Abstract
The development of a nosocomial pneumonia is facilitated by alterations in host innate pulmonary antibacterial defenses following surgical trauma, which can result in decreased pulmonary bacterial clearance and increased morbidity and mortality. In a murine model of postoperative nosocomial infection, surgical stress (laparotomy) decreased Escherichia coli clearance from the lungs of animals that underwent surgery. Consistent with previous studies, (i) pulmonary levels of tumor necrosis factor alpha at 6 h and of interleukin-1β (IL-1β), IL-6, and gamma interferon (IFN-γ) at 24 h post-bacterial infection (PBI) were decreased in animals that underwent laparotomy 24 h prior to E. coli infection (LAP/E. coli) compared to animals that received E. coli only; (ii) KC and macrophage inhibitory protein 2 were elevated at 6 h PBI in LAP/E. coli animals compared to E. coli-only animals; however, at 24 h PBI, levels were higher in the E. coli-only group; (iii) at 24 h PBI, monocyte chemoattractant protein 1 was lower in the LAP/E. coli group compared to the E. coli-only group; (iv) IL-10 levels were unaffected at all time points evaluated; and (v) the total number of neutrophils present in the lungs of LAP/E. coli animals at 6 h PBI was decreased in comparison to that in E. coli-only animals, resulting in decreased bacterial clearance and increased mortality in LAP/E. coli animals by 24 h PBI. Similar changes in cytokine profiles, pulmonary bacterial clearance, and mortality were consistent with reported findings in patients following surgical trauma. This model, therefore, provides a clinically relevant system in which the molecular and cellular mechanisms that lead to the development of nosocomial pneumonia can be further explored.
Nosocomial bacterial pneumonia is the second most common hospital-acquired infection (2, 40, 59) and is the leading cause of infection in hospital intensive care units (2, 40), accounting for anywhere from 31 to 47% of all intensive care unit nosocomial infections (72). Of even greater importance is that nosocomial pneumonia is the leading cause of death among all nosocomial infections (40). The most commonly cited pathogens responsible for nosocomial pneumonia are Staphylococcus aureus, Pseudomonas aeruginosa, Enterobacter species, Escherichia coli, and Klebsiella pneumoniae (2, 58).
Normal host defense against lung bacterial infection includes both innate and acquired immune responses (71). The primary function of the innate immune response is the elimination of foreign particles deposited on the surface of the airways and the rapid clearance of pathogens from the alveoli (68). Innate immunity includes pattern recognition molecules and receptors, complement (alternative and mannan-binding lectin pathways), antimicrobial peptides (such as defensins), leukocytes (e.g., neutrophils and monocytes/macrophages), and cytokines produced by leukocytes (71).
The first line of phagocytic defense against pathogens that gain access to the gas-exchanging airways is the alveolar macrophages (66). Pathogen-activated phagocytes, in turn, produce cytokines (proinflammatory, modulatory, and antiinflammatory) which have a critical role in the localization, reinforcement, and resolution of the host defense response (68). The cytokines secreted by phagocytes in response to infection include tumor necrosis factor alpha (TNF-α), interleukin-1β (IL-1β), IL-10, IL-12 (which stimulates gamma interferon [IFN-γ] from lymphocytes), and members of the C-X-C (IL-8 in patients or macrophage inhibitory protein 2 [MIP-2] and KC in mice) and C-C (i.e., monocyte chemoattractant protein 1 [MCP-1]) chemokine families (45). While the generation of this cytokine cascade is critical for clearance of microbial agents, a modulatory response is equally important to ensure that the intense acute inflammation does not cause too much damage to the host (23, 57).
Injury, either surgical or traumatic, is associated with acute impairment of the immune response (65), which is believed to be a major factor in the development of nosocomial pneumonia (1). Recent studies have shown that this impairment is characterized, in part, by suppressed macrophage regulatory and effector functions (14, 38), resulting in alterations in cytokine production and in the host's ability to clear bacterial pathogens from the pulmonary airspace (27, 47, 53, 54).
Within hours after surgery or trauma to tissue, an intense proinflammatory reaction occurs within the lungs of injured patients and is characterized by local production of proinflammatory cytokines (27, 38, 47) followed by an influx of activated neutrophils (30). IL-6, a mediator of the acute-phase response to injury and infection, is elevated postoperatively and has been reported to be directly related to the degree of tissue injury (4), while an increased production of TNF-α following surgery is considered to reflect the degree of surgical stress (3). Kato et al. (33) have also reported elevated plasma levels of IL-6, IL-8, and granulocyte colony-stimulating factor both during and after major abdominal surgery. However, during the early phase of the postoperative course (days 1 and 2 following a major surgery), the production of cytokines associated with the Th1 phenotype of CD4 T cells is dramatically impaired (7, 25, 26), with reductions in TNF-α, IFN-γ, and IL-2 ranging anywhere from 37 to 55%. Subsequently, during the late phase of the postoperative course (days 3 to 5), production of IL-2 and IFN-γ increased to preoperative levels, while TNF-α was still suppressed (26). In addition, patients with a profile of postoperative elevated IL-8 levels and increasing levels of IL-10 were more likely to develop a nosocomial pneumonia (47, 64). Prior to surgical incision, in patients receiving total intravenous anesthesia, levels of IL-1β, IL-4, IL-6, TNF-α, and IFN-γ did not differ from preinduction levels (25). However, by 24 h postoperatively, IL-1β, IL-4, IL-6, and TNF-α showed marked decreases. Our laboratory has also shown that even the stress of anesthesia can alter innate pulmonary cytokine responses (41, 56). However, anesthesia alone (i.e., halothane) does not appear to affect baseline phagocytic properties of monocytes (69).
Numerous studies have demonstrated that an unbalanced differentiation of Th1 and Th2 cells during an immune response may result in severely impaired defense against a variety of pathogens (61). Functional analysis of T lymphocytes obtained from patients after major surgery demonstrated impaired cytokine secretion involving both Th1- and Th2-type cytokines, including IFN-γ, IL-2, and IL-4. IL-10, while not suppressed early on postoperatively, was increased late after surgery, suggesting that the defect in T-cell cytokine secretion may be characterized by the production of the antiinflammatory cytokine IL-10 rather than by a strict differentiation to a Th1 or Th2 phenotype (10).
Decreased production of IL-1and IL-2 and increased monocyte prostaglandin E2 production after trauma have been suggested as two of the major monocyte dysfunctions that contribute to alterations in immune system responsiveness (16, 17, 44). Following injury or major surgery, elevated levels of prostaglandin E2 in monocytes have been shown to suppress monocyte antigen-presenting capacity and T-cell expression of IL-2 (16-18), along with a loss of cell surface HLA-DR molecules (74). In addition, major surgery altered neutrophil chemotaxis and adherence for up to 14 days postoperatively (42), and these characteristics have been reported to correlate positively with phagocytosis and bactericidal killing (43).
In order to identify underlying mechanisms responsible for perioperative alterations in host-pathogen interactions, we have developed a clinically relevant murine model of surgically induced suppression of pulmonary bacterial clearance. Considering that surgical trauma can impair a host's ability to resolve subsequent bacterial infection, we hypothesized that a postoperative challenge with a bacterial pathogen would result in (i) decreased pulmonary bacterial clearance, (ii) surgical suppression in cytokine profiles typically associated with innate antibacterial host defenses, and (iii) a decrease in influx and/or function of neutrophils and/or macrophages into the pulmonary airspace.
To test these hypotheses, leukocyte influx, proinflammatory cytokines (TNF-α, IL-1β, and IFN-γ), cytokines that promote the transition from acute to subacute or chronic inflammatory responses (e.g., IL-6 and IL-10), and several chemotactic cytokines (e.g., KC, MIP-2, and MCP-1) were assessed at various time points following bacterial infection (in surgery and nonsurgery animals), as these have been identified by our laboratory and by others (5, 14, 21, 47) as important mediators of antibacterial host defense. An understanding of the pathogenic mechanism(s) that leads to the development of pneumonia postsurgery will help identify potential strategies to decrease the morbidity, mortality, and cost that is associated with this surgical complication.
MATERIALS AND METHODS
All procedures performed on mice in this study were approved by the University at Buffalo's Institutional Animal Care and Use Committee and complied with all state, federal, and National Institutes of Health regulations.
Bacterial strain and culture.
