Abstract
Background & objectives:
In the traditional system of medicine in India Ashwagandha powder and Sidh Makardhwaj have been used for the treatment of rheumatoid arthritis. However, safety and efficacy of this treatment have not been evaluated. Therefore, the present study was carried out to evaluate the efficacy and safety of Ayurvedic treatment (Ashwagandha powder and Sidh Makardhwaj) in patients with rheumatoid arthritis.
Methods:
One hundred and twenty five patients with joint pain were screened at an Ayurvedic hospital in New Delhi, India. Eighty six patients satisfied inclusion criteria and were included in the study. Detailed medical history and physical examination were recorded. Patients took 5g of Ashwagandha powder twice a day for three weeks with lukewarm water or milk. Sidh Makardhwaj (100 mg) with honey was administered daily for the next four weeks. The follow up of patients was carried out every two weeks. The primary efficacy end point was based on American College of Rheumatology (ACR) 20 response. Secondary end points were ACR50, ACR70 responses, change from baseline in disease activity score (DAS) 28 score and ACR parameters. Safety assessments were hepatic function [alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP), bilirubin and ß2 microglobulin], renal function (urea and creatinine and NGAL) tests and urine mercury level.
Results:
The study was completed by 90.7 per cent (78/86) patients. Patients with moderate and high disease activity were 57.7 per cent (45/78) and 42.3 per cent (33/78), respectively. All patients were tested positive for rheumatoid factor and increased ESR level. Ashwagandha and Sidh Makardhwaj treatment decreased RA factor. A significant change in post-treatment scores of tender joint counts, swollen joint counts, physician global assessment score, patient global assessment score, pain assessment score, patient self assessed disability index score and ESR level were observed as compared to baseline scores. ACR20 response was observed in 56.4 per cent (44/78) patients (American College of Rheumatology criteria) and moderate response in 39.74 per cent (31/78) patients [European League Against Rheumatism (EULAR) criteria]. Ayurvedic treatment for seven weeks in rheumatoid arthritis patients showed normal kidney and liver function tests. However, increased urinary mercury levels were was observed after treatment.
Interpretation & conclusions:
The findings of the present study suggest that this Ayurvedic treatment (Ashwagandha powder and Sidh Makardhwaj) has a potential to be used for the treatment of rheumatoid arthritis. However, due to small sample size, short duration, non randomization and lack of a control group as study limitations, further studies need to be done to confirm these findings.
Keywords: Ashwagandha powder (Withania somnifera), clinical trial, efficacy, rheumatoid arthritis, safety, Sidh Makardhwaj
Rheumatoid arthritis (RA) is a chronic, systemic, inflammatory, progressive disease. The prevalence of rheumatoid arthritis has been reported to be 0.75 per cent in Indian population1. The disease is more common in women than in men and occurs between the ages of 40 and 60 yr2. It leads to irreversible joint damage and systemic complications, and is associated with substantial morbidity and increased mortality3,4. Patients with active RA suffer from significant decline in functional capacity and 40 per cent become work disabled within five years from onset of symptoms5. Direct and indirect costs are also enormous6.
The goals of RA management are to control pain and swelling, delay disease progression, minimize disability and improve quality of life. Non steroidal anti-inflammatory drugs (NSAIDs) have both analgesic and anti-inflammatory properties but do not change disease outcomes7 and side effects are gastrointestinal ulcers (15-20% of patients), ulcer with bleeding and perforations (2-4% of patients over 70 yr of age)8. Glucocorticoids have greater action on joint pain than NSAIDs but have numerous side effects including adrenal suppression, ulcers and osteoporosis9. Disease modifying antirheumatic drugs (DMARDs) reduce the progression of joint erosion but they have slow onsets and no analgesic activity. Methotrexate has been shown to cause pulmonary complications10.
Ayurveda is a widely practiced system of traditional medicine in India. It has been shown that 60-90 per cent of persons with arthritis use complementary and alternative medicine (CAM)11. Stress is widely recognized as an important risk factor in the aetiology of inflammatory rheumatic diseases12,13. Withania somnifera (Ashwagandha) exhibits anti-inflammatory, anti-tumour, anti-stress, antioxidant, immunomodulatory, haematopoietic and rejuvenating properties14,15. Thamaraiselvi et al16 have shown Ashwagandha powder to be effective in patients with RA. Sidh Makardhwaj is a formulation mentioned in Ayurvedic Formulary of India17. It is a sublimed product made from pure mercury, sulphur and gold. It is used in rheumatoid arthritis, and neurological disorders, as rasayana for vigour and longevity of life17. Though Ashwagandha and Sidh Makardhwaj have been used in the treatment of patients with RA for many decades, their safety and efficacy have not been evaluated. The present study was, therefore, undertaken to evaluate the efficacy and safety of Ayurvedic treatment i.e. Ashwagandha and Sidh Makardhwaj in patients with RA.
