Abstract
A transducer with an angled and focused aperture for intravascular ultrasound imaging has been developed. The acoustic stack for the angled-focused transducer was made of PMN-PT single crystal with one matching layer, one protective coating layer, and a highly damped backing layer. It was then press-focused to a desired focal length and inserted into a thin needle housing with an angled tip. A transducer with an angled and unfocused aperture was also made, following the same fabrication procedure, to compare the performance of the two transducers. The focused and unfocused transducers were tested to measure their center frequencies, bandwidths, and spatial resolutions. Lateral resolution of the angled-focused transducer (AFT) improved more than two times compared to that of the angled-unfocused transducer (AUT). A tissue-mimicking phantom in water and a rabbit aorta tissue sample in rabbit blood were scanned using AFT and AUT. Imaging with AFT offered improved contrast, over imaging with AUT, of the tissue-mimicking phantom and the rabbit aorta tissue sample by 23 dB and 8 dB, respectively. The results show that AFT has strong potential to provide morphological and pathological information of coronary arteries with high resolution and high contrast.
Keywords: Intravascular ultrasound (IVUS) imaging, Angled-focused transducer, High frequency transducer, Contrast, Coronary stenosis evaluation, Rabbit aorta
1. Introduction
Coronary artery diseases, which are the leading causes of death world-wide, were responsible for more than 7 million deaths in 2008 [1]. Atherosclerosis is the main cause of coronary heart disease [2]. Effective diagnosis of atherosclerotic coronary disease by identifying and quantifying atherosclerotic lesions has been one of the main issues in clinics. Evaluation of coronary stenosis may be performed using physiological assessment [3] and morphological assessment [4]. Coronary flow reserve or fractional flow reserve could be measured as an indirect and physiological assessment to quantify atherosclerotic lesions [3]. Computed tomography (CT), coronary angiography, and intravascular ultrasound (IVUS) have been used as a morphological approach to evaluate coronary stenosis [4]. IVUS is a catheter-based minimally invasive technique and has been constantly used to visualize the internal structure of coronary arteries for several years between clinicians [5,6]. A single frequency IVUS has a center frequency between 20 MHz and 40 MHz with a spatial resolution of 100 μm to 300 μm [7].
To improve the diagnostic capabilities of imaging modalities, research groups have been developing new approaches [8-12]. Our group has been intensively developing IVUS imaging transducers and systems [13-17]. A PMN-PT free-standing film approach was used to develop a high frequency IVUS transducer for a high frequency application [13]. A combined pulse inversion chirp coded tissue harmonic and fundamental imaging technique was used to increase tissue contrast with low side lobes [14]. A new material was also developed for the IVUS application [15]. An Integrated IVUS and fiber-based optical coherence tomography catheter was developed [16]. A new material in 1–3 composite configuration was used to develop an IVUS transducer for high frequency and wide bandwidth [17].
An aperture with a viewing angle and a focus may increase accessibility of transducers to a target region and improve image quality. The viewing angle of the aperture with respect to elevation direction can be achieved by placing an acoustic stack with a desired angle into the transducer housing. Before the integration of the acoustic stack and transducer housing, the curved surface is created by a press-focusing technique to produce the desired focus. An IVUS transducer, which is inside the sheath of a catheter, must pass through the artery to acquire arterial cross-sectional images. However, sometimes decreased lumen area within the artery, due to blood clots or atherosclerosis-induced blockages, becomes too narrow for the catheter to pass by. Conversely, a transducer with an angled aperture is able to visualize the region of interest, which cannot be obtained by typical side looking IVUS transducers, because images of blood clots or blockages, ahead of the transducer aperture, can be visualized by the angled sound field transmitted by an angled aperture. Focusing improves spatial resolution by concentrating ultrasound energy into a focal area [18,19]. Images with higher resolution may delineate morphological and pathological information of the coronary artery more effectively. Furthermore, IVUS catheters are disposable and the low fabrication cost of a single element transducer is another advantage compared to other types of transducers such as array type transducers which are more difficult to make and more expensive than single element transducers. Array type transducers are typically more bulky compared to single element transducers, which is another challenge for IVUS applications [20].
By considering these advantages, we have proposed and developed an angled-focused single element transducer for IVUS imaging. To compare the image quality obtained by an angled-focused transducer (AFT), an angled-unfocused transducer (AUT) was also fabricated and tested following the same experimental protocol. PMN-PT single crystal was chosen as a piezoelectric material and one matching layer was used for acoustic matching with one protective coating layer. Pulse-echo tests were performed to estimate the center frequency and bandwidth of AFT and AUT. Spatial resolution of both transducers was estimated using ultrasound B-mode images of 20 μm tungsten wire targets. An ex vivo tissue sample (a rabbit aorta) was imaged with the presence of rabbit blood in the lumen area using the developed transducers. Contrast of the images of a tissue-mimicking phantom and tissue sample was estimated and analyzed to characterize the performance of AFT.
