Abstract
Introduction
Atypical hemolytic uremic syndrome (aHUS) is a prototypic thrombotic microangiopathy attributable to complement dysregulation. In the absence of complement inhibition, progressive clinical deterioration occurs. We postulated that a biopsy of normal skin could corroborate the diagnosis of aHUS via the demonstration of vascular deposits of C5b-9.
Materials and methods
Biopsies of normal skin from 22 patients with and without aHUS were processed for routine light microscopy as well as immunofluorescent studies. An assessment was made for vascular C5b-9 deposition immunohistochemically and by immunofluorescence. The biopsies were obtained primarily from the forearm and or deltoid.
Results
Patients with classic features of atypical HUS showed insidious microvascular changes including loose luminal platelet thrombi except in two patients in whom a striking thrombogenic vasculopathy was apparent in biopsied digital ulcers. Extensive microvascular deposits of the membrane attack complex (MAC)/ C5b-9 were identified excluding one patient in whom eculizumab was initiated prior to biopsy. In 5 of the 7 patients where follow-up was available, the patients exhibited an excellent treatment response to eculizumab. Patients without diagnostic clinical features of atypical HUS failed to show significant vascular deposits of complement except two patients with TTP including one in whom a Factor H mutation was identified.
Conclusion
In a clinical setting where aHUS is an important diagnostic consideration, extensive microvascular deposition of C5b-9 supports the diagnosis of either aHUS or a subset of TTP patients with concomitant complement dysregulation; significant vascular C5b-9 deposition predicts clinical responsiveness to eculizumab.
Keywords: Atypical hemolytic uremic syndrome, aHUS, eculizumab
INTRODUCTION
Atypical hemolytic uremic syndrome (aHUS) is one of the prototypic thrombotic microangiopathy syndromes. The salient features include thrombi, endothelial cell damage, thrombocytopenia, and hemolysis [1]. It has significant overlapping features with thrombotic thrombocytopenic purpura (TTP) although mechanistically they are distinct [2]. Very critical to the pathogenesis of aHUS is dysregulation of complement activation.
There are two main complement pathways, namely the classic and alternate pathways Activation of either pathway initiates the formation of membrane attack complex (MAC) [3]. The MAC complex is composed of C5b, C6, C7, C8, and C9 and hence also falls under the designation of C5b-9; it can generate pores on target cells and activate intracellular processes including those associated with a prothrombotic state and, if numerous enough, cell death [4]. However, the MAC complex is inhibited by various regulatory proteins, of which the most important is CD59. The other complement inhibitors include CD46, Factor H, a cofactor of factor 1 and a decay accelerator of C3-convertase [5].
Factor H is the main inhibitor of complement in the fluid phase of non-activating surfaces. There are also five Factor H (FH) related proteins that may regulate complement activation which are located on chromosome 1q32 [6]. Genetic mutations in the aforesaid FH related proteins can predispose to disease including aHUS. The FH mutations can have variable clinical effects. The main polyanion binding surface recognition is located in the C-terminus area in FH. This area also has a C3d binding site. R1210C mutation of FH, present as a rare polymorphism in geographically separated human populations, is also an associated atypical HUS mutation [7]. The long pentraxin (PTX3) is an acute phase protein, which plays an important role in the innate immunity by binding to complement protein C1q, regulating apoptotic cell clearance and limiting tissue damage in inflammatory conditions. Two binding sites for PTX3 are located on FH. The primary binding site is located on FH domains 19 –20, which interact with the N-terminal domain of PTX3, while a secondary binding site on domain 7 binds the glycosylated PTX3 pentraxin domain. Surface-bound PTX3 enhances FH recruitment and iC3b deposition [8]. Mutations in FH impair the interaction of FH with PTX3 impairing important complement regulatory mechanisms. Complete Factor H deficiency causes dense deposit disease, which is attributed to massive amounts of C3b that accumulate in the kidney [9]. There are certain mutations that have been implicated in atypical HUS including FH, Factor 1, CD46, C3, Factor B, and thrombomodulin.
Although the skin is rarely involved in aHUS, it is the most easily accessible organ for biopsy. We postulated that a biopsy of normal skin could potentially reveal evidence of systemic, dysregulated complement activation characterized by microvascular deposition of MAC/C5b-9 deposition as the end point. We describe biopsy findings of normal skin procured from a cohort of 9 patients with diagnostic clinical features of atypical HUS and 13 patients without diagnostic features of aHUS but in whom sufficient clinical abnormalities prompted a biopsy to rule out aHUS.
MATERIALS AND METHODS
Biopsies of skin were procured from nine patients with HUS. Eight of these patients had diagnostic features of aHUS while one patient had Escherichia (E) coli triggered HUS. In addition, thirteen biopsies were obtained from patients who did not fulfill diagnostic clinical criteria of atypical HUS. In most cases biopsies were available for both routine light microscopy and immunofluorescent (IF) studies. The paraffin embedded samples were examined by routine hematoxylin and eosin (H and E) as well as an assessment for the deposition of MAC/C5b-9, C3d, and C4d was via a diaminobenzidene technique. MAC/C5b-9, C3d, and C4d within the epidermal basement membrane zone (BMZ), the BMZ of the eccrine coil, the elastic fibers in the dermis, and the elastic lamina of vessels were considered nonspecific staining patterns. The IF and immunohistochemical assay for evaluating deposition of MAC/C5b-9, C3d, and C4d conducted on fresh tissue has been previously described [10]. Our immunohistochemical protocol for the assessment of C3d, C4d and MAC/C5b-9 has been previously described and used (VisionBio Systems) for C3d and C4d and (Leica Microsystems) for MAC/C5b-9 [11, 12].
