Abstract
Introduction:
Antibiotic resistance is now a serious problem, although it was not so only a few years ago. The need of the hour is to give clear evidence of the efficacy of antibiotic use, or lack thereof, to the surgeon for a procedure as common as mandibular third molar surgery.
Aim:
This study aimed to evaluate whether postoperative combined amoxicillin and clavulanic acid in mandibular third molar extraction is effective in preventing inflammatory complications.
Study and Design:
The study was structured as a prospective randomized double-blind placebo-controlled clinical trial.
Materials and Methods:
A study was designed wherein the 96 units (two bilaterally similar impacted mandibular third molars per head in 48 patients) were randomly assigned to two treatment groups (Group I and Group II). Each patient served as his/her own control. Each patient received 625 mg of combined amoxicillin and clavulanic acid 1 h before surgery. In the case of third molars belonging to Group I, 625 mg of combined amoxicillin and clavulanic acid TDS was continued for 3 days; in Group II, placebo in similar-looking packs was continued for 3 days. The patients were evaluated on the third and seventh postoperative days for signs of clinical infection and for microbial load evaluation.
Statistical Analysis:
The data between the two groups were statistically analyzed by the two-tailed Fisher's exact test, with a 95% confidence interval.
Results:
The difference was not statistically significant between the test group and the control group with regard to erythema, dehiscence, swelling, pain, trismus, and infection based on microbial load. The data were statistically significant for alveolar osteitis, with the occurrence of alveolar osteitis (14.58%) in the placebo group.
Conclusion:
Postoperative antibiotics are recommended only for patients undergoing contaminated, long-duration surgery.
Keywords: Antibiotics, infection, mandibular third molars, randomized controlled trial
INTRODUCTION
The surgical extraction of impacted third molars is one of the most common oral surgery procedures carried out around the world. Owing to the nature and environment of the surgery, inflammation and infection associated with bacterial contamination are the most common postoperative complications.[1] Operations to extract third molars are considered Type II/clean-contaminated operations, as oral surgery is always carried out in a clean-contaminated environment where a large amount of bacteria exist and postoperative complications are usually associated with bacterial contamination and infections. Therefore, it seems reasonable to prescribe antibiotics to prevent and reduce the frequency of postoperative complications.
The subject of antibiotic prophylaxis in this type of surgery has been a matter of debate.[2] Some investigators consider complications after surgery to be due to the trauma of the procedure itself and not to infectious events and therefore do not think that antibiotics will be beneficial; they thus advocate the use of anti-inflammatory drugs.[3] Others recommend the use of antibiotic prophylaxis for significant reduction in postsurgical complications such as pain, trismus, delayed healing of the wound, and swelling on a case-by-case basis, where these symptoms are related to infection.[4]
On the other hand, because the incidence of postoperative complications is relatively low and usually not life-threatening, and the evidence produced by numerous underpowered clinical trials is considered controversial, there is no consensus if and how antibiotics should be used in third molar surgery.[5,6] The overall infection rate in dentoalveolar surgery is estimated at 1-5%, and the prescription of antibiotics in third molar surgery remains controversial.[7,8] Remarkably, despite an over-60-year-history of antibiotics use, there is no consensus regarding the use of systemic antibiotics in the setting of third molar surgery to prevent postoperative inflammatory complications.[9]
The present study was designed to determine if postoperative antibiotics are necessary to reduce the incidence of infection after mandibular third molar extraction. Also evaluated was whether the presence of microbial load at the suture site corresponds to clinical signs of infection.
MATERIALS AND METHODS
A prospective non-inferiority randomized double-blind placebo-controlled clinical trial study was designed wherein all healthy males and females were included who reported to the Department of Oral and Maxillofacial Surgery, Centre for Dental Education and Research, All India Institute of Medical Sciences, New Delhi, India with bilaterally similar impacted mandibular third molars between May 1, 2011, and December 31, 2012. Prior institutional ethical clearance (reference no. IESC/T-210/03.06.2011) and informed written consent from every patient were obtained, and the trial was registered (registration no. CTRI/2012/12/003239). The exclusion criteria were:
Local pathology—generalized or localized periodontitis; cyst or tumor associated with third molars; e.g. acute periodontitis
Allergy to penicillin
Immunocompromised state
Other systemic illness
Pregnancy
Noncompliance of the patient in taking postoperative medication
Refusal to give consent
Inability to appear for follow-up.
