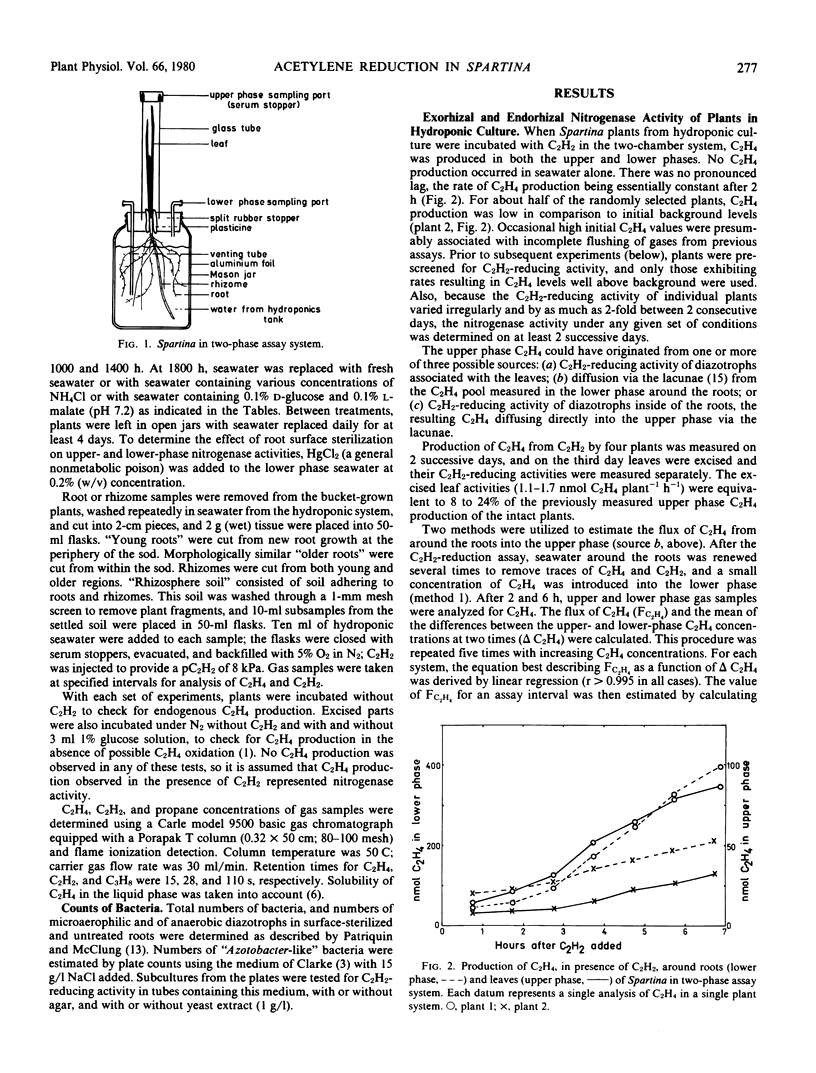
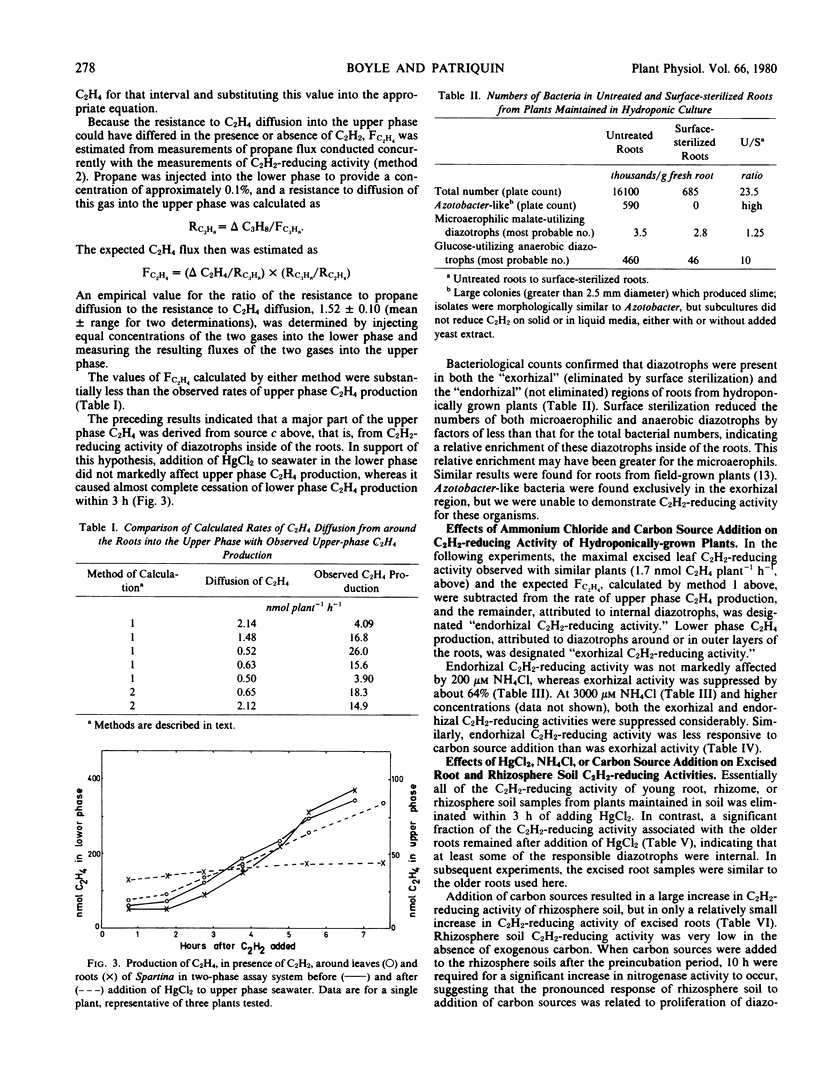
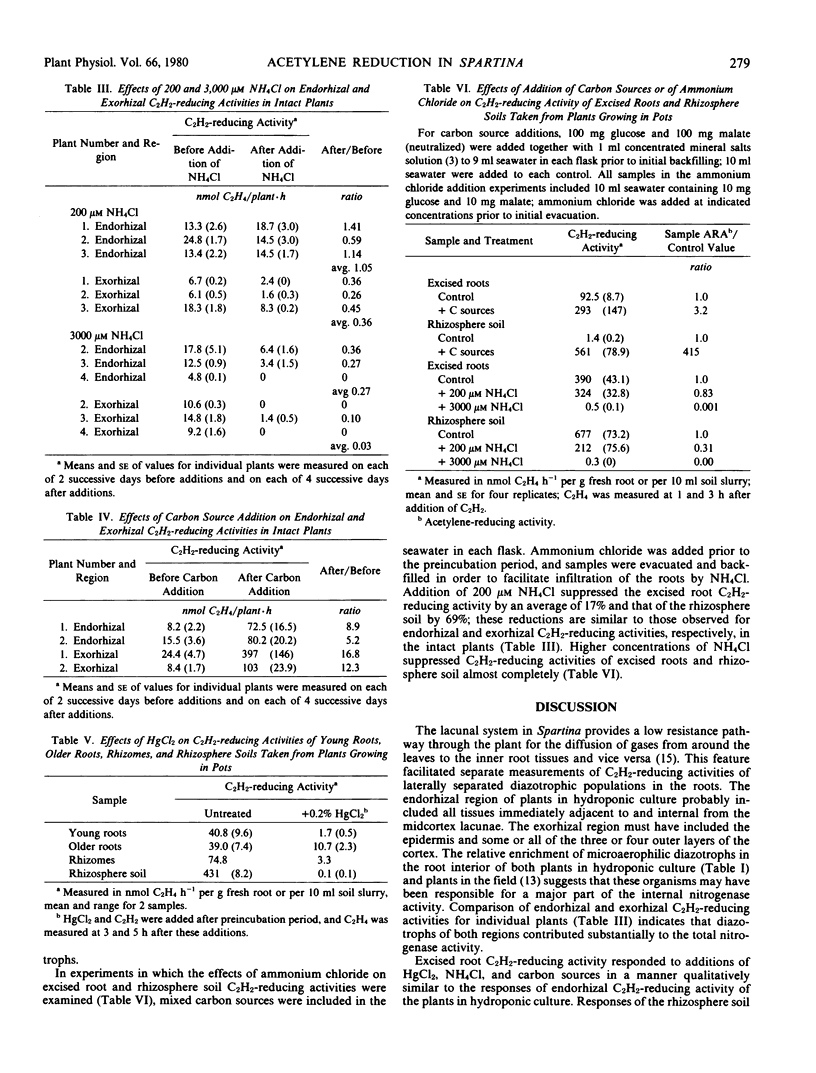
Abstract
Earlier studies indicated that bacteria responsible for nitrogenase activity of some grasses are located inside the roots. Those studies were conducted with excised roots in which a long, unexplained “lag phase” occurred before initiation of nitrogenase activity. When hydroponically maintained Spartina alterniflora Loisel. was incubated in a two-compartment system with acetylene, ethylene was produced following, at most, a 2-hour lag in both the upper (shoot) and lower (roots + water) phases. Ethylene production in the upper phase not attributable to leaf-associated acetylene-reducing activity or to diffusion of ethylene from around the roots is considered to represent “endorhizal acetylene-reducing activity,” the internally produced ethylene diffusing into the upper phase via the lacunae. Ethylene produced in the lower phase is designated “exorhizal acetylene-reducing activity.” The endorhizal acetylene-reducing activity, in comparison to exorhizal activity, was relatively insensitive to additions of HgCl2, NH4Cl, or carbon sources to the lower phase. Post-lag acetylene-reducing activity of roots excised from plants growing in soil responded to additions in a manner similar to that of endorhizal acetylene-reducing activity, whereas post-lag acetylene-reducing activity of rhizosphere soil responded in a manner similar to that of exorhizal acetylene-reducing activity.
