Abstract
Purpose
Conventional platinum based chemotherapy for advanced urothelial carcinoma is plagued by common resistance to this regimen. Several studies implicate the EGFR family of RTKs in urothelial carcinoma progression and chemoresistance. Many groups have investigated the effects of inhibitors of this family in patients with urothelial carcinoma. This review focuses on the underlying molecular pathways that lead to urothelial carcinoma resistance to EGFR family inhibitors.
Materials and Methods
We performed a PubMed® search for peer reviewed literature on bladder cancer development, EGFR family expression, clinical trials of EGFR family inhibitors and molecular bypass pathways. Research articles deemed to be relevant were examined and a summary of original data was created. Meta-analysis of expression profiles was also performed for each EGFR family member based on data sets accessible via Oncomine®.
Results
Many clinical trials using inhibitors of EGFR family RTKs have been done or are under way. Those that have concluded with results published to date do not show an added benefit over standard of care chemotherapy in an adjuvant or second line setting. However, a neoadjuvant study using erlotinib before radical cystectomy demonstrated promising results.
Conclusions
Clinical and preclinical studies show that for reasons not currently clear prior treatment with chemotherapeutic agents rendered patients with urothelial carcinoma with muscle invasive bladder cancer resistant to EGFR family inhibitors as well. However, EGFR family inhibitors may be of use in patients with no prior chemotherapy in whom EGFR or ERBB2 is over expressed.
Keywords: urinary bladder neoplasms, drug resistance, neoplasm invasiveness, EGFR tyrosine kinase inhibitor 324674, antineoplastic agents
CURRENT TREATMENT OPTIONS IN BLADDER CANCER
Of all urothelial malignancies 90% arise from the transitional epithelium and are classified as TCC (fig. 1).1 MIBC comprises 33% of initial cases of TCC while the remaining cases are classified as NMIBC.2 NMIBC is more easily treated and managed than MIBC. Standard of care treatment for NMIBC is TURBT followed by a single dose of intravesical chemotherapy or intravesical BCG.3 While this regimen yields a 5-year survival rate of 82% to 100%, the 2-year recurrence rate in these patients is 28% to 40%4,5 and as high as 80% in the subset of patients who initially present with high grade tumors (table 1).
Figure 1.
Bladder structural layers and EGFR family member expression. A, various structures of bladder and tissue layers from transitional epithelium, which is innermost layer, to adventitia, which is outermost layer. Urothelium is composed of transitional epithelium, basal lamina and submucosa. B and C, pattern of EGFR family member expression. B, in normal urothelium. C, in cancerous urothelium.
Table 1.
UC current staging, TNM classification and treatment options
| Stage | TNM Classification | Treatment Options
|
% Recurrence Risk | |
|---|---|---|---|---|
| Common | Other | |||
| 0 | Ta, N0, M0 or Tis, N0, M0 | TUR + fulguration, segmental cystectomy if aggressive | Intravesical thiotepa, mitomycin, doxorubicin or BCG | 28–40 |
| I | T1, N0, M0 | TUR + fulguration, segmental cystectomy if aggressive | Intravesical thiotepa, mitomycin, doxorubicin or BCG | 80 |
| II | T2a, N0, M0 or T2b, N0, M0 | RC, neoadjuvant chemotherapy for MIBC | Definitive radiation therapy with systemic chemotherapy | 50 |
| III | T3a, N0, M0, or T3b, N0, M0, or T4a, N0, M0 | RC, neoadjuvant chemotherapy for MIBC | Definitive radiation therapy with systemic chemotherapy | 50 |
| IV | T4b, N0, M0, or any T, N1–N3, M0, or any T, any N, M1 | Palliative care + clinical trials (most cases) | Radical cystectomy with pelvic lymph node dissection (some cases) | – |
| Recurrence | Any T, any N, any M | TUR + fulguration, segmental cystectomy if aggressive (low stage) | Radical cystectomy, neoadjuvant chemotherapy for MIBC (high stage) | Not applicable |
Approximately 15% to 30% of high grade NMIBC cases develop into MIBC,1 in addition to 30% with de novo MIBC presentation. MIBC is highly aggressive compared to NMIBC, correlating with a high rate of metastasis and mortality. Most patients who present with MIBC undergo RC alone. However, level 1 evidence supports the use of platinum based neoadjuvant chemotherapy followed by RC and urinary diversion or radiation therapy with accompanying chemotherapy.1,6 A multicenter, randomized, controlled clinical trial showed 77-month median survival (57% 5-year survival rate) in patients with nonmetastatic MIBC treated with neoadjuvant chemotherapy combined with cystectomy compared to 46-month median survival (43% 5-year survival rate) in those treated with cystectomy alone.7 The patient prognosis after surgery depends on the extent of invasion and whether lymph node metastases are present.8
Upon the development of metastasis cytotoxic chemotherapy with GC as a first line treatment is gaining acceptance.8 Although it is initially effective, average survival on this treatment is only 15 months with a 5-year survival rate of between 5% and 20%.8,9 Therefore, treatment to prevent the dissemination of bladder tumors to distant sites and treatments that sensitize patients with MIBC to chemotherapy are required at this time.
