Abstract
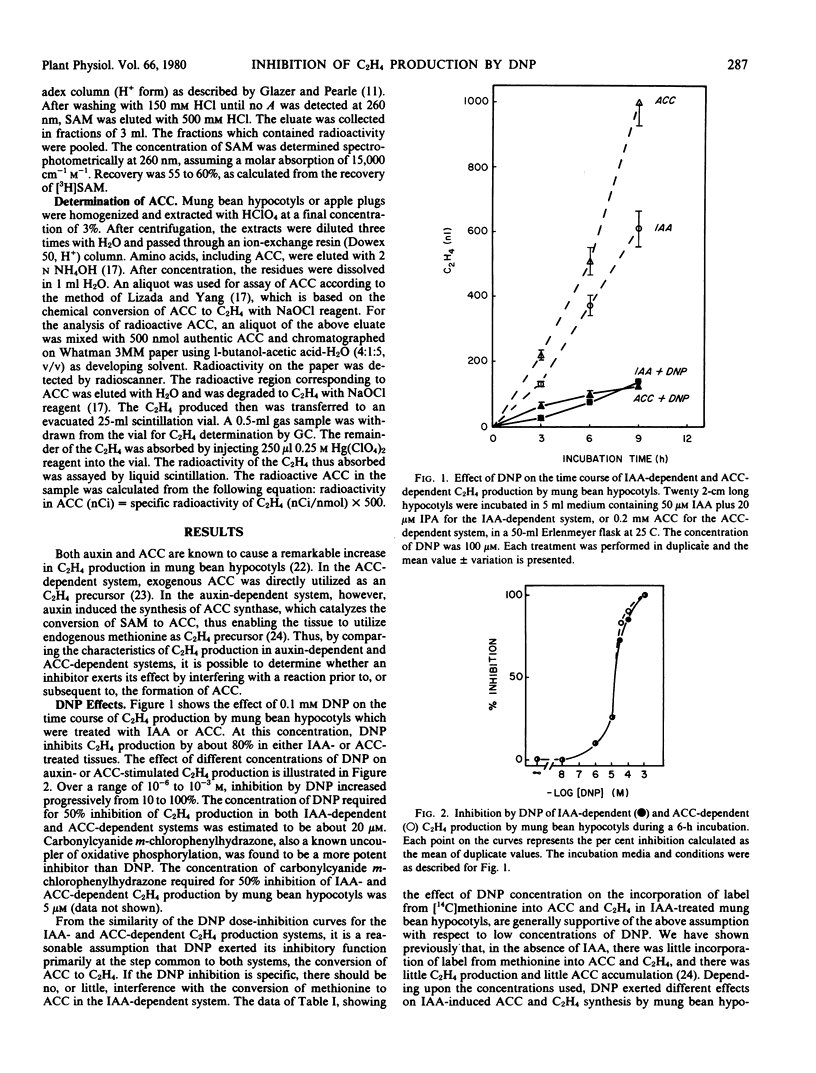
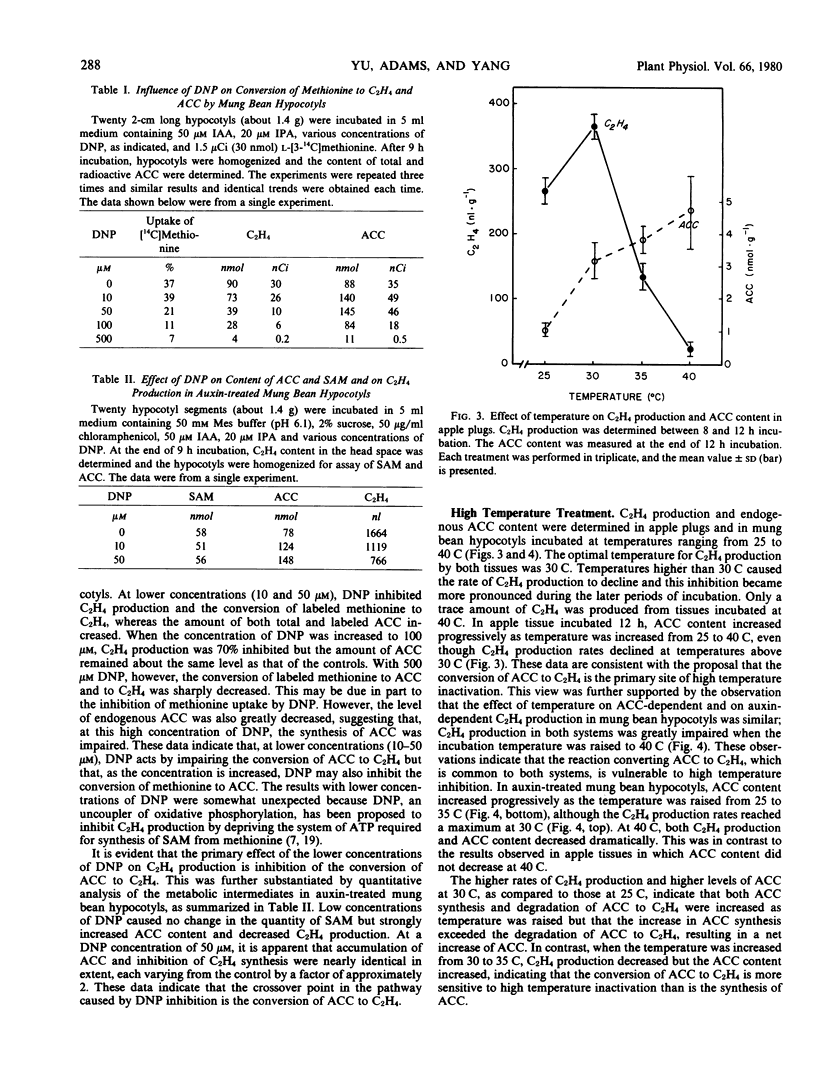
2,4-Dinitrophenol (DNP) and high temperature (35 to 40 C) are known to inhibit C2H4 production in various plant tissues. The present study was made to determine the step in the C2H4 biosynthetic pathway (methionine → S-adenosylmethionine [SAM] → 1-aminocyclopropane-1-carboxylic acid [ACC] → C2H4) at which these treatments exert their inhibitory effect. In mung bean hypocotyls the dose-inhibition curves for the effect of DNP on auxin-dependent C2H4 production (in which auxin exerts its effect by stimulating the conversion of SAM to ACC) and on ACC-dependent C2H4 production (in which ACC is directly utilized as precursor) were similar. It was concluded, therefore, that DNP at low concentrations (below 50 micromolar) exerted its effect primarily on the conversion of ACC to C2H4, a step which is common to both systems. This view was further substantiated by quantitative analysis of the intermediates in the biosynthetic sequence. DNP exerted little influence on the content of SAM but caused a significant increase in the ACC content and marked inhibition in C2H4 production, indicating that the conversion of ACC to C2H4 is the crossover point. At higher concentrations (above 100 micromolar), DNP inhibited the conversion of methionine to ACC and to C2H4, and this effect could be attributed to the inhibition of SAM synthesis.