Material & Methods
The present study was a prospective, open-label, non-randomized, outpatient-based, single centered drug trial conducted in the department of Pharmacology, All India Institute of Medical Sciences (AIIMS), New Delhi. It was conducted during October 2009 to December 2010 after obtaining approval from the Institute Ethics Committee. The study was registered with Clinical Trial Registry of India, (CTRI- 2009 000699). Single batches of Sidh Makardhwaj (Maharshi Ayurveda Pharmaceutical Pvt. Ltd., India), Ashwagandha and honey (Dabur, India) were procured for the entire study. Patients of either sex between the age group of 18 to 60 yr, who were diagnosed with rheumatoid arthritis by the American College of Rheumatology (ACR) 1987 criteria18, able to provide written informed consent were included in the study. Exclusion criteria were medical history of unstable angina, myocardial infarction, heart failure or stroke within three months of the study, uncontrolled hypertension (diastolic blood pressure >100 mm Hg), uncontrolled diabetes mellitus, alanine and aspartate aminotransferases (ALT and AST) > 2 x upper limit of normal, impaired renal function (creatinine ≥2.0 mg/dl), pregnancy/lactation, or patients on any other Ayurvedic drugs during the last 15 days.
The first 125 patients with joint pain were screened in the OPD at CGHS Ayurvedic Hospital, Lodhi Road, New Delhi during the study period. Eighty six patients satisfied the inclusion criteria and were willing to participate in the study, signed the informed consent. Detailed medical history, general physical examination and rheumatologic evaluation were recorded by the designated Ayurvedic physician. Laboratory tests were carried out as per protocol. Subsequently, all patients were examined by the physician at every visit during the trial.
Medications: The patients took 5g of Ashwagandha powder twice a day for three weeks with lukewarm water or milk. Sidh Makardhwaj (100 mg) with honey was administered daily for the next four weeks. Records of dispensed drugs were maintained in drug inventory form. Concurrent analgesics/NSAIDs in any form, oral, injectable or topical were not permitted. However, there was facility of rescue treatment with NSAIDs. Patients who received rescue medications were excluded from the study. Patients were allowed to continue with their ongoing lifestyle and diet.
Clinical assessment: The primary efficacy end point was the proportion of patients with a 20 per cent improvement as per ACR criteria (ACR20)19 response at the end of treatment. ACR 20 is defined as 20 per cent improvement in tender joint counts, swollen joint counts and 20 per cent improvement in 3 of the 5 areas, i.e. physician global assessment score, patient global assessment score, pain assessment score, patient self assessed disability index score, ESR18. Secondary end points included ACR50 and ACR70 responses, change from baseline in the Disease Activity Score in 28 joints (DAS28), categorical analyses of DAS28/European League Against Rheumatism (EULAR) response20, change from baseline in each of the ACR core set of parameters. Safety assessments included hepatic function [alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and bilirubin] and renal function (urea and creatinine) tests were analyzed separately using individual kit by semi auto analyzer (Mini techno, USA). Early sensitive marker of liver (ß2 microglobulin) and kidney (NGAL: neutrophil gelatinase associated lipocalin) were reported as the instructions of the manufacturer of ELISA assay kits (Logitech India Pvt, Delhi, India) to evaluate the effect of the Ashwagandha and Sidh Makardhwaj treatment. Urinary mercury level was estimated by ICP-AES (Jobin Horiba, JY 2000-2, France)21.
Sample size determination: The sample size was determined by the following specifications: (i) there would be an 80 per cent power for detecting a change from baseline in the DAS in 28 joints; (ii) 20 per cent improvement as per ACR criteria (ACR 20) response at fourth week; (iii) the test of the null hypothesis was conducted at a 2-sided 5 per cent significance level; (iv) acceptable margin of errow will be 5 per cent; and (v) population size of 200 and expected response of 10 per cent. Under these assumptions, the required sample size was 75 subjects. However, considering high dropout rate (20%) and lack of sufficient data from published rheumatoid arthritis drug trials using Ayurvedic medicines, it was then decided to enrol 90 patients.