2. Materials and methods
An angled-focused transducer (AFT) and an angled-unfocused transducer (AUT) were designed and fabricated. The entire fabrication process was the same for both transducers except press-focusing the aperture of AFT. Acoustic stacks for AFT and AUT were composed of a piezoelectric material (PM in Fig. 1), a matching layer (ML in Fig. 1), a backing layer (BL in Fig. 1), and a protective coating layer. The single crystal form of lead magnesium niobate-lead titanate solid solution (PMN-PT, IBULE Photonics, Incheon, Korea) was used as a piezoelectric material with the designed thickness of 40 μm and a square aperture size of 0.57 mm × 0.57 mm. The matching layer was made by blending Insulcast 501 epoxy (American Safety Technologies, Roseland, NJ) and 2–3 μm silver particles (Aldrich Chemical Co., Milwaukee, WI), which was cast over the piezoelectric material. The thickness of the matching layer was 10 μm. The backing layer, made by a conductive silver epoxy (E-Solder 3022, Von Roll Isola Inc., New Haven, CT), was molded on the back side of the piezoelectric material. The thickness of the backing layer was approximately 1 mm. The protective coating layer was formed at the very last step of the whole fabrication process. Parylene was deposited on top of the matching layer as the protective coating layer using a PDS 2010 Labcoater (Specialty Coating Systems, Indianapolis, IN). The thickness of the protective coating layer was 3 μm.
Fig. 1.
An angled-unfocused single element transducer for intravascular ultrasound (IVUS) imaging. The acoustic stack was composed of a matching layer (ML), PMN-PT piezoelectric material (PM), and backing layer (BL) as shown in the inset image. Aperture size was 0.57 mm × 0.57 mm. The length and the diameter of the stainless steel hypodermic needle were 45 mm and 1.06 mm, respectively. The angle between the aperture and the elevation direction (two dash–dot lines) was 60°. Only ML was exposed. PM and BL were immersed in epoxy. An SMA connector was attached at the other end of the needle.
The acoustic stack for an angled-focused transducer (AFT) was press-focused by using a spherical ball with 5 mm diameter to place the focus of the acoustic stack at approximately 2.5 mm. Therefore, the f-number of the acoustic stack was approximately 3.6. Stainless steel hypodermic needles with an outer diameter of 1.06 mm and a length of 45 mm were prepared. On one side of the stainless steel hypodermic needle, a circular hole, through which an acoustic stack was inserted later, with a diameter of approximately 0.9 mm was made. A silver wire with a thin insulating jacket was connected to the backing layer of the acoustic stacks using a conductive silver epoxy. The acoustic stack was placed into the circular hole on the stainless steel hypodermic needle at a 60° angle with respect to elevation direction as shown in Fig. 1. The gap between the acoustic stack and the stainless steel hypodermic needle was filled in by an insulating epoxy (Epo-Tek 301, Epoxy Technologies, Billerica, MA). Only the matching layer was exposed (bright dotted rectangle in Fig. 1) while the other parts of the acoustic stack were immersed in insulating epoxy surrounded by the stainless steel hypodermic needle. The exposed matching layer was sputtered with chrome/gold electrodes with a total thickness of approximately 150 nm for ground. The silver wire, which was connected to the backing layer of the acoustic stack, was attached to an SMA connector.
The performance of an angled-focused transducer (AFT) and an angled-unfocused transducer (AUT) was evaluated. Pulse-echo time response and echo spectrum were measured. A quartz block was used as a reflection target at the focus of the transducer for AFT. For AUT, a quartz block was located at 3.4 mm away from the transducer aperture. A pulser/receiver (5910PR, Panametrics, Waltham, MA) triggered the transducer to transmit a broadband pulse. The backscattered ultrasound echo was received and digitized by an analog-to-digital acquisition card (A/D card, CS122G1, DynamicsSignals LLC, Lockport, IL). Radiofrequency (RF) data, which was comprised of the emitted pulse and received backscattered ultrasound echo, was saved in computer storage to analyze the pulse-echo time domain waveform and its frequency spectrum. The upper and lower −6 dB cut-off frequencies were first measured from the frequency spectrum. The center frequency was calculated as a mean value of the −6 dB cut-off frequencies. Band-width was estimated as the fractional ratios between the difference of the two −6 dB cut-off frequencies and the center frequency.
Axial and lateral resolutions were measured after obtaining ultrasound B-mode images of a 20 μm tungsten wire using the angled-focused transducer (AFT) and the angled-unfocused transducer (AUT). To acquire an ultrasound B-mode image of a tungsten wire, each transducer scanned a 2 mm range with a step size of 5 μm. A tungsten wire was located near the focus of AFT or the natural focus of AUT, which was 3.4 mm. Axial and lateral resolutions were determined by considering the −6 dB widths on the point spread function plots.