EGFR FAMILY IN BLADDER CANCER
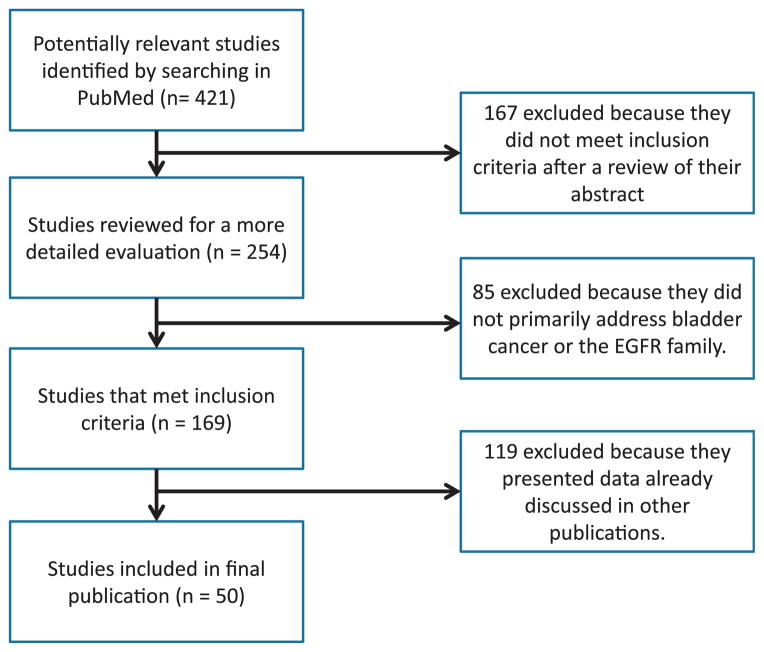
The 4 receptors that comprise the EGFR family are EGFR (ErbB1/Her1), human EGFR 2 (ERBB2/HER2/NEU), human EGFR 3 (ERBB3/HER3) and human EGFR 4 (ERBB4/HER4). A search of publications revealed 421 that discussed the EGFR family or bladder cancer (fig. 2). These receptors are stimulated by a number of growth factors, including EGF, transforming growth factor-α and amphiregulin for EGFR, the heregulins for ERBB3 and ERBB4, and HB-EGF for EGFR and ErbB4 (fig. 3).10 Interestingly HB-EGF may have different characteristics based on whether it is present in its membrane-anchored or soluble form.11 In contrast, ERBB2 is an orphan receptor that is believed to exist in a constitutively primed state,12 which binds to other activated EGFR family members. Each EGFR member consists of a ligand binding extracellular domain, a transmembrane domain and an intracellular tyrosine-kinase domain (fig. 4).
Figure 2.
For literature search strategy PubMed was initially queried for relevant publications meriting inclusion in bladder cancer discussion. After initial search 167 publications were removed because they did not meet inclusion criteria for review. During more thorough review of remaining 254 publications 85 were excluded because they did not primarily address bladder cancer or EGFR family of RTKs. Another 119 publications were excluded because they did not add new data to understanding of EGFR family role in MIBC.
Figure 3.
Pathway of all possible EGFR family dimer combinations showing various growth factors known to activate EGFR family and all possible dimer combinations. EGFR primarily signals through canonical SRC and RAS pathways leading to migration, invasion, adhesion, angiogenesis and survival of cancer cells. ERBB4 signals through STAT5 pathway, which leads to proliferation, apoptosis, differentiation and inflammation. EGF, epidermal growth factor. TGF-α, transforming growth factor-α.
Figure 4.
Structure of ErbB RTKs and inhibitors of each family member, representing most common isoform as described in AceView (http://www.ncbi.nlm.nih.gov/IEB/Research/Acembly/). Each family member also contains number of spliced variants not discussed in this review. Extracellular domain contains cleavable signaling sequence (SS ) in N-terminal end followed by 2 ligand interacting domains, L1 and L2, separated by 1 or 2 furin-like cysteine rich regions CR1 and CR2. In ERBB4 but not in other receptors coiled-coil region (CCR) is located between signaling sequence and L1 domains. Juxtamembrane domain (JM ) is region of many spliced alternative isoforms, especially in ERBB4. Tyrosine kinase (TK ) domain is present in all family members but in ERBB2 it is marked by dileucine domain (DLD) not found in others. ERBB2 and ERBB4 each contain 2 YLP motifs in C-terminal end that is not seen in other 2 members. Also shown are various EGFR family inhibitors. Antibodies cetuximab, trastuzumab and MM-121 work by binding to extracellular domain and inhibiting ligand binding, respectively. Note small molecule inhibitors gefitinib, erlotinib, lapatinib and dacomitinib. Arrows from each inhibitor indicate where these inhibitors bind on respective molecules.
The EGFR family of RTKs relies on dimerization among the 4 family members to transmit signal from the extracellular space into the cytoplasm, where downstream signaling cascades are activated (fig. 3). Upon ligand binding a conformational change is observed in the extracellular domain of the receptor, which enables dimerization with the other EGFR family members.13 Dimerization induces the dimer partners to undergo transactivation, causing phosphorylation of specific sites. These sites serve as docking sites for various adapter proteins that activate a host of pathways, including PKC, PI3K, RAS, SRC, ABL, PAK and STAT5 (fig. 3).14 Not surprisingly the recent TCGA study of 131 MIBC samples, 118 peripheral blood samples and 23 tumor adjacent, normal-appearing bladder samples revealed that changes that affected the PI3K/Akt/mTOR pathway and the RTK/RAS pathway occurred in 42% and 44% of bladder tumors, respectively.15
Epidermal Growth Factor Receptor
In normal urothelium EGFR is expressed in the basal layer and correlates with a less differentiated state of these cells.16 Due to the apparent role of EGFR in bladder cell dedifferentiation it is not surprising that EGFR over expression in UC has been reported frequently in the literature (table 2).17–19 A study of 56 samples of NMIBC or MIBC demonstrated that while EGFR over expression was modest in NMIBC (3 of 25 cases or 12%), it was more evident in MIBC (10 of 28 or 35%) as determined by IHC.19 A similar study used a cohort of 175 NMIBC and 70 MIBC cases.18 In accord with the rates in the previous study EGFR over expression was observed in 67 of 245 cases (27%).18 Furthermore, a study of 21 patients with NMIBC (3 or 14%) or MIBC (18 or 86%) showed that 14 (74%) were positive for EGFR staining by IHC while EGFR over expression was noted in 10 (53%).17 Lastly, HB-EGF has a prognostic role in patient survival. In a study of 121 NMIBC and MIBC specimens patients with predominantly nuclear expression of HB-EGF had 30% lower 5-year cancer specific survival than patients with predominantly nuclear expression of HB-EGF (p = 0.027).20
Table 2.