A human bacteremic isolate of E. coli CP9 (O4/K54/H5) was used as a model pathogen for these studies (62, 63). Briefly, E. coli CP9 was grown overnight in Luria-Bertani (LB) broth at 37°C. Prior to intranasal (i.n.) inoculation, bacteria were diluted such that a 30-μl inoculum contained an estimated concentration of 3 × 106 CFU. The actual titer of the inoculum was determined by plating serial 10-fold dilutions of a 100-μl sample of the challenge inoculum on LB agar plates and incubating at 37°C for 18 h.
Animal model of nosocomial pneumonia. (i) Surgical trauma (laparotomy).
Male, specific-pathogen-free CD-1 mice (Charles River, Wilmington, Mass.), age 5 to 6 weeks, were acclimated for 1 week prior to the beginning of the experiment. Anesthesia, 2% halothane in 50% O2, was delivered by nose cone throughout the duration of the surgery. A 2-cm longitudinal abdominal incision was made, the bowel was exteriorized, and the retroperitoneum was denuded by rubbing with a sterile cotton swab. The bowel was put back into the abdominal cavity, and the incision was closed with a 6-0 Ethilon suture. A solution of 0.1% bupivicaine HCl (Marcaine; Abbott Laboratories, North Chicago, Ill.), a local anesthetic that does not affect the host-response process (13), was injected subcutaneously around the periphery of the closed incision. A combination of imipenem and cilastin (Primaxin; Merck & Co., Inc., West Point, Pa.; 25 mg/kg of body weight; t1/2 = 1.1 h) was immediately administered (in a 1-ml volume of normal saline) into the scruff of the neck for fluid volume replacement and prevention of an opportunistic postoperative wound infection. The imipenem-cilastin solution was also given to nonsurgical animals (i.e., those that did not undergo laparotomy) on the same day as laparotomy as a control for any effects on the host response.
(ii) Bacterial challenge.
At 24 h postsurgery, mice were heavily sedated with 100 mg (intraperitoneal) of ketamine (Ketaset; Fort Dodge Animal Health, Fort Dodge, Overland Park, Kans.)/kg and inoculated i.n. with 30 μl of sterile normal saline or E. coli (∼3 × 106 CFU) and then allowed to recover. Pilot studies from our laboratory, in which bacterial lung titers were determined immediately following the E. coli challenge, demonstrated an approximate 33% efficiency of the i.n. challenge (3 × 106 CFU challenge versus 1 × 106 CFU harvested [data not shown]).
(iii) Experimental groups.
At each time period evaluated (0, 6, 24, and 48 h post-bacterial inoculation [PBI]), there were four independent groups with the following group designations: (i) −LAP/NS, no surgery followed 24 h later by i.n. normal saline; (ii) −LAP/CP9, no surgery followed 24 h later by i.n. E. coli; (iii) +LAP/NS, surgery followed 24 h later by i.n. normal saline; (iv) +LAP/CP9, surgery followed 24 h later by i.n. E. coli. In order to achieve a sufficient number of animals per group for statistical analysis, seven independent experiments were conducted with an average of 25 animals per experiment, with each of the four experimental groups included in every experiment.
(iv) Lung harvesting, myeloperoxidase (MPO), and cytokine analyses.
At 0, 6, 24, and 48 h PBI, mice were anesthetized with halothane as described above. When fully anesthetized, a longitudinal incision was made up the abdomen, cutting through the sternum and the neck musculature to expose the trachea. The vena cava was transected, and the pulmonary vasculature was flushed by slowly injecting 5 ml of 37°C Hanks' balanced salt solution (with Ca2+ and Mg2+; GIBCO BRL, Life Technologies, Grand Island, N.Y.) into the right ventricle. The rib cage was then cut away, and a 22-gauge catheter was inserted into the trachea and tied into place with a suture.
A bronchoalveolar lavage (BAL) was performed by injecting five 1-ml volumes of 37°C sterile normal saline through the catheter and into the lungs. The effluent was collected with a syringe, and the recovered BAL was centrifuged at 1,500 × g for 3 min at 4°C. Without disturbing the pellet, the supernatant was aliquoted and stored at −80°C for subsequent cytokine analyses. An aliquot was removed (100 μl) for E. coli titer determination. After the BAL was completed, the lungs and heart were removed en bloc and the heart was discarded. Lungs were weighed, suspended in homogenate buffer (150 mM NaCl, 15 mM Tris, 1 mM CaCl2 · 2H2O, 1 mM MgCl2 · 6H2O) plus 100× protease inhibitor cocktail 1 (Calbiochem, La Jolla, Calif.) to a total volume of 3 ml and homogenized on ice three times for 3 s using a Polytron PT-2000 homogenizer (Brinkman Instruments, Westbury, N.Y.). Aliquots of lung homogenates (100 μl) were removed for E. coli titer determinations. Homogenates were centrifuged at 40,000 × g for 10 min at 4°C, and the supernatants were aliquoted and frozen at −80°C until cytokine analyses could be conducted. The remaining pellets were processed for MPO activity measurement by resuspension in 1 ml of buffer containing 50 mM KH2PO4, 13.7 mM hexadecyltrimethylammonium bromide, and 5 mM EDTA, pH 6.0. Samples were then sonicated on ice for 60 s using a Sonifier Cell Disruptor 350 with a microtip probe (Branson Ultrasonics, Danbury, Conn.) and centrifuged at 40,000 × g for 10 min at 4°C. The supernatant was reserved, an additional 1 ml of buffer was added to the pellet, and the sonication procedure was repeated. The supernatants were pooled, aliquoted, and stored at −80°C until the MPO activity assay was performed.
MPO assay.
Sample supernatants (see above) were thawed and assayed for MPO activity by combining 100 μl of sample with 1.5 ml of assay buffer containing 50 mM KH2PO4, 176 μM H2O2, and 525 μM o-dianisidine hydrochloride (Sigma Chemical), pH 6.0, in a cuvette and continually recording the absorbance at 460 nm for 90 s using a DU-650 spectrophotometer (Beckman Instruments). The spectrophotometer was blanked with 50 mM phosphate buffer before reading the sample. MPO activity was expressed as the absorbance change (at 460 nm) (ABS) per minute over the linear portion of the curve and normalized to the total volume extracted from the lung.
BAL fluid differential cell count.
After the BAL fluid was centrifuged and samples were aliquoted, the remaining pellet was resuspended in 3 ml of cold phosphate-buffered saline (PBS) plus 0.1% azide, and the cell suspension was layered on 2 ml of cold 2% bovine serum albumin in PBS plus 0.1% azide and centrifuged at 150 × g for 10 min at 4°C. The supernatant was aspirated and the pellet was resuspended in 1 ml of cold PBS plus 0.1% azide, and a total cell count was performed with a Multisizer 3 Coulter Counter (Beckman Coulter, Fullerton, Calif.). Differential cell counts were performed on cytoslides prepared using a cytocentrifuge (Cytospin 3; Shandon Southern Instruments, Sewickley, Pa.) stained with Diff-Quik (Baxter, Detroit, Mich.) according to the manufacturer's instructions.
TNF-α WEHI bioassay.
TNF-α bioactivity was measured using the WEHI 164 subclone 13 fibrosarcoma cell line bioassay, as previously described (9). This assay is based on the specific cytotoxicity of TNF-α to WEHI cells, a cell line derived from a mouse fibrosarcoma (a generous gift from Steven L. Kunkel, Department of Pathology, University of Michigan, Ann Arbor). 3-[4,5-Dimethylthiazo-2-yl]-2,5-diphenyltetrazolium bromide was used as a cell viability indicator, being taken up and cleaved to a dark blue formazan product by active mitochondria, which was quantified spectrophotometrically. An increase in TNF-α concentration results in increased cell death and reduced absorbance at 570 nm (46). This assay has a detection limit of approximately 1 pg/ml (15).
Albumin ELISA.