AFT and AUT were used to image a tissue-mimicking phantom in water and an ex vivo tissue sample with the presence of blood in the luminal area. The tissue-mimicking phantom was made by mixing gelatin (9% by weight), 40 μm silica particles (1% by weight), and 5 μm silica particles (1% by weight) with water. A lumen was also made in the middle of the tissue-mimicking phantom. The tissue-mimicking phantom was solidified inside a cuvette. For imaging of the ex vivo tissue sample, a normal New Zealand white rabbit aorta (NRA) with fatty tissue and rabbit blood with Ethylenediaminetetraacetic acid (EDTA) were purchased from Sierra for Medical Science, Inc. (Whittier, CA). To image a tissue sample (a normal New Zealand white rabbit aorta (NRA)), it was fixed inside a cuvette by solidifying 9% gelatin solution together with the tissue sample (NRA). The fixed tissue sample (NRA) and gelatin block inside the cuvette is shown in Fig. 2. No ultrasound scatterers were presented in the gelatin block as shown.
Fig. 2.
A schematic view of the experimental setup. An angled-focused transducer or an angled-unfocused transducer was attached to a 3D translation stage. A tissue sample (a New Zealand white rabbit aorta (NRA)) was fixed in the middle of a cuvette by solidifying the tissue sample (NRA) and 9% gelatin together. The lumen of the tissue sample was filled with rabbit blood and was aligned with a transducer for imaging. The cuvette was placed on top of the stepper motor. Main control arranged the sequence of the motor controller, a function generator, and the A/D card. After the stepper motor turned one step as commanded by the motor controller, the function generator triggered a pulser/receiver to transmit a broadband pulse through the transducer. The transmitted pulse and received ultrasound backscattered echoes were digitized by the A/D card and saved in RF data storage. During a 360°-turn, 2564 scanlines were saved with 8200 samples each.
An imaging system for the angled-focused transducer and the angled-unfocused transducer was developed. A transducer was attached to the 3D translation stage to control the location of the transducer which was co-registered with the lumen wall of a tissue-mimicking phantom or tissue sample (a normal New Zealand white rabbit aorta (NRA)). The cuvette was rotated by a stepper motor (Zaber technologies, Inc., Vancouver, British Columbia, Canada) with 0.1404° step size to acquire 2564 scanlines during a 360° turn with 10 ms pause between consecutive steps. During the 10 ms pause, main control triggered a motor controller to rotate the stepper motor and the function generator to excite the pulser/receiver to acquire a new scanline. The transducer was connected to a pulser/receiver, which was operated in pulse-echo mode. Therefore, RF data consisted of the digitized pulse and echo signals from the A/D card. RF data were saved on RF data storage for off-line processing. One scanline had 8200 samples with the sampling rate of 1 GSamples/s, which corresponded to 4.1 μs along the A-line. The applied gain for receiving the echo was 26 dB.
RF data were scan converted to construct images of a tissue-mimicking phantom or a tissue sample (NRA). RF data was high-pass filtered to cancel DC offset. Quadrature demodulation was used to base-band the RF raw data followed by a low pass filter with a cut-off frequency of 0.5 × fc × BW MHz, where fc and BW are the center frequency and the bandwidth of the transducer, respectively. The scan conversion was performed with bilinear interpolation to construct two-dimensional cross-sectional images of a tissue-mimicking phantom or tissue sample (NRA). Reconstructed images were displayed with 50 dB dynamic range.
Quantitative analysis was performed on images of a tissue-mimicking phantom and tissue sample (NRA) obtained by an angled-focused transducer (AFT) and an angled-unfocused transducer (AUT). Seven kernels for feature region of interest (ROI) and one kernel for background ROI were chosen along the vertical center line of the tissue-mimicking phantom. For the tissue sample (NRA) images, eight kernels for feature ROI were determined by selecting regions, whose ultrasound speckle patterns represented the tissue sample (NRA). Kernels for background ROI of the tissue sample (NRA) images were chosen inside the luminal area next to the corresponding feature kernels, so the signal from background kernels were ultrasound echo signals from rabbit blood. The size of kernels was 200 μm × 200 μm. Contrast of evenly spaced seven feature kernels were calculated with one background kernel using the tissue-mimicking phantom images. Contrast of eight kernels was estimated using the tissue sample (NRA) images. Contrast was calculated as
where Sf and Sb were the mean signals of each kernel at the feature and the background ROIs, respectively. Contrast was plotted in dB scale with respect to the locations of the kernels. Mean and standard deviation of contrast of seven kernels in tissue-mimicking phantom images and eight kernels in tissue sample (NRA) images were also calculated.