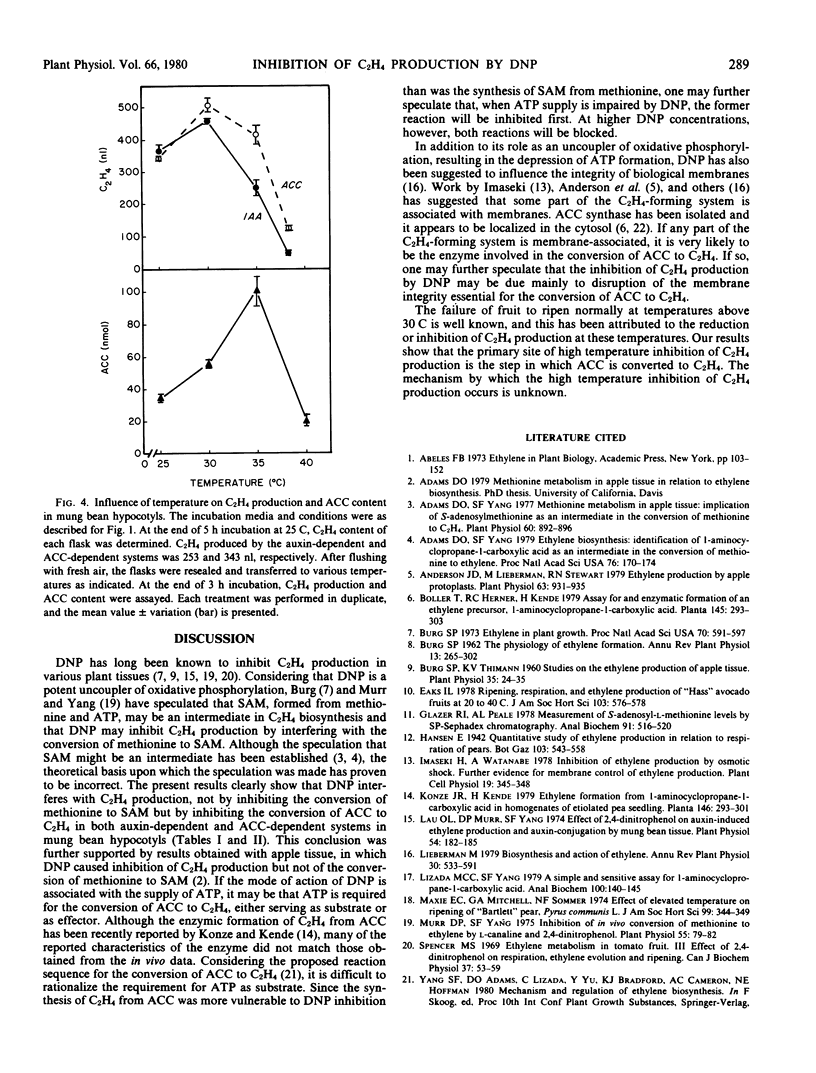
The optimal temperature for maximal C2H4 production by apple tissue and mung bean hypocotyl is about 30 C. An increase in temperature to 35 C caused an accumulation of endogenous ACC, whereas C2H4 production was greatly reduced. These results suggest that the conversion of ACC to C2H4 is highly vulnerable to high temperature inhibition.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams D. O., Yang S. F. Ethylene biosynthesis: Identification of 1-aminocyclopropane-1-carboxylic acid as an intermediate in the conversion of methionine to ethylene. Proc Natl Acad Sci U S A. 1979 Jan;76(1):170–174. doi: 10.1073/pnas.76.1.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams D. O., Yang S. F. Methionine metabolism in apple tissue: implication of s-adenosylmethionine as an intermediate in the conversion of methionine to ethylene. Plant Physiol. 1977 Dec;60(6):892–896. doi: 10.1104/pp.60.6.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson J. D., Lieberman M., Stewart R. N. Ethylene production by apple protoplasts. Plant Physiol. 1979 May;63(5):931–935. doi: 10.1104/pp.63.5.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P. Ethylene in plant growth. Proc Natl Acad Sci U S A. 1973 Feb;70(2):591–597. doi: 10.1073/pnas.70.2.591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burg S. P., Thimann K. V. Studies on the Ethylene Production of Apple Tissue. Plant Physiol. 