Pulmonary injury was assessed by measuring levels of albumin present in BAL fluid by direct enzyme-linked immunosorbent assay (ELISA), following the procedure of Nemzek et al. (50). The polyclonal rabbit anti-mouse albumin antibody used in the assay was a generous gift from Daniel G. Remick (Department of Pathology, University of Michigan). Briefly, the antibody and BAL samples were diluted in borate buffer (120 mM NaCl, 50 mM H3BO3, and 16 mM NaOH), and 50 μl of the appropriate sample or standard was added to the wells of a 96-well Nunc Maxisorb plate (Immunoplate; Nunc, Neptune, N.J.) and incubated overnight at 4°C. Upon termination of incubation, the solution was aspirated from the wells and the wells were washed five times with wash buffer (PBS plus 0.5% Tween 20; 300 μl per well). To block nonspecific binding sites, 150 μl of casein buffer (Pierce) was added to all wells and the plate was incubated at room temperature for 1 h on a nutator. Following incubation, the plates were washed as previously described and 50 μl of the rabbit anti-mouse albumin (diluted in PBS with 0.01% rabbit serum) was added to each well. Plates were then incubated on the nutator for 1 h at room temperature. Plates were washed, and 50 μl of goat anti-rabbit immunoglobulin G-horseradish peroxidase (Jackson ImmunoResearch Laboratories, West Grove, Pa.) was added to each well and incubated for 1 h at room temperature. Plates were washed, and 100 μl of substrate solution (3:1 substrate reagent A/substrate reagent B ratio; PharMingen, San Diego, Calif.) was added to each well. Plates were then incubated for 30 min in the dark at room temperature. The reaction was stopped with 2 N sulfuric acid (100 μl per well), and plates were immediately read on a SPECTRAmax 190 microplate spectrophotometer (Molecular Devices, Sunnyvale, Calif.) with the absorbency set at 450 and 570 nm.
Cytokine ELISAs.
Immunogenic IL-6, IL-10, IFN-γ, KC, MCP-1, and MIP-2 concentrations from BAL fluid, or IL-1β concentrations from lung homogenate, were determined with commercially available ELISA kits from R&D Systems (Minneapolis, Minn.) or from PharMingen following the manufacturers' instructions.
Statistical analysis.
Two-factor analyses of variance were performed to test for interaction between surgery and bacterial infection with respect to lung injury (i.e., albumin), neutrophil infiltration (i.e., MPO), and inflammation (i.e., cytokine) parameters. Between-group comparisons on the effect of surgery and bacteria were performed at each harvest time point for significant differences from their respective saline control groups using Tukey's test for pairwise comparisons. Categorical (mortality) data were compared by a chi-square analysis of contingency tables. Experimental groups (n = 8 to 12 animals per group) at each time period were evaluated. Values are expressed as means ± standard errors of the mean, with a P value of <0.05 considered significant.
RESULTS
Pulmonary bacterial clearance.
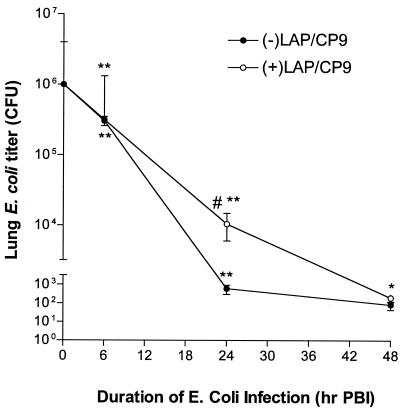
The average titer of the E. coli challenge inoculum from all experiments (n = 7) was 3.8 × 106± 2.7 × 105 CFU. At 6 h PBI, animals in both E. coli-challenged groups had E. coli titers that were lower than the challenge inoculum (−LAP/CP9, 3.0 × 105± 4.5 × 104; +LAP/CP9, 3.2 × 105± 6.6 × 104). At 24 h PBI, the −LAP/CP9 group demonstrated effective clearance (6.13 × 102± 3.17 × 102 CFU). Conversely, E. coli clearance in the +LAP/CP9 group was impaired (1.04 × 104± 0.44 × 104 CFU; P < 0.05) (Fig. 1). By 48 h PBI, E. coli titers in all groups were not measurable. Therefore, at 24 h after E. coli inoculation, animals that did not undergo laparotomy were able to more effectively clear the bacterial burden (as measured by the number of pulmonary CFU), compared to clearance in animals that underwent laparotomy.
FIG. 1.
Pulmonary bacterial clearance. Bacterial counts in combined lung homogenate and BAL fluid are expressed as CFU at 6, 24, and 48 h PBI. #, P < 0.05 compared to −LAP/CP9 at the same time point; *, P < 0.05 compared to t = 0 h (i.e., prior to i.n. E. coli); **, P < 0.001 compared to t = 0 h.
Lung injury.
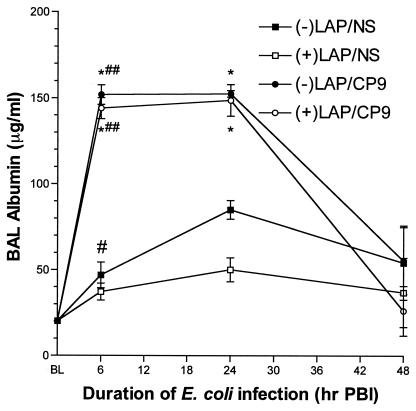
The amount of albumin present in the BAL fluid is an accurate assessment of the level of damage done to the alveolar capillary wall (49). In our model, lung injury peaks at 6 to 24 h PBI and begins to resolve by 24 to 48 h PBI. E. coli pulmonary infection clearly resulted in an increase in alveolar capillary wall injury at 6 and 24 h PBI in both −LAP/CP9 animals (151.9 ± 5.7 μg/ml and 152.5 ± 11.9 μg/ml) and +LAP/CP9 animals (144.0 ± 6.1 μg/ml and 148.7 ± 9.2 μg/ml) (Fig. 2). Predictably, albumin levels were low in the −LAP/NS and +LAP/NS groups. By 48 h PBI, albumin levels in all groups had returned to baseline. Therefore, laparotomy alone or followed by i.n. E. coli did not result in any additional lung injury beyond what was produced by E. coli alone.
FIG. 2.
Albumin measurements in BAL fluid at baseline (BL), 6, 24, and 48 h PBI. #, P < 0.01 compared to BL; ##, P < 0.001 compared to BL; *, P < 0.001 compared to respective saline control group at the same time point.
Mortality.
No mortality was observed in any of the experimental groups at 6 h PBI. This was in marked contrast to results with +LAP/CP9 animals that survived longer than 6 h PBI. By 24 h PBI, the mortality in the +LAP/CP9 animals was 26% (21 of 80 animals), compared to 6.0% (5 of 80 animals) in the +LAP/NS group (P < 0.01). No mortality occurred in any of the experimental groups between 24 and 48 h PBI. The increase in the number of deaths in the +LAP/CP9 group at >6 h PBI and <24 h PBI coincided with the less-effective clearance of E. coli (relative to that in −LAP/CP9 animals) seen in this group at the same time point.
Leukocyte influx. (i) Whole-lung MPO assay.
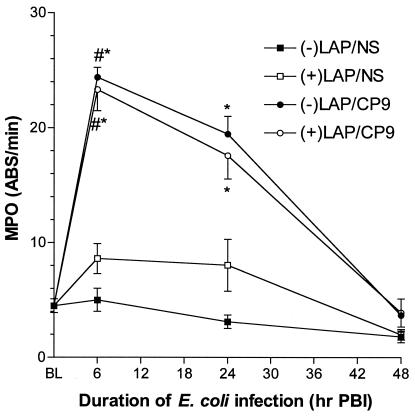
MPO is a marker for neutrophil infiltration into the lung. MPO levels in −LAP/CP9 and in +LAP/CP9 animals were increased at both 6 h PBI (−LAP/CP9, 24.4 ± 0.86 ABS/min; +LAP/CP9, 23.3 ± 1.84 ABS/min) and 24 h PBI (−LAP/CP9, 19.5 ± 1.54 ABS/min; +LAP/CP9, 17.6 ± 2.05 ABS/min), with no differences observed between the −LAP/CP9 and +LAP/CP9 groups at any time point tested (Fig. 3). Predictably, MPO levels were low in the −LAP/NS and +LAP/NS groups. By 48 h PBI, MPO levels had returned to baseline. Therefore, laparotomy had no demonstrable effect on lung MPO activity in infected or uninfected animals.
FIG. 3.
MPO activity in lung homogenates at baseline (BL), 6, 24, and 48 h PBI. #, P < 0.001 compared to BL; *, P < 0.01 compared to respective saline control at the same time point.
(ii) BAL fluid cell counts.