3. Results
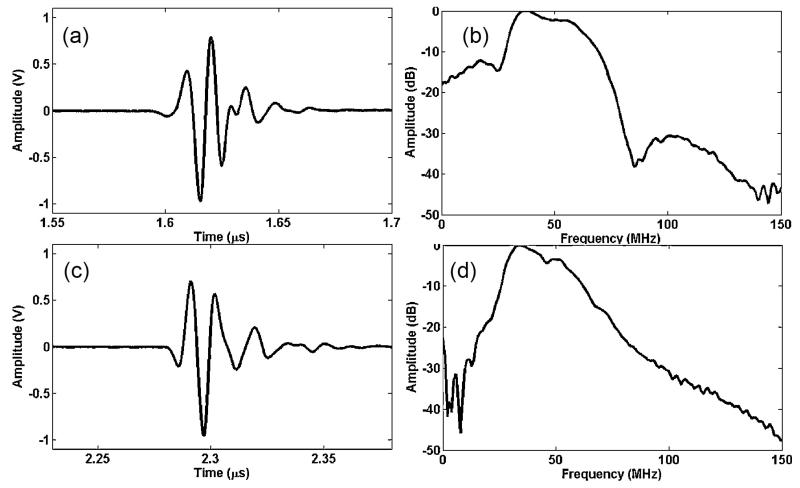
Echo waveforms, measured at the focus of an angled-focused transducer (AFT) and near the natural focus of an angled-unfocused transducer (AUF), are shown in Fig. 3(a) and (c), respectively. Corresponding frequency spectra of measured echo waveforms are presented in Fig. 3(b) and (d). The center frequency (fc) and −6 dB bandwidth (BW) of AFT were measured as 47 MHz and 72%, respectively (Fig. 3(a) and (b)). fc and BW of AUT were estimated at 42 MHz and 68% (Fig. 3(c) and (d)).
Fig. 3.
(a) Pulse-echo waveform of the angled-focused transducer and (b) the spectrum of the echo signal. Measured center frequency and bandwidth were 47 MHz and 72%. (c) Pulse-echo waveform of the angled-unfocused transducer and (d) the spectrum of the echo signal. Center frequency and bandwidth were 42 MHz and 68%.
Ultrasound B-mode images of 20 μm tungsten wire obtained by an angled-focused transducer (AFT) and an angled-unfocused transducer (AUT) are shown in Fig. 4(a) and (d), respectively. Axial and lateral beam profiles are shown in Fig. 4(b) and (c) for AFT and Fig. 4(e) and (f) for AUT. For AFT, the axial and lateral resolutions were estimated as 25 μm and 120 μm (Fig. 4(b) and (c)). The axial and lateral resolutions of AUT were measured as 25 μm and 270 μm as shown in Fig. 4(e) and (f).
Fig. 4.
Ultrasound B-mode scan of 20 μm tungsten wire obtained by (a) the angled-focused transducer (AFT) and (d) the angled-unfocused transducer (AUT). A total of 400 scanlines were obtained during the imaging of the 2 mm width. Images in (a) and (d) are scaled and displayed in 50 dB dynamic range. (b) Axial and (c) lateral beam profiles obtained from (a) indicate that the axial resolution and the lateral resolutions of AFT are 25 μm and 120 μm, respectively. (e) Axial and (f) lateral beam profiles of AUT are shown. Spatial resolutions were measured as 25 μm for the axial direction and 270 μm for the lateral direction.
Images of a tissue-mimicking phantom obtained by the angled-focused transducer (AFT) and the angle-unfocused transducer (AUT) are presented in Fig. 5(a) and (b), respectively. Speckle patterns due to 5 μm and 40 μm silica particles are clearly shown. Locations of feature kernels from one to seven and a background kernel are shown in Fig. 5(a) and (b) as bright squares and grey squares, denoted as B. In the luminal area of Fig. 5(a) and (b), no scattering is detected because the lumen was filled with water. In Fig. 5(c), calculated contrast was plotted with respect to the location of the seven feature kernels. Average and standard deviation of contrast, calculated at seven locations in the images obtained by AFT and AUT (Fig. 5(a) and (b)), were 32.9 ± 4.2 dB and 9.8 ± 1.1 dB, respectively.
Fig. 5.
Tissue-mimicking phantom images obtained by (a) an angled-focused transducer (AFT) and (b) an angled-unfocused transducer (AUT). In (a) and (b), bright squaresare the locations of seven kernels for the feature region of interest (ROI) and one grey square, denoted as B, indicates the location of a kernel for background ROI. (c) Contrastvs. locations of kernels was plotted in dB scale. Tissue sample (a normal New Zealand white rabbit aorta (NRA)) images, obtained by (d) AFT and (e) AUT are presented. In (d) and (e), eight feature kernels (dark squares) and corresponding background kernels (bright squares) are shown in clock wise direction. (f) Contrasts were obtained at eightlocations and plotted. All images are displayed in 50 dB dynamic range. All kernels were 200 μm × 200 μm in size.
The tissue sample of a New Zealand white rabbit aorta (NRA) was imaged by an angled-focused transducer and an angled-unfocused transducer as shown in Fig. 5(d) and (e). In the images in Fig. 5(d) and (e), dark squares indicate eight locations of feature kernels and bright squares indicate corresponding background kernels. Numbers from one to eight indicate the location of each kernel. The bright ring of strong signal with a diameter of approximately 4 mm indicates the tissue sample (NRA). Inside the luminal area, significant ultrasound backscattering from rabbit blood is also observed. Fatty tissue, which surrounds the tissue sample (NRA), is also clearly detected. Contrast in dB scale is plotted with respect to the locations of feature and background kernels. Mean and standard deviation of contrast, obtained using kernels in the images in Fig. 5(d) and (e), were −9.5 ± 3.8 dB and −17.3 ± 3.7 dB, respectively.