RESULTS
Cases of diagnostic atypical HUS
5 women and 4 men from 25 years to 71 years of age (mean age of 46 years) comprised the 9 patients of the study group. One patient had classic E. coli triggered HUS. Clinical features common to all 9 patients included renal insufficiency, abnormalities in liver function tests, hemolysis, and thrombocytopenia. Among underlying medical conditions were myelofibrosis, colonic adenocarcinoma, membranous glomerulonephritis, idiopathic pancreatitis, sepsis in the setting of chorioaamnionitis and sepsis induced by retention of products of conception.
In two patients from the cohort, both females, cutaneous lesions were observed on examination. One patient, a 24-year-old women presented with sepsis and DIC secondary to chorioamnionitis in the 16th week of pregnancy. She was found to have retiform purpura on the left 4th toe. The other patient had cutaneous ulcers of the second toe.
Subsequent to the biopsy, clinical follow-up was available for 7 of the patients. Five of these patients received eculizumab and exhibited a dramatic response with improvement of renal function along with normalization of anemia and thrombocytopenia. In regards to the other two patients, one received conservative management only and the other patient responded well to plasma exchange.
Of the 5 patients who received eculizumab, one patient, a 59-year-old male, had been diagnosed with membranous glomerulonephritis and developed renal failure at age 33 followed by recurrent renal graft failure attributed to calcineurin-inhibitor-related renal thrombotic microangiopathy. He had a successful salvage of the second graft by discontinuation of the calcineurin-inhibitor and initiation of eculizumab. A diagnosis was made of calcineurin inhibitor aHUS. One patient, a 71-year-old female exhibited a clinical picture of pancreatitis without an apparent etiology coincident with microangiopathic hemolytic anemia, thrombocytopenia, hypocomplementemia (decreased serum C3 concentration), and a normal ADAMTS13 (a disintegrin and metalloproteinase with a thrombospondin type I motif member 13) activity. Atypical HUS seemed to be the most plausible diagnosis. A biopsy of the normal skin confirmed the diagnosis of aHUS and the patient exhibited significant laboratory and clinical improvement after 4-5 weeks of treatment with eculizumab. Another patient, a 52-year-old female with myelofibrosis and severe refractory hemolytic anemia and thrombocytopenia responded very well to eculizumab after few weeks of therapy. One patient, a 34-year-old female developed hemolysis and renal failure in the setting of sepsis induced by retention of products of conception. The patient was resistant to plasmapheresis but had a striking response to eculizumab. The last patient, a 24-year-old female with E. coli sepsis and DIC in the setting of chorioamnionitis and ulcerated toe responded very well to treatment.
Light microscopic findings
5 of the 9 patient biopsies were obtained from normal skin of the forearm and or deltoid. In one patient the biopsy was procured from both the toe ulcer as well as the normal arm skin. In one patient only a biopsy of the toe ulcer was obtained and in another patient a normal skin biopsy from the lower extremity was obtained. In one patient the biopsy site was not specified.
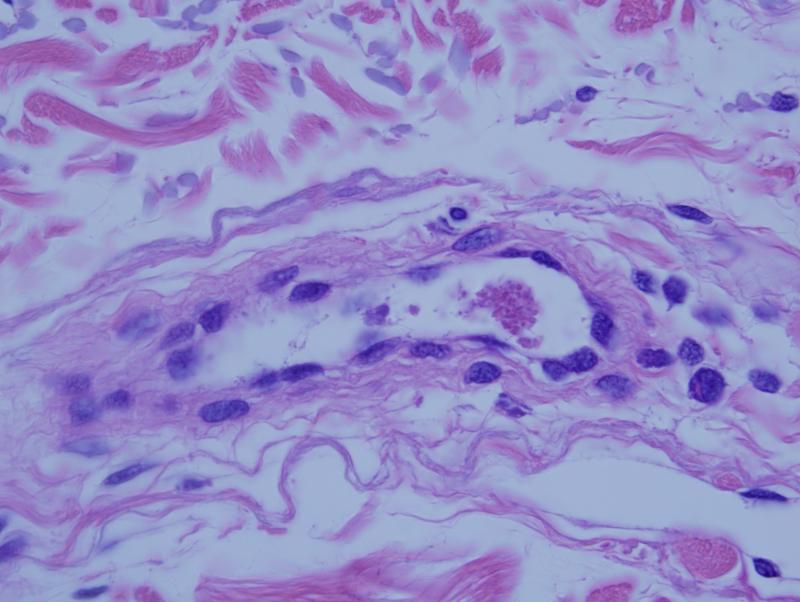
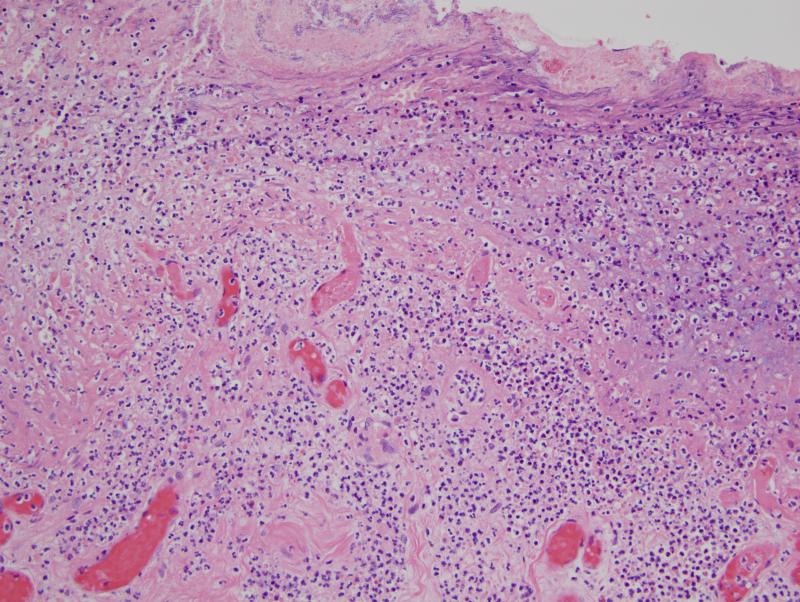
In most biopsies subtle abnormalities pointing toward an underlying procoagulant state were identified. There was focal endothelial cell detachment with loose small intraluminal thrombi comprising platelets admixed with fibrin (figure 1). Vasculitic changes were never seen. In biopsies from cutaneous ulcerative lesions, a marked occlusive pauci-inflammatory thrombogenic vasculopathy with ischemic alterations of the skin was observed (figure 2).