Study design
Routine radiographic investigations and oral prophylaxis were carried out preoperatively in all the patients. Forty-eight patients with bilaterally similar impacted mandibular third molars were included in the study (total of 96 experimental units, power 80%, alpha error 5%). All patients received 625 mg of combined amoxicillin and clavulanic acid 1 h before surgery. Following extraction, they were randomly assigned to two treatment groups. Each patient served as his/her own control. This was done to ensure similar biologic response to drug and placebo in a patient and also to increase statistical power. The patients and the operator were blinded to the drugs. The amoxicillin and clavulanic acid combination and the placebo were dispensed in similar-looking packs by the hospital pharmacist and were labelled as drug A and drug B. Each experimental unit received either drug A or B for the first site, as per random table. After 3 weeks, for the other side, the experimental unit received the drug A if B was given previously or B if A was given previously [Graph 1].
Graph 1.
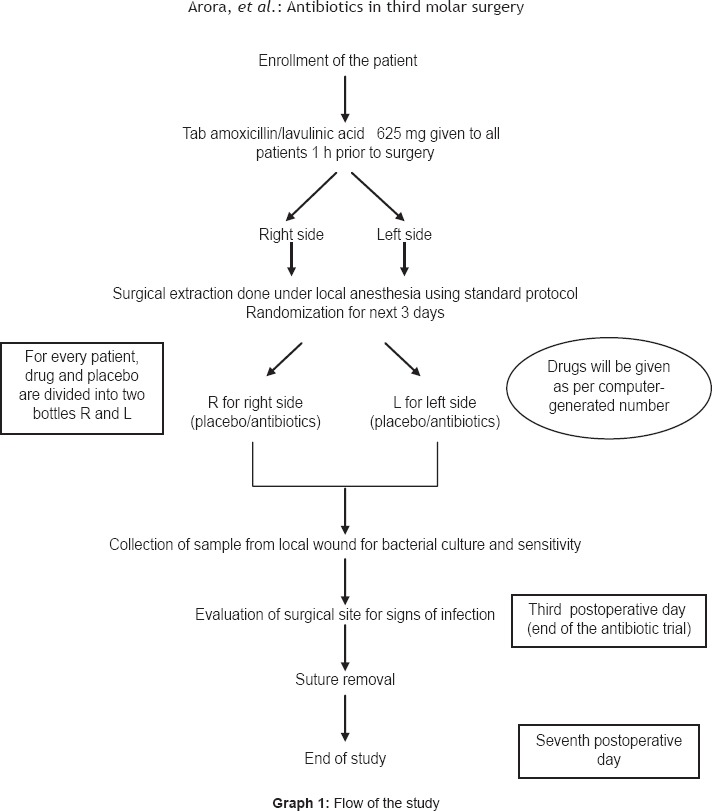
Flow of the study
The drugs were provided to the investigator by the Head of Department, who was blinded to the patients. Each packet contained the postoperative medication for a single patient, which was predetermined by a computer-generated random-number table. Using this method of randomization, the patients according to their sequence of enrolment received their postoperative medication in the corresponding prepacked packet. After the patients were assigned to two groups, 625 mg of combined amoxicillin and clavulanic acid was continued for 3 days in Group I and placebo in similar-looking packs was continued for 3 days in Group II. The randomization code was secured with the Head of the Department in a sealed envelope to be broken as per Good Clinical Practice (GCP) guidelines or in case of serious adverse outcome. The code was revealed to the investigator at the end of the trial. The operating surgeon, investigator, statistician, and patients were blinded with regard to which patient received what medication.