1960 Jan;35(1):24–35. doi: 10.1104/pp.35.1.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glazer R. I., Peale A. L. Measurement of S-adenosyl-L-methionine levels by SP Sephadex chromatography. Anal Biochem. 1978 Dec;91(2):516–520. doi: 10.1016/0003-2697(78)90538-9. [DOI] [PubMed] [Google Scholar]
- Lau O. L., Murr D. P., Yang S. F. Effect of 2,4-Dinitrophenol on Auxin-induced Ethylene Production and Auxin Conjugation by Mung Bean Tissue. Plant Physiol. 1974 Aug;54(2):182–185. doi: 10.1104/pp.54.2.182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lizada M. C., Yang S. F. A simple and sensitive assay for 1-aminocyclopropane-1-carboxylic acid. Anal Biochem. 1979 Nov 15;100(1):140–145. doi: 10.1016/0003-2697(79)90123-4. [DOI] [PubMed] [Google Scholar]
- Murr D. P., Yang S. F. Inhibition of in Vivo Conversion of Methionine to Ethylene by l-Canaline and 2,4-Dinitrophenol. Plant Physiol. 1975 Jan;55(1):79–82. doi: 10.1104/pp.55.1.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SPENCER M. S. Ethylene metabolism in tomato fruit. III. Effect of 2,4-dinitrophenol on respiration, ethylene evolution, and ripening. Can J Biochem Physiol. 1959 Jan;37(1):53–59. [PubMed] [Google Scholar]