Figure 1.
This patient with classic features of atypical HUS had a biopsy of apparently normal skin procured from the forearm. There is a deeper seated small intravascular thrombus comprising fibrin admixed with platelets.
Figure 2.
In this 41 year old female with diagnostic features of atypical HUS, pedal necrosis developed. A biopsy shows a striking pauci-inflammatory thrombogenic vasculopathy with resultant ischemic undermining of the skin as revealed by concomitant ulceration.
Evaluation of complement deposition on formalin fixed biopsy material
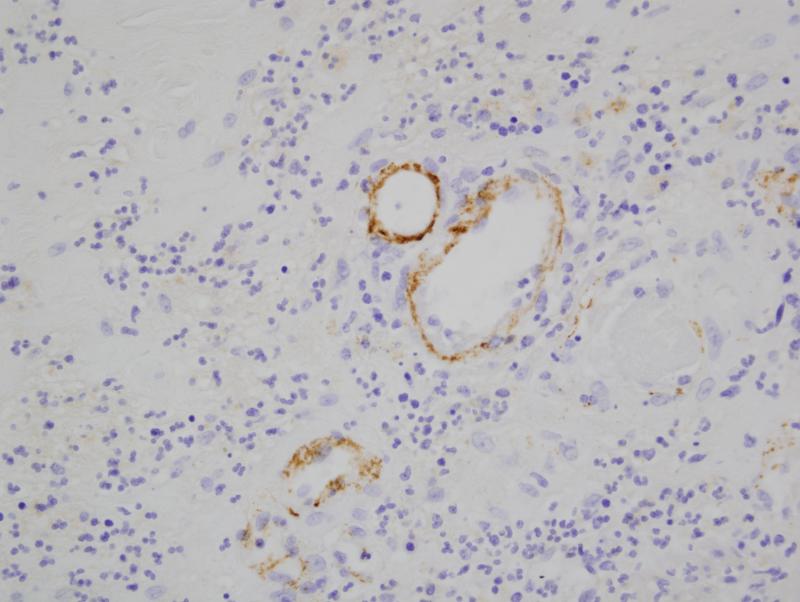
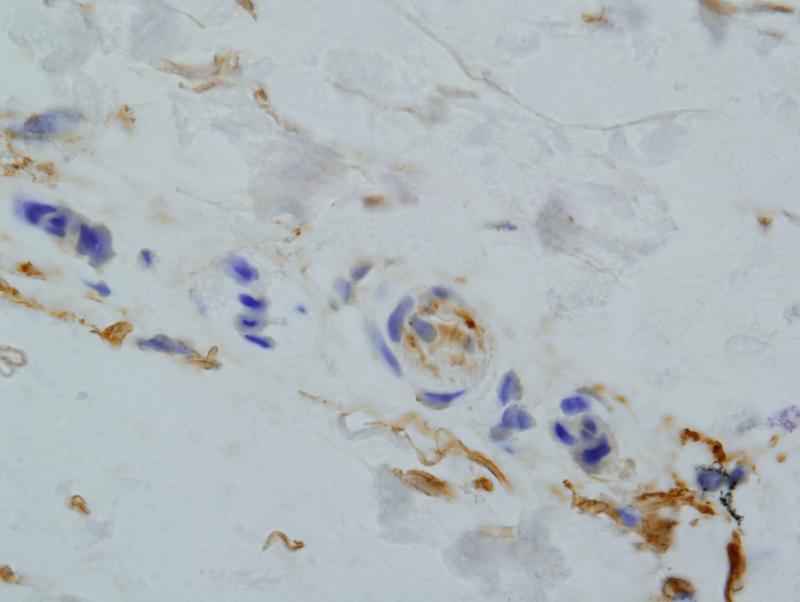
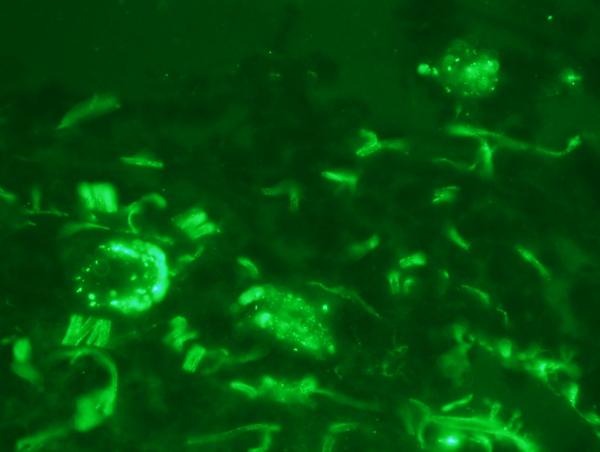
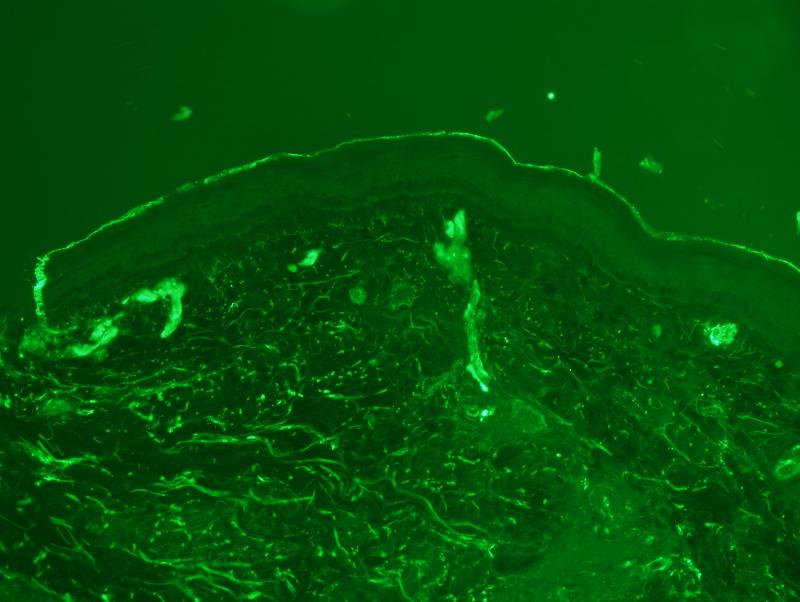
There was extensive intense +3/3granular deposition for C3d, C4d (figure 3), and C5b-9 (figure 4) involving many vessels throughout the dermis. In certain cases, the vascular deposits were most conspicuous in the deeper dermis in apposition to the eccrine coil and in the subcutaneous fat. The staining involved the endothelium and abluminal aspect of the vessel with variable involvement of the remainder of the vessel wall and surrounding perivascular tissue. As previously mentioned deposition for any of these aforesaid components of complement activation along the dermal-epidermal junction (DEJ) and within elastic tissue including the elastic lamina of blood vessels was not considered pathogenetically significant. In one case, significant deposits of complement were not seen in the random normal skin biopsy of the arm. This patient had HUS rather than aHUS. In another patient the biopsy was performed subsequent to the initiation of eculizumab therapy. In this particular biopsy the extent of MAC/C5b-9 was mild to moderate and significantly less than those cases in which the eculizumab was started after the biopsy.
Figure 3.