Ninety-six similar drug-dispensing packets were sequentially numbered for the concealed allocation of patients to the trial groups. Each packet contained the postoperative medication for a single patient, which was predetermined by a computer-generated random-number table. Amoxicillin and clavulanic acid in tablets (Group I) and lactulose powder (Group II) dispensed in hard gelatin capsules of similar color by the hospital pharmacist were prepacked in these packets. By this method of randomization, the patients according to their sequence of enrolment received their postoperative medication in the corresponding prepacked packets.
Surgery
All patients were given a prophylactic dose of combined amoxicillin and clavulanic acid 625 mg 1 h before operation. All operations were done by the same surgeon and assistant using the same set of instruments under local anesthesia consisting of 2% lignocaine hydrochloride with 1:200,000 adrenaline. In both groups, the site was prepared with 5% povidone-iodine solution, and a conventional Ward's incision was made to reflect the flap. In the conventional group, a mucoperiosteal flap was raised with a periosteal (Molt's no. 9) elevator to expose one of the impacted teeth and the surrounding bone. A no. 6 carbide round bur in a straight handpiece was used at 40,000 rpm for trephination and guttering at the buccal or distal aspect of the tooth, or both. A straight fissure bur was used to section the tooth when needed. At all times, cutting of bone and tooth was accompanied by copious irrigation with chilled saline solution. Primary closure was done with 3-0 Vicryl® sutures (Ethicon, Somerville, NJ, USA). Analgesics were given as required and chlorhexidine mouthwash was prescribed to every patient for 2 weeks postoperatively, and patients were kept on a semisolid diet. Suture removal was carried out after 1 week. Each patient was taken up for extraction of the impacted third molar on the other side after 3-4 weeks. The same procedure was carried out but with administration of the drug labelled B if A was given previously or A if B was given previously.
Follow-up evaluation
Primary efficacy variables were the development of local infection and evolution of the inflammatory parameters throughout the study period, determined in a blinded way by a single-blinded investigator on the third and seventh postoperative days. The following signs of infection were looked out for: Erythema, dehiscence, swelling, pain, trismus, alveolar osteitis, and infection based on microbial load. Suture site aspirate was collected using a needle and syringe where 1 mL sterile saline solution was injected into the soft tissue incision site and aspirated back. The objective was to evaluate the local microbial load in both the groups on the third and seventh postoperative days and establish whether local microbial load corresponds to clinical outcome.
Statistical analysis
Data collected for every patient included: Age, gender, oral hygiene status, type of impaction, whether the patient received postoperative antibiotics or not, signs of infection, and presence or absence of microbial load in the postoperative period. Data were tabulated in Microsoft Excel (Microsoft, Redmond, WA, USA) and analyzed for statistical difference between the two groups by the two-tailed Fisher's exact test with a 95% confidence interval using Stata version 8 (Statacorp, College Station, TX, USA).
RESULTS
Of the 353 patients screened for inclusion criteria, 48 patients with bilaterally similar impacted mandibular third molars were included in the study [Graph 2]. The mean age of the patients was 26.4 years with standard deviation (SD) +5.9. The age range was 18-40 years. Out of 48 patients, 32 were males and 16 were females. The difficulty scores of teeth in each group were assessed using Pederson's difficulty index. Eighty-four sites (87.5%) were operated in less than 30 min and 12 (12.5%) required more than 30 min. There was no statistically significant difference between the two groups in terms of any patient characteristic [Table 1].
Graph 2.
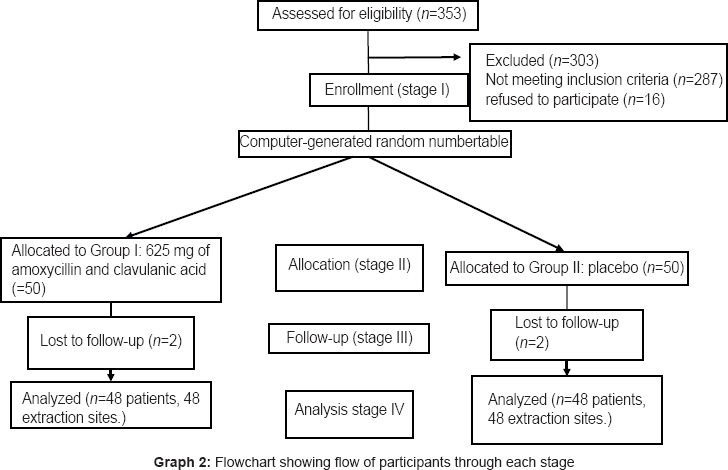
Flowchart showing flow of participants through each stage
Table 1.