Statistical analysis: Chi-square test was applied for patients showing improvement/cure after therapy. Laboratory measurements were compared with baseline using an analysis of variance.
Results
Efficacy evaluation of Ashwagandha and Sidh Makardhwaj: A total of 90.7 per cent (78/86) patients adhered to study protocol and completed seven weeks of treatment (three weeks of Ashwagandha powder followed by four weeks of Sidh Makardhwaj). Eight patients prematurely discontinued due to lack of efficacy and refusal of continued treatment (2 = due to unknown reason, 2 = shifted to allopathic medication, 4 = concomitant use of other medication). There were 57.7 per cent (45/78) female and 42.3 per cent (33/78) male subjects and mean ages were 45.7 ± 8.6 (range 19-59) and 49.8 ± 7.9 (range, 25-59) yr, respectively. At baseline, majority of patients tested positive for rheumatoid factor (RF), its values in male and female patients were 31.2 ± 3.1 and 50.9 ± 16.1 IU/ml, respectively and post-treatment levels were 22.1 ± 1.4 and 41.7 ± 14.4 IU/ml. The levels of ESR (erythrocyte sedimentation rate) at baseline in male and female were 28.8 ± 3.3 and 43.8 ± 16.16.3 mm/h and post-treatment levels were 21.6 ± 1.9 and 35.4 ± 14.3 mm/h, respectively. The results showed significant decrease in post-treatment levels of ESR and RA factor as compared to baseline levels in male and female (Table I).
Table I.
Clinical features in rheumatoid arthritis patients (male=33, female=45) on Ashwagandha and Sidh Makardhwaj treatment
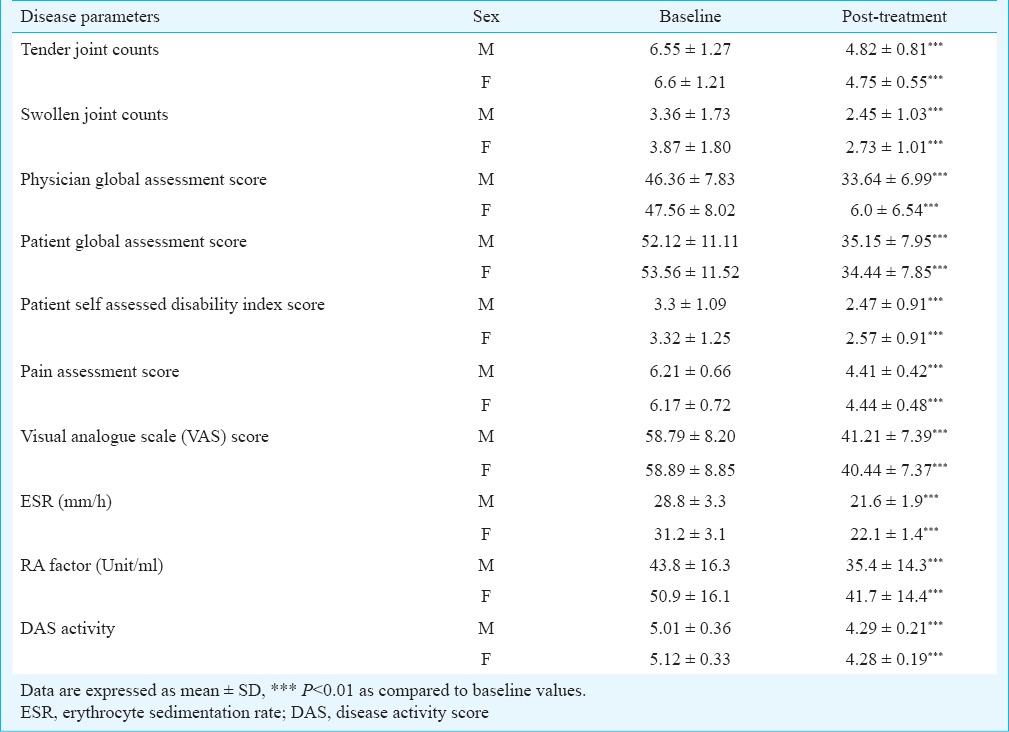
Seven weeks treatment effectiveness was assessed by (i) ACR 20 response; (ii) change in DAS28 (baseline DAS28 to 4 wk DAS28); (iii) non response, moderate and good response according to the EULAR response criteria; and (iv) the proportion of patients achieving disease remission (DAS28 < 2.6), according to the EULAR criteria.