There are extensive fine granular deposits of C5b-9 involving the endoluminal aspect of the vessel. Additional background of staining of elastic tissue is noted and not of diagnostic value.
Figure 4.
There is prominent localization of C4d to involve the abluminal aspect of the capillary. Similar deposits were noted in venules and small arteries. The deposition pattern was conspicuous superficially but was also noted throughout the dermis.
Immunofluorescent studies conducted on frozen tissue
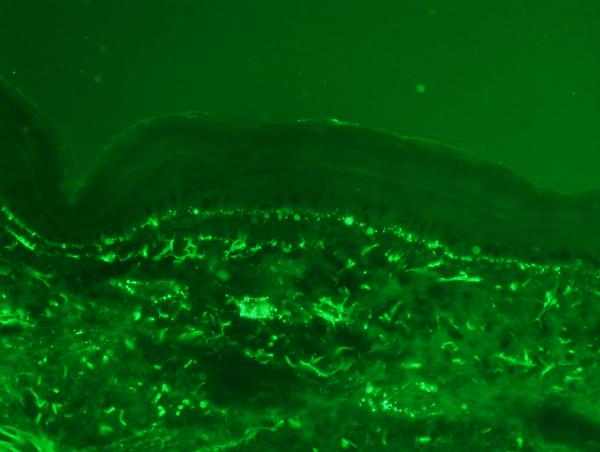
In all cases except two, there was extensive granular deposition of MAC/C5b-9 in vessels throughout the dermis (figure 5a, figure 5b). The vascular deposits could be accentuated in the deeper dermis and involve the subcutaneous fat. Deposition along the dermal-epidermal junction, highlighting elastic fibers including the elastic lamina of blood vessels and within the basement membrane zone of the eccrine coil were not considered significant. There was pronounced fibrinogen deposition within vessels in all biopsies (figure 6).
Figure 5a and 5b.
The immunofluorescent studies show a striking pattern of granular vascular staining for C5b-9 within the dermis. The deposition along the dermal epidermal junction and within elastic fibers is not significant in this particular clinical context and is not of any diagnostic significance.
Figure 6.
There is highlighting of vessels for fibrinogen despite the lack of fibrin deposition noted light microscopically.
Patients without diagnostic clinical features of atypical HUS
10 females and 3 males ranging in age from 18 to 64 years of age (mean age of 40) comprised the cohort of patients without clinical aHUS features although one or more had isolated features that could be seen in aHUS. However, in none of these cases were the clinical presentation and laboratory findings diagnostic of aHUS; the patients were felt to have other conditions apart from aHUS. The various illnesses in these 13 patients are described briefly in Table 1.
Table 1.