Demographic details of study sample (n=48)

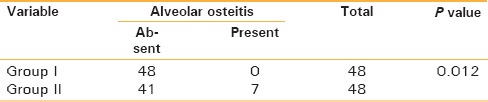
The incidence of postoperative complications (erythema and dehiscence) in the study was 6.25% in Group I as compared to 16.67% in Group II. There was no statistically significant difference (P = 0.199) in the incidence of infection in both the groups. The presence of swelling and pain was statistically insignificant, with P values of 0.132 and 0.66 respectively. This shows that there was no significant difference in the occurrence of swelling and pain in Group I and Group II patients. Swelling was the most common symptom, followed by local pain, in both the groups. The association of trismus to postoperative antibiotic regime was found to be nonsignificant statistically, with a P value of 0.294. Alveolar osteitis was not noted in any site in the short antibiotic regime group (Group I), while signs of alveolar osteitis were noted in seven sites (14.58%) in the placebo group (Group II). Alveolar osteitis was found in 28.57% of distoangular impactions and 20% of horizontal impaction in Group II. Data were statistically significant, with a P value of 0.012, and there was difference in the distribution of alveolar osteitis between Group I and Group II patients [Table 2].
Table 2.
Distribution of patients characteristics according to alveolar osteitis in Group-I and Group-II
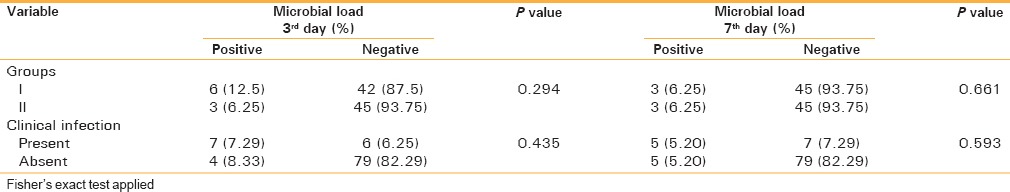
Suture site aspirates were positive for microbes in nine patients on the third day (six patients in Group I and three patients in Group II) and in six patients on the seventh day (3 patients in Group I and three patients in Group II). There was no statistically significant difference in the microbial load distribution among both the groups, nor was there any difference in relation to the suture site and presence of clinical infection, on both the third and seventh days [Table 3]. No Gram-positive bacteria were isolated from culture. The organisms were mainly Gram-negative bacteria, namely Pseudomonas and enterobacteria (Citrobacter, E. coli).
Table 3.
Distribution of the 3rd and 7th day microbial load according to groups and presence of clinical infection
DISCUSSION
Infections are currently the leading global burden of disease. Antibiotic resistance is now a serious problem, which was not the case 50 years ago.[10,11,12] Thus, we have decided to undertake the study to compare postoperative infection in patients receiving postoperative placebo and antibiotics after mandibular third molar extraction. Swelling was the most common symptom, followed by local pain in both the groups. This is in concordance with the findings of other studies.[13,14] The reason for swelling in the study can be attributed to surgical trauma, as it was not associated with any discharge or foul smell.
