Abstract
High altitude (HA), defined as approximately 3000–5000 m, considerably alters physiological and psychological parameters within a few hours. Chronic HA-mediated hypoxia (5000 m) results in permanent neuronal damage to the human brain that persists for one year or longer, even after returning to sea level. At HA, there is a decrease in barometric pressure and a consequential reduction in the partial pressure of oxygen (PO2), an extreme environmental condition to which humans are occasionally exposed. This condition is referred to as hypobaric hypoxia (HBH), which represents the most unfavourable characteristics of HA. HBH causes the disruption of oxygen availability to tissue. However, no review article has explored the impact of HBH on cognitive functions or the potential therapeutic agents for HBH. Therefore, the present review aimed to describe the impact of HBH on both physiological and cognitive functions, specifically learning and memory. Finally, the potential therapeutic agents for the treatment of HBH-induced cognitive impairment are discussed.
Keywords: high altitude, hypoxia, therapeutics
Introduction
High altitude (HA)
Exposure to HA, or approximately 3000–5000 m, considerably alters physiological and psychological parameters within a few hours (1,2). At HA, individuals are commonly confronted with disorders such as acute mountain sickness (AMS) (3), HA pulmonary oedema (HAPE) (4), and HA cerebral oedema (HACE)(5). The learning and memory deficits induced by HA exposure draw special concern because these deficits incapacitate or compromise an individual’s performance of highly demanding mental functions. Prolonged exposure of human volunteers to HA decreased their verbal working memory (6). At HA, there is a decrease in barometric pressure and a consequential reduction in the partial pressure of oxygen (PO2), an extreme environmental condition to which humans are occasionally exposed (7). This state in which the partial pressure of oxygen is reduced is termed hypobaric hypoxia (HBH), which reflects the most unfavourable characteristics of HA. HBH causes an imbalance of oxygen availability to tissue, causing severe physiological and psychological dysfunction in humans and other animals. Here, we summarise some of the experimental data concerning the effect of HBH on physiological functions.
Hypobaric hypoxia (HBH)
HBH exposure leads to severe abnormalities in physiological and psychological functions. Exposure to HBH is known to cause sleep disturbances (8), hypophagia (9), oxidative stress (10), and alterations of acetylcholine neurotransmitter (11). Chronic exposure to HBH for 31 days via gradual decompression in a hypobaric chamber from sea level to the altitude equivalent of 8848 m resulted in significant changes in mood (12). Exposure to HBH ranging between 4 200 and 4700 m for a duration from a few hours to almost one month significantly affected mood and cognitive performance in an elevation-dependent manner. The severity of these effects dramatically increases at 4700 m altitude (13) due to an imbalance in physiological activities, resulting in impaired cognitive functions, specifically learning and memory functions. The negative effects of HBH on cognitive functions depend on the duration of exposure and the altitude. However, before describing the effects of HBH on cognitive functions, it is essential to describe in the context of learning and memory.
Cognitive Functions
Cognitive functions comprise learning and memory functions. Learning is defined as the acquisition of knowledge via experience or study. Memory is defined as the process by which individuals retain newly acquired information over time. The formation of a memory “engram”, a representation of memory in the brain, involves time-dependent consolidation (14). Based on the period in which information can be sustained, memory is categorised into three fundamental types or stages: sensory, short-term and longterm memory (15). The incoming information is initially and very briefly maintained in sensory storage, either as visual sensory memory (iconic memory) or auditory sensory memory (echoic memory). Most of the information in sensory memory is lost within a fraction of second, thus enabling only a small portion of this information to be transmitted to short-term memory storage. The processing of incoming sensory information occurs in the cortical areas that are responsible for the initial perception of sensory stimuli, i.e., in the primary auditory and visual cortices (Figure 1) (16).
Figure 1.
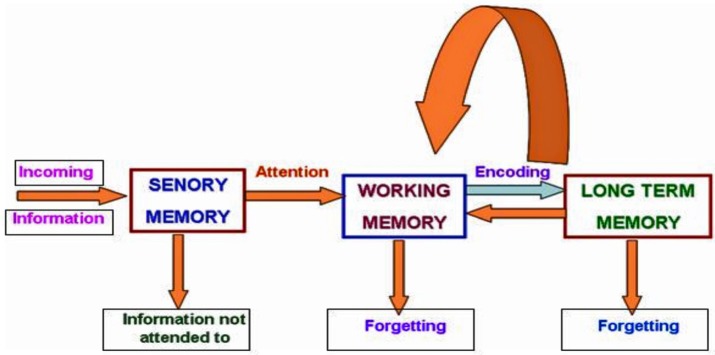
Schematic diagram of the types of memory.