Roles of EGFR receptor family in UC progression
| Receptor | Presence vs Normal Tissue
|
Differentiation Role | Disease Progression Correlations | |
|---|---|---|---|---|
| NMIBC | MIBC | |||
| EGFR: | Causes cell dedifferentiation | EGFR protein responds to high epidermal growth factor in urine27 + drives cells proliferation + growth39 | ||
| No. pts | 230 | 336 | ||
| Fold change | 0.73 | 1.49 | ||
| p Value | 0.525 | 0.034 | ||
| ERBB2: | Forms heterodimers with other ErbB family members. | Correlates with muscle invasive metastases, + first + second recurrences,21 may increase tumor response to growth factors in urine32 | ||
| No. pts | 230 | 336 | ||
| Fold change | 1.94 | 1.55 | ||
| p Value | 0.586 | <0.001 | ||
| ERBB3: | Forms heterodimers which primarily signal the PI3K pathway | ErbB3 protein responds to heregulins in urine,27 correlates with tumor size, No. + histological grade21 | ||
| No. pts | 204 | 283 | ||
| Fold change | 1.94 | 1.53 | ||
| p Value | <0.001 | 0.004 | ||
| ERBB4: | Causes cell differentiation | Correlated with stage in 1 study32 | ||
| No. pts | 204 | 283 | ||
| Fold change | 0.06 | 0.71 | ||
| p Value | 0.065 | <0.001 | ||
Overall meta-analysis of 5 studies in Oncomine of superficial TCC21–25 and 6 of MIBC21–26 comprising a total of 566 samples revealed that EGFR expression did not significantly differ in normal tissue compared to superficial TCC (p = 0.525). However, it was over expressed in MIBC compared to normal tissue (1.49-fold change, p = 0.034, table 2). While these studies indicate that EGFR is over expressed in bladder cancer, by looking closely at the large cohort series it can be determined that EGFR over expression is more common and occurs more frequently in MIBC than in NMIBC.
Despite the presence of EGFR mutations in many other cancers a survey of 11 UC cell lines and 75 primary tumors demonstrated no mutations when analyzed by automated sequencing.27 A specific probe of exons 19 and 21 via quantitative polymerase chain reaction of formalin fixed, paraffin embedded primary tumors from 21 patients, primarily MIBC, revealed the same result.17 Furthermore, a study of 28 urothelial primary adenocarcinomas28 and another study in 8 cell lines29 showed no mutations in EGFR, indicating that mutations in EGFR are rare in primary UC. However, in the TCGA study there was a 9% incidence of EGFR amplification in 131 MIBC samples.15
Although to our knowledge the EGFR mutation rate in distant metastases is unknown, a study of 17 patients with MIBC (total of 22 primary tumors and 24 associated metastases) showed strong concordance (mean 75%) between the chromosomal aberrations in the primary tumors and their associated metastases.30 Since mutations and gene amplifications of EGFR are a rare event in UC, it was hypothesized that EGFR over expression is due to deregulation of the protein recycling and degradation pathways.17,29 Specifically, endophilin A1, which regulates EGFR endocytosis, was commonly down-regulated in bladder tumors, providing another plausible mechanism of EGFR over expression.31 Despite these findings EGFR expression has not been determined to be an independent predictor of disease progression or mortality.
ERBB2
Although ERBB2 is an orphan receptor with no identified ligand and, thus, it cannot form active homodimers, ERBB2 transmits signals by forming heterodimers with other members of the EGFR family.32 ERBB2 is normally expressed on the superficial and intermediate layers of the urothelium.19 During wound healing ERBB2 promotes migration and re-epithelialization of the damaged tissue.10 ERBB2 expression correlates with metastatic MIBC as well as tumor recurrence18 while co-expression of ERBB2 and P53 increased the probability of nodal metastases (table 2).33 However, a study of 70 primary TCCs revealed that ERBB2 over expression is indiscriminate of disease with or without metastasis.32 Of these 70 cases 9 of 19 nonmetastatic tumors (47%) and 18 of 51 metastatic tumors (35%) over expressed ERBB2 as determined by moderate (2+) or high (3+) staining by IHC.32 Meta-analysis of 5 studies of superficial TCC21–25 and 6 of MIBC21–26 reported in Oncomine, comprising a total of 566 samples, showed that ERBB2 expression did not significantly differ in normal tissue compared to superficial TCC (p = 0.586). However, it was significantly over expressed in MIBC compared to normal tissue (1.55-fold change, p <0.0001).
While ERBB2 amplification is a common occurrence in other cancers, in a study of 73 primary UCs gene amplification was present in only 3% to 9% of cases, including 53 NMIBC and 20 MIBC samples.2 However, unlike EGFR, ERBB2 mRNA levels were highly up-regulated in 18 NMIBC and MIBC samples compared to normal tissue.34 A TCGA study of 131 MIBC bladder tumors corroborated this finding, indicating that ERBB2 mutation or amplification was present in 9% of samples, similar to levels in breast cancer, but with more mutations and fewer amplifications in bladder than in breast tumors.15 A study seeking to correlate the EGFR family expression profile with the patient prognosis demonstrated that the predictive value of ERBB2 when co-expressed with EGFR or ERBB3 may be due to its ability to increase the response of tumors to growth factors in urine.18 Indeed, a previous study showed that ERBB2 could slow the degradation of EGFR molecules that were bound to ligand.35 HER2 amplification was reported to be more common in associated metastases than in their corresponding primary tumors. Specifically in a study of 150 MIBC cases the HER2 amplification rate in metastases was 15.3% compared to 8.7% in primary tumors (p = 0.0003).36
ERBB3
