Abstract
A plasmid vector encoding the cholera toxin B subunit (pCtB) was evaluated as an intradermal genetic adjuvant for a model DNA vaccine expressing the human papillomavirus type 16 L1 capsid gene (p16L1) in mice. p16L1 was coadministered with plasmid pCtB or commercial polypeptide CtB as a positive control. Coadministration of pCtB induced a significant increment of specific anti-L1 immunoglobulin A (IgA) antibodies in cervical secretions (P < 0.05) and fecal extracts (P < 0.005). Additionally, coadministration of pCtB enhanced the production of interleukin-2 and gamma interferon by spleen cells but did not affect the production of interleukin-4, suggesting a Th1-type helper response. Furthermore, improved CD8+ T-cell-mediated cytotoxic activity was observed in mice vaccinated with the DNA vaccine with pCtB as an adjuvant. This adjuvant effect was comparable to that induced by the CtB polypeptide. These results indicate that intradermal coadministration of pCtB is an adequate means to enhance the mucosa-, Th1-, and CD8+-mediated cytotoxic responses induced by a DNA vaccine.
Cholera toxin (CT), the enterotoxin produced by Vibrio cholerae, is a potent immunoadjuvant (26). CT is composed of two structurally and functionally different subunits, the toxic A subunit (CtA) and the cell-binding B subunit (CtB). Biologically active CtB assembles into pentamers and binds with a high affinity to the cellular receptor GM1 ganglioside (35), which is expressed by a wide range of nucleated cells, including epithelial cells, lymphocytes, and antigen-presenting cells (APCs). CtB is recognized as a mucosal adjuvant itself (12). When CtB is applied simultaneously with heterologous antigens, it induces the stimulation of a mucosal response to the admixed antigen (15).
The adjuvant capacity of CT for orally administered antigens has long been recognized (26). More recently, CtB has been used to enhance immune responses to antigens delivered by novel immunization routes (16, 20). In particular, application of CtB onto mouse skin has been proved to induce potent humoral and cellular responses against the coadministered antigen (1).
The use of DNA vaccines represents a novel strategy for the induction of specific mucosal immune responses. Like traditional vaccines, the ability of DNA vaccines to elicit mucosal responses can potentially be improved by the use of adjuvants. Coadministration of CT has been used to increase the specific immunoglobulin G (IgG) (8) and mucosal IgA (24) responses mediated by DNA vaccines. However, the use of CtB alone as an adjuvant for DNA vaccines is still limited. In addition, use of the CT-coding sequences as genetic adjuvants for DNA vaccines has recently been proposed as an innovative approach that has produced promising results (2) and that deserves further investigation.
Plasmid DNA encoding suitable antigens can readily and economically be constructed and produced in large quantities. The final product retains an intrinsic characteristic of DNA: stability. This is of particular interest for vaccines meant to be applied in developing countries, since it would reduce the need for costly cold storage. For that reason, designing and testing of plasmids containing coding sequences with the potential of enhancing responses against DNA vaccines is an attractive approach nowadays.
In the present work we investigated the capacity of the CtB-coding sequence to enhance the humoral and cellular responses mediated by a DNA vaccine. To do this, a plasmid containing the CtB gene was constructed and used as an adjuvant for a model DNA vaccine containing the L1 gene from human papillomavirus (HPV) type 16 (HPV-16) (32). Mice were immunized intradermally with the DNA vaccine and the plasmid expressing CtB (pCtB), commercial CtB polypeptide, CT holotoxin, or a translational fusion plasmid expressing a biologically inactive CtB. Our study showed that coadministration of pCtB enhances the production of fecal and genital IgA antibodies. Furthermore, pCtB induced a Th1-type response associated with an increment on CD8+-T-cell-specific cytotoxic activity against L1-expressing cells. The adjuvant capacity of pCtB was comparable to that of the CtB polypeptide. The fusion plasmid did not show adjuvant activity. These results suggest that the plasmid expressing the CtB gene can be used as a strong adjuvant for intradermally administered DNA vaccines not only to enhance mucosal responses but also to improve Th1-mediated cellular responses.
MATERIALS AND METHODS
Construction of plasmids.
All reagents used for the isolation and amplification of DNA were purchased from Gibco BRL (Life Technologies Inc., Gaithersburg, Md.). Genomic DNA was isolated from cultures of the V. cholerae El Tor biotype and was used as the template for PCRs. The complete sequence coding for the enterotoxin B-subunit mature polypeptide was amplified with the following oligonucleotides: CtBF (5′-TCA GGC GGC CGC CAT ATG CAC ATG AGG CAC CT-3′) and CtBR (5′-TCA GTC TAG ATT AAT TTG CCA TAC TAA TTG C-3′). To facilitate cloning, NotI and XbaI restriction sites were incorporated into primers CtBF and CtBR, respectively; the sequences are shown in italics. The primers were designed to amplify a fragment spanning from position 187 to position 512 (GenBank accession no. K01170) (25). Amplified fragments were digested with NotI and XbaI to generate cohesive ends and inserted into the NotI and XbaI sites of the pCDNA3 expression vector (Invitrogen Co., La Jolla, Calif.), under the control of the human cytomegalovirus immediate-early promoter, to generate plasmid pCtB.
The L1 gene was amplified from a wild-type HPV-16 genome by PCR with the following oligonucleotides: L1F (5′-CGG TAC CTA GTT CCA GGT CTC CAC-3′) and L1R (5′-CCT CGA GAT ATA CAC AAC CAA ACA AC-3′). Primers L1F and L1R amplify a 1,672-bp fragment containing the entire L1-coding sequence. Recognition sites for KpnI and XhoI were incorporated into primers L1F and L1R, respectively, and are indicated in italics. Amplified L1 fragments were digested with KpnI and XhoI and inserted into the corresponding sites of the pCDNA3 vector to create the p16L1 expression construct.
The L1 gene was also amplified with the L1F and L1R′ oligonucleotides. Use of primer L1R′ (5′-CGC GGC CGC GCGT TTA GCA GTT GTA GAG-3′) leads to the amplification of a truncated L1 sequence (1,612 bp) that lacks the natural termination signal. A NotI site was incorporated into L1R′ and is indicated in italics. The truncated L1 fragment was subcloned into the KpnI and NotI sites of plasmid pCtB to generate translational fusion plasmid pL1tB.
The integrity of the DNA of the CtB and L1 inserts from all constructs was corroborated by sequencing with an ABI PRISM 310 Genetic Analyzer PE (Applied Biosystems, Foster City, Calif.).
In vitro plasmid expression.
