990;22:39–47. doi: 10.1016/0022-2828(90)90970-d. doi:10.1016/0022-2828(90)90970-D. [DOI] [PubMed] [Google Scholar]
- [24].Chunn JL, Molina JG, Mi T, Xia Y, Kellems RE, Blackburn MR. Adenosine-dependent pulmonary fibrosis in adenosine deaminase-deficient mice. J Immunol. 2005;175:1937–46. doi: 10.4049/jimmunol.175.3.1937. [DOI] [PubMed] [Google Scholar]
- [25].Ramkumar V, Stiles GL, Beaven MA, Ali H. The A3 adenosine receptor is the unique adenosine receptor which facilitates release of allergic mediators in mast cells. J Biol Chem. 1993;268:16887–90. [PubMed] [Google Scholar]
- [26].Tornheim K, Lowenstein JM. The purine nucleotide cycle. Control of phosphofructokinase and glycolytic oscillations in muscle extracts. J Biol Chem. 1975;250:6304–14. [PubMed] [Google Scholar]
- [27].Chinopoulos C. Which way does the citric acid cycle turn during hypoxia? The critical role of α-ketoglutarate dehydrogenase complex. J Neurosci Res. 2013;91:1030–43. doi: 10.1002/jnr.23196. doi:10.1002/jnr.23196. [DOI] [PubMed] [Google Scholar]
- [28].Meyer RA, Dudley GA, Terjung RL. Ammonia and IMP in different skeletal muscle fibers after exercise in rats. J Appl Physiol. 1980;49:1037–41. doi: 10.1152/jappl.1980.49.6.1037. [DOI] [PubMed] [Google Scholar]
- [29].Van Bilsen M, van der Vusse GJ, Coumans WA, de Groot MJ, Willemsen PH, Reneman RS. Degradation of adenine nucleotides in ischemic and reperfused rat heart. Am J Physiol. 1989;257:H47–54. doi: 10.1152/ajpheart.1989.257.1.H47. [DOI] [PubMed] [Google Scholar]
- [30].Manfredi JP, Holmes EW. Control of the purine nucleotide cycle in extracts of rat skeletal muscle: Effects of energy state and concentrations of cycle intermediates. Arch Biochem Biophys. 1984;233:515–29. doi: 10.1016/0003-9861(84)90475-2. doi:10.1016/0003-9861(84)90475-2. [DOI] [PubMed] [Google Scholar]
- [31].Garlick PB, Davies MJ, Hearse DJ, Slater TF. Direct detection of free radicals in the reperfused rat heart using electron spin resonance spectroscopy. Circ Res. 1987;61:757–60. doi: 10.1161/01.res.61.5.757. [DOI] [PubMed] [Google Scholar]
- [32].Thompson-Gorman SL, Zweier JL. Evaluation of the role of xanthine oxidase in myocardial reperfusion injury. J Biol Chem. 1990;265:6656–63. [PubMed] [Google Scholar]