The third member of the EGFR family, ERBB3, lacks intrinsic kinase activity, although it has a kinase domain. Upon binding to its ligands, Heregulin 1 and 2, ERBB3 forms heterodimers and homodimers but only the former are capable of transmitting signals, predominantly through the PI3K/AKT pathway (fig. 3).37 In normal urothelium ERBB3 is expressed primarily on superficial cells but several studies demonstrated low grade expression of ERBB3 throughout the urothelium.19 ERBB3 over expression may have a more inclusive effect on UC. A correlative IHC study in 245 patients, including 47 with NMIBC (19%), 118 with local MIBC (48%) and 80 with metastatic MIBC (33%), showed a positive association of ERBB3 with tumor size, number of tumors and histological grade while EGFR correlated only with tumor size and ERBB2 correlated only with tumor grade.18 Furthermore, ERBB3 and ERBB2 were good predictors of first tumor recurrence.18 In contrast, in a study in 73 patients with NMIBC or MIBC ERBB3 was under expressed in MIBC compared to NMIBC and it correlated strongly with ERBB2 expression (table 2).2 However, ERBB3 was not significantly over or under expressed in NMIBC. Despite this last report an Oncomine meta-analysis of 4 studies of superficial TCC22–25 and 5 of MIBC22–26 comprising a total of 487 samples revealed that ERBB3 expression was significantly up-regulated in superficial TCC (1.94-fold change, p <0.0001) and in MIBC (1.53-fold change, p = 0.004) compared to normal tissue (table 2). Significantly a study of 131 MIBC samples demonstrated that mutations in ERBB3 were present in 6% of bladder tumors with similar levels of mutation having been previously reported.15
ERBB4
In contrast to the other members of this family, ERBB4 mediates differentiation in epithelial tissues, including the mammary gland.38 A number of alternately spliced forms of ERBB4 were identified38 that have unique roles in mammary gland development and differentiation as well as growth inhibition.39,40 The 2 sites in ERBB4 where variations are introduced by alternative splicing are the juxtamembrane domain and the cytoplasmic domain.38 The 2 juxtamembrane isoforms are identified as JM-a and JM-b, which differ by the insertion of 23 (JM-a) or 13 (JM-b) alternative amino acids in the proximal extracellular domain N-terminal to the transmembrane domain, while the cytoplasmic isoforms are CYT-1 and CYT-2, of which the latter has a 16 amino acid deletion containing a PI3K binding motif.41,42 Since these variations are in different ERBB4 domains, there are 4 possible combinations, including JMa-/CYT-1, JM-a/CYT-2, JM-b/CYT-1 and JM-b/CYT-2 (fig. 4).38 The JM-a ERBB4 isoform juxtamembrane domain is cleaved in regulated fashion by metalloproteases.38,43 The membrane bound 80 kDa cytoplasmic domain can be further cleaved by γ-secretase, which then allows the cytoplasmic domain to translocate to the nucleus, where it is believed to affect transcription.43 Additionally, while the CYT-1 and CYT-2 isoforms can bind the adapter protein Shc, only the CYT-1 isoform can activate the PI3K/AKT signaling cascade.38
In the bladder ERBB4 is normally expressed in the superficial layer of the urothelium and it correlates with the more differentiated phenotype (table 2).19,32 It was reported that most bladder tumors, NMIBC as well as MIBC, under express ERBB4 as a whole with this under expression becoming more frequent with disease progression.2,32 In agreement with this finding an Oncomine meta-analysis of 4 studies of superficial TCC22–25 and 5 of MIBC22–26 comprising a total of 487 samples showed that ERBB4 expression was unchanged in superficial TCC compared to normal tissue (p = 0.065). However, it was significantly under expressed in MIBC compared to normal tissue (−0.71 fold change, p <0.0001, table 2). A study of 18 samples from patients with NMIBC or MIBC indicated that the JM-a/CYT-1 and JM-a/CYT-2 splice variants of ERBB4 were over expressed in tumor tissues compared with samples of normal urothelium.34 Interestingly it was suggested that the ability of the JM-a extracellular isoform to be cleaved by metalloproteases enables the cytoplasmic domain to function in a ligand independent manner.38 This could then allow for unregulated activation of the Shc/RAS/MAPK pathway and, for the CYT-1 isoform, the PI3K/AKT pathway.
Therefore, overall EGFR and ERBB2 can be significantly over expressed in MIBC but not in NMIBC compared to normal urothelium while ERBB3 is over expressed in each. In contrast, ERBB4 is significantly under expressed in MIBC but not in NMIBC compared to normal tissue. While these results indicate the significance of the RTKs in UC progression, it is important to keep in mind that data sets such as Oncomine only report mRNA data, which only correlates to protein levels by approximately 40%.44
EGFR AND ERBB2 INHIBITOR CLINICAL TRIALS IN BLADDER CANCER
More recently clinical trials of bladder cancer have used EGFR inhibitors alone or combined with cytotoxic chemotherapy to explore new therapeutic strategies in patients with recurrent and metastatic MIBC. This has included using the inhibitors as neoadjuvant therapy in patients with MIBC treated with RC, that is those with localized disease, as well as for first and second line therapy for recurrent disease (table 3). However, only a few studies described are discussed in this review because many are ongoing and the data collected from some that are complete are as yet unreleased.
Table 3.
Clinical trials of EGFR family targeted therapy alone or combined with platinum based chemotherapy
| ClinicalTrials.gov Identifier (disease) |
Drug | Status | EGFR/ERBB2 Inclusion Requirement |
Target No. Pts |
Median OS | EGFR/ERBB2 Pos (%) | Therapy |
|---|---|---|---|---|---|---|---|
| Not applicable (MIBC) | GC or MVAC | Completed | None | 405 | GC 14 vs MVAC 15.2 mos | Unknown | 1st Line systemic chemotherapy for no prior systemic therapy |
| NCT00380029 (MIBC) | Erlotinib | Closed | None | 20 | 10 Survivors (50%) 24.8 mos vs standard of care 63%, 60 mos | Unknown | Neoadjuvant for T2 muscle invasive disease |
| NCT00014144 (MIBC) | Gefitinib | Closed | None | 31 | 3 vs Standard of care 15.1 mos | EGFR (25), ERBB2 (18), EGFR/ERBB2 (10) | 2nd Line for advanced TCC + failed previous chemotherapy |
| NCT00041106 (MIBC) | Gefitinib + GC | Completed | None | 54 | 15.1 vs Standard of care 15.1 mos | Unknown | Initial for advanced TCC |
| NCT01374789 (MIBC) | Panitumumab + GC | Closed | None | 124 | Not reported | Not reported | 1st Line for locally advanced or metastatic disease |
| NCT00949455 (MIBC) | Lapatinib | Unknown | Confirmed 2+ or 3+ IHC EGFR or ERBB2 staining | 204* | Not reported | EGFR +/or ERBB2 (100) | Maintenance lapatinib after 1st line chemotherapy |
| NCT00005831 (MIBC) | Trastuzumab, carboplatin, paclitaxel + GC | Completed | ERBB2 over expression, gene amplification or elevated serum levels | 109 | 14.1 vs Standard of care 15 mos | ERBB2 (100) | 2nd Line for recurrent disease after local therapy or disease incurable by local therapy |