Differential BAL fluid cell counts assess neutrophil influx into the pulmonary airspace. Laparotomy alone (i.e., +LAP/NS) did not increase polymorphonuclear monocytes (PMNs) in BAL fluid (Table 1). At both 6 and 24 h PBI, PMNs were predominant in CP9-challenged animals (both with and without laparotomy). While E. coli induced robust PMN extravasation into the lung, this effect was more than twofold greater at 6 h PBI in animals that received only i.n. E. coli (−LAP/CP9; 14,330 ± 1,325) than in animals that underwent laparotomy (+LAP/CP9; 6,127 ± 1,865) (P < 0.001). BAL fluid PMNs were still elevated at 24 h PBI, with no difference between −LAP/CP9 animals and +LAP/CP9 animals (11,770 ± 5,541 versus 11,770 ± 4,050, respectively). By 48 h PBI, PMNs were at preexperimental levels and macrophages were the predominant cell type in all groups. Interestingly, in a comparison between the number of macrophages per group between time points, there was a greater number of macrophages in the +LAP/CP9 group, as well as the NS/CP9 group, at 48 h PBI compared to levels at 6 h PBI (P < 0.05 and P < 0.001, respectively.). Thus, while i.n. E. coli produced a marked influx of PMNs into the pulmonary airspace that was evident at 6 h postinoculation, this effect was diminished by more than twofold in animals that underwent laparotomy prior to E. coli inoculation.
TABLE 1.
Cellular composition of BAL fluida
| BAL | Total no. of leukocytes (103) | Total no. of PMNs (103) | Total no. of Mφs (103) |
|---|---|---|---|
| 6 h postinfection | |||
| −LAP/NS | 604 ± 28 | 52 ± 21 | 90 ± 11 |
| +LAP/NS | 552 ± 111 | 145 ± 68 | 75 ± 14 |
| −LAP/CP9 | 15,000 ± 1,410* | 14,330 ± 1,325#,** | 87 ± 26 |
| +LAP/CP9 | 6,672 ± 1,865** | 6,127 ± 1,800** | 87 ± 23 |
| 24 h postinfection | |||
| −LAP/NS | 2,078 ± 969 | 47 ± 33 | 342 ± 177 |
| +LAP/NS | 922 ± 628 | 3 ± 2 | 153 ± 115 |
| −LAP/CP9 | 13,570 ± 5,624 | 11,770 ± 5,541 | 479 ± 179## |
| +LAP/CP9 | 13,170 ± 4,385 | 11,770 ± 4,050 | 325 ± 112 |
| 48 h postinfection | |||
| −LAP/NS | 3,839 ± 1,981 | 978 ± 869 | 688 ± 350 |
| +LAP/NS | 2,484 ± 719 | 365 ± 106 | 410 ± 129 |
| −LAP/CP9 | 14,170 ± 5,689 | 1,613 ± 1,143 | 1,204 ± 390## |
| +LAP/CP9 | 8,790 ± 2,252 | 338 ± 166 | 610 ± 214 |
Data are means ± standard errors of the means of 8 to 12 mice per group at each time point. Mice received 3 × 106 CFU of E. coli i.n. at time zero, preceded 24 h earlier by laparotomy (LAP) or normal saline (NS). Cell counts and differentials were done on BAL fluid as described in Materials and Methods. Mφ, macrophage. *, P < 0.05 compared to saline control group at the same time point; **, P < 0.001 compared to saline control group at the same time point; #, P < 0.001 compared to +LAP/CP9 at the same time point; ##, P < 0.05 compared to same group at 6 h postinfection.
Proximal acute response cytokines: TNF-α, IL-1β, and IL-6.
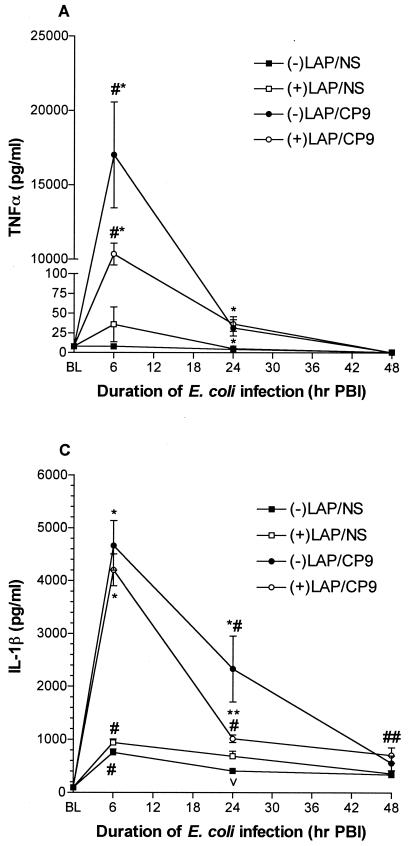
TNF-α is an essential component for bacterial clearance in many pulmonary disorders (51). At 6 h PBI, TNF-α bioactivity was greatly increased in −LAP/CP9 animals compared to +LAP/CP9 animals (17,010.0 ± 3,556.0 versus 10,340.0 ± 736.0 pg/ml), and +LAP/NS levels were slightly elevated compared to −LAP/NS levels (35.8 ± 22.2 versus 8.0 ± 1.4 pg/ml; P < 0.05) (Fig. 4A). At 24 h PBI, TNF-α levels in CP9-challenged animals had decreased dramatically (−LAP/CP9, 31.6 ± 10.4 pg/ml; +LAP/CP9, 31.9 ± 4.8 pg/ml). By 48 h PBI, TNF-α levels in all groups had returned to baseline.
FIG. 4.
Proximal acute response cytokines. (A) TNF-α (A), IL-6 (B), and IL-β (C) measurements in BAL fluid and lung homogenate (IL-1β only) at baseline (BL), 6, 24, and 48 h PBI. #, P < 0.001 compared to BL; ##, P < 0.05 compared to BL; *, P < 0.001 compared to respective saline control group at the same time point; **, P < 0.01 compared to +LAP/CP9 at the same time point; 218, P < 0.01 compared to BL; ▴, P < 0.05 compared to respective saline control group at the same time point.
The pattern of IL-1β responses was similar to that of TNF-α (11, 36). At 6 h PBI, IL-1β levels were markedly increased in both −LAP/CP9 (4,664.0 ± 468.8 pg/ml) and +LAP/CP9 (4,203.0 ± 304.5 pg/ml) animals, with no difference between the −LAP/NS and +LAP/NS groups (Fig. 4B). At 24 h PBI, IL-1β levels in the −LAP/CP9 group were still elevated in comparison to the +LAP/CP9 group (2,331.0 ± 621.9 versus 1,014.0 ± 71.5 pg/ml; P < 0.05). By 48 h PBI, IL-1β levels in all groups approached baseline.
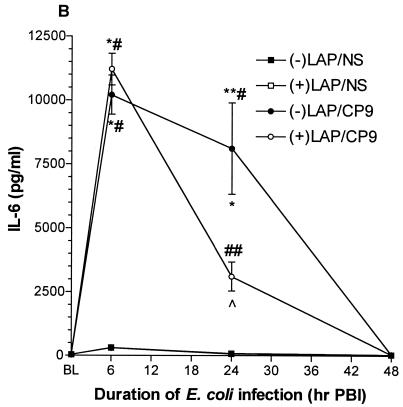
TNF-α and IL-1β are potent inducers of IL-6, an integral mediator of the acute-phase response to injury and infection (24, 33). At 6 h PBI, IL-6 levels were dramatically increased in both −LAP/CP9 (10,204.0 ± 765.9 pg/ml) and +LAP/CP9 (11,212.0 ± 616.9 pg/ml) groups, with no difference between the −LAP/NS and +LAP/NS groups (Fig. 4C). Like IL-1β, at 24 h PBI IL-6 levels in the −LAP/CP9 group were still elevated in comparison to the +LAP/CP9 group (8,112.0 ± 1,787.0 versus 3,108.0 ± 565.8 pg/ml; P < 0.01). By 48 h PBI, IL-6 levels in all groups had returned to baseline.
These results showed that during the acute response period evaluated, levels of TNF-α, IL-1β, and IL-6 were all elevated in E. coli-challenged animals at 6 h PBI. By 24 h PBI, +LAP/CP9 animals exhibited a decreased responsiveness (i.e., a decrease in cytokine levels) compared to −LAP/CP9 animals, with levels in all groups at baseline by 48 h PBI.
Leukocyte chemoattractants: KC, MIP-2, and MCP-1.