The second stage, short-term memory, is responsible for the acquisition and conscious processing of information to be subsequently transmitted to long-term memory storage or forgotten (15). The period for which short-term memory is responsible for maintaining information is elastic, varying from seconds to 10–20 minutes, depending on the type of information and the task used to assess memory. The third stage of memory, long-term memory, is considered persistent memory (16). The maintenance of long-term memory depends on synaptic plasticity. Long-term memories can be disturbed under stress or disease conditions. Particularly, HBH strongly disturbs memory in altitude-dependent manner. At present, few mechanisms underlying the HBH-mediated deficits in learning and memory function have been identified.
Effects of HBH on Cognitive Functions
Hypobaric hypoxia (HBH) at HA is known to disrupt cognitive functions in humans (13). Cognitive functions, such as learning and memory, are adversely affected by exposure to HBH (11). Short-term memory decreased following exposure of human volunteers to acute, mild or moderate hypoxia for 1 h at 4 400 m, and these effects were exacerbated at increasing altitudes (17). Studies of mountaineers and volunteers subjected to simulated HBH have suggested that impairments in psychomotor function and visual reaction time were associated with HA hypoxia (18). Titus et al., 2007 suggested that hippocampal dendrite atrophy following exposure to HBH could represent a potential mechanism underlying these cognitive deficits (19). Exposure to acute HBH induced cognitive deficits, along with oxidative stress (20). At the behavioural level, postnatal exposure to HA hypoxia at 7000 m for 19 days impaired spatial memory (21).
Further, Barhwal et al., 2007 (22), and 2009 (23), reported that 3 and 14 days after exposure to HBH impaired working and reference memory, respectively, via increased oxidative stress and apoptotic cell death. In another study, Barhwal et al. 2009 (24), identified the role of the L-type calcium channel in spatial memory impairment induced by exposure to HBH for 3 or 7 days. In addition, learning ability and memory retrieval were both impaired at 25 000 ft (25). Maiti et al. 2008 (26), and 2008 (27), suggested that spatial memory was impaired after 3 or 7 days of exposure to HBH, which was associated with neuronal damage. Rats exhibited significant deficits in spatial working memory following exposure to HBH for 3 days (28). Hota et al. 2008 (29), demonstrated that memory retrieval was delayed after exposure to HBH for 14 days due to glutamate-mediated neuronal excitotoxicity. Also, Baitharu et al. 2012 (30), and 2013 (31), found that corticosterone and glucocorticoid may serve as factors that cause memory impairment after exposure to HBH at 25000 ft for 7 days. Several lines of evidence reported the effect of HBH on cholinergic system. Muthuraju et al. 2009 (32), reported that the effects of exposure to HBH for 7 days on relearning and memory retrieval depend on the impairment of cholinergic systems. In another study, Muthuraju et al. 2010 (33), examined learning ability after exposure to HBH and revealed impairment in learning ability and an increased level of acetylcholinesterase activity. Further more, the alteration of acetylcholine synthesis following HBH exposure causes impairments in memory ability and memory consolidation (11,34). Other studie that targets to evaluate role of HBH on hippocampal neurons. Hippocampal neurodegeneration leads to cognitive impairment following exposure to HBH (35). Based on the available evidences, we conclude that the HBH-mediated learning and memory impairments, could be involvement of oxidative stress, alter in acetylcholine system, glutamate-mediated excitotoxicity, and corticosterone imbalance. Among these studies, some investigators evaluated the ability of potential therapeutic agents to ameliorate HBH-induced learning and memory impairments.