| Not applicable (MIBC) | Lapatinib | Completed | Confirmed 1+, 2+ or 3+ IHC EGFR or ERBB2 staining | 59 | All pts 17.9 wks, EGFR/ERBB2 over expression 30.3 wks, standard of care 15.1 mos | EGFR (51), ERBB2 (42), EGFR/ERBB2 (22) | 2nd Line for advanced TCC + failed platinum based therapy |
| NCT01245660 (MIBC) | Lapatinib | Terminated due to low enrollment | None | 3* | Not applicable | Not applicable | Neoadjuvant lapatinib before RC |
| NCT01828736 (MIBC) | Trastuzumab, GC + carboplatin/cisplatin | Closed | Confirmed 3+ IHC ERBB2 staining or IHC 2+ ERBB2 IHC staining + pos fluorescence in situ hybridization | 61 | Not reported | ERBB2 (100) | 2nd Line for failed previous chemotherapy for nonmetastatic disease |
| NCT00004856 (MIBC) | Trastuzumab | Terminated | Confirmed 3+ IHC ERBB2 staining or HER2 amplification on fluorescence in situ hybridization | 40* | Not reported | ERBB2 (100) | 2nd Line for failed previous chemotherapy |
| NCT01956253 (MIBC) | Neratinib | Closed | None | 1 | Not reported | Not reported | Efficacy evaluation of neratinib for metastatic bladder Ca harboring HER2-GRB7 fusion |
| NCT00238420 (MIBC) | Paclitaxel with/without trastuzumab | Closed | None | 88 | Not reported | Not reported | Local after TURBT + not RC candidate |
| NCT01353222 (MIBC or NMIBC) | DN24-02 | Open | Confirmed 1+, 2+ or 3+ IHC ERBB2 staining | 180* | Not reported | ERBB2 (100) | 1st Line adjuvant for ERBB2 + UC requiring RC |
Number of recruited participants may differ.
Neoadjuvant Therapy for Primary MIBC
A phase II study sought to determine whether 4 weeks of neoadjuvant erlotinib before RC would improve the survival of patients with MIBC.45 The 20 patients enrolled in this study had clinical stage T2 disease and previously underwent TURBT but EGFR status was not a consideration. Significantly after erlotinib administration and at surgery it was found that 5 of the 20 patients (25%) had no detectable disease remaining (pT0) and 7 (35%) had experienced clinical down staging (pT1 or less). At a mean followup of 24.8 months 10 of the 20 patients (50%) were still alive and showed no evidence of disease. Therefore, as the investigators noted, EGFR inhibition in the neoadjuvant setting can have beneficial effects in patients undergoing RC for MIBC.
Efficacy
EGFR inhibitors as therapy for recurrent disease
A number of studies using the EGFR inhibitor gefitinib have been performed in combination with or after chemotherapy. A phase II study by SWOG using gefitinib as single agent salvage therapy was performed in 31 patients in whom conventional chemotherapy for metastatic TCC had previously failed.46 Although EGFR status was not a condition of eligibility for this study, almost half of the pretreatment biopsies expressed strong EGFR staining. Despite this the median OS in patients in this study was 3 months and median progression-free survival was 2 months. In this group and at the dose used (500 mg) toxicity was high with grade 4 cardiovascular ischemia in 4 of 31 patients (13%).
In contrast, a phase II study using the same dose of gefinitib combined with GC treatment was performed in chemotherapy naïve patients by CALGB (Cancer and Leukemia Group B).47 Patients were considered eligible for study if they had histologically confirmed metastatic MIBC and had not previously undergone any systemic therapies, including chemotherapy. Again EGFR status was not part of the eligibility criteria. Median survival in study patients was 15.1 months and median time to progression was 7.4 months. Although gefitinib was well tolerated in this patient group, there was no improvement in the response rate or survival compared to those in a historical control with GC alone.8,9
The results of these studies indicate that resistance to gefitinib develops after or in conjunction with chemoresistance. It is also possible that chemotherapy naïve patients are better able to tolerate gefitinib, although a separate study may be required to test that hypothesis.
ERBB2 inhibitor as chemosensitizing agent
A phase II trial using the humanized monoclonal ERBB2 antibody trastuzumab in combination with paclitaxel, carboplatin and gemcitabine enrolled 109 patients with local or metastatic MIBC and histologically proven transitional or squamous cell carcinomas that were incurable with local therapy and were chemotherapy naïve for advanced disease.48 Patients were also required to have shown ERBB2 over expression to be eligible for the trial. Although the trial was careful to exclude those patients who would not benefit from the addition of trastuzumab and had an initial response rate of 70%, the median survival for those enrolled in the trial was 14.1 months48 compared to 15 months in patients receiving standard of care for metastatic MIBC.8,9 Therefore, it is possible that resistance to trastuzumab therapy sets in rapidly and nullifies the initial positive response. Another 2 phase II trials in patients with HER2 positive bladder cancer were initiated, including for trastuzumab alone and trastuzumab with chemotherapy (ClinicalTrials.gov NCT00004856 and NCT01828736, respectively). However, the results of these trials have not yet been reported.
Dual EGFR/ERBB2 inhibitor as second line therapy for MIBC
A phase II study in 59 patients with local or metastatic MIBC sought to determine the efficacy of the dual EGFR/ERBB2 inhibitor lapatinib as second line therapy after disease progression while on prior platinum based chemotherapy.49 An objective response rate of greater than 10% was observed in only 1.7% of patients but 31% achieved stable disease. Median time to progression and OS in this post-chemotherapy population was 8.6 and 17.9 weeks, respectively. The objective response rate and stable disease correlated with EGFR over expression (p = 0.029). In addition, OS was significantly prolonged in patients who had EGFR or ERBB3 over expressing tumors (p = 0.001). Therefore, dual inhibition of EGFR/ERBB2 seems to be more effective for UC than single agents alone, even in chemotherapy resistant patients in whom EGFR or ERBB3 is over expressed. These results are in accord with those in tissue culture and animal models of bladder cancer progression, in which dual EGFR/ERBB2 inhibition appeared to be more effective than single kinase inhibition.50
Additional trials of diverse EGFR/ERBB2 inhibitors are ongoing or recently concluded. As these results become available the role of EGFR family inhibitors in MIBC will become more apparent.