B16FO cells (a murine melanoma-derived cell line) were transfected with plasmid p16L1, pCtB, or pL1tB (all of which express a neomycin [G418] resistance gene) by using the FuGENE 6 transfection reagent (Roche Diagnostics, Mannheim GmbH, Germany). B16FO cells (3 × 105) were cultured in Dulbecco modified Eagle medium (DMEM) supplemented with 10% heat-inactivated fetal calf serum, 100 U of penicillin per ml, and 100 μg of streptomycin per ml at 37°C for 24 h. The FuGENE reagent (10 μl) was mixed with 200 μl of serum-free DMEM and incubated for 5 min at room temperature. Plasmid DNA (1.5 μg) was diluted in 100 μl of serum-free DMEM, gently mixed with the FuGENE reagent solution, and incubated for 15 min at room temperature. Supplemented DMEM (200 μl) was added to the FuGENE reagent-DNA mixture and added dropwise to the cells growing in 2.5 ml of supplemented DMEM. The cells were incubated for 48 h at 37°C in a 5% CO2 atmosphere. G418 was then added at a final concentration of 2 mg/ml. Transfected cells were maintained under continuous selective pressure for 3 to 4 weeks. Expression of the L1 and CtB proteins was studied by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 10 and 14% polyacrylamide gels, respectively. A total of 106 cells were washed with ice-cold phosphate-buffered saline (PBS) three times. The cell pellets were diluted 1:1 in double-strength sample buffer 120 mM Tris [pH 6.8], 0.1% sodium dodecyl sulfate, 10% glycerol, 1% bromophenol blue, 100 mM dithiothreitol ([DTT]) and boiled for 3 min. Samples along with molecular weight markers were resolved by electrophoresis (Life Technologies Inc.). After electrophoresis, the proteins were electroblotted onto nitrocellulose membranes. Nonspecific binding sites were saturated by incubating the membrane overnight at 4°C in Tris-buffered saline (TBS; 10 mM Tris [pH 7.5], 0.9% NaCl) containing 3% bovine serum albumin (BSA) and 0.01% sodium azide. The L1 protein was detected by using a rabbit antipapillomavirus antibody (Dako Co., Carpinteria, Calif.) and the CT B subunit was detected with a rabbit anti-CT antibody (Sigma, St. Louis, Mo.), which was used as a probe to react with the B subunit of CT (Sigma), by enzyme-linked immunosorbent assay (ELISA). After incubation with alkaline phosphatase-conjugated swine anti-rabbit secondary antibody (Dako Co.), the bands were developed by incubation with Sigma Fast 5-bromo-4-chloro-3-indolylphosphate-Nitro Blue Tetrazolium alkaline phosphatase substrate (Sigma).
In vivo plasmid expression.
B16FO (H-2b) cells (105) were inoculated intradermally into the backs of C57BL/6 (H-2b) mice, and the mice were monitored for tumor formation. Twenty days after inoculation, the tumors were transfected by injection with 100 μg of plasmid p16L1, pCtB, or pL1tB. The mice were killed 48 h after plasmid injection, the tumors were recovered, and 100-mg tumor samples were obtained and homogenized in 1 ml of Trizol reagent (Life Technologies Inc.) with a power homogenizer. Total RNA was purified by phenol-chloroform separation and isopropyl alcohol precipitation. Specific RNA sequences were reverse transcribed to cDNA and then amplified by PCR with the SuperScript One-Step RT-PCR system (Life Technologies Inc.). The mRNA sequence specific for HPV-16 L1 was amplified with primers L1f (5′-CTA GTT CCA GGT CTC CAC-3′) and L1r (5′-CAT ATA CAC AAC CAA ACA AC-3′), and the CtB mRNA sequence was amplified with primers CtBf (5′-TCA CAT ATG CAC ATG AGG CAC CT-3′) and CtBr (5′-TCA GTT AAT TTG CCA TAC TAA TTG C-3′). mRNA from pL1tB-transfected cells was amplified by using primers L1f and L1r, primers CtBf and CtBr, or primers L1f and CtBr.
GM1 ganglioside-binding assay.
For the GM1 ganglioside-binding assay, cultures of pCtB- and pL1tB-transfected B16FO cells, which had been maintained continuously under selective pressure with G418 for more than 4 weeks, were used. The cells (106) were washed three times with ice-cold PBS. The cells were resuspended in lysis buffer containing protease inhibitors (50 mM Tris-Cl, 150 mM NaCl, 0.02% sodium azide, 1% Triton X-100, 0.5 mM phenylmethylsulfonyl fluoride, 10 μg of aprotinin per ml, 10 μg of pepstatin per ml, 100 μg of leupeptin per ml). Cell lysates were concentrated with an Amicon Centriprep-10 concentrator (Millipore Corp., Billerica, Mass.) and diluted in PBS to a final concentration of 5 μg/ml. The binding assay was performed basically as described elsewhere (40). Ninety-six-well low-binding polystyrene plates were coated with 100 μl of 2 μM GM1 ganglioside (Sigma) in PBS at room temperature overnight. During this time the plates were covered to avoid evaporation. After the plates were washed four times with PBS, nonspecific binding sites were blocked with 200 μl of 2% BSA in PBS for 2 h at 37°C. After the plates were washed, increasing concentrations of either cell lysates or CtB polypeptide (Sigma) diluted in PBS containing 2% BSA were added. Nontransfected cell lysates were included as negative controls. To inhibit the assembly of B subunits into pentamers, the reducing agent DTT was added to samples of cell lysates and CtB before the samples were added to the plates. After incubation at 37°C for 2 h, bound proteins were detected by using a rabbit anti-CT antibody (Sigma), followed by an anti-rabbit alkaline phosphatase-conjugated secondary antibody (Dako Co.). The color reaction was stopped by the addition of 50 μl of 3 M NaOH, and the absorbance at 450 nm was determined and recorded. The mean values from four independent experiments are reported.
Mice, immunization, and sample collection.
Animals were used in accordance with the Research Animals Use and Care Guidelines of the Institute of Biomedical Research. Groups of female C57BL/6 mice (age, 6 to 8 weeks) were immunized intradermally on days 0 and 14 with p16L1 (100 μg), pCtB (100 μg), pL1tB (100 μg), commercial CtB polypeptide (10 μg; Sigma), or commercial CT holotoxin (10 μg; Sigma). An additional group was immunized with 100 μl of sterile, contaminant-free PBS (Roche Diagnostics) as a negative control. For the adjuvant capacity study, mice received 100 μg of p16L1 coadministered with either different concentrations of pCtB (1, 10, 100, 250, or 500 μg), 10 μg of CtB polypeptide, or 10 μg of CT. Cervical secretions were collected by washing the genital tract three times with 30 μl of sterile, contaminant-free PBS. Secretions were cleared of cell debris and tissue fragments by centrifugation at 13,000 × g for 5 min and were stored at −70°C. Fecal pellets were collected and weighed; the final volume was adjusted to 100 mg/ml with PBS containing 0.01% sodium azide. Fecal pellets were suspended by shaking with a vortex mixer (Barnstead/Thermolyne, Dubuque, Iowa) and cleared of fecal debris by centrifugation at 13,000 × g for 5 min, and the supernatants were collected and stored at −70°C. Spleens were obtained from mice that had been killed by cervical dislocation, and spleen cells were extracted by perfusion with RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, 100 U of penicillin per ml, and 100 μg of streptomycin per ml and used immediately.
Detection of antibodies by ELISA.
The presence of antibodies was determined by ELISA with either baculovirus-derived HPV-16 virus-like particles (VLPs; kindly provided by John Schiller, National Institutes of Health, Bethesda, Md.) or commercial CtB. Extraction and purification of VLPs have been described elsewhere (7). Plates were coated overnight at 4°C with 500 ng of either purified VLPs or CtB diluted in 100 μl of PBS per well. The plates were washed four times with TBS-T (6.5 g of Tris base per liter, 27.5 g of NaCl per liter, 0.1% Tween 20). Nonspecific binding sites were blocked by adding 200 μl of blocking solution (TBS-T containing 2% BSA) to each well for 2 h at 37°C. Cervical secretions and fecal samples were diluted 1:10 in blocking solution. All samples were serially diluted twofold down the microtiter plate. Bound antibodies were detected with anti-mouse IgA-alkaline phosphatase-conjugated secondary antibody (Dako Co.). Endpoint titers were defined as the reciprocal of the highest dilution with an absorbance value greater than three times the mean absorbance value for the control mice vaccinated with PBS. Samples from nonresponders were assigned a value of one-half the lowest dilution tested.
Determination of IL-2, IFN-γ, and IL-4 production.
A total of 106 spleen cells were stimulated with purified HPV-16 VLPs for 48 h in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 2 mM l-glutamine. Conditioned medium was collected at 12, 24, and 48 h poststimulation. Medium was cleared from the particulate matter by centrifugation and was assayed immediately. The concentrations of interleukin-2 (IL-2), gamma interferon (IFN-γ), and IL-4 were determined with a ChemiKine Human Interleukin-2, IFN-γ, or Interleukin-4 sandwich ELISA kit (Chemicon International, Temecula, Calif.), according to the instructions of the manufacturer. All medium samples were assayed in duplicate. Conditioned medium from nonstimulated cells was included as a negative control.