Potential Therapeutic Agents
It has been clearly demonstrated that HBH causes learning and memory impairments. However, few studies have identified therapeutic agents that improve cognitive functions under HBH conditions. The factors that are involved in the HBH-mediated learning and memory impairments may include neuronal damage, oxidative stress and neurotransmitter alterations. Jayalakshmi et al. 2007 (28), reported that N-acetyl cysteine (NAC) administration during HBH exposure ameliorated the hypoxia-induced impairments in spatial working memory function. In addition, NAC supplementation decreased oxidative stress, increased the antioxidant status, and reduced free radical production during HBH. Other study suggests that acetyl-L-carnitine supplementation improved the spatial working memory deficits of rats chronically exposed to HBH (24). Additionally, Barhwal et al. 2009 (23) suggested that L-type calcium channels and glutamate receptors play an important role in learning and memory functions during HBH. They reported that isradipine, an L-type calcium channel blocker, may serve as a useful therapeutic agent to improve spatial memory during HBH. Further more, bacosides ameliorated the memory impairment induced by exposure to HBH (25). Also, bacosides upregulated NCAM, enhanced cytochrome c oxidase activity, and improved memory during HBH. Hota et al. 2008 (29), concluded that ceftriaxone ameliorated HBH-induced cognitive impairment. Alternatively, Baitharu et al. 2012 (30,31), suggested that corticosterone plays a role in learning and memory functions during HBH. They reported that the administration of the corticosterone synthesis inhibitor metyrapone and Withania somnifera root extract improved cognitive functions during HBH. Besides, Muthuraju et al. 2009, 2010, and 2011 reported that physostigmine and galantamine ameliorated the increase in the level of acetylcholinesterase during and after HBH exposure and enhanced cholinergic system function. This facilitation of the cholinergic system may help to improve learning and memory during HBH. Prasad et al. 2013, reported that Quercetin reverses cognitive impairment by reducing the level of oxidative stress. Furthermore, Vishal et al. 2012 (36), found that an enriched environment prevented HBH-induced memory impairment. Based on these studies, the aforementioned therapeutic agents may ameliorate HBH-induced cognitive impairment.
Conclusion
The current review has collectively considered the importance of several important factors associated with the effect of HBH on cognitive functions and the potential therapeutic agents. Recently, the disruption of cognitive functions associated with HBH has been considered a serious mental health issue. The experiments and findings that have associated HBH with learning and memory deficits should be of great interest to researchers, particularly with respect to the future development of interventions. Additionally, this review of the literature emphasises the importance of considering the direct impacts of HBH on cognitive function. Accumulating experimental evidence implicates oxidative stress, the cholinergic system dysfunction, and alterations in the glutamate and corticosterone levels in the effects of HBH. Animal data confirm that the aforementioned therapeutic agents ameliorate oxidative stress and modulate the neurotransmitter and corticosterone levels. In the future, further investigations must confirm these changes during HBH and must determine the efficacy of these therapeutic agents against HBH-mediated abnormalities.
Acknowledgments