Clinical features of patients without aHUS
| Patient | Age | Sex | Clinical Features |
|---|---|---|---|
| 1 | 63 | M | CMML, S/P Bone marrow transplant |
| 2 | 45 | F | Non-small cell lung cancer, Isolated Renal Insufficiency |
| 3 | 48 | F | Alagille syndrome |
| 4 | 54 | F | Pancreatitis, bowel perforated and sepsis |
| 5 | 31 | F | HELLP Syndrome |
| 6 | 35 | F | HELLP Syndrome |
| 7 | 21 | F | SLE flare-up, hemolysis and renal dysfunction |
| 9 | 27 | M | TTP, Non-detectable ADAMS13 |
| 8 | 18 | F | TTP, ADAMS13 <10 |
| 10 | 22 | F | TTP, Non-detectable ADAMS13 |
| 11 | 51 | F | TTP, Non-detectable ADAMS13 |
| 12 | 31 | F | Clinically Classic TTP, Normal ADAMS13 |
| 13 | 64 | M | MF with transformation to AML and JAK-2 mutation, S/P allogenic stem cell transplant, chronic procoagulant state manifested by Budd Chiari syndrome |
Alagille syndrome (pulmonary atresia, pulmonary hypertension, hemolytic anemia, chronic thrombocytopenia and renal failure), CMML-chronic myelomonocytic leukemia, F-female, HELLP syndrome (hemolysis, elevated liver enzymes, low platelet count), M-male, MF-myelofibrosis, S/P-status post, SLE-systemic lupus erythematous, TTP-thrombotic thrombocytopenic purpura.
None of these patients received eculizumab except three patients with diagnostic features of TTP. As these patients are unusual and raise consideration in regards to a TTP/aHUS overlap they will be considered in greater detail. One patient was a 27-old-male who had classic TTP with no detectable ADAMTS13 on the basis of autoantibodies directed at ADAMTS13. He had a skin biopsy of the left thigh to rule out atypical HUS, which revealed extensive deposits of MAC/C5b-9 demonstrated both by immunohistochemistry and immunofluorescence. As the patient was refractory to other therapies for TTP including intravenous gammaglobulin and plasmapharesis, he received eculizumab with a rapid and striking improvement. This case has been previously reported [13]. The patient was subsequently discovered to have a complement regulatory protein mutation implicated in aHUS (figure 7). The second patient was an 18-year-old female admitted with severe abdominal pain, anemia and thrombocytopenia. Her course was complicated by slurred speech, dyspnea and chest pain. She was found to have hemolysis and received IVIg and steroids with no success. ADAMS13 activity was <10 and she began to have episodes of abnormal mental status. In the meanwhile a skin biopsy was performed which showed significant complement deposits and eculizumab was initiated along with plasmapheresis. However, she had no response to therapy and after three days she developed seizures followed by cardiac arrest and death. An autopsy was performed and revealed diffuse microthrombi in the heart, lungs, liver and kidneys as well as gross hemorrhage through the entire GI tract, consistent with a diagnosis of TTP. The third patient, a 22-year-old woman presented with right-sided weakness and was found to have anemia and thrombocytopenia while she was 2 month pregnant and subsequently was diagnosed with TTP and received plasma exchange and IVIg without response. She developed a gradually rising creatinine and was started on dialysis. A skin biopsy was done which did not show evidence of complement deposition. However, she was given eculizumab with no response. Over the next few days, she became lethargic and developed respiratory deterioration and cardiac arrest. An autopsy showed multifocal cardiac myocyte necrosis and several organizing thrombi within the cardiac arterioles, renal glomerular arterioles and brain capillaries.
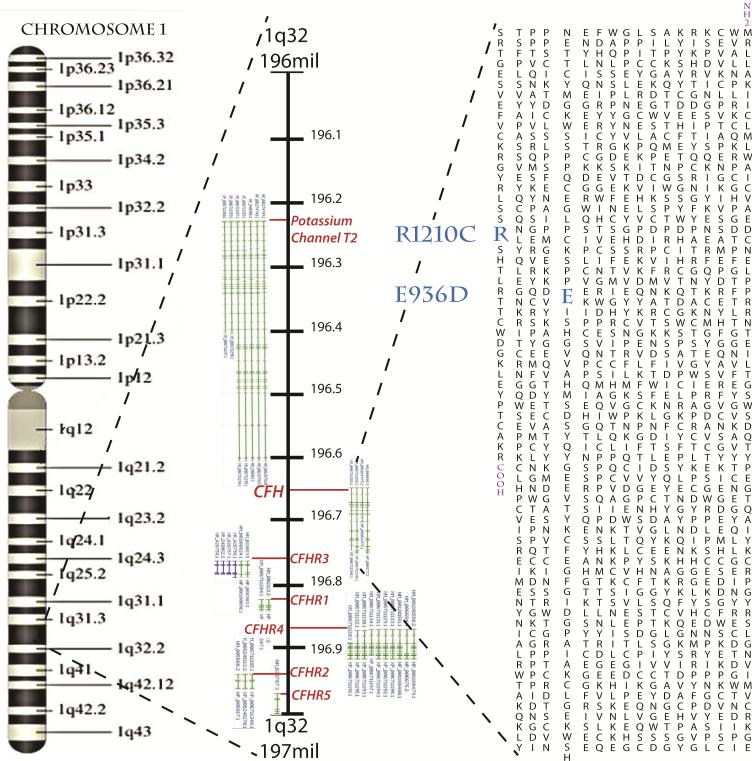
Figure 7.
A gene map showing the locations of the two factor H mutations linked to complement deposition abnormalities found in patients in this study. CFH is found on Chromosome 1 at 196,621,008-196,716,634 base pairs. The related protein weighs 155kd and has three C3b binding domains. The E936D mutation falls in the 16th of 20 complement control regions and may perturb protein folding necessary to spatially join the two termini of CFH. The more drastic R1210C where a charged residue is replaced with an uncharged residue falls in the 20th of 20 complement control regions, which makes up a glycosoaminoglycan binding region, and may cause problems with intracellular metabolism and secretion. Complement control regions 19 and 20 are vital for binding C3b.