CTL assay.
Spleen cells were cultured at 37°C in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, 100 U of penicillin per ml, 100 μg of streptomycin per ml, 10 U of IL-2 (Life Technologies Inc.) per ml, and 2 mM l-glutamine. A total of 108 splenocytes were restimulated with purified HPV-16 VLPs for 5 days. The cytotoxic T-lymphocyte (CTL) assay was performed in 96-well round-bottom plates with HPV-16 L1-expressing B16FO cells as the target; untransfected B16FO cells were used as controls. Restimulated splenocytes were incubated with 104 target cells for 4 h at different effector cell/target cell ratios. Cytotoxicity was evaluated by measuring the activity of cytosolic lactate dehydrogenase, which is released upon cell lysis, by the Cytotox 96 nonradioactive cytotoxicity assay (Promega Co., Madison, Wis.), according to the instructions of the manufacturer. The maximum levels of release of lactate dehydrogenase from effector cells and target cells were measured for inclusion in cytotoxicity calculations. The absorbance values for the background control (culture medium) were subtracted from all experimental and control values. Corrected absorbance values were used to calculate the percent cytotoxicity by the following formula: [(experimental release − effector cell spontaneous release − target cell spontaneous release)/(target maximum release − spontaneous release)] × 100. The final cytotoxicity value represents the mean for 10 individual mice per group.
Proliferation assay.
A total of 2 × 105 spleen cells were seeded in triplicate wells of 96-well round-bottom plates in RPMI 1640 medium supplemented with 10% heat-inactivated fetal calf serum, 100 U of penicillin per ml, 100 μg of streptomycin per ml, and 2 mM l-glutamine and incubated in the presence or absence of purified HPV-16 VLPs for 5 days at 37°C. During the final 18 h of culture the cells were pulsed with 1 μCi of tritiated thymidine (Amersham, Arlington Heights, Ill.) per well. The cells were harvested onto fiberglass filters (Schleicher & Schuell, Inc., Keene, N.H.), which were left to dry overnight before liquid scintillation counting (EcoLume; ICN, Costa Mesa, Calif.). The stimulation index was calculated as the mean number of counts for the VLP-stimulated cells divided by the mean number of counts for the nonstimulated cells.
Depletion of T-lymphocyte subpopulations.
Columns and reagents were purchased from Miltenyi Biotech GmbH (Gladbach, Germany), and antibodies were purchased from Pharmingen (Becton Dickinson Co., San Jose, Calif.). Paramagnetic microbeads conjugated to either monoclonal rat anti-mouse CD8a (Ly-2) or rat anti-mouse CD4 (L3T4) antibodies were used to deplete CD8+ and CD4+ cells, respectively. A total of 108 antigen-activated splenocytes were resuspended in 90 μl of PBS supplemented with 2 mM EDTA and 0.5% BSA (column buffer), and the mixture was incubated with 10 μl of paramagnetic microbeads for 15 min at 4°C. Magnetically labeled cells were washed with 2 ml of column buffer by centrifugation at 2,500 × g for 5 min, and 107 cells were resuspended in 500 μl of degassed column buffer. LS+ columns were attached to a magnet and activated with 3 ml of degassed column buffer; cells were applied to the column, and effluent was collected and considered either a CD4+- or a CD8+-depleted population.
The efficiency of cell depletion was assessed by flow cytometry with a FACSCalibur flow cytometer (Becton Dickinson Co.). Subpopulations were determined by using APC-anti-mouse CD3e (CD3 ɛ chain), phycoerythrin-anti-mouse CD8a (Ly-2), or biotin-anti-mouse CD4 (L3T4) antibodies. Cytometry analysis was performed with the CellQuest program. Only samples with depletion efficiencies ≥99% were used for the cytotoxicity assays.
Statistical analysis.
The Wilcoxon signed-rank test and Student's t test were used to analyze the significance of the differences between the experimental and the control groups. All tests were two-tailed, and basic significance was considered a P value of 0.05.
RESULTS
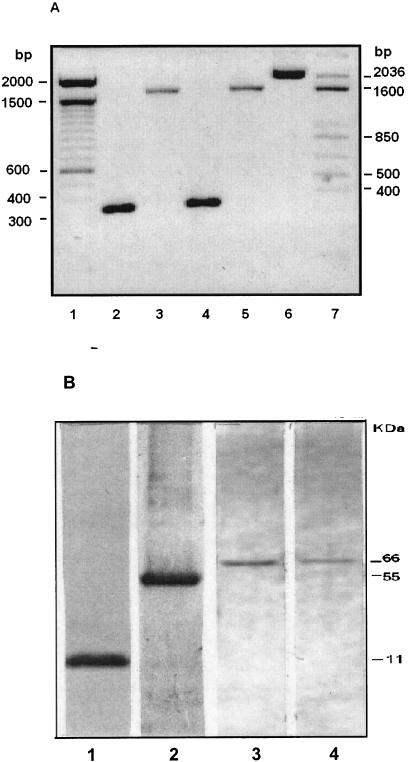
In vivo and in vitro expression of plasmids. Plasmids were constructed to encode and deliver the CtB polypeptide from V. cholerae and the HPV-16 L1 protein into mammalian cells, separate or as part of a fusion. The integrities of the cloned sequences were verified by sequencing the entire genes and flanking vector sequences. In vivo expression of the plasmids was evaluated upon transfection of murine melanoma tumors with either p16L1, pCtB, or pL1tB. RNA was isolated, reverse transcribed into cDNA, and then amplified. A fragment of 325 bp, which corresponded to the expected size of the CtB sequence, was amplified from pCtB-transfected cells (Fig. 1A, lane 2), whereas the entire 1,672-bp L1 gene was amplified from p16L1-transfected cells (Fig. 1A, lane 3). Fragments of the expected molecular size for CtB and L1 were amplified from pL1tB-transfected cells by using primers specific for CtB (Fig. 1A, lane 4) or L1 (Fig. 1A, lane 5). Furthermore, a fragment of approximately 2,000 bp was amplified from pL1tB-transfected cells (Fig. 1A, lane 6) by using a combination of L1-specific forward primers and CtB-specific reverse primers, indicating that the L1 and CtB genes are transcribed as a single sequence. Expression of the L1 protein and CtB polypeptide was investigated in cells transfected in vitro and was demonstrated by Western blotting. Bands of the expected molecular mass for monomeric CtB (11 kDa) and L1 (55 kDa) were detected in protein extracts from cells transfected with plasmids pCtB (Fig. 1B, lane 1) and p16L1 (Fig. 1B, lane 2), which indicates that the proteins were translated in vitro. Protein extracts from cells transfected with pL1tB showed the presence of a 66-kDa product that reacted with both anti-L1 (Fig. 1B, lane 3) and anti-CtB (Fig. 1B, lane 4) antibodies, suggesting that the proteins were expressed as a fusion.
FIG. 1.
Analysis of L1 and CtB gene expression in mammalian cells. pCtB, p16L1, or pL1tB was injected directly into B16FO cell-induced tumors in mice (A) or transfected into B16FO cells in vitro (B). Production of specific mRNAs and proteins were analyzed 48 h later. (A) Total RNA was isolated from injected tumor cells; reverse transcribed to cDNA; and then amplified by PCR with CtB-specific (lanes 2 and 4), L1-specific (lanes 3 and 5), or L1 forward-CtB reverse (lane 6) oligonucleotides. Lanes 1 and 7, DNA size markers; lane 2, reverse transcription-PCR products from tumors transfected with pCtB; lane 3, product from tumors transfected with p16L1; lanes 4, 5, and 6, products from tumors transfected with pL1tB. (B) Protein extracts from B16FO cells transfected in vitro with pCtB (lane 1), p16L1 (lane 2), or pL1tB (lanes 3 and 4) were separated by electrophoresis; and proteins were detected after Western blotting with antibodies against the L1 protein (lanes 2 and 3) or the CtB polypeptide (lanes 1 and 4).