There were significant changes in post-treatment scores of tender joint counts, swollen joint counts, physician global assessment score, patient global assessment score, pain assessment score, patient self assessed disability index score and ESR level as compared to baseline scores in male and female patients (Table I). ACR 20 response was observed in 48.5 per cent (16/33) male and 62.2 per cent (28/45) female patients.
In our study, DAS28 score in male and female patients at baseline were 5.01±0.36 and 5.12 ± 0.33, respectively and at post-treatment were 4.29 ± 0.21 and 4.28 ± 0.19 showing significant decrease in DAS28 score. DAS28 score of higher than 5.1 is indicative of high disease activity, whereas a DAS28 below 3.2 indicates low disease activity18. A total of 45 (57.7%) patients were in moderate disease activity with DAS28 score between 3.2 and 5.1. High disease activity was observed in 42.3 per cent (33/78) patients with DAS28 score >5.1.
In moderate disease activity, DAS28 improvement over the time in the range of 0.6-1.2 was observed in 55.6 per cent (25/45) patients and <0.6 improvement over the time in DAS28 was observed in 44.4 per cent (20/45) patients. In high disease activity, >1.2 improvement over the time in DAS28 was observed in 18.2 per cent (6/33) patients and DAS28 improvement over the time in the range of 0.6-1.2 was observed in 81.8 per cent (27/33) patients. In summary, Sidh Makardhwaj treatment for four weeks in rheumatoid arthritis patients showed moderated response in 39.74 per cent (31/78) patients.
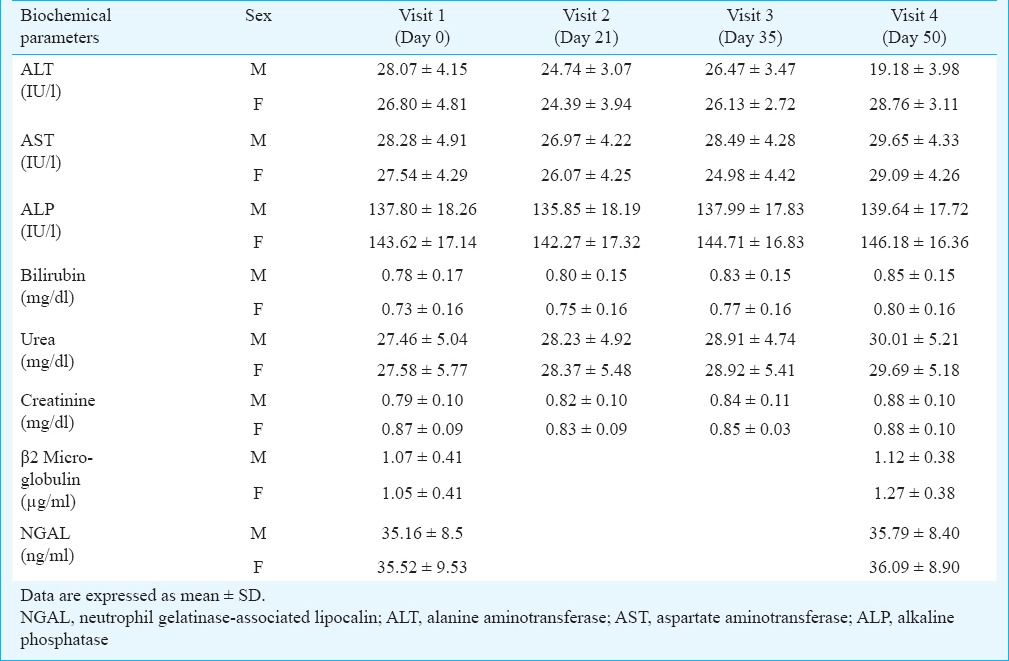
Safety evaluation of Ashwagandha and Sidh Makardhwaj: There was no significant change in serum levels of ALT, AST, ALP, bilirubin, urea, creatinine, ß2MG and NGAL at post-treatment as compared to baseline (Table II). Urinary mercury level increased from 6.9 ± 1.3 to 32.5 ± 2.4 and 8.2 ± 1.6 to 41.7 ± 3.1 µg/l in male and female patients, respectively. Mercury levels increased significantly after Ayurvedic treatment as compared to baseline level.