Light microscopy
In 9 patients, the biopsies were from the forearm or deltoid. In two patients the biopsy was performed from normal abdominal skin and in another patient the biopsy was from normal skin of the lower extremity. In one patient the biopsy site was not specified.
Significant abnormalities were not seen excluding two cases in which there were focal chronic microvascular changes correlating with significant vascular deposits of MAC/C5b-9 both patients having TTP.
Evaluation of complement on formalin fixed biopsy material
Significant deposition for C3d, C4d, and MAC/C5b-9 was not observed in any of the cases except two cases of TTP including one where the patient had a complement regulatory gene mutation defining what is in essence of an overlapping TTP and aHUS picture clinically.
Direct immunofluorescent studies
In all cases excluding the two patients with TTP, vascular deposition for MAC/C5b-9 was not observed. Deposition along the DEJ, on elastic fibers including the elastic lamina of blood vessels and within the BMZ of the eccrine coil was not considered significant and was observed in most biopsies.
DISCUSSION
The skin biopsy has potential to play a very important role in the diagnosis of aHUS. Paradoxically, clinical features indicative of cutaneous involvement in the setting of aHUS is rarely described. Although the exact defect in complement regulation cannot be elucidated in every case, the common mechanism of vascular injury is MAC/C5b-9 deposition and has important therapeutic implications with respect to the implementation of eculizumab. Eculizumab is a monoclonal antibody that binds C5, prohibiting its division into C5a and C5b; C5a acts as an anaphylatoxin, and C5b initiates the formation of the MAC/C5b-9 with the coordination of C6-C9. Prevention of the formation of C5a and MAC/C5b-9 inhibits complement-mediated thrombotic microangiopathy [14-20]. Eculizumab first received approval for the treatment of patients with paroxysmal nocturnal hemoglobinuria (PNH) in 2007 [14, 19-21] followed by multiple retrospective and prospective trials enrolling adult, adolescent or pediatric patients with aHUS. These trials led to an accelerated approval for the use of eculizumab for the treatment of pediatric and adult patients with aHUS in 2011. Eculizumab resulted in elimination of the requirement for dialysis or plasma therapy, sustained improvement in the estimated glomerular filtration rate (eGFR), and hematologic parameters that correlate with aHUS disease activity [14-18, 22-24].
Two patients in our series had skin lesions in the context of symptomatic atypical HUS emphasizing that the skin is a targeted organ site despite the infrequency with which cutaneous involvement has been reported. There are only two prior reports of cutaneous lesions in the setting of atypical HUS. One child presented shortly after birth with thrombocytopenia, hemolytic anemia and acute renal failure. His course was complicated by multiple intestinal perforations and cutaneous ulcers. The patient responded very quickly to eculizumab. Subsequent investigations revealed antibodies to factor H [15]. An additional study described a 4-year-old Indian girl who developed gangrene of the fingertips 2 days after initial presentation of aHUS. Factor H autoantibodies were identified. Renal function continued to decline despite daily plasma exchanges, and she was started on peritoneal dialysis 5 days after admission. The patient died. Another patient developed end-stage renal disease due to aHUS in the fourth month after birth. A de novo activating C3 mutation was found. At age 9 months, she developed ischemic changes in fingers of both hands and several toes, which did not improve with plasma exchange therapy. The patient had an almost complete response to the eculizumab. All nonnecrotic digits rapidly regained perfusion. The three already gangrenous fingers healed with loss of the distal phalanges [25]. In our study, both patients with cutaneous lesions were women whereby there was a predilection for acral involvement manifesting as ulcers and background livedo. Both of these patients were treated with eculizumab with an excellent clinical response.
In our study, the biopsies in patients with atypical HUS were largely performed on normal appearing skin; in this regard the light microscopic abnormalities were very subtle. In the vast majority of cases only mild microvascular changes were identified. In particular, we noted some degree of basement membrane zone (BMZ) reduplication as well as small loose intraluminal platelet thrombi. Despite the relative paucity of changes observed by routine light microscopy, all patients except two showed prominent MAC/C5b-9 with variable deposition of C3d and C4d involving the endothelium as well as the vessel wall. The principle caliber of vessels affected was capillaries, venules, and small arterioles. A virtually identical pattern was seen by direct IF testing in terms of intensity of MAC/C5b-9 as well as the relative distribution of C5b-9. Any staining of the elastic tissue of the dermis especially within the elastic lamina of blood vessels and epithelial basement membrane zone was interpreted as being nonspecific. A significant staining pattern had to be endothelial-based and/or at least abluminal, affecting smaller caliber vessels, namely capillaries, venules, and small arterioles. In one patient with clinical features of atypical HUS, the biopsy was performed while the eculizumab therapy was initiated. In this particular biopsy the extent of MAC/C5b-9 was mild to moderate, which is most likely related to the fact that the patient was receiving treatment. A comparable degree of microvascular changes and complement deposition was not apparent in most cases that were not clinically diagnostic of aHUS. A number of biopsies were performed on patients who had symptoms encountered in aHUS but had other reasons such as systemic lupus erythematous, HELLP, and TTP as the etiologic basis of the multiorgan thrombotic diathesis and or hemolysis and or did not have enough symptoms and abnormal lab findings to render a diagnosis of aHUS. Overall, there was either no or minimal and focal complement deposition in qualitative and quantitative contrast to those patients with aHUS who exhibited prominent deposition. Interestingly, in one patient with HUS triggered by E. coli infection, complement deposition was not identified on a normal skin biopsy.