GM1 ganglioside-binding assay.
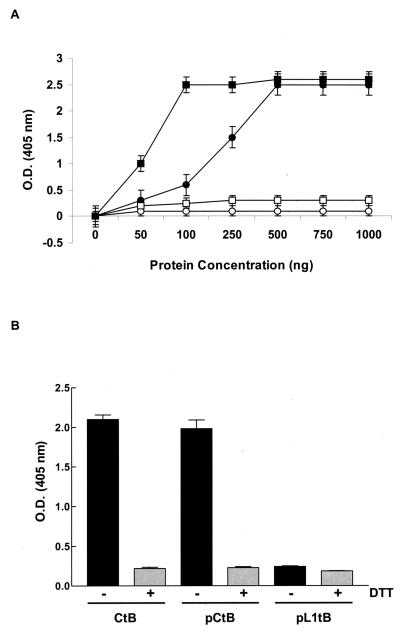
In order to bind to its cellular receptor, GM1 ganglioside, CtB must assemble into pentamers; interestingly, only this biologically active pentameric molecule expresses adjuvant activity (35). The capacity of plasmid-expressed CtB to bind to GM1 ganglioside was evaluated in the present study. Increasing concentrations of whole-cell protein extracts from cells transfected with plasmid pCtB or pL1tB were assessed in a GM1 ganglioside-binding assay. Commercial CtB polypeptide was included as a positive control. As shown in Fig. 2A, CtB produced by pCtB-transfected cells bound to GM1 ganglioside, suggesting that plasmid-produced CtB assembles into pentamers. In this assay, the maximum levels of binding were detected with commercial CtB at a concentration of 100 ng and protein extracts from cells transfected with pCtB at 500 ng. Unlike pCtB, pL1tB-derived CtB showed a significantly lower capacity of binding to GM1 ganglioside; this suggests that the conformation of the CtB polypeptide was modified by the presence of the fused L1 protein, hampering the expression of a biologically active CtB. As expected, no binding was detected in protein extracts form nontransfected cells. It is known that binding of CtB to GM1 ganglioside can be inhibited in vitro by addition of the reducing agent DTT (5). Therefore, to demonstrate the biological activity of pCtB-derived CtB, a GM1 ganglioside-binding assay was performed in the presence or absence of DTT with 100 ng of commercial CtB and 500 ng of protein extract from transfected cells. These protein concentrations yielded the maximum levels of binding in the previous assay (Fig. 2A). Binding of both commercial and plasmid-derived CtB was inhibited by the presence of DTT (Fig. 2B). This observation indicates that the CtB produced by the plasmid is biologically active.
FIG. 2.
GM1 ganglioside binding of CtB produced by plasmids pCtB and pL1tB. (A) Increasing concentrations of either whole-cell protein extracts from cells transfected with pCtB (closed circles), pL1tB (open squares), or commercial CtB (closed squares) were tested by a GM1 ELISA. Protein extracts from nontransfected cells (open circles) were used as negative controls. (B) The biological activity of plasmid-produced CtB was analyzed by a GM1 ganglioside-binding assay. A total of 500 ng of protein extracts from cells transfected with plasmid pCtB or pL1tB was tested in the presence or absence of DTT, as indicated. Commercial CtB (100 ng) was included as a positive control. Each point represents the mean of four independent assays. Error bars represent the standard errors of the means. O.D., optical density.
Evaluation of mucosal antibody responses.
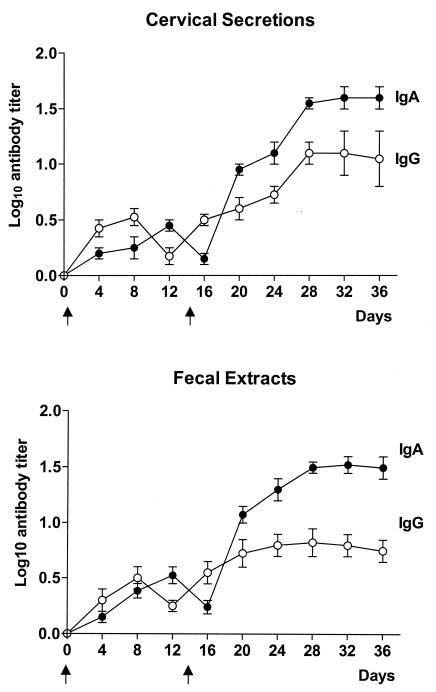
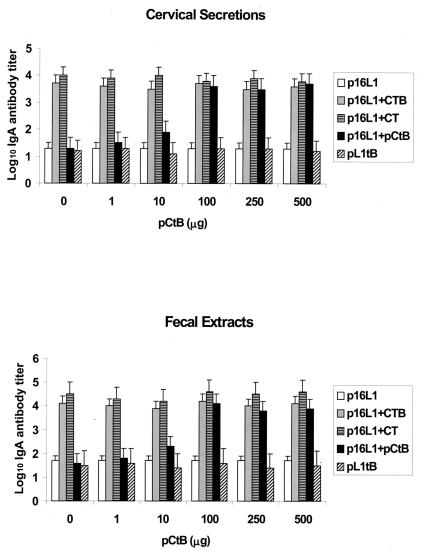
Antibodies produced on mucosal surfaces are believed to be the major line of defense against viruses infecting mucosal membranes. HPV-16 infects the anogenital mucosa. Therefore, we first investigated the development of mucosal antibodies in cervical secretions and fecal extracts after intradermal administration of a DNA vaccine against HPV-16. Specific antibodies were not detected in PBS-vaccinated controls (data not shown). Figure 3 shows that IgA antibodies were produced in mice after the initial vaccination. The level of antibodies increased following a boosting inoculation, reaching maximum levels in cervical secretions and fecal extracts at days 28 and 32, respectively. IgG antibodies were produced concurrently with the IgA antibody response. Maximal titers of IgG antibodies were detected at day 28, although the magnitude of the response was significantly lower than that of IgA antibodies (for cervical secretions, P = 0.001; for fecal extracts, P = 0.0001). Our results indicate that vaccination with p16L1 mainly elicits a mucosal IgA response. Therefore, we next evaluated the adjuvant potential of pCtB for the induction of IgA antibodies in cervical secretions and fecal extracts of vaccinated mice. Mice were vaccinated with p16L1 alone or with p16L1 coadministered with commercial CtB, CT, or different concentrations of pCtB. A group of mice was immunized with plasmid pL1tB. As expected, the presence of commercial CtB polypeptide or CT protein induced a significant increment of IgA titers in both cervical secretions (P < 0.0001) and fecal extracts (P < 0.0001) (Fig. 4). A dose-dependent adjuvant effect of pCtB was observed. Coadministration of 100 μg of pCtB prompted a significant increment in the mean antibody titer induced by plasmid p16L1 alone in both cervical secretions (P < 0.0001) and fecal extracts (P < 0.0001). At this concentration, pCtB demonstrated an adjuvant capacity comparable to those of the CtB polypeptide and the CT protein (Fig. 4).
FIG. 3.
Kinetics of IgA and IgG responses in cervical secretions and fecal extracts after intradermal immunization with plasmid p16L1. C57BL/6 mice were immunized with 100 μg of plasmid p16L1 at the indicated times (arrows). Cervical secretions and fecal extracts were obtained every 4 days and were assayed for the presence of anti-L1 IgA and IgG antibodies by ELISA. Each point represents the mean for 10 mice assayed independently. Error bars indicate standard errors of the means.
FIG. 4.