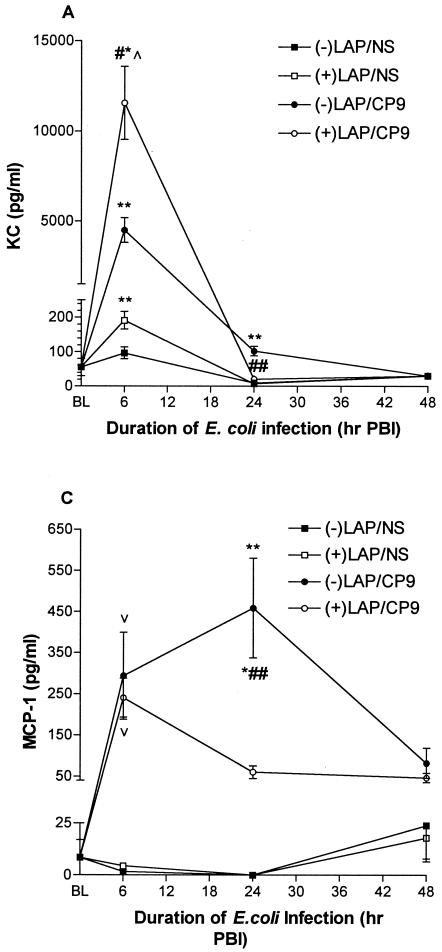
The C-X-C chemokines KC and MIP-2 enhance neutrophil recruitment, activation, and bacterial clearance (21, 70). At 6 h PBI, KC levels were markedly lower in the −LAP/CP9 group compared to the +LAP/CP9 group (4,496.0 ± 692.2 versus 11,554.0 ± 2,026.0 pg/ml; P < 0.001), with no difference between the −LAP/NS and +LAP/NS groups (Fig. 5A). However, in contrast to levels at 6 h PBI, KC levels at 24 h PBI in the +LAP/CP9 group were lower in comparison to the −LAP/CP9 group (20.1 ± 3.8 versus 101.9 ± 13.7 pg/ml; P < 0.001). By 48 h PBI, KC levels in all groups had returned to baseline.
FIG. 5.
Leukocyte chemoattractants. KC (A), MIP-2 (B), and MCP-1 (C) measurements in BAL fluid at baseline (BL), 6, 24, and 48 h PBI. #, P < 0.001 compared to −LAP/CP9 at the same time point; ##, P < 0.05 compared to BL; *, P < 0.001 compared to respective saline control at the same time point; **, P < 0.001 compared to +LAP/CP9 at the same time point; ▴, P < 0.001 compared to BL; 218, P < 0.05 compared to respective saline control at the same time point.
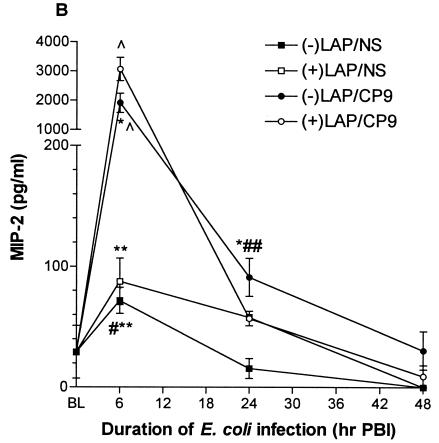
At 6 h PBI, MIP-2 levels were increased in both the −LAP/CP9 animals (1,911.0 ± 331.1 pg/ml) and the +LAP/CP9 animals (3,063.0 ± 402.1 pg/ml), with no difference between the −LAP/NS and +LAP/NS groups (Fig. 5B). Similar to KC, MIP-2 levels in the +LAP/CP9 group at 24 h PBI were lower in comparison to the −LAP/CP9 group (57.0 ± 6.0 and 91.1 ± 15.6 pg/ml, respectively). By 48 h PBI, MIP-2 levels in all groups had returned to baseline.
MCP-1 is produced by various cell types in response to a number of stimuli, including IL-1β and TNF-α (5, 55, 60). This C-C chemokine recruits and activates macrophages, lymphocytes, and eosinophils (45). At 6 h PBI, MCP-1 levels were increased in both the −LAP/CP9 animals (293.8 ± 105.8 pg/ml) and the +LAP/CP9 animals (240.6 ± 46.9 pg/ml), with no difference between the −LAP/NS and +LAP/NS groups (Fig. 5C). At 24 h PBI, MCP-1 levels in the −LAP/CP9 group had increased, while levels in the levels in the +LAP/CP9 group had decreased (458.8 ± 121.3 versus 60.3 ± 15.2 pg/ml; P < 0.001). At 48 h PBI, MCP-1 levels in the −LAP/CP9 group were still elevated in comparison to baseline (82.0 ± 36.4 versus 3.0 ± 1.2 pg/ml; P < 0.05), while the remaining groups had returned to baseline.
These results show that during the acute response period evaluated, levels of KC, MIP-2, and MCP-1 were all elevated in E. coli-challenged animals at 6 h PBI. By 24 h PBI, +LAP/CP9 animals exhibited a decreased responsiveness (i.e., a decrease in cytokine levels) compared to −LAP/CP9 animals, with levels in all groups (except MCP-1in the −LAP/CP9 group) at baseline by 48 h PBI.
Modulating cytokines: IFN-γ and IL-10.
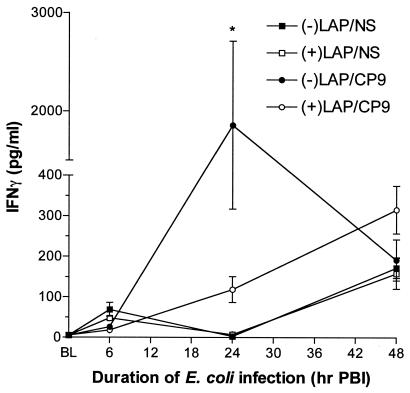
IFN-γ secreted by lymphocytes enhances leukocyte antibacterial effector functions (29). At 6 h PBI, IFN-γ levels in all experimental groups did not differ from baseline. By 24 h PBI, IFN-γ levels in both the −LAP/CP9 and +LAP/CP9 groups had increased. However, levels in the +LAP/CP9 group were substantially lower compared to the E. coli-only group (118.3 ± 31.9 versus 1,857.0 ± 854.5 pg/ml; P < 0.05) (Fig. 6). At 48 h PBI, IFN-γ levels in all groups did not differ from baseline. The decrease in IFN-γ levels in the +LAP/CP9 group at 24 h PBI coincided with the less-effective clearance of E. coli (relative to that in −LAP/CP9 animals) seen in this group at the same time point.
FIG. 6.
IFN-γ measurements in BAL fluid at baseline (BL), 6, 24, and 48 h PBI. *, P < 0.05 compared to +LAP/CP9 at the same time point.
IL-10 can directly suppress neutrophil and macrophage effector functions and can also down-regulate the expression of TNF-α, IFN-γ, and select chemokines (28, 32, 35, 53). IL-10 levels in the BAL fluid remained low at all time points tested, with no differences between experimental groups.
DISCUSSION
Postoperative alterations in host immune functions after major surgery have been well described (1, 7, 17, 18, 25, 26, 39, 42, 44), and several studies have proposed a causal relationship between surgical or traumatic injury and the subsequent development of infectious complications (34, 37). When delivered endotracheally, lipopolysaccharide (LPS) in the cell walls of gram-negative bacteria induces the release of TNF-α from pulmonary macrophages and neutrophils, and the resulting extensive inflammatory response is believed to be responsible, in part, for the high mortality associated with nosocomial infections (20). In addition, mice that were depleted of alveolar macrophages and then infected with K. pneumoniae showed an increase in lung and plasma levels of TNF-α and MIP-1α along with impaired bacterial clearance despite adequate recruitment of neutrophils (6).
Similarly, in our model of nosocomial pneumonia, bacterial clearance was impaired in mice that underwent laparotomy and, 24 h later, received an i.n. inoculation of E. coli. This effect was not seen in animals that received i.n. E. coli only, without prior surgery. In addition, animals that underwent laparotomy followed by E. coli inoculation experienced a more-than-fourfold increase in mortality (between 6 and 24 h postinfection) compared to mice that did not undergo surgery, despite no demonstrable increase in damage to the alveolar capillary wall or differences in pulmonary neutrophil influx in surviving animals.
When the bacterial burden in the lungs is above the level that the resident alveolar macrophages can successfully phagocytize, the recruitment and activation of neutrophils within pulmonary airspaces becomes critical to the successful resolution of a bacterial infection. BAL reflects the extravasation of neutrophils into the pulmonary airspaces, while MPO activity assesses neutrophil influx into the lung parenchyma. Mice that underwent surgery, followed by an i.n. E. coli inoculation, had fewer neutrophils in the airspaces at 6 h PBI and less of these cells in the lung at 24 h PBI than animals that received i.n. E. coli without any prior surgery. Patients at risk for nosocomial pneumonia often experience endotoxemia with a concomitant neutrophil dysfunction (8, 67). Results from a recent study that used a rat model of endotoxemia-associated pneumonia showed an inhibition of airway neutrophil accumulation in response to intratracheal LPS instillation into endotoxemic rats (73) along with inhibited vascular permeability, which began to subside at 6 h following LPS instillation. At that time, neutrophils may be released from the microvasculature and migrate into the airspaces. However, these previously sequestered neutrophils may display an altered response to a subsequent stimulus (19, 76), such as a decreased responsiveness to pulmonary inflammatory stimuli. Despite a difference between models as to what predisposing conditions may increase susceptibility for the development of a nosocomial pneumonia, the previous sequence of events could help reconcile our observed differences between MPO activity and vascular permeability. Although production of the neutrophil chemoattractants KC and MIP-2 were not initially altered, these C-X-C chemokines were reduced at 24 h PBI.