POSSIBLE CAUSES OF MIBC RESISTANCE TO INHIBITORS OF EGFR FAMILY OF RTKS
Overall the clinical studies performed in patients with MIBC using EGFR family inhibitors demonstrate a lack of efficacy of this treatment combined with or after chemotherapy over the results of chemotherapy alone. However, notably this apparent inefficacy may be due to a lack of screening for the presence of an EGFR family member in the inclusion criteria rather than the inefficacy of the study drugs. Analysis of the results demonstrate that EGFR family inhibitors were fairly effective as neoadjuvant therapy in patients with MIBC undergoing RC as first line therapy for localized disease. They were partially effective in chemotherapy naïve patients with metastatic MIBC in whom EGFR or ERBB3 was over expressed. However, their effects in combination with chemotherapy or as salvage therapy in patients in whom chemotherapy had failed were far less obvious. It is important to note that EGFR and/or ERBB2 over expression/activation was not a criterion in some of the studies. In studies in which the status of these RTKs was determined the response rate was improved in patients with EGFR/ERBB2 over expressing tumors. Therefore, it is reasonable to assume that if the receptor was not over expressed, the corresponding inhibitors failed to have an effect. However, a lack of EGFR/ERBB2 expression alone may not be the only cause of resistance of patients to inhibitors of the EGFR family. As studies of other types of cancer have revealed, multiple other causes can lead to resistance to these inhibitors.
CONCLUSIONS
This analysis demonstrates that EGFR and ERBB2 inhibitors are not effective in all patients with bladder cancer. However, at the same time the literature supports the idea that a small cohort of patients with MIBC (those with EGFR and/or ERBB2 positive tumors) respond to EGFR and/or ERBB2 inhibitors initially. This stresses the importance of screening for the presence of EGFR family RTKs in any clinical trial of the role of EGFR family inhibitors. Recurrence is likely caused by the activation of bypass pathways that activate downstream targets or by mutations in downstream targets that decouple them from RTKs. As noted mutations in EGFR are rare in UC but mutations in related genes may drive the resistance of these tumors to EGFR/ERBB2 inhibitors.
Notably for reasons not currently clear clinical and preclinical studies show that prior treatment with chemotherapeutic agents rendered patients with UC (those with MIBC) resistant to EGFR family inhibitors as well. A likely cause is that chemotherapy may suppress EGFR or ERBB2 expression, thereby rendering tumors resistant to EGFR family inhibitors. This speculation is supported by the observation that EGFR family inhibitors were particularly successful in causing MIBC down staging in a neoadjuvant setting in patients before they underwent RC for localized disease.
What this tells us is that EGFR family inhibitors will be particularly useful in patients with no prior chemotherapy in whom EGFR or ERBB2 is over expressed. While this limits the number of patients who may benefit from this treatment, these results assure us that EGFR family inhibitors will fill a niche that would serve a long-standing need in the treatment of UC. However, much work still must be done to fully understand the conditions under which EGFR family inhibitors would be effective in patients with MIBC.
Acknowledgments
Supported by resources from the Veterans Affairs Northern California Health Care System, Sacramento, California.
Abbreviations and Acronyms
- BCG
bacillus Calmette-Guérin
- EGFR
epidermal growth factor receptor
- GC
gemcitabine and cisplatin
- HB-EGF
heparin-binding epidermal growth factor-like growth factor
- HER
human EGFR
- IHC
immunohistochemistry
- MIBC
muscle invasive bladder cancer
- NMIBC
nonMIBC
- OS
overall survival
- PI3K
phosphatidylinosital-4, 5-bisphosphate 3-kinase
- RC
radical cystectomy
- RTK
receptor tyrosine kinase
- TCC
transitional cell carcinoma
- TCGA
The Cancer Genome Atlas
- TURBT
transurethral resection of bladder tumor
- UC
urothelial carcinoma
Footnotes
The contents reported/presented within do not represent the views of the Department of Veterans Affairs or the United States Government.
References
- 1.Vishnu P, Mathew J, Tan WW. Current therapeutic strategies for invasive and metastatic bladder cancer. Oncol Targets Ther. 2011;4:97. doi: 10.2147/OTT.S22875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Amsellem-Ouazana D, Bièche I, Tozlu S, et al. Gene expression profiling of ERBB receptors and ligands in human transitional cell carcinoma of the bladder. J Urol. 2006;175:1127. doi: 10.1016/S0022-5347(05)00317-4. [DOI] [PubMed] [Google Scholar]
- 3.Brincks EL, Risk MC, Griffith TS. PMN and anti-tumor immunity—the case of bladder cancer immunotherapy. Semin Cancer Biol. 2013;23:183. doi: 10.1016/j.semcancer.2013.02.002. [DOI] [PubMed] [Google Scholar]
- 4.Böhle A, Jocham D, Bock PR. Intravesical bacillus Calmette-Guerin versus mitomycin C for superficial bladder cancer: a formal meta-analysis of comparative studies on recurrence and toxicity. J Urol. 2003;169:90. doi: 10.1016/S0022-5347(05)64043-8. [DOI] [PubMed] [Google Scholar]
- 5.Han RF, Pan JG. Can intravesical bacillus Calmette-Guérin reduce recurrence in patients with superficial bladder cancer? A meta-analysis of randomized trials. Urology. 2006;67:1216. doi: 10.1016/j.urology.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 6.Winquist E, Kirchner TS, Segal R, et al. Neoadjuvant chemotherapy for transitional cell carcinoma of the bladder: a systematic review and meta-analysis. J Urol. 2004;171:561. doi: 10.1097/01.ju.0000090967.08622.33. [DOI] [PubMed] [Google Scholar]