Full text
PDF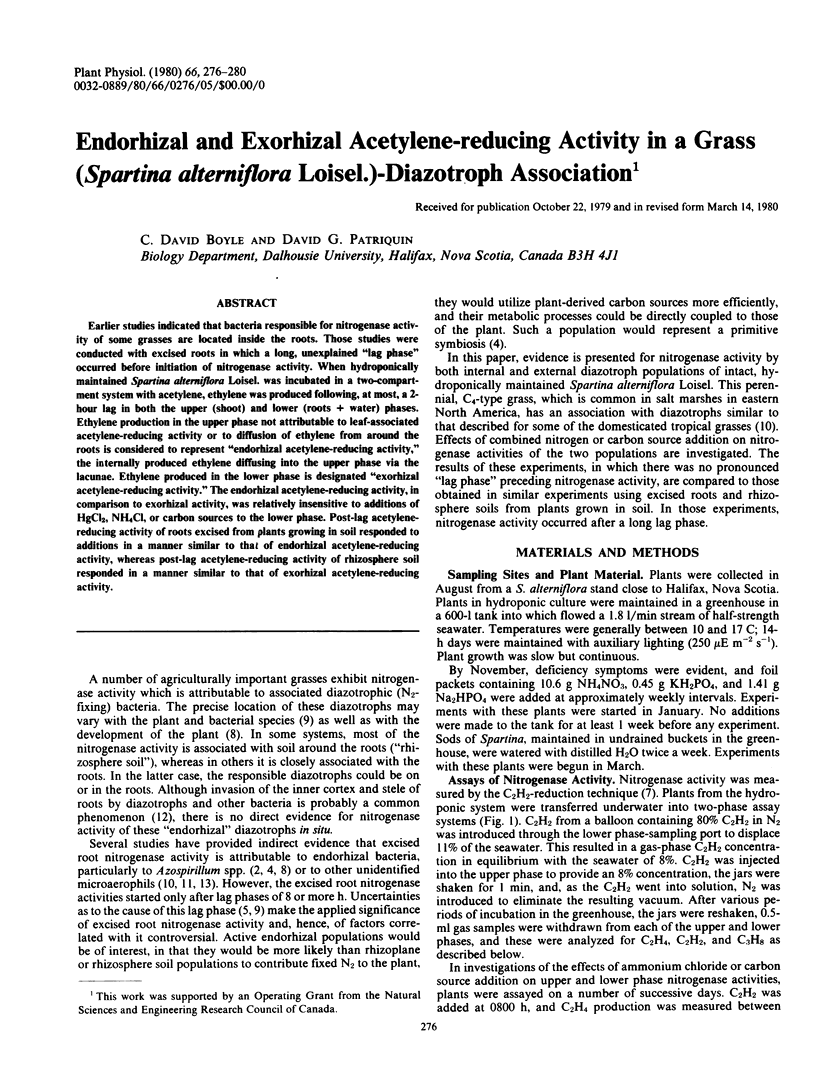

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Evans H. J., Barber L. E. Biological nitrogen fixation for food and fiber production. Science. 1977 Jul 22;197(4301):332–339. doi: 10.1126/science.197.4301.332. [DOI] [PubMed] [Google Scholar]
- Flett R. J., Hamilton R. D., Campbell N. E. Aquatic acetylene-reduction techniques: solutions to several problems. Can J Microbiol. 1976 Jan;22(1):43–51. doi: 10.1139/m76-006. [DOI] [PubMed] [Google Scholar]
- Hardy R. W., Holsten R. D., Jackson E. K., Burns R. C. The acetylene-ethylene assay for n(2) fixation: laboratory and field evaluation. Plant Physiol. 1968 Aug;43(8):1185–1207. doi: 10.1104/pp.43.8.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patriquin D. G., Döbereiner J. Light microscopy observations of tetrazolium-reducing bacteria in the endorhizosphere of maize and other grasses in Brazil. Can J Microbiol. 1978 Jun;24(6):734–742. doi: 10.1139/m78-122. [DOI] [PubMed] [Google Scholar]
- Von Bülow J. F., Döbereiner J. Potential for nitrogen fixation in maize genotypes in Brazil. Proc Natl Acad Sci U S A. 1975 Jun;72(6):2389–2393. doi: 10.1073/pnas.72.6.2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- deBont J. A. Bacterial degradation of ethylene and the acetylene reduction test. Can J Microbiol. 1976 Jul;22(7):1060–1062. doi: 10.1139/m76-155. [DOI] [PubMed] [Google Scholar]