In two cases of TTP, there were prominent deposits of C5b-9 in smaller caliber vessels with one patient demonstrating a very positive response to eculizumab. This particular patient was very interesting based on fulfilling diagnostic features of TTP including an absent ADAMSTS13 along with a genetic mutation implicated in aHUS. Indeed his first cousin had developed aHUS as a child. Whether or not a similar overlapping aHUS and TTP picture was operational in the other TTP patient exhibiting extensive vascular MAC/C5b-9 is unclear. It should be emphasized that there is likely a subset of patients with TTP who may have genetic mutations which define an aHUS clinical phenotype (figure 7). Conversely, authors have shown genetic variations in the ADAMTS13 gene to partially explain the reduced activity known to occur in some patients with aHUS. In their study they found reduced complement and ADAMTS13 activity in over 60% and 50% of patients, respectively [26]. Severe ADAMTS13 deficiency leads to generation of massive platelet thrombi, which might contribute to complement activation [27]. Increased levels of C3a and MAC/C5b-9 were observed in TTP during acute episodes, as compared with healthy controls. Decreased complement C3 levels indicative of complement consumption occurred in 15% of acute TTP patients. Significant decrease of complement activation products C3a and C5b-9 was observed during plasma exchange. The sustained presence of anti-ADAMTS13 inhibitory antibodies in complete remission was associated with increased complement activation [26].
In the majority of cases in this study, the biopsies were of normal skin from the forearm or deltoid. In one patient, a biopsy was only performed from the toe ulcer and in another patient biopsies were taken from both the normal deltoid skin and a toe ulcer. In two patients, biopsies were performed only on the lower extremity. Two patients had a biopsy of normal abdominal skin. We recommend the deltoid area to reduce the incidence of false positivity, which may occur when utilizing sun exposed skin or lower extremity skin. Ultraviolet light exposure can lead to complement activation, especially if the patient is taking a photosensitizing drug or is applying a contactant with photosensitizing properties. In the same vein, it is well established that the lower extremity vessels are preferential sites for immune complex entrapment, reflective of hydrostatic pressure. Hence, there may be a low level of insidious complement activation occurring within the lower extremity microvasculature leading to a higher incidence of false positive results.
In summation, the skin biopsy is of value in establishing a diagnosis of aHUS even in the absence of clinically discernible skin lesions. From a light microscopic perspective, subtle alterations include chronic microvascular alterations with basement membrane zone reduplication along with evidence of a procoagulant state. The microvascular deposition of MAC/C5b-9 through the dermis and subcutaneous fat as identified by both immunohistochemistry and by direct immunofluorescence utilizing non-lesional skin appears to be a reproducible pattern and is a reliable marker of aHUS and likely a subset of patients with TTP. It is important to appreciate patterns of MAC/C5b-9 deposition that are not pathologically relevant to avoid overdiagnosis of aHUS and eculizumab responsive variants of TTP.
ADDENDUM
C.M. Magro: study concept and design, analysis and interpretation of data, writing the first draft of the manuscript, contribution to revisions, and approval of final version to be published. S. Momtahen, J.J. Mulvey, and A.H. Yassin: study concept and design, interpretation of the data, and revision of the intellectual content. B.R. Kaplan: study concept and design, analysis and interpretation of data, contribution to revisions, and approval of final version to be published.
ACKNOWLEDGEMENTS
We would like to thank Dr. Ann Zimrin for additional clinical information on select cases in this series and nurse practitioner “Lori Prendergas” who performed many of the skin biopsies. We acknowledge support from The NIH Medical Scientist Training Program (MSTP; grant GM07739), and The Cornell Pharmacology Program (Training grant T32 CA062948) to J.J.M.
Footnotes
All authors and staff in a position to control the content of this CME activity and their spouses/life partners (if any) have disclosed that they have no financial relationships with, or financial interests in, any commercial organizations pertaining to this educational activity
REFERENCES
- 1.Noris M, Remuzzi G. Atypical hemolytic-uremic syndrome. N Engl J Med. 2009;361:1676–87. doi: 10.1056/NEJMra0902814. [DOI] [PubMed] [Google Scholar]
- 2.Tsai HM. Thrombotic thrombocytopenic purpura and the atypical hemolytic uremic syndrome: an update. Hematol Oncol Clin North Am. 2013;27:565–84. doi: 10.1016/j.hoc.2013.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Meri S. Complement activation in diseases presenting with thrombotic microangiopathy. Eur J Intern Med. 2013;24:496–502. doi: 10.1016/j.ejim.2013.05.009. [DOI] [PubMed] [Google Scholar]
- 4.Tegla CA, Cudrici C, Patel S, et al. Membrane attack by complement: the assembly and biology of terminal complement complexes. Immunol Res. 2011;5:45–60. doi: 10.1007/s12026-011-8239-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kirschfink M. Targeting complement in therapy. Immunol Rev. 2001;180:177–89. doi: 10.1034/j.1600-065x.2001.1800116.x. [DOI] [PubMed] [Google Scholar]