- 7.Grossman HB, Natale RB, Tangen CM, et al. Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med. 2003;349:859. doi: 10.1056/NEJMoa022148. [DOI] [PubMed] [Google Scholar]
- 8.Zachos I, Konstantinopoulos PA, Tzortzis V, et al. Systemic therapy of metastatic bladder cancer in the molecular era: current status and future promise. Expert Opin Investig Drugs. 2010;19:875. doi: 10.1517/13543784.2010.496450. [DOI] [PubMed] [Google Scholar]
- 9.Witjes JA, Compérat E, Cowan NC, et al. EAU guidelines on muscle-invasive and metastatic bladder cancer: summary of the 2013 guidelines. Eur Urol. 2014;65:778. doi: 10.1016/j.eururo.2013.11.046. [DOI] [PubMed] [Google Scholar]
- 10.Bindels EM, van der Kwast TH, Izadifar V, et al. Functions of epidermal growth factor-like growth factors during human urothelial reepithelialization in vitro and the role of erbB2. Urol Res. 2002;30:240. doi: 10.1007/s00240-002-0260-7. [DOI] [PubMed] [Google Scholar]
- 11.Adam RM, Danciu T, McLellan DL, et al. A nuclear form of the heparin-binding epidermal growth factor-like growth factor precursor is a feature of aggressive transitional cell carcinoma. Cancer Res. 2003;63:484. [PubMed] [Google Scholar]
- 12.Olayioye MA, Neve RM, Lane HA, et al. The ErbB signaling network: receptor heterodimerization in development and cancer. EMBO J. 2000;19:3159. doi: 10.1093/emboj/19.13.3159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jathal MK, Chen L, Mudryj M, et al. Targeting ErbB3: the new RTK(id) on the prostate cancer block. Immunol Endocr Metab Agents Med Chem. 2011;11:131. doi: 10.2174/187152211795495643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- 15.Cancer Genome Atlas Research Network. Comprehensive molecular characterization of urothelial bladder carcinoma. Nature. 2014;507:315. doi: 10.1038/nature12965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Messing E, Gee JR, Saltzstein DR, et al. A phase 2 cancer chemoprevention biomarker trial of isoflavone G-2535 (genistein) in presurgical bladder cancer patients. Cancer Prev Res (Phila) 2012;5:621. doi: 10.1158/1940-6207.CAPR-11-0455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chaux A, Cohen JS, Schultz L, et al. High epidermal growth factor receptor immunohistochemical expression in urothelial carcinoma of the bladder is not associated with EGFR mutations in exons 19 and 21: a study using formalin-fixed, paraffin-embedded archival tissues. Hum Pathol. 2012;43:1590. doi: 10.1016/j.humpath.2011.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chow NH, Chan SH, Tzai TS, et al. Expression profiles of ErbB family receptors and prognosis in primary transitional cell carcinoma of the urinary bladder. Clin Cancer Res. 2001;7:1957. [PubMed] [Google Scholar]
- 19.Chow NH, Liu HS, Yang HB, et al. Expression patterns of erbB receptor family in normal urothelium and transitional cell carcinoma. An immunohistochemical study. Virchows Arch. 1997;430:461. doi: 10.1007/s004280050056. [DOI] [PubMed] [Google Scholar]
- 20.Kramer C, Klasmeyer K, Bojar H, et al. Heparin-binding epidermal growth factor-like growth factor isoforms and epidermal growth factor receptor/ErbB1 expression in bladder cancer and their relation to clinical outcome. Cancer. 2007;109:2016. doi: 10.1002/cncr.22627. [DOI] [PubMed] [Google Scholar]
- 21.Blaveri E, Simko JP, Korkola JE, et al. Bladder cancer outcome and subtype classification by gene expression. Clin Cancer Res. 2005;11:4044. doi: 10.1158/1078-0432.CCR-04-2409. [DOI] [PubMed] [Google Scholar]
- 22.Dyrskjøt L, Kruhøffer M, Thykjaer T, et al. Gene expression in the urinary bladder: a common carcinoma in situ gene expression signature exists disregarding histopathological classification. Cancer Res. 2004;64:4040. doi: 10.1158/0008-5472.CAN-03-3620. [DOI] [PubMed] [Google Scholar]
- 23.Lee JS, Leem JS, Lee SY, et al. Expression signature of E2F1 and its associated genes predict superficial to invasive progression of bladder tumors. J Clin Oncol. 2010;28:2660. doi: 10.1200/JCO.2009.25.0977. [DOI] [PubMed] [Google Scholar]
- 24.Modlich O, Prisack HB, Pitschke G, et al. Identifying superficial, muscle-invasive, and metastasizing transitional cell carcinoma of the bladder: use of cDNA array analysis of gene expression profiles. Clin Cancer Res. 2004;10:3410. doi: 10.1158/1078-0432.CCR-03-0134. [DOI] [PubMed] [Google Scholar]
- 25.Sanchez-Carbayo M, Socci ND, Lozano J, et al. Defining molecular profiles of poor outcome in patients with invasive bladder cancer using oligonucleotide microarrays. J Clin Oncol. 2006;24:778. doi: 10.1200/JCO.2005.03.2375. [DOI] [PubMed] [Google Scholar]
- 26.National Cancer Institute. [Accessed July 17, 2014];The Cancer Genome Atlas Data Portal. Available at http://tcga-data.nci.nih.gov/tcga/
- 27.Blehm KN, Spiess PE, Bondaruk JE, et al. Mutations within the kinase domain and truncations of the epidermal growth factor receptor are rare events in bladder cancer: implications for therapy. Clin Cancer Res. 2006;12:4671. doi: 10.1158/1078-0432.CCR-06-0407. [DOI] [PubMed] [Google Scholar]
- 28.Alexander RE, Montironi R, Lopez-Beltran A, et al. EGFR alterations and EML4-ALK rearrangement in primary adenocarcinoma of the urinary bladder. Mod Pathol. 2014;27:107. doi: 10.1038/modpathol.2013.132. [DOI] [PubMed] [Google Scholar]