4. Discussion
An angled-focused transducer (AFT) for the imaging of a tissue-mimicking phantom and an ex vivo tissue sample (a New Zealand white rabbit aorta (NRA)) yielded an improvement of approximately 23 dB and 8 dB in contrast of obtained images over an angled-unfocused transducer (AUT). This result corresponds to the human perception when seeing image pairs of the tissue mimicking phantom (Fig. 5(a) and (b)) and the ex vivo tissue sample (NRA) (Fig. 5(d) and (e)). Particularly, the interface between the tissue sample (NRA) and rabbit blood in the lumen is much more clearly delineated in the image (Fig. 5(d)), obtained by AFT, than the interface in the image (Fig. 5(e)), obtained by AUT. This observation is closely related to the improved lateral resolution of AFT over AUT by a factor of two or more. Because focusing of an aperture concentrates ultrasound energy into the region of interest [19], focusing is highly recommended for the future fabrication of transducers for IVUS imaging.
In this study, we focused the aperture of a transducer at 2.5 mm, which resulted in an f-number of 3.6 because the effective aperture size of the square aperture was calculated as approximately 0.69 mm. The selection of a focus was determined after considering the diameter of typical human coronary arteries, which range from approximately 3.5 to 4.5 mm [21]. The selection of aperture size and focal distance in conjunction with the dimension of imaging targets is of importance because image quality degradation due to the fast divergence in the propagation of the focused sound field may pose a problem. Imaging range for IVUS is usually less than 5 mm in radius according to many clinical and laboratory IVUS imaging practices [22-27]. Tissue-mimicking phantom study demonstrated that focusing increased image quality at least up to 6 mm in radius as shown in Fig. 5(a)-(c). Therefore, the appropriate focusing of an aperture resulted in the increase of image quality without inducing fast divergence of the sound field.
Obtained two-dimensional images of a tissue-mimicking phantom or rabbit aorta tissue sample using transducers with an angled aperture did not represent the same cross-section, compared to images obtained by conventional side looking transducers. Because of the 30° angle between the transducer aperture and lumen wall, obtained images were skewed by 30° and projected onto a two-dimensional plane. Image registration to obtain true location information should be performed as a future work. The outer diameter of the current transducers was 1.06 mm. The outer diameter of a transducer should be smaller than 0.7 mm to insert the transducer inside the sheath for clinical applications. To increase the bandwidth of a transducer, which results in higher axial resolution, two or three matching layers may be employed as a future work.
5. Conclusions
We have designed and developed a transducer with an angled and focused aperture for intravascular ultrasound imaging. Lateral resolution improved from 270 μm to 120 μm, showing a capability to visualize fine structure of imaging targets. Focusing of the transducer aperture improved contrast of a tissue-mimicking phantom in water and a rabbit aorta tissue sample with the presence of rabbit blood, resulting in improvements of 23 dB and 8 dB in contrast over images obtained by a transducer with an unfocused aperture. A transducer with angled and focused aperture demonstrates convincing ability to visualize morphological information of the coronary artery for the diagnosis of atherosclerotic coronary stenosis.
Acknowledgments
This work was supported by International Collaborative R&D Program (N01150049) funded by the Ministry of Trade, Industry & Energy (MOTIE), Korea (N01150049, Developing high frequency bandwidth [40–60 MHz] high resolution image system and probe technology for diagnosing cardiovascular lesion). This work was supported in part by ONR-NICOP Research Grant (Award No. N62909-14-N029) and Korean R&D program (14-SF-EE-01) funded by the Ministry of Trade, Industry & Energy (MOTIE) and Defense Acquisition Program Administration (DAPA), Korea. This work was also supported in part by the National Institutes of Health under grant No. P41-EB002182.
Biographies
Biographies

Dr. Sangpil Yoon is postdoctoral fellow at University of Southern California. He received BS and MS degrees in mechanical engineering from Yonsei University, Seoul, Korea and in aerospace engineering from Georgia Institute of Technology, Atlanta, Georgia, and his PhD degree in mechanical engineering from University of Texas at Austin, Austin, Texas in 2012. His current research interests include IVUS imaging system development, acoustic-transfection using high frequency ultrasound, and fundamental research of cell physiology using ultrasound.

Jay A. Williams serves as Resource Manager of the NIH Resource on Medical Ultrasonic Transducer Technology. Mr. Williams joined the group in March 2002 just prior to their move to the University of Southern California in August of that year. He has also been the webmaster for the Resource Center, http://bme.usc.edu/UTRC, since the move to USC. Some of his accomplishments include: 128 element 30 MHz composite 30 μm pitch phased arrays, 256 element 30 MHz composite 50 μm pitch linear arrays, 64 element 35 MHz composite 50 μm pitch linear arrays, 8 element 35–60 MHz annular arrays, very light-weight (<0.3 gram 40–60 MHz, <0.2 gram 80–100 MHz) high frame-rate b-scan transducers, 100–150 MHz sputtered ZnO transducers, and 10 MHz composite HIFU catheter transducers.