An important component of our study was to find any significant association between clinical infection (erythema, dehiscence, swelling, pain, and trismus) and the microbial load at the operated site. We did not find any significant association between clinical signs of infection and the microbial load. It was seen that there were patients with early signs of clinical infection but negative microbial load, while some patients had no clinical signs of infection but microbial load was found to be positive. The bacteria isolated from the suture site aspirates included predominantly Gram-negative bacteria namely– Pseudomonas, enterobacteria (Citrobacter, E. coli). The presence of Gram-negative bacteria could be due to contamination of the sample during collection or due to the use of combined amoxicillin and clavulanic acid, which acts predominantly on Gram-positive bacteria. The presence of these bacteria in the absence of clinical infection may indicate less virulent strains of bacteria or that the bacterial colony count was not sufficient to overcome the host resistance and cause infection. It is noteworthy that no case in the study had active pus discharge or abscess formation requiring incision and drainage. Tenderness and dehiscence are not proven indicators of surgical site infection; however, that does not invalidate the result, as the same criteria were used to evaluate surgical site infection in both the groups. All the cases that were counted as infection were cases of erythema and wound dehiscence, which could simply be due to surgical trauma/edema.
Alveolar osteitis or dry socket is the most frequently reported sequela and may affect 25-30% of the patients undergoing removal of impacted mandibular third molars.[15,16] Surgical trauma, age, and gender are known risk factors for the development of alveolar osteitis and other postoperative complications.[17] Alveolar osteitis was seen in seven sites (14.58%) in the placebo group in comparison to 0% in the antibiotic group in the study, which is in accordance with the findings from the studies by Delilbasi[16] and Blum.[17] Alveolar osteitis was found in 28.57% of distoangular impactions and in 20% of mesioangular impactions in Group II, where more bone cutting and a longer duration of surgery was required compared to other types of molar impactions, with a P value of 0.012 signifying that the data are statistically significant. In these cases, a postoperative antibiotic for 3 days is recommended to prevent the incidence of alveolar osteitis, as seen in our study.
There are, however, certain limitations of our study, which need to be addressed. First, the small sample size does not permit us to draw major conclusions. Second, quantification of the infection/inflammatory diagnostic tests was not performed. Despite these limitations, the study does standardize the two groups in terms of type of impaction using Pederson's difficulty index and the treatment provided via the same surgeon and same approach. The antibiotic regimen was the same in terms of the dose, duration, route, and type of antibiotics used. The proper protocol for randomization was followed to prevent bias and distortion of results. In conclusion, the present study does not completely answer the question of whether postoperative antibiotics are required in mandibular third molar removal but does provide strong evidence to supplement the available literature more clearly.
The following conclusions have been drawn from the present study:
Swelling and pain following third molar surgery are the most common findings in our study. Neither correlates with the postoperative antibiotic regime
Postoperative antibiotics for 3 days are required in distoangular, horizontal impactions, in difficult impaction (covered by bone), and in cases where the duration of surgery exceeds 30 min to reduce the incidence of alveolar osteitis
Decision for postoperative antibiotic regime should be made according to tooth position, the bone surrounding the tooth, the presence or absence of pathology, and the estimated duration of surgery.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
REFERENCES
- 1.Ataoğlu H, Oz GY, Candirli C, Kiziloğlu D. Routine antibiotic prophylaxis is not necessary during operations to remove third molars. Br J Oral Maxillofac Surg. 2008;46:133–5. doi: 10.1016/j.bjoms.2006.11.005. [DOI] [PubMed] [Google Scholar]