Table II.
Safety evaluation of liver and kidney of rheumatoid arthritis patients (male=33, female=45) on treatment with Ashwagandha and Sidh Makardhwaj
In our study, significant improvements were observed in patients’ tender joint counts, swollen joint counts, ESRs, physician's rating of disease activity, physical function and pain. For the ACR20 response, there was a moderate improvement while ACR50 and ACR70 responses were not observed. In agreement with earlier reports15, Ashwagandha and Sidh Makardhwaj were found to be effective and safe treatment for patients with RA. Seven weeks after starting Ayurvedic treatment, mean DAS28 scores significantly decreased in male and female patients. Only 39.74 per cent of the patients were EULAR responders (moderate). The results of the present study are in concordance with several studies on Ayurvedic treatment22,23,24,25,26,27,28.
Sidh Makardhwaj has been used in the Indian System of Medicine with claimed efficacy and safety29. Ayurvedic physicians often avoid prescribing it as a medicine particularly for longer periods. But there has always been ambiguity in use of this mercury sulphide containing Ayurvedic medicine and often associated with the question of toxicity. The US Environmental Protection Agency (EPA) has adopted a reference dose (RfD) for methyl mercury of 0.1 µg/kg body weight/day30. The total mercury content of Sidh Makardhwaj formulation used in the present study was 35454.2 µg/g. The calculated total ingested mercury per day was 3545.4 µg (Sidh Makardhwaj dose = 100 mg per day). Thus, in its therapeutic dose, the per day ingested mercury was many fold higher than the reference dose. It was observed that with this high concentration of mercury in Sidh Makardhwaj given for 28 days did not cause significant change in liver and kidney functions of the patients. Urinary mercury levels were significantly increased after Ayurvedic treatment. Hence, mercury present in Sidh Makardhwaj was slowly eliminated from the body.
Lande in 1927 used aurothioglucose for the first time in the treatment of RA31. In another study, Forestier in 193532 used gold thiopropanol sodium sulphanate in 550 cases of RA with beneficial results. Gold containing drugs comprise a class of distinctive anti-arthritic agents (DMARDs) used when NSAIDs are insufficient to treat severe cases of rheumatoid or psoriatic arthritis. The reported remissions of rheumatoid arthritis was of the order of 30 per cent with gold therapy33. Gold content in Sidh Makardhwaj formulation used in the present study was 29.1 mg/g. The calculated total ingested gold per day was 2.91 mg (Sidh Makardhwaj dose = 100 mg per day). Thus, the per day ingested gold in Sidh Makardhwaj was in therapeutic dose.
There were a few limitations of our study. This was a seven week study with a sample size of 78 patients carried out in a single Ayurvedic hospital at New Delhi. Randomization was not done. Also, there was a lack of standard therapy group as a control group due to ethical issues.
In conclusion, our findings demonstrated the potential efficacy and safety of Ashwagandha (3 wk) and Sidh Makardhwaj (4 wk) in the treatment of RA. Ashwagandha and Sidh Makardhwaj should be further tested in long term randomized placebo controlled trial to establish its clinical use.
Acknowledgment
Authors thank the chief medical officer of CGHS, Ayurvedic hospital, New Delhi, for recruitment of patients, and the staff of the CGHS, Ayurvedic hospital, New Delhi, for their assistance. Authors acknowledge the support provided by Drs Sudhir Sarangi and Prafful in improving the quality of manuscript. The financial support by Central Council for Research in Ayurveda and Sidha (CCRAS), Department of AYUSH, Ministry of Health and Family Welfare, Government of India, for this research work is duly acknowledged (F. No. Z31014/04/2009/EMR-CCRAS).