He currently has a patent, no. 7695784, covering post positioning for interdigital bonded composites, USC file 3829. He audited courses in Business Management, Computer Science, Mechanical Engineering, Architecture, and Physics at the Pennsylvania State University, 1975–1977. He also achieved honors in both Analog Electronics at Radio Semiconductor, 1984, and Digital Electronics at Control Data Institute Multi-skills Center, 1987. He had worked in industry for 25 years in a variety of technical fields such as Mass Spectrometry, Microwave Telecommunication, Liquid Chromatography, Digital Electronics, Ultrasound, and Information Technology. In 1990 he began working in ultrasound at Blatek, Inc., State College, PA, serving 8½ years in engineering then 2½ years in management, including the last 5 years as IS/IT Manager, and last 2 years as Quality System Manager establishing their first ISO 9001 and FDA CGMP (21CFR820) quality system. He has now has been in the ultrasound field for more than 24 years.

Bong Jin Kang received his M.S. degrees in the Department of Electrical Engineering and Biomedical Engineering from the University of Southern California, Los Angeles, CA, in 2008 and 2010, respectively. He is currently a PhD candidate in the Department of Biomedical Engineering, University of Southern California, Los Angeles, CA. His current research is focused on high-frequency array-based ultrasound imaging system for small animal imaging.

Changhan Yoon received his M.S. PhD degrees of Electronic Engineering in 2009 and 2013, respectively, from Sogang University, Seoul, Korea. He is currently a post-doctoral research associate in the NIH Resource Center for Medical Ultrasonic Transducer Technology at the University of Southern California, Los Angeles, CA. His main research interests include medical ultrasound and photoacoustic imaging, system, clinical applications, and ultrasound microbeam.

Nestor E. Cabrera-Munoz obtained a B.S. degree in Mechanical-Electrical Engineering from Monterrey Institute of Technology (ITESM Campus Monterrey, Mexico) in 2005 and an M.S. degree in Biomedical Engineering from the University of Southern California (Los Angeles, CA) in 2010. He is now pursuing a PhD degree in Biomedical Engineering at the University of Southern California focusing his research on high-frequency ultrasonic transducers and arrays. He counts with professional R&D and project management experience in the areas of HVAC and automotive engineering. His current interests include the design, modeling, fabrication and testing of ultrasonic transducers and arrays for medical and NDT applications.

Jong Seob Jeong received his B.S. and M.S. degrees in electronic engineering from Sogang University, Seoul, Korea, in 1999 and 2001, respectively, and his PhD degree in biomedical engineering from the University of Southern California, Los Angeles, CA, in 2010. From 2010 to 2011, he pursued post-doctoral research at the NIH Resource Center for Medical Ultrasonic Transducer technology, Department of Biomedical Engineering, University of Southern California, Los Angeles, CA. He is currently an assistant professor in the Department of Medical Biotechnology, Dongguk University, Seoul, Korea. His research interests include medical signal processing and design, modeling, and fabrication of ultrasonic single-element and array transducers for diagnosis and therapy.

Sang Goo received a degree in Chemical Engineering and Forest Scientific Technology in Seoul National University (SNU) and Masters in Chemical Engineering from Yonsei University (1991), both of which is located in Korea. He returned to SNU for a PhD in Chemical Engineering (1995).
He is the founder and current CEO of iBULE Photonics in Incheon, Korea. He is responsible for the growth of PMN-PT single crystal which is considered to be a key component in medical ultrasonic transducers, naval sonars, and commercial sensors, while he manages the production of these final-end products utilizing PMN-PT technology. His job is also involved with practical applications of energy harvesting with piezoelectric energy.

K. Kirk Shung obtained a B.S. degree in electrical engineering from Cheng-Kung University in Taiwan in 1968; an M.S. degree in electrical engineering from the University of Missouri, Columbia, MO, in 1970; and a PhD degree in electrical engineering from the University of Washington, Seattle, WA, in 1975. He taught at The Pennsylvania State University, University Park, PA, for 23 years before moving to the Department of Biomedical Engineering, University of Southern California, Los Angeles, CA, as a professor in 2002.
He is the Dean’s professor at University of Southern California. He has been the director of the NIH Resource on Medical Ultrasonic Transducer Technology since 1997. Dr. Shung is a life fellow of IEEE and a fellow of the Acoustical Society of America and the American Institute of Ultrasound in Medicine. He is a founding fellow of the American Institute of Medical and Biological Engineering. He received the IEEE Engineering in Medicine and Biology Society Early Career Award in 1985 and was the coauthor of a paper that received the best paper award for the IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control (UFFC) in 2000. He was elected an outstanding alumnus of Cheng-Kung University in Taiwan in 2001. He was selected as the distinguished lecturer for the IEEE UFFC society for 2002–2003. He received the Holmes Pioneer Award in Basic Science from the American Institute of Ultrasound in Medicine in 2010. He was selected to receive the academic career achievement award from the IEEE Engineering in Medicine and Biology Society in 2011. Dr. Shung has published more than 400 papers and book chapters. He is the author of the textbook Principles of Medical Imaging, published by Academic Press in 1992, and the textbook Diagnostic Ultrasound: Imaging and Blood Flow Measurements, published by CRC Press in 2005. He co-edited the book Ultrasonic Scattering by Biological Tissues, published by CRC Press in 1993. He is an associate editor of the IEEE Transactions on Ultrasonics, Ferroelectrics, and Frequency Control and a member of the editorial board of Ultrasound in Medicine and Biology. Dr. Shung’s research interests are in ultrasonic transducers, high-frequency ultrasonic.