The proximal cytokine IL-1β was also decreased 24 h PBI. This regulator of the host antibacterial response, together with TNF-α, plays an important role in neutrophil recruitment and activation by promoting increased expression of molecules on the surface of endothelial cells that are required for the initial attachment and activation of leukocytes prior to migration into the lung (36). TNF-α and IL-1β also play an important role in activating other resident cells of the lungs to produce inflammatory cytokines (i.e., C-X-C chemokines) for an optimal innate antibacterial response (78).
In general, members of the C-X-C chemokine family (e.g., MIP-2 and KC) are responsible for chemotaxis of neutrophils, while the C-C chemokines (e.g., MCP-1) are involved in monocyte chemotaxis (65). The leukocyte stimulatory and chemotactic properties attributed to chemokines led to the prediction that these molecules may enhance the microbicidal functions of neutrophils and macrophages (52). In our study, both KC and MIP-2 were markedly elevated at 6 h PBI in both of the E. coli-challenged groups, with levels in animals that underwent prior laparotomy slightly higher than in the nonsurgery group. By 24 h PBI, KC and MIP-2 levels had returned to baseline values in the laparotomy animals and, although markedly decreased, KC and MIP-2 levels in E. coli-only animals were still elevated in comparison to baseline levels in the saline group. These results may, in part, account for the enhanced bacterial clearance seen in the −LAP/CP9 group in comparison to the +LAP/CP9 group at 24 h PBI, which also showed decreased clearance and decreased KC and MIP-2 levels at this same time point. Interestingly, in vivo inhibition of MIP-2 bioactivity with anti-murine MIP-2 serum has been shown to result in decreased lung neutrophil influx and bacterial clearance in murine Klebsiella pneumonia (21).
The production of IL-6 is also stimulated by TNF-α and IL-1β and is believed to reflect the intensity of the level of the initial acute inflammatory response (24). Additionally, IL-6 is responsible for systemic responses that protect the host by activating acute-phase reactants and promoting the transition of the acute inflammatory response to a less injurious subacute or chronic response (31). The observation in our model that the CXC chemokines and IL-6 are decreased in the BAL fluid at 24 h PBI suggests that the initial antibacterial response was not as robust following laparotomy compared to the response in mice without surgical trauma, which could play a role in the decreased bacterial clearance observed at 24 h PBI in the +LAP/CP9 group.
While KC and MIP-2 are produced early on in the inflammatory response, MCP-1 production is usually delayed but sustained (77). In our model, MCP-1 levels were elevated in CP9-challenged animals at 6 h PBI, with levels in the −LAP/CP9 group peaking at 24 h PBI. In contrast, MCP-1 levels in the +LAP/CP9 group had decreased by 24 h PBI. Optimal MCP-1 production requires increased TNF and IL-1β levels and oxidant stimulation of endothelial cells from neutrophils as they migrate through the alveolar capillary wall following the initial response. This monotactic chemokine plays an important role in the recruitment and activation of new blood monocytes into the lung and is an important component of the transition to a less injurious subacute or chronic antibacterial response. In our model, the transition from an acute to a subacute response can be reflected by the increased number of macrophages present in the BAL fluid of −LAP/CP9 animals at 24 and 48 h PBI and could account for the more efficient bacterial clearance and decreased incidence of mortality seen in this group, specifically at the 24-h PBI time point. Conversely, the number of macrophages in the +LAP/CP9 group remained relatively unchanged throughout the course of the experiment, which may be indicative of these animals' inability to mount an effective proinflammatory response to a subsequent bacterial infection following the initial surgical injury.
IFN-γ plays an important protective role in innate immune responses against common bacterial pathogens by increasing the effector function of neutrophils and macrophages and enhancing leukocyte killing activity by a TNF-α-dependent mechanism (48, 75). Clinically, traumatic injury suppresses IFN-γ production, and there is some evidence that daily treatment with IFN-γ immediately following trauma results in fewer deaths (12). However, there are no published reports that have been able to demonstrate a connection between surgically induced inhibition of IFN-γ and bacterial clearance. In our model, surgery followed by i.n. E. coli resulted in decreased levels of IFN-γ in BAL fluid, compared to levels in animals that received E. coli only, at 24 h PBI. IFN-γ is produced in lymphocytes, primarily in response to IL-12 released from resident alveolar macrophages, and administration of IL-12 protects animals against a pulmonary K. pneumoniae challenge (22). Impaired production of IFN-γ, a key cytokine involved in host antibacterial defense may, in part, be responsible for the decreased clearance of E. coli from the lung following E. coli challenge.
In summary, we have developed a clinically relevant murine model of postoperative nosocomial pneumonia that is characterized by decreased pulmonary bacterial clearance and increased mortality. Alterations in neutrophil recruitment and cytokine production that are clearly associated with impairment in the host antibacterial defense are also present. Additionally, cytokine production is consistent with previously reported findings in patients following surgical trauma. Interestingly, there are no differences in lung injury (as assessed by albumin extravasation into the air spaces) between surviving animals that underwent surgery 24 h prior to E. coli inoculation and those that received E. coli only. There was, however, an increased mortality rate in the former group, which may be related to changes in the acute antibacterial host response (e.g., an exaggerated proinflammatory response) or toxic products from bacteria that are not optimally cleared from the lungs. While more research is needed to establish the specific molecular and cellular mechanisms that are responsible for the development and progression of a nosocomial pneumonia, we speculate that the modulatory effect of surgical stress on cytokine production may cause changes in recruitment and regulation of effector functions of neutrophils and/or alveolar macrophages, thereby impairing innate host antibacterial immunity.
Acknowledgments
This work was supported by NIH 1 RO1 AI46534 (P.A.M. and P.R.K.), HL69763 (T.A.R.), and the John R. Oishei Foundation.