We thank all researcher those who are sacrificing their knowledge in high altitude research.
Footnotes
Conflict of interest
None.
Funds
None.
Authors’ Contributions
Conception and design, drafting of the article, collection and assembly of data: SM, SP
References
- 1.Ou LC, Faulkner C, Tam V, Leiter JC. Liver function in rats acclimatized to a simulated altitude of 5500 m. High Alt Med Biol. 2013;14(4):375–3782. doi: 10.1089/ham.2011.1083. doi: 10.1089/ham.2011.1083 . [DOI] [PubMed] [Google Scholar]
- 2.Smirl JD, Lucas SJ, Lewis NC, duManoir GR, Smith KJ, Bakker A, et al. Cerebral pressure-flow relationship in lowlanders and natives at high altitude. J Cereb Blood Flow Metab. 2014;34(2):248–257. doi: 10.1038/jcbfm.2013.178. doi: 10.1038/jcbfm.2013.178 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.MacInnis MJ, Carter EA, Freeman MG, Pandit BP, Siwakoti A, Subedi A, et al. A prospective epidemiological study of acute mountain sickness in Nepalese pilgrims ascending to high altitude (4380 m) PLoS One. 2013;8(10):e75644. doi: 10.1371/journal.pone.0075644. doi: 10.1371/journal.pone.0075644 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bhagi S, Srivastava S, Singh SB. High-altitude Pulmonary Edema: Review. J Occup Health. 2014;56(4):235–243. doi: 10.1539/joh.13-0256-ra. doi: org/10.1539/joh.13-0256-RA . [DOI] [PubMed] [Google Scholar]
- 5.Basnyat B, Murdoch DR. High-altitude illness. Lancet. 2003;361(9373):1967–1974. doi: 10.1016/S0140-6736(03)13591-X. doi: 10.1016/S0140-6736(03)13591-X . [DOI] [PubMed] [Google Scholar]
- 6.Yan X, Zhang J, Gong Q, Weng X. Prolonged high-altitude residence impacts verbal working memory: an fMRI study. Exp Brain Res. 2011;208(3):437–445. doi: 10.1007/s00221-010-2494-x. doi: 10.1007/s00221-010-2494-x . [DOI] [PubMed] [Google Scholar]
- 7.Pagani M, Salmaso D, Sidiras GG, Jonsson C, Jacobsson H, Larsson SA, et al. Impact of acute hypobaric hypoxia on blood flow distribution in brain. Acta Physiol (Oxf) 2011;202(2):203–9. doi: 10.1111/j.1748-1716.2011.02264.x. doi: 10.1111/j.1748-1716.2011.02264.x . [DOI] [PubMed] [Google Scholar]
- 8.Ray K, Dutta A, Panjwani U, Thakur L, Anand JP, Kumar S. Hypobaric hypoxia modulates brain biogenic amines and disturbs sleep architecture. Neurochem Int. 2011;58(1):112–118. doi: 10.1016/j.neuint.2010.11.003. doi: 10.1016/j.neuint.2010.11.003 . [DOI] [PubMed] [Google Scholar]
- 9.Simler N, Grosfeld A, Peinnequin A, Guerre-Millo M, Bigard AX. Leptin receptor-deficient obese Zucker rats reduce their food intake in response to hypobaric hypoxia. Am J Physiol Endocrinol Metab. 2006;290(3):E591–597. doi: 10.1152/ajpendo.00289.2005. doi: 10.1152/ajpendo.00289.2005 . [DOI] [PubMed] [Google Scholar]
- 10.Arya A, Sethy NK, Singh SK, Das M, Bhargava K. Cerium oxide nanoparticles protect rodent lungs from hypobaric hypoxia-induced oxidative stress and inflammation. Int J Nanomedicine. 2013;8:4507–4520. doi: 10.2147/IJN.S53032. doi: 10.2147/IJN.S53032 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Muthuraju S, Maiti P, Pati S, Solanki P, Sharma AK, Singh SB, et al. Role of cholinergic markers on memory function of rats exposed to hypobaric hypoxia. Eur J Pharmacol. 2011;672(1–3):96–105. doi: 10.1016/j.ejphar.2011.08.023. doi: 10.1016/j.ejphar.2011.08.023 . [DOI] [PubMed] [Google Scholar]
- 12.Bolmont B, Thullier F, Abraini JH. Relationships between mood states and performances in reaction time, psychomotor ability, and mental efficiency during a 31-day gradual decompression in a hypobaric chamber from sea level to 8848 m equivalent altitude. Physiol Behav. 2000;71(5):469–476. doi: 10.1016/s0031-9384(00)00362-0. doi: 10.1016/S0031-9384(00)00362-0 . [DOI] [PubMed] [Google Scholar]