Dr. Hyung Ham Kim is an Ultrasound Research Sales Manager at Analogic Corporation. He received his BS degree in Electrical Engineering from Korea Advanced Institute of Science and Technology, Daejeon, Korea in 1993, his MS degree in Electrical Engineering from Seoul National University, Seoul, Korea in 1995, and his MS and PhD degrees in Biomedical Engineering from the University of Southern California, Los Angeles, CA in 2006 and 2010, respectively. His current research interests include the design and fabrication of high frequency transducers/arrays for high resolution ultrasound imaging and therapeutic applications.
References
- [1].Mendis S, Puska P, Norrving B. Global Atlas on Cardiovascular Disease Prevention and Control. World Health Organization; 2011. [Google Scholar]
- [2].Ross R. Atherosclerosis—an inflammatory disease. New Engl. J. Med. 1999;340:115–126. doi: 10.1056/NEJM199901143400207. [DOI] [PubMed] [Google Scholar]
- [3].Kern MJ, Lerman A, Bech JW, De Bruyne B, Eeckhout E, Fearon WF, et al. Physiological assessment of coronary artery disease in the cardiac catheterization laboratory: a scientific statement from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Circulation. 2006;114:1321–1341. doi: 10.1161/CIRCULATIONAHA.106.177276. [DOI] [PubMed] [Google Scholar]
- [4].Leber AW, Knez A, von Ziegler F, Becker A, Nikolaou K, Paul S, et al. Quantification of obstructive and nonobstructive coronary lesions by 64-slice computed tomography: a comparative study with quantitative coronary angiography and intravascular ultrasound. J. Am. College Cardiol. 2005;46:147–154. doi: 10.1016/j.jacc.2005.03.071. [DOI] [PubMed] [Google Scholar]
- [5].Mintz GS, Nissen SE, Anderson WD, Bailey SR, Erbel R, Fitzgerald PJ, et al. American College of Cardiology Clinical Expert Consensus Document on Standards for Acquisition, Measurement and Reporting of Intravascular Ultrasound Studies (IVUS). A report of the American College of Cardiology Task Force on Clinical Expert Consensus Documents. J. Am. Coll. Cardiol. 2001;37:1478–1492. doi: 10.1016/s0735-1097(01)01175-5. [DOI] [PubMed] [Google Scholar]
- [6].Garcia-Garcia HM, Costa MA, Serruys PW. Imaging of coronary atherosclerosis: intravascular ultrasound. Eur. Heart J. 2010;31:2456–2469. doi: 10.1093/eurheartj/ehq280. [DOI] [PubMed] [Google Scholar]
- [7].Jansen K, van Soest G, van der Steen AF. Intravascular photoacoustic imaging: a new tool for vulnerable plaque identification. Ultrasound Med. Biol. 2014;40:1037–1048. doi: 10.1016/j.ultrasmedbio.2014.01.008. [DOI] [PubMed] [Google Scholar]
- [8].Demirci U, Ergun AS, Oralkan O, Karaman M, Khuri-Yakub BT. Forward-viewing CMUT arrays for medical imaging. IEEE Trans. Ultrason. Ferroelect. Freq. Control. 2004;51:887–895. doi: 10.1109/tuffc.2004.1320749. [DOI] [PubMed] [Google Scholar]
- [9].Yamaguchi T, Terashima M, Akasaka T, Hayashi T, Mizuno K, Muramatsu T, et al. Safety and feasibility of an intravascular optical coherence tomography image wire system in the clinical setting. Am. J. Cardiol. 2008;101:562–567. doi: 10.1016/j.amjcard.2007.09.116. [DOI] [PubMed] [Google Scholar]
- [10].Yoo H, Kim JW, Shishkov M, Namati E, Morse T, Shubochkin R, et al. Intra-arterial catheter for simultaneous microstructural and molecular imaging in vivo. Nat. Med. 2011;17:1680–1684. doi: 10.1038/nm.2555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Wang B, Karpiouk A, Yeager D, Amirian J, Litovsky S, Smalling R, et al. In vivo intravascular ultrasound-guided photoacoustic imaging of lipid in plaques using an animal model of atherosclerosis. Ultrasound Med. Biol. 2012;38:2098–2103. doi: 10.1016/j.ultrasmedbio.2012.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Yeager D, Chen YS, Litovsky S, Emelianov S. Intravascular photoacoustics for image-guidance and temperature monitoring during plasmonic photothermal therapy of atherosclerotic plaques: a feasibility study. Theranostics. 2013;4:36–46. doi: 10.7150/thno.7143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [13].Li X, Wu W, Chung Y, Shih WY, Shih WH, Zhou Q, et al. 80-MHz intravascular ultrasound transducer using PMN-PT free-standing film. IEEE Trans. Ultrason. Ferroelectrics Freq. Control. 