REFERENCES
- 1.Angele, M. K., and E. Faist. 2002. Clinical review: immunodepression in the surgical patient and increased susceptibility to infection. Crit. Care 6:298-305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anonymous. 2000. National Nosocomial Infections Surveillance (NNIS) system report, data summary from January 1992-April 2000, issued June 2000. Am. J. Infect. Control 28:429-448. [DOI] [PubMed] [Google Scholar]
- 3.Aosasa, S., S. Ono, H. Mochizuki, H. Tsujimoto, S. Osada, E. Takayama, S. Seki, and H. Hiraide. 2000. Activation of monocytes and endothelial cells depends on the severity of surgical stress. World J. Surg. 24:10-16. [DOI] [PubMed] [Google Scholar]
- 4.Biffl, W. L., E. E. Moore, F. A. Moore, and V. M. Peterson. 1996. Interleukin-6 in the injured patient. Ann. Surg. 224:647-664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Brieland, J. K., C. M. Flory, M. L. Jones, G. R. Miller, D. G. Remick, J. S. Warren, and J. C. Fantone. 1995. Regulation of monocyte chemoattractant protein-1 gene expression and secretion in rat pulmonary alveolar macrophages by lipopolysaccharide, tumor necrosis factor-α, and interleukin 1β. Am. J. Respir. Cell Mol. Biol. 12:104-109. [DOI] [PubMed] [Google Scholar]
- 6.Brough-Holub, E., G. B. Toews, F. Van Iwaarden, R. M. Strieter, S. L. Kunkel, R. Paine, and T. J. Standiford. 1997. Alveolar macrophages are required for protective pulmonary defenses in murine Klebsiella pneumoniae: elimination of alveolar macrophages increases neutrophil recruitment but decreases bacterial clearance and survival. Infect. Immun. 65:1139-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Brune, I. B., W. Wilke, T. Hensler, B. Holzmann, and J. Siewert. 1999. Downregulation of T helper type 1 immune response and altered pro-inflammatory and anti-inflammatory T cell cytokine balance following conventional but not laparoscopic surgery. Am. J. Surg. 177:55-60. [DOI] [PubMed] [Google Scholar]
- 8.Buffone, V., J. L. Meakins, and N. V. Christou. 1984. Neutrophil function in surgical patients: relationship to adequate bacterial defenses. Arch. Surg. 119:39-43. [DOI] [PubMed] [Google Scholar]
- 9.Davidson, B. A., P. R. Knight, J. D. Helinski, N. D. Nader, T. P. Shanley, and K. J. Johnson. 1999. The role of tumor necrosis factor-α in the pathogenesis of aspiration pneumonitis in rats. Anesthesiology 91:486-499. [DOI] [PubMed] [Google Scholar]
- 10.Del Prete, G., M. de Carli, F. Almerigogna, M. Guidizi, R. Biagiotti, and S. Romagnani. 1993. Human IL-10 is produced by both type 1 helper (Th1) and type 2 helper (Th2) cell clones and inhibits their antigen-specific proliferation and cytokine production. J. Immunol. 150:353-360. [PubMed] [Google Scholar]
- 11.Dianarillo, C. A. 1989. Interleukin-1 and its biologically related cytokines. Adv. Immunol. 44:153-205. [DOI] [PubMed] [Google Scholar]
- 12.Dries, D. J. 1996. Interferon gamma in trauma-related infections. Intensive Care Med. 22:S462-S467. [DOI] [PubMed] [Google Scholar]
- 13.Eldor, J. 2003. Local anesthetic antibacterial activity. Anaesthesia 58:926-928. [DOI] [PubMed] [Google Scholar]
- 14.Endlich, B., D. Armstrong, J. Brodsky, M. Novotny, and T. A. Hamilton. 2002. Distinct temporal patterns of macrophage-inflammatory protein-2 and KC chemokine gene expression in surgical injury. J. Immunol. 186:3586-3594. [DOI] [PubMed] [Google Scholar]
- 15.Eskandari, M. K., D. T. Nguyen, S. L. Kunkel, and D. G. Remick. 1990. WEHI 164 subclone 13 assay for TNF: sensitivity, specificity, and reliability. Immunol. Investig. 19:69-79. [DOI] [PubMed] [Google Scholar]
- 16.Faist, E., A. Mewes, C. C. Baker, T. Strasser, S. S. Alkan, P. Rieber, and G. Heberer. 1987. Prostaglandin E2 (PGE2)-dependent suppression of interleukin α (IL-2) production in patients with major trauma. J. Trauma 27:837-848. [DOI] [PubMed] [Google Scholar]
- 17.Faist, E., A. Mewes, T. Strasser, A. Walz, S. Alkan, C. Baker, W. Ertel, and G. Heberer. 1988. Alteration of monocyte function following major injury. Arch. Surg. 123:287-292. [DOI] [PubMed] [Google Scholar]
- 18.Faist, E., T. S. Kupper, C. C. Baker, I. H. Chaudry, and A. E. Baue. 1986. Depression of cellular immunity after major injury: its association with posttraumatic complications and its reversal with immunomodulation. Arch. Surg. 121:1000-1005. [DOI] [PubMed] [Google Scholar]
- 19.Frevert, C. W., A. E. Warber, and L. Kobzik. 1994. Defective pulmonary recruitment of neutrophils in a rat model of endotoxemia. Am. J. Respir. Mol. Cell Biol. 11:716-723. [DOI] [PubMed] [Google Scholar]
- 20.Gordon, S. B., and R. C. Read. 2002. Macrophage defenses against respiratory tract infections. Br. Med. Bull. 61:45-61. [DOI] [PubMed] [Google Scholar]
- 21.Greenberger, M. J., R. M. Strieter, S. L. Kunkel, J. M. Danforth, L. L. Laichalk, D. C. McGillicudy, and T. J. Standiford. 1996. Neutralization of macrophage inhibitory protein-2 attenuates neutrophil recruitment and bacterial clearance in murine Klebsiella pneumonia. J. Infect. Dis. 173:159-165. [DOI] [PubMed] [Google Scholar]
- 22.Greenberger, M., S. L. Kunkel, R. M. Strieter, N. Lukas, J. Bramson, J. Gauldie, F. Graham, M. Hitt, J. Danforth, and T. J. Standiford. 1996. IL-12 gene therapy protects mice in lethal Klebsiella pneumonia. J. Immunol. 157:3006-3012. [PubMed] [Google Scholar]
- 23.Haslett, C. 1997. Granulocyte apoptosis and inflammatory disease. Br. Med. Bull. 53:669-683. [DOI] [PubMed] [Google Scholar]
- 24.Heinrich, P. C., J. V. Castell, and T. Andus. 1990. Interleukin-6 and the acute phase response. Biochem. J. 265:621-636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Helmy, S. A. K., M. A. M. Wahby, and M. El-Nawaway. 1999. The effect of anesthesia and surgery on plasma cytokine production. Anesthesia 54:733-738. [DOI] [PubMed] [Google Scholar]
- 26.Hensler, T., H. Hecker, K. Heeg, C. Heidecke, H. Bartels, W. Barthlen, H. Wagner, J. Siewert, and B. Holzmann. 1997. Distinct mechanisms of immunosuppression as a consequence of major surgery. Infect. Immun. 65:2283-2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Holmes, M. C., P. Zhang, S. Nelson, W. R. Summer, and G. J. Bagby. 2002. Neutrophil modulation of the pulmonary chemokine response to lipopolysaccharide. Shock 18:555-560. [DOI] [PubMed] [Google Scholar]
- 28.Howard, M., A. O'Garra, H. Ishida, R. de Waal Malefyt, and J. de Vries. 1992. Biological properties of interleukin 10. J. Clin. Immunol. 12:239-247. [DOI] [PubMed] [Google Scholar]
- 29.Jenson, W., R. Rose, A. Wasserman, T. H. Kalb, K. Anton, and G. H. Remold. 1987. In vitro activation of the antibacterial activity of human pulmonary macrophages by recombinant γ interferon. J. Infect. Dis. 155:574-577. [DOI] [PubMed] [Google Scholar]
- 30.Jiminez, M. F., R. W. Watson, J. Parodo, D. Evans, D. Foster, M. Steinberg, O. D. Rotstein, and J. C. Marshall. 1997. Dysregulated expression of neutrophil apoptosis in the systemic inflammatory response syndrome. Arch. Surg. 132:1263-1269. [DOI] [PubMed] [Google Scholar]
- 31.Kaplinski, G., V. Marin, F. Montero-Julian, A. Mantovani, and C. Farnarier. 2003. IL-6: a regulator of the transition from neutrophil to monocyte recruitment during inflammation. Trends Immunol. 24:25-29. [DOI] [PubMed] [Google Scholar]
- 32.Kasama, T., R. Streiter, N. W. Lukacs, M. D. Burdick, and S. L. Kunkel. 1994. Regulation of neutrophil-derived chemokine expression by IL-10. J. Immunol. 152:3559-3569. [PubMed] [Google Scholar]
- 33.Kato, M., H. Suzuki, M. Murakami, M. Akama, S. Matsukawa, and Y. Hashimoto. 1997. Elevated plasma levels of interkeukin-6, interleukin-8, and granulocyte colony-stimulating factor during and after major abdominal surgery. J. Clin. Anesth. 9:293-298. [DOI] [PubMed] [Google Scholar]