- 13.Shukitt-Hale B, Banderet LE, Lieberman HR. Elevation-dependent symptom, mood, and performance changes produced by exposure to hypobaric hypoxia. Int J Aviat Psychol. 1998;8(4):319–334. doi: 10.1207/s15327108ijap0804_1. [DOI] [PubMed] [Google Scholar]
- 14.McGaugh Memory -a Century of Consolidation. Science. 2000;287(5451):248–251. doi: 10.1126/science.287.5451.248. doi: 10.1126/science.287.5451.248 . [DOI] [PubMed] [Google Scholar]
- 15.Santini E, Huynh TN, Klann E. Mechanisms of translation control underlying long-lasting synaptic plasticity and the consolidation of long-term memory. Prog Mol Biol Transl Sci. 2014;122:131–167. doi: 10.1016/B978-0-12-420170-5.00005-2. doi: 10.1016/B978-0-12-420170-5.00005-2 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kandel ER, Dudai Y, Mayford MR. The molecular and systems biology of memory. Cell. 2014;157(1):163–186. doi: 10.1016/j.cell.2014.03.001. doi: 10.1016/j.cell.2014.03.001 . [DOI] [PubMed] [Google Scholar]
- 17.Du JY, Li XY, Zhuang Y, Wu XY, Wang T. Effects of acute mild and moderate hypoxia on human short memory. Space Med Med Eng (Beijing) 1999;12(4):270–273. [PubMed] [Google Scholar]
- 18.Li XY, Wu XY, Fu C, Shen XF, Yang CB, Wu YH. Effects of acute exposure to mild or moderate hypoxia on human psychomotor performance and visual-reaction time. Space Med Med Eng (Beijing) 2000;13(4):235–239. [PubMed] [Google Scholar]
- 19.Titus AD, Shankaranarayana Rao BS, Harsha HN, Ramkumar K, Srikumar BN, Singh SB, et al. Hypobaric hypoxia-induced dendritic atrophy of hippocampal neurons is associated with cognitive impairment in adult rats. Neuroscience. 2007;145(1):265–278. doi: 10.1016/j.neuroscience.2006.11.037. doi: 10.1016/j.neuroscience.2006.11.037 . [DOI] [PubMed] [Google Scholar]
- 20.Shi Q, Fu J, Ge D, He Y, Ran J, Liu Z, et al. Huperzine A ameliorates cognitive deficits and oxidative stress in the hippocampus of rats exposed to acute hypobaric hypoxia. Neurochem Res. 2012;37(9):2042–2052. doi: 10.1007/s11064-012-0826-x. doi: 10.1007/s11064-012-0826-x . [DOI] [PubMed] [Google Scholar]
- 21.Simonová Z, Sterbová K, Brozek G, Komárek V, Syková E. Postnatal hypobaric hypoxia in rats impairs water maze learning and the morphology of neurones and macroglia in cortex and hippocampus. Behav Brain Res. 2003;141(2):195–205. doi: 10.1016/s0166-4328(02)00366-2. doi: 10.1016/S0166-4328(02)00366-2 . [DOI] [PubMed] [Google Scholar]
- 22.Barhwal K, Singh SB, Hota SK, Jayalakshmi K, Ilavazhagan G. Acetyl-L-carnitine ameliorates hypobaric hypoxic impairment and spatial memory deficits in rats. Eur J Pharmacol. 2007;570(1–3):97–107. doi: 10.1016/j.ejphar.2007.05.063. doi: 10.1016/j.ejphar.2007.05.063 . [DOI] [PubMed] [Google Scholar]
- 23.Barhwal K, Hota SK, Baitharu I, Prasad D, Singh SB, Ilavazhagan G. Isradipine antagonizes hypobaric hypoxia induced CA1 damage and memory impairment: Complementary roles of L-type calcium channel and NMDA receptors. Neurobiol Dis. 2009;34(2):230–244. doi: 10.1016/j.nbd.2009.01.008. [DOI] [PubMed] [Google Scholar]
- 24.Barhwal K, Hota SK, Jain V, Prasad D, Singh SB, Ilavazhagan G. Acetyl-l-carnitine (ALCAR) prevents hypobaric hypoxia-induced spatial memory impairment through extracellular related kinasemediated nuclear factor erythroid 2-related factor 2 phosphorylation. Neuroscience. 2009;30;161(2):501–514. doi: 10.1016/j.neuroscience.2009.02.086. doi: 10.1016/j.neuroscience.2009.02.086 . [DOI] [PubMed] [Google Scholar]
- 25.Hota SK, Barhwal K, Baitharu I, Prasad D, Singh SB, Ilavazhagan G. Bacopa monniera leaf extract ameliorates hypobaric hypoxia induced spatial memory impairment. Neurobiol Dis. 2009;34(1):23–39. doi: 10.1016/j.nbd.2008.12.006. doi: 10.1016/j.nbd.2008.12.006 . [DOI] [PubMed] [Google Scholar]