References
- 1.Malaviya AN, Kapoor SK, Singh RR, Kumar A, Pande I. Prevalence of rheumatoid arthritis in the adult Indian population. Rheumatol Int. 1993;13:131–4. doi: 10.1007/BF00301258. [DOI] [PubMed] [Google Scholar]
- 2.Alghuweri A, Marafi A, Alhiary M. Use of serological markers for evaluation patients with rheumatoid arthritis. Int J Biol Med Res. 2012;3:1397–8. [Google Scholar]
- 3.Gabriel SE, Crowson CS, O’Fallon WM. Comorbidity in arthritis. J Rheumatol. 1999;26:2475–9. [PubMed] [Google Scholar]
- 4.Gabriel SE, Crowson CS, Kremers HM, Doran MF, Turesson C, O’Fallon WM, et al. Survival in rheumatoid arthritis: a population-based analysis of trends over 40 years. Arthritis Rheum. 2003;48:54–68. doi: 10.1002/art.10705. [DOI] [PubMed] [Google Scholar]
- 5.Young A, Dixey J, Kulinskaya E, Cox N, Davies P, Devlin J, et al. Which patients stop working because of rheumatoid arthritis? Results of five years’ follow up in 732 patients from the early RA study (ERAS) Ann Rheum Dis. 2002;61:335–40. doi: 10.1136/ard.61.4.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aggarwal A, Chandran S, Misra R. Physical, psychosocial and economic impact of rheumatoid arthritis: a pilot study of patients seen at a tertiary care referral centre. Natl Med J India. 2006;19:187–91. [PubMed] [Google Scholar]
- 7.Ofman JJ, Badamgarav E, Henning JM, Knight K, Laine L. Utilization of nonsteroidal anti-inflammatory drugs and antisecretory agents: a managed care claims analysis. Am J Med. 2004;116:835–42. doi: 10.1016/j.amjmed.2004.02.028. [DOI] [PubMed] [Google Scholar]
- 8.Madhok R, Kerr H, Capell HA. Recent advances: Rheumatology. BMJ. 2000;321:882–5. doi: 10.1136/bmj.321.7265.882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gotzsche PC, Johansen HK. Short-term low-dose corticosteroids vs placebo and nonsteroidal antiinflammatory drugs in rheumatoid arthritis. Cochrane Database Syst Rev. 2002;2:CD000189. doi: 10.1002/14651858.CD000189. [DOI] [PubMed] [Google Scholar]
- 10.Dawson JK, Graham DR, Desmond J, Fewins HE, Lynch MP. Investigation of the chronic pulmonary effects of low-dose oral methotrexate in patients with rheumatoid arthritis: a prospective study incorporating HRCT scanning and pulmonary function tests. Rheumatology (Oxford) 2002;41:262–7. doi: 10.1093/rheumatology/41.3.262. [DOI] [PubMed] [Google Scholar]
- 11.Rao JK, Mihaliak K, Kroenke K, Bradley J, Tierney WM, Weinberger M. Use of complementary therapies for arthritis among patients of rheumatologists. Ann Intern Med. 1999;131:409–16. doi: 10.7326/0003-4819-131-6-199909210-00003. [DOI] [PubMed] [Google Scholar]
- 12.Cutolo M, Straub RH. Stress as a risk factor in the pathogenesis of rheumatoid arthritis. Neuroimmunomodulation. 2006;13:277–82. doi: 10.1159/000104855. [DOI] [PubMed] [Google Scholar]
- 13.Ho RC, Fu EH, Chua AN, Cheak AA, Mak A. Clinical and psychosocial factors associated with depression and anxiety in Singaporean patients with rheumatoid arthritis. Int J Rheum Dis. 2011;14:37–47. doi: 10.1111/j.1756-185X.2010.01591.x. [DOI] [PubMed] [Google Scholar]
- 14.Mishra LC, Singh BB, Dagenais S. Scientific basis for the therapeutic use of Withania somnifera (ashwagandha): a review. Altern Med Rev. 2000;5:334–46. [PubMed] [Google Scholar]
- 15.Puri AS, Sharma D, Bector NP. Role of Withania somnifera (Ashwagandha) in various types of arthropathies. Indian J Med Res. 1968;56:1581–3. [Google Scholar]
- 16.Thamaraiselvi T, Brindha S, Kaviyarasi NS, Annadurai B, Gangwar SK. Anti-arthritic effect of Amukkara (Withania somnifera) choornam in patients with rheumatoid arthritis. Int J Adv Biol Res. 2012;2:174–6. [Google Scholar]
- 17.New Delhi: Ministry of Health & Family Welfare, Government of India; 2005. Ayurvedic Formulary of India, Parts I & II. [Google Scholar]