- 29.Flaig TW, Su LJ, McCoach C, et al. Dual epidermal growth factor receptor and vascular endothelial growth factor receptor inhibition with vandetanib sensitizes bladder cancer cells to cisplatin in a dose- and sequence-dependent manner. BJU Int. 2009;103:1729. doi: 10.1111/j.1464-410X.2009.08367.x. [DOI] [PubMed] [Google Scholar]
- 30.Hovey RM, Chu L, Balazs M, et al. Genetic alterations in primary bladder cancers and their metastases. Cancer Res. 1998;58:3555. [PubMed] [Google Scholar]
- 31.Majumdar S, Gong EM, Di Vizio D, et al. Loss of Sh3gl2/endophilin A1 is a common event in urothelial carcinoma that promotes malignant behavior. Neoplasia. 2013;15:749. doi: 10.1593/neo.121956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Røtterud R, Nesland JM, Berner A, et al. Expression of the epidermal growth factor receptor family in normal and malignant urothelium. BJU Int. 2005;95:1344. doi: 10.1111/j.1464-410X.2005.05497.x. [DOI] [PubMed] [Google Scholar]
- 33.Tsai YS, Tzai TS, Chow NH, et al. Frequency and clinicopathologic correlates of ErbB1, ErbB2, and ErbB3 immunoreactivity in urothelial tumors of upper urinary tract. Urology. 2005;66:1197. doi: 10.1016/j.urology.2005.06.117. [DOI] [PubMed] [Google Scholar]
- 34.Junttila TT, Laato M, Vahlberg T, et al. Identification of patients with transitional cell carcinoma of the bladder overexpressing ErbB2, ErbB3, or specific ErbB4 isoforms: real-time reverse transcription-PCR analysis in estimation of ErbB receptor status from cancer patients. Clin Cancer Res. 2003;9:5346. [PubMed] [Google Scholar]
- 35.Kokai Y, Myers JN, Wada T, et al. Synergistic interaction of p185c-neu and the EGF receptor leads to transformation of rodent fibroblasts. Cell. 1989;58:287. doi: 10.1016/0092-8674(89)90843-x. [DOI] [PubMed] [Google Scholar]
- 36.Fleischmann A, Rotzer D, Seiler R, et al. Her2 amplification is significantly more frequent in lymph node metastases from urothelial bladder cancer than in the primary tumours. Eur Urol. 2011;60:350. doi: 10.1016/j.eururo.2011.05.035. [DOI] [PubMed] [Google Scholar]
- 37.Sergina NV, Rausch M, Wang D, et al. Escape from HER-family tyrosine kinase inhibitor therapy by the kinase-inactive HER3. Nature. 2007;445:437. doi: 10.1038/nature05474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Junttila TT, Sundvall M, Määttä JA, et al. Erbb4 and its isoforms: selective regulation of growth factor responses by naturally occurring receptor variants. Trends Cardiovasc Med. 2000;10:304. doi: 10.1016/s1050-1738(01)00065-2. [DOI] [PubMed] [Google Scholar]
- 39.Muraoka-Cook RS, Feng SM, Strunk KE, et al. ErbB4/HER4: role in mammary gland development, differentiation and growth inhibition. J Mammary Gland Biol Neoplasia. 2008;13:235. doi: 10.1007/s10911-008-9080-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jones FE, Golding JP, Gassmann M. ErbB4 signaling during breast and neural development: novel genetic models reveal unique ErbB4 activities. Cell Cycle. 2003;2:555. [PubMed] [Google Scholar]
- 41.Soule HD, Vazguez J, Long A, et al. A human cell line from a pleural effusion derived from a breast carcinoma. J Natl Cancer Inst. 1973;51:1409. doi: 10.1093/jnci/51.5.1409. [DOI] [PubMed] [Google Scholar]
- 42.Wu HC, Hsieh JT, Gleave ME, et al. Derivation of androgen-independent human LNCaP prostatic cancer cell sublines: role of bone stromal cells. Int J Cancer. 1994;57:406. doi: 10.1002/ijc.2910570319. [DOI] [PubMed] [Google Scholar]
- 43.Ni CY, Murphy MP, Golde TE, et al. gamma-Secretase cleavage and nuclear localization of ErbB-4 receptor tyrosine kinase. Science. 2001;294:2179. doi: 10.1126/science.1065412. [DOI] [PubMed] [Google Scholar]
- 44.Schwänhausser B, Busse D, Li N, et al. Global quantification of mammalian gene expression control. Nature. 2011;473:337. doi: 10.1038/nature10098. [DOI] [PubMed] [Google Scholar]
- 45.Pruthi RS, Nielsen M, Heathcote S, et al. A phase II trial of neoadjuvant erlotinib in patients with muscle-invasive bladder cancer undergoing radical cystectomy: clinical and pathological results. BJU Int. 2010;106:349. doi: 10.1111/j.1464-410X.2009.09101.x. [DOI] [PubMed] [Google Scholar]
- 46.Petrylak DP, Tangen CM, Van Veldhuizen PJ, Jr, et al. Results of the Southwest Oncology Group phase II evaluation (study S0031) of ZD1839 for advanced transitional cell carcinoma of the urothelium. BJU Int. 2010;105:317. doi: 10.1111/j.1464-410X.2009.08799.x. [DOI] [PubMed] [Google Scholar]
- 47.Philips GK, Halabi S, Sanford BL, et al. A phase II trial of cisplatin (C), gemcitabine (G) and gefitinib for advanced urothelial tract carcinoma: results of Cancer and Leukemia Group B (CALGB) 90102. Ann Oncol. 2009;20:1074. doi: 10.1093/annonc/mdn749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hussain MH, MacVicar GR, Petrylak DP, et al. Trastuzumab, paclitaxel, carboplatin, and gemcitabine in advanced human epidermal growth factor receptor-2/neu-positive urothelial carcinoma: results of a multicenter phase II National Cancer Institute trial. J Clin Oncol. 2007;25:2218. doi: 10.1200/JCO.2006.08.0994. [DOI] [PubMed] [Google Scholar]
- 49.Wülfing C, Machiels JP, Richel DJ, et al. A single-arm, multicenter, open-label phase 2 study of lapatinib as the second-line treatment of patients with locally advanced or metastatic transitional cell carcinoma. Cancer. 2009;115:2881. doi: 10.1002/cncr.24337. [DOI] [PubMed] [Google Scholar]
- 50.Quesnelle KM, Grandis JR. Dual kinase inhibition of EGFR and HER2 overcomes resistance to cetuximab in a novel in vivo model of acquired cetuximab resistance. Clin Cancer Res. 2011;17:5935. doi: 10.1158/1078-0432.CCR-11-0370. [DOI] [PMC free article] [PubMed] [Google Scholar]