Adjuvant effect of plasmid pCtB. Mice were vaccinated with 100 μg of p16L1 in the presence and absence of the following adjuvants: commercial CTB polypeptide (p16L1 + CTB), commercial CT (p16L1 + CT), or different concentrations of plasmid pCtB (p16L1 + pCtB), as indicated. A group of mice received 100 μg of plasmid pL1tB. All mice were given a booster immunization at day 14. IgA responses were measured in cervical secretions and fecal extracts collected at day 28. Bars represent the mean values for 10 mice assessed independently. Error bars indicate the standard errors of the means.
It has been demonstrated that bacterial elements of plasmid vectors, such as CpG sequences, have adjuvant effects (23). Therefore, to address the question of whether the adjuvant effect observed was due to these bacterial components, we constructed plasmid pL1tB, which drives the expression of a biologically inactive L1-CtB fusion. Accordingly, the IgA response elicited by the pL1tB fusion plasmid was significantly lower than that produced by coadministration of pL1tB and pCtB, CtB, or CT (P < 0.0001). In fact, the response elicited by pL1tB was similar to that produced by immunization with p16L1 alone (P > 0.05). These results indicate that the adjuvant effect was due to the presence of functional CtB expressed by pCtB rather than to bacterial adjuvants.
It is known that CtB is a potent mucosal immunogen that induces strong urogenital antibody responses in humans (33, 34). Thus, to determine if vaccination with pCtB was able to induce mucosal antibody responses in mice and to investigate if the presence of anti-CtB antibodies could affect the response against the coadministered antigen, mice were immunized with adjuvant pCtB, CtB, or CT alone or with each adjuvant together with plasmid p16L1. The titers of antibodies against L1 and CtB were evaluated. As shown in Table 1, IgA antibodies against CtB were detected in all vaccinated animals. Nevertheless, antibodies against L1 were observed only in animals immunized with p16L1 and an adjuvant. Interestingly, anti-L1 antibody titers remained high regardless of the presence of anti-CtB antibodies. This observation suggests that in our model intrinsic CtB immunogenicity does not alter the production of antibodies against the product of the coadministered plasmid DNA.
TABLE 1.
Evaluation of induction of mucosal antibodies against CtB and L1 by plasmid vaccination
| Inoculum | Log10 titer(P valuea)
|
|
|---|---|---|
| IgA anti-L1 | IgA anti-CTB | |
| Cervical secretions | ||
| pCtB | 0.5 | 1.9 |
| pCtB + p16L1 | 3.2 (0.001) | 1.8 (0.2) |
| CtB | 0.4 | 2.6 |
| CtB + p16L1 | 3.5 (0.0008) | 2.4 (0.07) |
| CT | 0.5 | 3.5 |
| CT + p16L1 | 4.1 (0.0005) | 3.1 (0.05) |
| Fecal extracts | ||
| pCtB | 0.3 | 2.1 |
| pCtB + p16L1 | 3.5 (0.004) | 1.8 (0.1) |
| CtB | 0.2 | 3.8 |
| CtB + p16L1 | 4.0 (0.0007) | 3.4 (0.07) |
| CT | 0.2 | 4.0 |
| CT + p16L1 | 4.9 (0.001) | 4.4 (0.07) |
P values were calculated by Student's t test to compare antibody titers induced by vaccination with the different adjuvants alone versus vaccination with the adjuvants plus plasmid p16L1.
Analysis of cell-mediated immune responses.
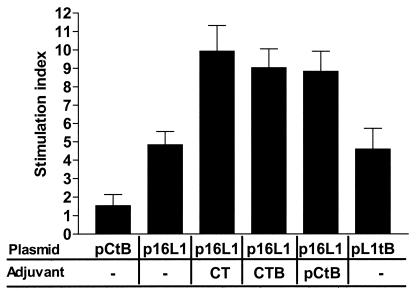
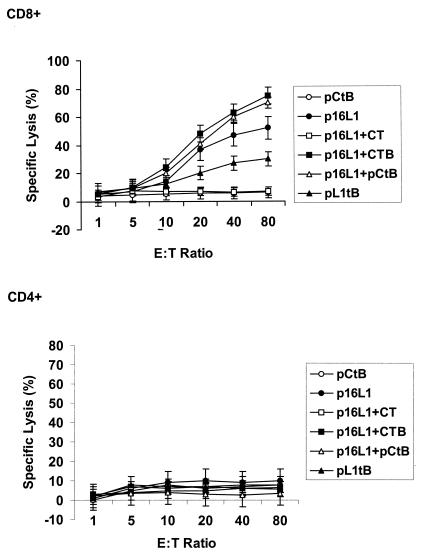
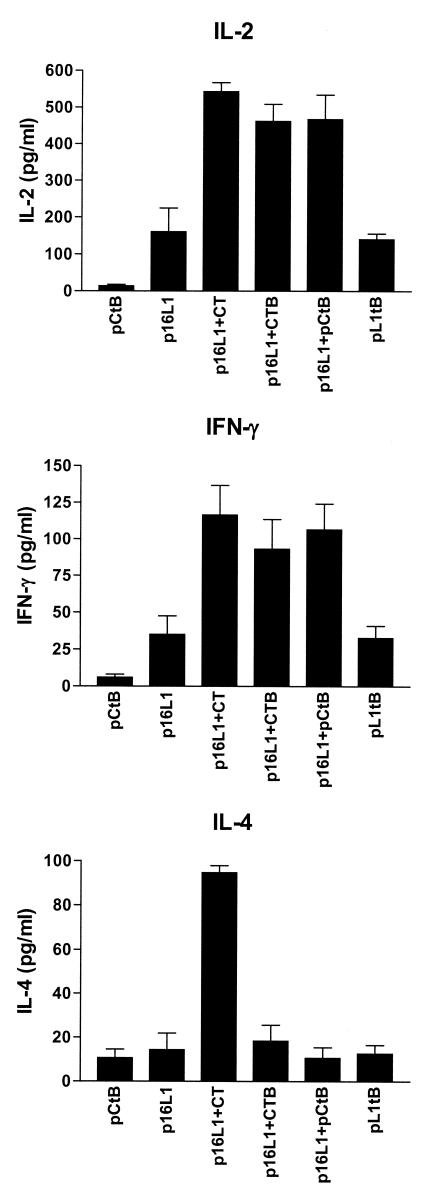
To assess the effect of pCtB on the generation of a cell-mediated immune response, we first evaluated the specific proliferation of lymphocytes derived from vaccinated mice. Spleen cells were isolated after intradermal vaccination. The lymphoproliferative responses after restimulation with HPV-16 VLPs are shown in Fig. 5. Vaccination with plasmid p16L1 or pL1tB induced a proliferative response significantly higher than that observed in pCtB-immunized mice (P < 0.01). Lymphoproliferation was significantly increased by the presence of both commercial and plasmid adjuvants (P < 0.0001). This observation indicates that CtB potentiates not only antibodies but also T-cell immune responses. We have previously demonstrated that DNA vaccination evokes a CD8+-T-cell response against cells expressing L1 (32). To examine the potential effect of the coadministration of pCtB on CTL activity, we evaluated the cytolysis of target cells expressing L1 by CD4+ and CD8+ lymphocytes from immunized and control mice using a nonradioactive assay. Lysis of L1-expressing cells was not mediated by CD4+ lymphocytes (Fig. 6). On the other hand, specific lysis of target cells by CD8+ lymphocytes was not observed in mice immunized with PBS or pCtB alone (Fig. 6). A low level of CD8+-mediated CTL activity was observed in mice immunized with fusion plasmid pL1tB. As expected, CTL activity was induced by immunization with p16L1. Interestingly, a significant increase in the level of specific lysis was observed in mice that received p16L1 coadministered with either pCtB (P < 0.005) or commercial CtB (P < 0.004). Our results show that CTL activity is mediated by CD8+ cells and that it is augmented by the presence of pCtB. We next investigated the type of Th response induced by the vaccine with an adjuvant. Vaccination with p16L1 or pL1tB caused moderate levels of secretion of IL-2 and IFN-γ (Fig. 7), which seems to be in accordance with the modest CTL response observed. The levels of production of IL-2 and IFN-γ were significantly increased in spleen cells from mice vaccinated with p16L1 and pCtB (P < 0.005), CtB (P < 0.005), or CT (P < 0.001). In contrast, neither pCtB nor CtB induced the secretion of IL-4 (Fig. 7). These results suggest that DNA vaccination against L1 antigen induces a Th1-type response concomitant with CD8+ CTL activity and that both responses are enhanced by coadministration of pCtB. Interestingly, coadministration of CT stimulated the production of IL-4, suggesting that the holotoxin is able to activate both Th1- and Th2-type responses.