2011;58:2281–2288. doi: 10.1109/TUFFC.2011.2085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Park J, Li X, Zhou Q, Shung KK. Combined chirp coded tissue harmonic and fundamental ultrasound imaging for intravascular ultrasound: 20-60 MHz phantom and ex vivo results. Ultrasonics. 2013;53:369–376. doi: 10.1016/j.ultras.2012.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Yan X, Lam KH, Li X, Chen R, Ren W, Ren X, et al. Lead-free intravascular ultrasound transducer using BZT-50BCT ceramics. IEEE Trans. Ultrason. Ferroelectrics Freq. Control. 2013;60:1272–1276. doi: 10.1109/TUFFC.2013.2692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Li X, Li J, Jing J, Ma T, Liang S, Zhang J, et al. Integrated IVUS-OCT Imaging for atherosclerotic plaque characterization. IEEE J. Select. Top. Quant. Electron. Publ. IEEE Lasers Electro-opt. Soc. 2014;20:7100108. doi: 10.1109/JSTQE.2013.2274724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Li X, Ma T, Tian J, Han P, Zhou Q, Shung KK. Micromachined PIN-PMN-PT crystal composite transducer for high-frequency intravascular ultrasound (IVUS) imaging. IEEE Trans. Ultrason. Ferroelectrics Freq. Control. 2014;61:1171–1178. doi: 10.1109/TUFFC.2014.3016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Foster FS, Pavlin CJ, Harasiewicz KA, Christopher DA, Turnbull DH. Advances in ultrasound biomicroscopy. Ultrasound Med. Biol. 2000;26:1–27. doi: 10.1016/s0301-5629(99)00096-4. [DOI] [PubMed] [Google Scholar]
- [19].Zhou Q, Lau S, Wu D, Shung KK. Piezoelectric films for high frequency ultrasonic transducers in biomedical applications. Prog. Mater. Sci. 2011;56:139–174. doi: 10.1016/j.pmatsci.2010.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Wang Y, Stephens DN, O’Donnell M. Optimizing the beam pattern of a forward-viewing ring-annular ultrasound array for intravascular imaging. IEEE Trans. Ultrason. Ferroelectrics Freq. Control. 2002;49:1652–1664. doi: 10.1109/tuffc.2002.1159845. [DOI] [PubMed] [Google Scholar]
- [21].Dodge JT, Jr., Brown BG, Bolson EL, Dodge HT. Lumen diameter of normal human coronary arteries. Influence of age, sex, anatomic variation, and left ventricular hypertrophy or dilation. Circulation. 1992;86:232–246. doi: 10.1161/01.cir.86.1.232. [DOI] [PubMed] [Google Scholar]
- [22].Nissen SE, Gurley JC, Grines CL, Booth DC, McClure R, Berk M, et al. Intravascular ultrasound assessment of lumen size and wall morphology in normal subjects and patients with coronary artery disease. Circulation. 1991;84:1087–1099. doi: 10.1161/01.cir.84.3.1087. [DOI] [PubMed] [Google Scholar]
- [23].Maehara A, Mintz GS, Bui AB, Walter OR, Castagna MT, Canos D, et al. Morphologic and angiographic features of coronary plaque rupture detected by intravascular ultrasound. J. Am. College Cardiol. 2002;40:904–910. doi: 10.1016/s0735-1097(02)02047-8. [DOI] [PubMed] [Google Scholar]
- [24].Kawasaki M, Bouma BE, Bressner J, Houser SL, Nadkarni SK, MacNeill BD, et al. Diagnostic accuracy of optical coherence tomography and integrated backscatter intravascular ultrasound images for tissue characterization of human coronary plaques. J. Am. College Cardiol. 2006;48:81–88. doi: 10.1016/j.jacc.2006.02.062. [DOI] [PubMed] [Google Scholar]
- [25].Nasu K, Tsuchikane E, Katoh O, Vince DG, Virmani R, Surmely JF, et al. Accuracy of in vivo coronary plaque morphology assessment: a validation study of in vivo virtual histology compared with in vitro histopathology. J. Am. College Cardiol. 2006;47:2405–2412. doi: 10.1016/j.jacc.2006.02.044. [DOI] [PubMed] [Google Scholar]
- [26].Thim T, Hagensen MK, Wallace-Bradley D, Granada JF, Kaluza GL, Drouet L, et al. Unreliable assessment of necrotic core by virtual histology intravascular ultrasound in porcine coronary artery disease. Circulation Cardiovasc. Imag. 2010;3:384–391. doi: 10.1161/CIRCIMAGING.109.919357. [DOI] [PubMed] [Google Scholar]
- [27].Levine GN, Bates ER, Blankenship JC, Bailey SR, Bittl JA, Cercek B, et al. 2011 ACCF/AHA/SCAI Guideline for Percutaneous Coronary Intervention. A report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Society for Cardiovascular Angiography and Interventions. J. Am. College Cardiol. 2011;58:e44–e122. doi: 10.1016/j.jacc.2011.08.007. [DOI] [PubMed] [Google Scholar]