- 2.Piecuch JF, Arzadon J, Lieblich SE. Prophylactic antibiotics for third molar surgery: A supportive opinion. J Oral Maxillofac Surg. 1995;53:53–60. doi: 10.1016/0278-2391(95)90502-2. [DOI] [PubMed] [Google Scholar]
- 3.Poeschl PW, Eckel D, Poeschl E. Postoperative prophylactic antibiotic treatment in third molar surgery–a necessity? J Oral Maxillofac Surg. 2004;62:3–9. doi: 10.1016/j.joms.2003.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Zeitler DL. Prophylactic antibiotics for third molar surgery: A dissenting opinion. J Oral Maxillofac Surg. 1995;53:61–4. doi: 10.1016/0278-2391(95)90503-0. [DOI] [PubMed] [Google Scholar]
- 5.Halpern LR, Dodson TB. Does prophylactic administration of systemic antibiotics prevent postoperative inflammatory complications after third molar surgery? J Oral Maxillofac Surg. 2007;65:177–85. doi: 10.1016/j.joms.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 6.Shapiro M, Townsend TR, Rosner B, Kass EH. Use of antimicrobials drugs in general hospitals: Patterns of prophylaxis. N Engl J Med. 1979;301:351–5. doi: 10.1056/NEJM197908163010703. [DOI] [PubMed] [Google Scholar]
- 7.Slama TG, Amin A, Brunton SA, File Tm, Jr, Milkovich G, Rodvold KA, et al. Council for Appropriate and Rational Antibiotic Therapy (CARAT). A clinician's guide to the appropriate and accurate use of antibiotics: The council for appropriate and Rational Antibiotic Therapy (CARAT) criteria. Am J Med. 2005;118(Suppl 7A):1S–6S. doi: 10.1016/j.amjmed.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 8.Toia F, D’Arpa S, Massenti MF, Amodio E, Pirrello R, Moschella F. Perioperative antibiotic prophylaxis in plastic surgery: A prospective study of 1,100 adult patients. J Plast Reconstr Aesthet Surg. 2012;65:601–9. doi: 10.1016/j.bjps.2011.11.038. [DOI] [PubMed] [Google Scholar]
- 9.Hill M. No benefit from prophylactic antibiotics in third molar surgery. Evid Based Dent. 2005;6:10. doi: 10.1038/sj.ebd.6400307. [DOI] [PubMed] [Google Scholar]
- 10.Ren YF, Malmstrom HS. Effectiveness of antibiotic prophylaxis in third molar surgery: A meta-analysis of randomized controlled clinical trials. J Oral Maxillofac Surg. 2007;65:1909–21. doi: 10.1016/j.joms.2007.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Siddiqi A, Morkel JA, Zafar S. Antibiotic prophylaxis in third molar surgery: A randomized double-blind placebo-controlled clinical trial using split-mouth technique. Int J Oral Maxillofac Surg. 2010;39:107–14. doi: 10.1016/j.ijom.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 12.Arteagoitia I, Diez A, Barbier L, Santamaría G, Santamaría J. Efficacy of amoxicillin/clavulanic acid in preventing infectious and inflammatory complications following impacted mandibular third molar extraction. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2005;100:e11–8. doi: 10.1016/j.tripleo.2005.03.025. [DOI] [PubMed] [Google Scholar]
- 13.Lacasa JM, Jiménez JA, Ferrás V, Bossom M, Sóla-Morales O, García-Rey C, et al. Prophylaxis versus pre-emptive treatment for infective and inflammatory complications of surgical third molar removal: A randomized, double-blind, placebo-controlled, clinical trial with sustained release amoxicillin/clavulanic acid (1000/62.5 mg) Int J Oral Maxillofac Surg. 2007;36:321–7. doi: 10.1016/j.ijom.2006.11.007. [DOI] [PubMed] [Google Scholar]
- 14.Monaco G, Tavernese L, Agostini R, Marchetti C. Evaluation of antibiotic prophylaxis in reducing postoperative infection after mandibular third molar extraction in young patients. J Oral Maxillofac Surg. 2009;67:1467–72. doi: 10.1016/j.joms.2008.12.066. [DOI] [PubMed] [Google Scholar]
- 15.Limeres J, Sanromán J, Tomás I, Diz P. Patients’ perception of recovery after third molar surgery following postoperative treatment with moxifloxacin versus amoxicillin and clavulanic acid: A randomized, double-blind, controlled study. J Oral Maxillofac Surg. 2009;67:286–91. doi: 10.1016/j.joms.2008.06.061. [DOI] [PubMed] [Google Scholar]
- 16.Delilbasi C, Altundal H, Dergin G, Istanbul KS. Comparison of different regimens for the prevention of alveolar osteitis. OHDMBSC. 2004;3:41–4. [Google Scholar]
- 17.Blum IR. Contemporary views on dry socket (alveolar osteitis): A clinical appraisal of standardization, aetiopathogenesis and management: A critical review. Int J Oral Maxillofac Surg. 2002;31:309–17. doi: 10.1054/ijom.2002.0263. [DOI] [PubMed] [Google Scholar]