FIG. 5.
Specific lymphoproliferative response induced by intradermal coadministration of p16L1 with adjuvants. Mice were given two intradermal immunizations with pCtB, pL1tB, or p16L1 alone or coadministered with either CT, CTB, or pCtB, as indicated. Spleen cells were collected 14 days after the second immunization. Proliferation was assessed after restimulation with HPV-16 VLPs. The results express the mean values for 10 mice per group and are representative of two independent experiments. Error bars indicate the standard errors of the means.
FIG. 6.
Induction of CTL activity by the DNA vaccine with adjuvants. Spleen cells were obtained from mice vaccinated with p16L1, pL1tB, pCtB, or a combination of p16L1 with pCtB or commercial CTB and CT. Splenocytes were stimulated with HPV-16 L1 antigen for 5 days, CD4+ or CD8+ lymphocytes were depleted, and the remaining cells were tested for CTL activities. Specific lysis of HPV-16 L1-expressing B16FO cells was evaluated at different effector cell/target cell (E:T) ratios, as indicated. Data represent the mean values for 10 mice per group assayed individually and are representative of two independent experiments. Error bars represent the standard errors of the means.
FIG. 7.
Production of cytokines by spleen cells from vaccinated mice. Spleen cells were isolated from mice vaccinated with pCtB, pL1tB, or p16L1 alone and in combination with pCtB or commercial CTB and CT. Cells were stimulated with HPV-16 L1 antigen for 48 h; and the production of IL-2, IFN-γ, and IL-4 was evaluated in spleen cell cultures by quantitative ELISA. Error bars represent the standard errors of the means of duplicate experiments.
DISCUSSION
In the present work we have investigated the effect of the coadministration of the CtB-coding sequence on humoral and cellular immune responses after intradermal immunization with a DNA vaccine. The results indicate that the inclusion of pCtB enhances not only the humoral mucosal response but also the response mediated by Th1-type cells and CTLs.
Commercial CtB polypeptide or CT holotoxin was used as the positive control in all experiments. They induced significant increments in fecal and cervical IgA antibody titers compared with the antibody titers in animals vaccinated with plasmid p16L1 alone. The capacity of CtB to induce high levels of serum IgG (4, 9, 10, 19, 38), fecal IgA (19, 20), and cervical IgA (14, 38) antibodies against different antigens has been demonstrated extensively. However, the effects of CtB and CT as adjuvants for a DNA vaccine have been less well documented. CT has been administered intranasally before DNA vaccination (21) and as an intramuscular booster (39) or an intranasal booster (44). Additionally, coadministration of CT has been demonstrated to increase the specific IgG (8) and mucosal IgA (24) responses mediated by DNA vaccines. However, most of those studies focused on the use of the whole CT. Here we demonstrated that the use of CtB subunit alone during both priming and booster intradermal immunizations strongly enhances the immune responses mediated by a DNA vaccine. In comparison with whole CT, which is a powerful toxin in humans, the CtB subunit is considered safe for administration to humans (17, 18); therefore, it may be a suitable adjuvant for DNA vaccines meant to activate human mucosal immunity.
DNA vaccines may afford a series of advantages over traditional vaccines, including greater stability. To be in consonance, the adjuvants used for DNA vaccines should offer equal advantages. Accordingly, the use of plasmids encoding molecules with demonstrated adjuvant capacities would be a suitable alternative to improve the efficacies of DNA vaccines without decreasing their advantages for commercial development. Previous work (32) indicated that application of an L1 gene-based DNA vaccine was able to induce genital IgA antibodies. Here we demonstrated that coadministration of a plasmid containing the CtB-coding sequence significantly increased specific IgA antibody titers in cervical secretions and fecal extracts. Earlier studies have established that DNA vaccine-mediated IgG responses can be enhanced by the use of plasmid vectors encoding the CT A and B subunits (2) or expressing the catalytic A subunit of CT (3). However, the adjuvant capacity of the CtB sequence alone for mucosal IgA responses has not been previously documented. The adjuvant effect of CtB for IgA antibodies observed herein might be associated with the capacity of CtB to stimulate isotype switching (27, 42). Interestingly, experimental evidence shows that induction of IgA switching by CtB depends on the presence of IL-2 as a cofactor (22). Accordingly, in this work we found that coadministration of pCtB prompted the production of IL-2 by antigen-stimulated spleen cells. Hence, we assume that enhancement of IL-2 production might be assisting IgA switching in our model. To corroborate this hypothesis the study of other factors involved in CtB-mediated IgA switching, such as transforming growth factor β1 (22), is warranted.
Bacterial vector backbones usually contain CpG motifs, which are acknowledged to possess immunostimulatory properties (23). Interestingly, it has been documented that the addition of either CpG or CT produces a similar specific fecal IgA response (19). Thus, to address the question of whether the adjuvant effect observed in this work was due to the presence of CpG sequences rather than to the CtB gene, we constructed a translational fusion plasmid to drive the expression of the L1 protein fused to the N terminus of the CtB polypeptide. The fused genes were properly transcribed and translated, but the fusion protein was demonstrated to have lost its capacity to bind to GM1 ganglioside. Concurrently with this, it has been demonstrated that fusion of oligonucleotides to the N-terminal fragment of the CtB gene not only modifies the affinity of CtB for GM1 ganglioside but also decreases the oral immunogenicity of the B subunit, suggesting a loss of biological activity (11). In accordance with this, we observed that coadministration of the fusion plasmid failed to induce an adjuvant effect, indicating that the enhancement of the immune reaction was not a consequence of the presence of bacterial CpG motifs.
Along with the IgA response, addition of pCtB had an adjuvant effect on specific lymphoproliferative responses, together with the production of IL-2 and IFN-γ by stimulated T cells, but had no effect on the production of IL-4, which suggests the development of a Th1-type cellular response. Unlike pCtB and CtB, CT stimulated the production of IL-4. Our observations are in agreement with those from recent work in which coadministration of CtB onto skin favored the development of a vigorous Th1 cellular response (1), while the CT holotoxin was associated with the induction of a Th2-type response (13, 43). Nevertheless, more recent findings propose that Th1-Th2 polarization may depend not only on the adjuvant but also on the antigen coadministered (36). Consistent with this, administration of the HPV-16 L1 antigen, in the form of VLPs, prompts both Th1 and Th2 responses in mice (28), chimpanzees (29), and humans (31). In contrast, immunization of rhesus macaques with a plasmid DNA expressing the HPV-16 L1 antigen favors a strong Th1 response (41). The latter observation coincides with our finding that vaccination with plasmid p16L1 induces Th1 responses but seems to contradict the idea of the antigen as a regulator of the Th1-Th2 profile. Instead, our data appear to indicate that, at least in this case, the vaccine delivery system in combination with the adjuvant, rather than the antigen, balances the Th1-Th2 profile toward a Th1 response. This is an interesting observation, since it has been established that a Th1 cytokine pattern is strongly associated with the natural clearance of cervical HPV infections in women (37). Thus, we consider that the potential of p16L1 with pCtB as an adjuvant as a tool for the elimination of already established infections deserves further studies.