- 6.Male DA, Ormsby RJ, Ranganathan S, et al. Complement factor H: sequence analysis of 221 kb of human genomic DNA containing the entire fH, fHR-1 and fHR-3 genes. Mol Immunol. 2000;37:41–52. doi: 10.1016/s0161-5890(00)00024-9. [DOI] [PubMed] [Google Scholar]
- 7.Martinez-Barricarte R, Pianetti G, Gautard R, et al. European Working Party on the Genetics of HUS. The complement factor H R1210C mutation is associated with atypical hemolytic uremic syndrome. J Am Soc Nephrol. 2008;19:639–46. doi: 10.1681/ASN.2007080923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deban L, Jarva H, Lehtinen MJ, et al. Binding of the long pentraxin PTX3 to factor H: interacting domains and function in the regulation of complement activation. J Immunol. 2008;181:8433–40. doi: 10.4049/jimmunol.181.12.8433. [DOI] [PubMed] [Google Scholar]
- 9.Martínez-Barricarte R, Heurich M, Valdes-Cañedo F, et al. Human C3 mutation reveals a mechanism of dense deposit disease pathogenesis and provides insights into complement activation and regulation. J Clin Invest. 2010;120:3702–12. doi: 10.1172/JCI43343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vasil KE, Magro CM. Cutaneous vascular deposition of C5b-9 and its role as a diagnostic adjunct in the setting of diabetes mellitus and porphyria cutaneatarda. J Am Acad Dermatol. 2007;56:96–104. doi: 10.1016/j.jaad.2006.05.013. [DOI] [PubMed] [Google Scholar]
- 11.Chapin J, Weksler B, Magro C, et al. Eculizumab in the treatment of refractory idiopathic thrombotic thrombocytopenic purpura. Br J Haematol. 2012;157:772–4. doi: 10.1111/j.1365-2141.2012.09084.x. [DOI] [PubMed] [Google Scholar]
- 12.Rother RP, Rollins SA, Mojcik CF, et al. Discovery and development of the complement inhibitor eculizumab for the treatment of paroxysmal nocturnal hemoglobinuria. Nat Biotechnol. 2007;25:1256–64. doi: 10.1038/nbt1344. [DOI] [PubMed] [Google Scholar]
- 13.Ariceta G, Arriyabalaga B, Aguirre M, et al. Eculizumab in the treatment of aHUS in infants. Am J Kidney Dis. 2012;59:707–10. doi: 10.1053/j.ajkd.2011.11.027. [DOI] [PubMed] [Google Scholar]
- 14.Chatelet V, Frémeaux-Bacchi V, Lobbedez T, et al. Safety and long-term efficacy of eculizumab in a renal transplant patient with recurrent atypical hemolytic-syndrome. Am J Transplant. 2009;9:2644–5. doi: 10.1111/j.1600-6143.2009.02817.x. [DOI] [PubMed] [Google Scholar]
- 15.Gruppo RA, Rother RP. Eculizumab for congenital atypical hemolytic–uremic syndrome. N Engl J Med. 2009;360:544–6. doi: 10.1056/NEJMc0809959. [DOI] [PubMed] [Google Scholar]
- 16.Zimmerhackl LB, Hofer J, Cortina G, et al. Prophylactic eculizumab after renal transplantation in atypical hemolytic–uremic syndrome. N Engl J Med. 2010;362:1746–8. doi: 10.1056/NEJMc1001060. [DOI] [PubMed] [Google Scholar]
- 17.Zuber J, Fakhouri F, Roumenina LT, et al. Use of eculizumab for atypical haemolytic uraemic syndrome and C3 glomerulopathies. Nat Rev Nephrol. 2012;8:643–57. doi: 10.1038/nrneph.2012.214. [DOI] [PubMed] [Google Scholar]
- 18.Zuber J, Quintrec ML, Krid S, et al. Eculizumab for atypical hemolytic uremic syndrome recurrence in renal transplantation. Am J Transplant. 2012;12:3337–54. doi: 10.1111/j.1600-6143.2012.04252.x. [DOI] [PubMed] [Google Scholar]
- 19.Hillmen P, Young NS, Schubert J, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006;355:1233–43. doi: 10.1056/NEJMoa061648. [DOI] [PubMed] [Google Scholar]
- 20.Brodsky RA, Young NS, Antonioli E, et al. Multicenter phase 3 study of the complement inhibitor eculizumab for the treatment of patients with paroxysmal nocturnal hemoglobinuria. Blood. 2008;111:1840–7. doi: 10.1182/blood-2007-06-094136. [DOI] [PubMed] [Google Scholar]
- 21.Kelly RJ, Hill A, Arnold LM, et al. Long-term treatment with eculizumab in paroxysmal nocturnal hemoglobinuria: sustained efficacy and improved survival. Blood. 2011;117:6786–92. doi: 10.1182/blood-2011-02-333997. [DOI] [PubMed] [Google Scholar]
- 22.Legendre CM, Licht C, Muus P, et al. Terminal Complement Inhibitor Eculizumab in Atypical Hemolytic–Uremic Syndrome. N Eng J Med. 2013;368:2169–81. doi: 10.1056/NEJMoa1208981. [DOI] [PubMed] [Google Scholar]
- 23.Malina M, Gulati A, Bagga A, et al. Peripheral gangrene in children with atypical hemolytic uremic syndrome. Pediatrics. 2013;131:e331–5. doi: 10.1542/peds.2012-0903. [DOI] [PubMed] [Google Scholar]
- 24.Feng S, Eyler SJ, Zhang Y, et al. Partial ADAMTS13 deficiency in atypical hemolytic uremic syndrome. Blood. 2013;122:1487–93. doi: 10.1182/blood-2013-03-492421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Noris M1, Mescia F, Remuzzi G. STEC-HUS, atypical HUS and TTP are all diseases of complement activation. Nat Rev Nephrol. 2012;8:622–33. doi: 10.1038/nrneph.2012.195. [DOI] [PubMed] [Google Scholar]
- 26.Réti M1, Farkas P, Csuka D, et al. Complement activation in thrombotic thrombocytopenic purpura. J Thromb Haemost. 2012;10:791–8. doi: 10.1111/j.1538-7836.2012.04674.x. [DOI] [PubMed] [Google Scholar]
- 27.Morgan HP, Schmidt CQ, Guariento M, et al. Structural basis for engagement by complement factor H of C3b on a self-surface. Nat Struct Mol Biol. 2011;18:463–70. doi: 10.1038/nsmb.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]