- 18.American College of Rheumatology Subcommittee on Rheumatoid Arthritis Guidelines. Guidelines for the management of rheumatoid arthritis: 2002 Update. Arthritis Rheum. 2002;46:328–46. doi: 10.1002/art.10148. [DOI] [PubMed] [Google Scholar]
- 19.Albert DA, Huang G, Dubrow G, Brensinger CM, Berlin JA, Williams HJ. Criteria for improvement in rheumatoid arthritis: alternatives to the American College of Rheumatology 20. J Rheumatol. 2004;31:856–66. [PubMed] [Google Scholar]
- 20.Fransen J, van Riel PL. The Disease Activity Score and the EULAR response criteria. Clin Exp Rheumatol. 2005;23:S93–9. [PubMed] [Google Scholar]
- 21.Anthemidis AN, Zachariadis GA, Michos CE, Stratis JA. Time-based on-line preconcentration cold vapour generation procedure for ultra-trace mercury determination with inductively coupled plasma atomic emission spectrometry. Anal Bioanal Chem. 2004;379:764–9. doi: 10.1007/s00216-004-2593-2. [DOI] [PubMed] [Google Scholar]
- 22.Soni A, Patel K, Gupta SN. Clinical evaluation of Vardhamana Pippali Rasayana in the management of Amavata (Rheumatoid Arthritis) Ayu. 2011;32:177–80. doi: 10.4103/0974-8520.92555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Baria R, Joshi N, Pandya D. Clinical efficacy of Panchamuladi Kaala Basti (enema) in the management of Amavata (Rheumatoid Arthritis) Ayu. 2011;32:90–4. doi: 10.4103/0974-8520.85737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mahto RR, Dave AR, Shukla VD. A comparative study of Rasona Rasnadi Ghanavati and Simhanada Guggulu on Amavata with special reference to Rheumatoid arthritis. Ayu. 2011;32:46–54. doi: 10.4103/0974-8520.85724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lekurwale PS, Pandey K, Yadaiah P. Management of Amavata with ‘Amrita Ghrita: A clinical study. Ayu. 2010;31:430–5. doi: 10.4103/0974-8520.82033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krishna KP. The efficacy of Ayurvedic treatment for rheumatoid arthritis: cross-sectional experiential profile of a longitudinal study. Int J Ayurveda Res. 2011;2:8–13. doi: 10.4103/0974-7788.83177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chopra A, Saluja M, Tillu G. Ayurveda-modern medicine interface: a critical appraisal of studies of Ayurvedic medicines to treat osteoarthritis and rheumatoid arthritis. J Ayurveda Integr Med. 2010;1:190–8. doi: 10.4103/0975-9476.72620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chopra A, Saluja M, Tillu G, Venugopalan A, Narsimulu G, Handa R, et al. Comparable efficacy of standardized Ayurveda formulation and hydroxychloroquine sulfate (HCQS) in the treatment of rheumatoid arthritis (RA): a randomized investigator-blind controlled study. Clin Rheumatol. 2012;31:259–69. doi: 10.1007/s10067-011-1809-z. [DOI] [PubMed] [Google Scholar]
- 29.Kapoor RC. Some observations on the metal-based preparations in the Indian Systems of Medicine. Indian J Tradit Know. 2010;9:562–75. [Google Scholar]
- 30.Washington, D.C: Office of Science and Technology, Office of Water, US. Environmental Protection Agency; 2001. [accessed on March 19, 2012]. U.S. Environmental Protection Agency. Water quality criterion for the protection of human health: methylmercury. Available from: http://water.epa.gov/water404.cfm . [Google Scholar]
- 31.Nagender RP, Pena-Mendez EM, Havel J. Gold and nano-gold in medicine: overview, toxicology and perspectives. J Appl Biomed. 2009;7:75–91. [Google Scholar]
- 32.Forestier J. Rheumatoid arthritis and its treatment by gold salts. J Lab Clin Med. 1935;20:827–40. [Google Scholar]
- 33.Lehman AJ, Esdaile JM, Klinkhoff AV, Grant E, Fitzgerald A, Canvin J METGO Study Group. A 48-week, randomized, double-blind, double-observer, placebo-controlled multicenter trial of combination methotrexate and intramuscular gold therapy in rheumatoid arthritis: results of the METGO study. Arthritis Rheum. 2005;52:1360–70. doi: 10.1002/art.21018. [DOI] [PubMed] [Google Scholar]