Cytotoxic cell-mediated immunity is likely to play a central role in eliminating HPV-infected cells, hindering disease progression. A previous study (32) provided evidence that DNA vaccination induces the generation of a cytotoxic response mediated by CD8+ cells. Here we found that coadministration of pCtB enhances such a cytotoxic response. To our knowledge, there are no previous reports on the enhancing effect of the CtB gene on CD8+-cytotoxic-cell activity mediated by DNA vaccination. In an earlier work (2) an increase in CTL responses was reported to be mediated by coadministration of the A and B subunit-coding sequences together; however, the effect of the CtB gene alone was not tested. Inasmuch as IL-2 is known to stimulate activation of CD8+ cells, the adjuvant effect of pCtB on CD8+ CTL responses may be associated with the higher concentrations of IL-2 produced in mice immunized with the DNA vaccine and pCtB. However, we cannot underestimate the participation of other cytokines; hence, a better characterization of the stimulatory molecules produced as a response to the vaccine would be necessary to understand the mechanism involved in CTL activation.
The mechanisms by which plasmid DNA is expressed and the gene product is presented to the immune system are not completely understood. It has been proposed that a small number of professional APCs are directly transfected with the injected DNA (30). Transfected APCs may traffic to regional lymphoid tissue, where they can activate B and T cells. Accordingly, it has been reported that plasmid DNA can be isolated from lymph node-derived and skin-derived dendritic cells after intradermal immunization (6). These observations lead us to assume that the adjuvant effect of pCtB might be mediated by the transfection of dermal APCs, which might have expressed the gene and transported the product to lymphoid tissue, where it might have interacted with its receptor on B and T cells, inducing the effects described. Further experiments are being conducted to determine the feasibility of the proposed mechanism.
In conclusion, our results indicate that the CtB-coding sequence can be used as an adjuvant to enhance immune responses to intradermally coadministered DNA vaccines. In particular, we have demonstrated that the adjuvant effect elicited by plasmid pCtB is comparable to that elicited by the CtB polypeptide. Therefore, pCtB is a good candidate as a genetic adjuvant of DNA vaccines meant to target the mucosal and cellular immune systems.
Acknowledgments
We thank John Schiller (National Institutes of Health) for the donation of recombinant baculovirus, Georgina Diaz Herrera for excellent technical assistance, and Isabel Perez Montfort for reviewing the English version of the manuscript.
This study was partially supported by DGAPA, National University of Mexico (grant IN225798).
REFERENCES
- 1.Anjuere, F., A. George-Chandy, F. Audant, D. Rousseau, J. Holmgren, and C. Czerkinsky. 2003. Transcutaneous immunization with cholera toxin B subunit adjuvant suppresses IgE antibody responses via selective induction of Th1 immune responses. J. Immunol. 170:1586-1592. [DOI] [PubMed] [Google Scholar]
- 2.Arrington, J., R. P. Braun, L. Dong, D. H. Fuller, M. D. Macklin, S. W. Umlauf, S. J. Wagner, M. S. Wu, L. G. Payne, and J. R. Haynes. 2002. Plasmid vectors encoding cholera toxin or the heat-labile enterotoxin from Escherichia coli are strong adjuvants for DNA vaccines. J. Virol. 76:4536-4546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Bagley, K. C., M. T. Shata, D. Y. Onyabe, A. L. DeVico, T. R. Fouts, G. K. Lewis, and D. M. Hone. 2003. Immunogenicity of DNA vaccines that direct the coincident expression of the 120kDa glycoprotein of human immunodeficiency virus and the catalytic domain of cholera toxin. Vaccine 21:3335-3341. [DOI] [PubMed] [Google Scholar]
- 4.Bergquist, C., T. Lagergard, M. Lindblad, and J. Holmgren. 1995. Local and systemic antibody responses to dextran-cholera toxin B subunit conjugates. Infect. Immun. 63:2021-2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Biet, F., L. Kremer, I. Wolowczuk, M. Delacre, and C. Locht. 2003. Immune response induced by recombinant Mycobacterium bovis BCG producing the cholera toxin B subunit. Infect. Immun. 71:2933-2937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Casares, S., K. Inaba, T. D. Brumeanu, R. M. Steinman, and C. A. Bona. 1997. Antigen presentation by dendritic cells after immunization with DNA encoding a major histocompatibility complex class II-restricted viral epitope. J. Exp. Med. 186:1481-1486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cubie, H. A., M. Plumstead, W. Zhang, O. de Jesus, L. A. Duncan, and M. A. Stanley. 1998. Presence of antibodies to human papillomavirus-like particles (VLPs) in 11-13-year-old schoolgirls. J. Med. Virol. 56:210-216. [PubMed] [Google Scholar]
- 8.Cui, Z., and R. J. Mumper. 2003. The effect of co-administration of adjuvants with a nanoparticle-based genetic vaccine delivery system on the resulting immune responses. Eur. J. Pharm. Biopharm. 55:11-18. [DOI] [PubMed] [Google Scholar]
- 9.Czerkinsky, C., M. W. Russell, N. Lycke, M. Lindblad, and J. Holmgren. 1989. Oral administration of a streptococcal antigen coupled to cholera toxin B subunit evokes strong antibody responses in salivary glands and extramucosal tissues. Infect. Immun. 57:1072-1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dertzbaugh, M. T., and C. O. Elson. 1993. Comparative effectiveness of the cholera toxin B subunit and alkaline phosphatase as carriers for oral vaccines. Infect. Immun. 61:48-55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dertzbaugh, M. T., and C. O. Elson. 1993. Reduction in oral immunogenicity of cholera toxin B subunit by N-terminal peptide addition. Infect. Immun. 61:384-390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Elson, C. O. 1989. Cholera toxin and its subunits as potential oral adjuvants. Immunol. Today 146:29-33. [DOI] [PubMed] [Google Scholar]
- 13.Gagliardi, M. C., F. Sallusto, M. Marinaro, A. Langenkamp, A. Lanzavecchia, and M. T. De Magistris. 2000. Cholera toxin induces maturation of human dendritic cells and licences them for Th2 priming. Eur. J. Immunol. 30:2394-2403. [DOI] [PubMed] [Google Scholar]
- 14.Haneberg, B., D. Kendall, H. M. Amerongen, F. M. Apter, J. P. Kraehenbuhl, and M. R. Neutra. 1994. Induction of specific immunoglobulin A in the small intestine, colon-rectum, and vagina measured by a new method for collection of secretions from local mucosal surfaces. Infect. Immun. 62:15-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Holmgren, J., N. Lycke, and C. Czerkinsky. 1993. Cholera toxin and cholera B subunit as oral-mucosal adjuvant and antigen vector systems. Vaccine 11:1179-1184. [DOI] [PubMed] [Google Scholar]
- 16.Isaka, M., Y. Yasuda, M. Mizokami, S. Kozuka, T. Taniguchi, K. Mayano, J.-I. Maeyama, K. Mizuno, K. Morokuma, K. Ohkuma, N. Goto, and K. Tochikubo. 2001. Mucosal immunization against hepatitis B virus by intranasal co-administration of recombinant hepatitis surface antigen and recombinant cholera toxin B subunit as an adjuvant. Vaccine 19:1460-1466. [DOI] [PubMed] [Google Scholar]