- 26.Maiti P, Singh SB, Mallick B, Muthuraju S, Ilavazhagan G. High altitude memory impairment is due to neuronal apoptosis in hippocampus, cortex and striatum. J Chem Neuroanat. 2008;36(3–4):227–238. doi: 10.1016/j.jchemneu.2008.07.003. doi: 10.1016/j.jchemneu.2008.07.003 . [DOI] [PubMed] [Google Scholar]
- 27.Maiti P, Muthuraju S, Ilavazhagan G, Singh SB. Hypobaric hypoxia induces dendritic plasticity in cortical and hippocampal pyramidal neurons in rat brain. Behav Brain Res. 2008;189(2):233–243. doi: 10.1016/j.bbr.2008.01.007. doi: 10.1016/j.bbr.2008.01.007 . [DOI] [PubMed] [Google Scholar]
- 28.Jayalakshmi K, Singh SB, Kalpana B, Sairam M, Muthuraju S, Ilavazhagan G. N-acetyl cysteine supplementation prevents impairment of spatial working memory functions in rats following exposure to hypobaric hypoxia. Physiol Behav. 2007;92(4):643–650. doi: 10.1016/j.physbeh.2007.05.051. doi: 10.1016/j.physbeh.2007.05.051 . [DOI] [PubMed] [Google Scholar]
- 29.Hota SK, Barhwal K, Ray K, Singh SB, Ilavazhagan G. Ceftriaxone rescues hippocampal neurons from excitotoxicity and enhances memory retrieval in chronic hypobaric hypoxia. Neurobiol Learn Mem. 2008;89(4):522–532. doi: 10.1016/j.nlm.2008.01.003. doi: 10.1016/j.nlm.2008.01.003 . [DOI] [PubMed] [Google Scholar]
- 30.Baitharu I, Deep SN, Jain V, Barhwal K, Malhotra AS, Hota SK, et al. Corticosterone synthesis inhibitor metyrapone ameliorates chronic hypobaric hypoxia induced memory impairment in rat. Behav Brain Res. 2012;228(1):53–65. doi: 10.1016/j.bbr.2011.11.030. doi: 10.1016/j.bbr.2011.11.030 . [DOI] [PubMed] [Google Scholar]
- 31.Baitharu I, Deep SN, Jain V, Prasad D, Ilavazhagan G. Inhibition of glucocorticoid receptors ameliorates hypobaric hypoxia induced memory impairment in rat. Behav Brain Res. 2013;240:76–86. doi: 10.1016/j.bbr.2012.11.005. doi: 10.1016/j.bbr.2012.11.005 . [DOI] [PubMed] [Google Scholar]
- 32.Muthuraju S, Maiti P, Solanki P, Sharma AK, Amitabh, Singh SB, et al. Acetylcholinesterase inhibitors enhance cognitive functions in rats following hypobaric hypoxia. Behav Brain Res. 2009;203(1):1–14. doi: 10.1016/j.bbr.2009.03.026. doi: 10.1016/j.bbr.2009.03.026 . [DOI] [PubMed] [Google Scholar]
- 33.Muthuraju S, Maiti P, Solanki P, Sharma AK, Singh SB, Prasad D, et al. Cholinesterase inhibitors ameliorate spatial learning deficits in rats following hypobaric hypoxia. Exp Brain Res. 2010;203(3):583–592. doi: 10.1007/s00221-010-2266-7. doi: 10.1007/s00221-010-2266-7 . [DOI] [PubMed] [Google Scholar]
- 34.Muthuraju S, Maiti P, Solanki P, Sharma AK, Pati S, Singh SB, et al. Possible role of cholinesterase inhibitors on memory consolidation following hypobaric hypoxia of rats. Int J Neurosci. 2011;121(5):279–288. doi: 10.3109/00207454.2011.556279. doi: 10.3109/00207454.2011.556279 . [DOI] [PubMed] [Google Scholar]
- 35.Prasad J, Baitharu I, Sharma AK, Dutta R, Prasad D, Singh SB. Quercetin reverses hypobaric hypoxiainduced hippocampal neurodegeneration and improves memory function in the rat. High Alt Med Biol. 2013;14(4):383–94. doi: 10.1089/ham.2013.1014. doi: 10.1089/ham.2013.1014 . [DOI] [PubMed] [Google Scholar]
- 36.Jain V, Baitharu I, Prasad D, Ilavazhagan G. Enriched environment prevents hypobaric hypoxia induced memory impairment and neurodegeneration: role of BDNF/PI3K/GSK3β pathway coupled with CREB activation. PLoS One. 2013;8(5):e62235. doi: 10.1371/journal.pone.0062235. doi: 10.1371/journal.pone.0062235 . [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]