- 17.Jertborn, M., A. M. Svennerholm, and J. Holmgren. 1992. Safety and immunogenicity of an oral recombinant cholera B subunit-whole cell vaccine in Swedish volunteers. Vaccine 10:130-132. [DOI] [PubMed] [Google Scholar]
- 18.Jertborn, M., I. Nordstrom, A. Kilander, C. Czerkinsky, and J. Holmgren. 2001. Local and systemic immune responses to rectal administration of recombinant cholera toxin B subunit in humans. Infect. Immun. 69:4125-4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jiang, W., H. J. Baker, and B. F. Smith. 2003. Mucosal immunization with helicobacter, CpG DNA, and cholera toxin is protective. Infect. Immun. 71:40-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.John, M., E. A. Bridges, A. O. Miller, S. B. Calderwood, and E. T. Ryan. 2002. Comparison of mucosal and systemic humoral immune responses after transcutaneous and oral immunization strategies. Vaccine 20:2720-2726. [DOI] [PubMed] [Google Scholar]
- 21.Kadowaki, S., Z. Chen, H. Asanuma, C. Aizawa, T. Kurata, and S. Tamura. 2000. Protection against influenza virus infection in mice immunized by administration of hemagglutinin-expressing DNAs with electroporation. Vaccine 18:2779-2788. [DOI] [PubMed] [Google Scholar]
- 22.Kim, P.-H., L. Eckmann, W. J. Lee, W. Han, and M. F. Kagnoff. 1998. Cholera toxin and cholera toxin B subunit induce IgA switching through the action of TGF-β1. J. Immunol. 160:1198-1203. [PubMed] [Google Scholar]
- 23.Krieg, A. M., A. K. Yi, S. Matson, T. J. Waldschmidt, S. A. Bishop, R. Teasdale, G. A. Koretzky, and D. M. Klinman. 1995. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature (London) 374:546-549. [DOI] [PubMed] [Google Scholar]
- 24.Kuklin, N., M. Daheshia, K. Karem, E. Manickan, and B. T. Rouse. 1997. Induction of mucosal immunity against herpes simplex virus by plasmid DNA immunization. J. Virol. 71:3138-3145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lockman, H., and J. B. Kaper. 1983. Nucleotide sequence analysis of the A2 and B subunits of Vibrio cholerae enterotoxin. J. Biol. Chem. 258:13722-13726. [PubMed] [Google Scholar]
- 26.Lycke, N., and J. Holmgren. 1986. Strong adjuvant properties of cholera toxin on gut mucosal immune responses to orally presented antigens. Immunology 59:301-308. [PMC free article] [PubMed] [Google Scholar]
- 27.Lycke, N., and W. Strober. 1989. Cholera toxin promotes B cell isotype differentiation. J. Immunol. 142:3781-3787. [PubMed] [Google Scholar]
- 28.Marais, D., J. A. Passmore, J. Maclean, R. Rose, and A. L. Williamson. 1999. A recombinant human papillomavirus (HPV) type 16 L1-vaccinia virus murine challenge model demonstrates cell-mediated immunity against HPV virus-like particles. J. Gen. Virol. 80:2471-2475. [DOI] [PubMed] [Google Scholar]
- 29.Palker, T. J., J. M. Monteiro, M. M. Martin, C. Kakareka, J. F. Smith, J. C. Cook, J. G. Joyce, and K. U. Jansen. 2001. Antibody, cytokine and cytotoxic T lymphocyte responses in chimpanzees immunized with human papillomavirus virus-like particles. Vaccine 19:3733-3743. [DOI] [PubMed] [Google Scholar]
- 30.Pardoll, D. M., and A. M. Beckerleg. 1995. Exposing the immunology of naked DNA vaccines. Immunity 3:165-169. [DOI] [PubMed] [Google Scholar]
- 31.Pinto, L. A., J. Edwards, P. E. Castle, C. D. Harro, D. R. Lowy, J. T. Schiller, D. Wallace, W. Kopp, J. W. Adelsberger, M. W. Baseler, J. A. Berzofsky, and A. Hildesheim. 2003. Cellular immune responses to human papillomavirus (HPV)-16 L1 in healthy volunteers immunized with recombinant HPV-16 L1 virus-like particles. J. Infect. Dis. 188:327-328. [DOI] [PubMed] [Google Scholar]
- 32.Rocha-Zavaleta, L., J. E. Alejandre, and A. Garcia-Carranca. 2002. Parenteral and oral immunization with a plasmid DNA expressing the human papillomavirus 16-L1 gene induces systemic and mucosal antibodies and cytotoxic T lymphocyte responses. J. Med. Virol. 66:86-95. [DOI] [PubMed] [Google Scholar]
- 33.Rudin, A., E. L. Johansson, C. Bergquist, and J. Holmgren. 1998. Differential kinetics and distribution of antibodies in serum and nasal and vaginal secretions after nasal and oral vaccination of humans. Infect. Immun. 66:3390-3396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rudin, A., G. C. Riise, and J. Holmgren. 1999. Antibody responses in the lower respiratory tract and male urogenital tract in humans after nasal and oral vaccination with cholera toxin B subunit. Infect. Immun. 67:2884-2890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sack, D. A., S. Huda, P. K. Neogi, R. R. Daniel, and W. M. Spira. 1980. Microtiter ganglioside enzyme-linked immunosorbent assay for vibrio and Escherichia coli heat-labile enterotoxins and antitoxin. J. Clin. Microbiol. 11:35-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Sasaki, K., M. Kato, T. Takahashi, S. Ochi, Y. Ichinose, K. Shiraki, Y. Asano, M. Iwanaga, and T. Tsuji. 2003. Live varicella vaccine polarizes the mucosal adjuvant action of cholera toxin or its B subunit on specific Th1-type helper T cells with a single nasal coadministration in mice. J. Med. Virol. 70:329-335. [DOI] [PubMed] [Google Scholar]
- 37.Scott, M., D. P. Stites, and A.-B. Moscicki. 1999. Th1 cytokine patterns in cervical human papillomavirus infection. Clin. Diagn. Lab. Immunol. 6:751-755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shen, X., T. Lagergard, Y. Yang, M. Lindblad, M. Fredriksson, and J. Holmgren. 2000. Systemic and mucosal immune responses in mice after mucosal immunization with group B streptococcus type III capsular polysaccharide-cholera toxin B subunit conjugated vaccine. Infect. Immun. 68:5749-5755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Stratford, R., G. Douce, F. Bowe, and G. Dougan. 2001. A vaccination strategy incorporating DNA priming and mucosal boosting using tetanus toxin fragment (TeTC). Vaccine 20:516-525. [DOI] [PubMed] [Google Scholar]
- 40.Svennerholm, A. M., J. Holmgren, R. Black, M. Levine, and M. Merson. 1983. Serologic differentiation between antitoxin responses to infection with Vibrio cholerae and enterotoxin-producing Escherichia coli. J. Infect. Dis. 147:514-522. [DOI] [PubMed] [Google Scholar]
- 41.Tobery, T. W., J. F. Smith, N. Kuklin, D. Skulsky, C. Ackerson, L. Huang, L. Chen, J. C. Cook, W. L. McClements, and K. U. Jansen. 2003. Effect of vaccine delivery system on the induction of HPV-16L1-specific humoral and cell-mediated immune responses in immunized rhesus macaques. Vaccine 21:1539-1547. [DOI] [PubMed] [Google Scholar]
- 42.Whitmore, A. C., D. M. Prowse, G. Haughton, and L. W. Arnold. 1991. Ig isotype switching in B lymphocytes: the effect of T cell-derived interleukins, cytokines, cholera toxin, and antigen on isotype switch frequency of a cloned B cell lymphoma. Int. Immunol. 3:95-103. [DOI] [PubMed] [Google Scholar]
- 43.Xu-Amano, J., H. Kiyono, R. J. Jackson, H. F. Staats, K. Fujihashi, P. D. Burrows, C. O. Elson, S. Pillai, and J. R. McGhee. 1993. Helper T cell subsets for immunoglobulin A responses: oral immunization with tetanus toxoid and cholera toxin as adjuvant selectively induces Th2 cells in mucosa associated tissues. J. Exp. Med. 178:1309-1320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yoshizawa, I., Y. Soda, T. Mizuochi, S. Yasuda, T. A. Rizvi, T. Mizuochi, T. Takemori, and Y. Tsunetsugu-Yokota. 2001. Enhancement of mucosal immune response against HIV-1 Gag by DNA immunization. Vaccine 19:2995-3003. [DOI] [PubMed] [Google Scholar]