- 34.Keane, R. M., W. Birmingham, C. M. Shatnet, R. A. Winchurch, and A. M. Munster. 1983. Prediction of sepsis in the multitraumatic patient by assays of lymphocyte responsiveness. Surg. Gynecol. Obstet. 156:163-167. [PubMed] [Google Scholar]
- 35.Laichalk, L. L., J. M. Danforth, and T. J. Standiford. 1996. Interleukin-10 inhibits neutrophil phagocytic and bactericidal activity. FEMS Immunol. Med. Microbiol. 15:181-187. [DOI] [PubMed] [Google Scholar]
- 36.Le, J., and J. Vilcek. 1987. TNF and IL-1: cytokines with multiple overlapping biological activities. Lab. Investig. 56:2342-2382. [PubMed] [Google Scholar]
- 37.Levy, E. M., S. A. Alharbi, G. Grindlinger, and P. H. Black. 1984. Changes in mitogen responsiveness lymphocyte subsets after traumatic injury: relation to development of sepsis. Clin. Immunol. Immunopathol. 32:224-233. [DOI] [PubMed] [Google Scholar]
- 38.Lin, E., S. E. Calvano, and S. F. Lowry. 2000. Inflammatory cytokines and cell response in surgery. Surgery 127:117-126. [DOI] [PubMed] [Google Scholar]
- 39.Little, D., M. Regan, R. M. Keane, and D. Bouchier-Hayes. 1993. Perioperative immune modulation. Surgery 114:87-91. [PubMed] [Google Scholar]
- 40.Maloney, S. A., and W. R. Jarvis. 1995. Epidemic nosocomial pneumonia in the intensive care unit. Clin. Chest Med. 16:209-223. [PubMed] [Google Scholar]
- 41.Markovic, S. N., P. R Knight, and D. M. Marusko. 1993. Inhibition of interferon stimulation of natural killer cell activity in mice anesthetized with halothane or isoflurane. Anesthesiology 78:700-706. [DOI] [PubMed] [Google Scholar]
- 42.Meakins, J. L. 1988. Host defense mechanisms in surgical patients: effect of surgery and trauma. Acta Chir. Scand. Suppl. 550:43-53. [PubMed] [Google Scholar]
- 43.Meakins, J. L., J. B. Pietsch, O. Bubenick, R. Kelly, H. Rode, J. Gordon, and L. D. MacLean. 1977. Delayed hypersensitivity: indicator of acquired failure of host defenses in sepsis and trauma. Ann. Surg. 186:241-250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Miller-Graziano, C. L., M. Fink, J. Y. Wu, G. Szabo, and K. Kodys. 1988. Mechanisms of altered monocyte prostaglandin E2 production in severely injured patients. Arch. Surg. 123:293-299. [DOI] [PubMed] [Google Scholar]
- 45.Moore, T. A., and T. J. Standiford. 1998. The role of cytokines in bacterial pneumonia: an inflammatory balancing act. Proc. Assoc. Am. Phys. 110:297-305. [PubMed] [Google Scholar]
- 46.Mosmann, T. 1983. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J. Immunol. Methods 65:55-63. [DOI] [PubMed] [Google Scholar]
- 47.Muehlstedt, S. G., C. J. Richardson, M. A. West, M. Lyte, and J. L. Rodriguez. 2001. Cytokines and the pathogenesis of nosocomial pneumonia. Surgery 130:602-611. [DOI] [PubMed] [Google Scholar]
- 48.Murray, H. W. 1996. Current and future clinical implications of interferon-gamma in host antimicrobial defense. Intensive Care Med. 22:S456-S461. [DOI] [PubMed] [Google Scholar]
- 49.Nader-Djalal, N., P. R. Knight, B. A. Davidson, and Kent Johnson. 1997. Hyperoxia exacerbates microvascular lung injury following acid aspiration. Chest 112:1607-1614. [DOI] [PubMed] [Google Scholar]
- 50.Nemzek, J. A., D. R. Calls, S. J. Ebong, D. E. Newcomb, G. L. Bolgos, and D. G. Remick. 2000. Immunopathology of a two-hit murine model of acid aspiration lung injury. Am. J. Physiol. Lung Cell. Mol. Physiol. 278:L512-L520. [DOI] [PubMed] [Google Scholar]
- 51.O'Grady, N. P., H. L. Preas, J. Pugin, C. Fiuza, M. Tropea, D. Reda, S. M. Banks, and A. F. Suffredini. 2001. Local inflammatory responses following bronchial endotoxin instillation in humans. Am. J. Respir. Crit. Care Med. 163:1591-1598. [DOI] [PubMed] [Google Scholar]
- 52.Oppenheim, J. J., C. O. C. Zachariae, N. Mukaida, and K. Matsushima. 1991. Properties of the novel proinflammatory supergene intercrine cytokine family. Annu. Rev. Immunol. 9:617-647. [DOI] [PubMed] [Google Scholar]
- 53.Oswald, I. P., R. T. Gazzinelli, A. Sher, and S. L. James. 1992. IL-10 synergizes with IL-4 and transforming growth factor to inhibit macrophage cytotoxic activity. J. Immunol. 148:3578-3582. [PubMed] [Google Scholar]
- 54.Oswald, I. P., T. A. Wynn, A. Sher, and S. L. James. 1992. Interleukin 10 inhibits macrophage microbicidal activity by blocking endogenous production of tumor necrosis factor-α required as a costimulatory factor for interferon-γ-induced activation. Proc. Natl. Acad. Sci. USA 89:8676-8680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Paine, R., III, M. W. Rolfe, T. J. Standiford, M. D. Burdick, B. J. Rollins, and R. M. Strieter. 1993. MCP-1 expression by rat type II alveolar epithelial cells in primary culture. J. Immunol. 150:4561-4570. [PubMed] [Google Scholar]
- 56.Penna, A. M., K. J. Johnson, J. Camiller, and P. R. Knight. 1990. Alterations in influenza A virus specific immune injury in mice anesthetized with halothane or ketamine. Intervirology 31:188-196. [DOI] [PubMed] [Google Scholar]
- 57.Pennington, J. E. 1992. Immunological perspectives in prevention and treatment of nosocomial pneumonia. Intensive Care Med. 18(Suppl. 1):S35-S38. [DOI] [PubMed] [Google Scholar]
- 58.Richards, M. J., J. R. Edwards, D. H. Culver, and R. P. Gaynes. 1999. Nosocomial infections in medical intensive care units in the United States. National nosocomial infections surveillance system. Crit. Care Med. 27:887-892. [DOI] [PubMed] [Google Scholar]
- 59.Rodriguez, J. L., J. K. Gibbons, L. G. Bitzer, R. E. Deckert, S. M. Steinberg, and L. M. Flint. 1991. Pneumonia: incidence, risk factors, and outcome in injured patients. J. Trauma 31:907-912. [PubMed] [Google Scholar]
- 60.Rollins, B. J., T. Yoshimura, E. J. Leonard, and J. S. Pober. 1990. Cytokine-activated human endothelial cells synthesize and secrete a monocyte chemoattractant, MCP-1/JE. Am. J. Pathol. 136:1229-1233. [PMC free article] [PubMed] [Google Scholar]
- 61.Romagnani, S. 1994. Lymphokine production by human T cells in disease states. Annu. Rev. Immunol. 12:227-257. [DOI] [PubMed] [Google Scholar]
- 62.Russo, T. A., G. Sharma, C. R. Brown, and A. A. Campagnari. 1995. The loss of the O4 antigen moiety from the lipopolysaccharide of an extraintestinal isolate of Escherichia coli has only minor effects on serum sensitivity and virulence in vivo. Infect. Immun. 63:1263-1269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Russo, T. A., J. E. Guenther, S. Wenderoth, and M. M. Frank. 1993. Generation of isogenic K54 capsule-deficient Escherichia coli strains through a TnphoA-mediated gene disruption. Mol. Microbiol. 9:357-364. [DOI] [PubMed] [Google Scholar]
- 64.Salo, M. 1992. Effects of anesthesia and surgery on the immune response. Acta Anaesthesiol. Scand. 36:201-220. [DOI] [PubMed] [Google Scholar]
- 65.Schluger, N. W., and W. N. Rom. 1997. Early responses to infection: chemokines as mediators of inflammation. Curr. Opin. Immunol. 9:504-508. [DOI] [PubMed] [Google Scholar]
- 66.Sibille, Y., and H. Y. Reynolds. 1990. Macrophages and polymorphonuclear neutrophils in lung defense and injury. Am. Rev. Respir. Dis. 141:471-501. [DOI] [PubMed] [Google Scholar]
- 67.Simms, H. H., and R. D'Amico. 1997. Posttraumatic auto-oxidative polymorphonuclear neutrophil receptor injury predicts the development of nosocomial infection. Arch. Surg. 132:171-177. [DOI] [PubMed] [Google Scholar]
- 68.Standiford, T. J., R. M. Strieter, M. J. Greenberger, and S. L. Kunkel. 1996. Expression and regulation of chemokines in acute bacterial pneumonia. Biol. Signals 5:203-208. [DOI] [PubMed] [Google Scholar]
- 69.Stevenson, G. W., S. Hall, J. Rudnick, G. Alvord, J. Rossio, W. Urba, J. B. Leventhal, P. Miller, F. Seleny, and H. C. Stevenson. 1987. Halothane anesthesia decreases human monocyte hydrogen peroxide generation: protection of monocytes by activation with gamma interferon. Immunopharmacol. Immunotoxicol. 9:489-510. [DOI] [PubMed] [Google Scholar]
- 70.Tsai, W. C., R. M. Strieter, J. M. Wilkowski, K. A. Bucknell, M. D. Burdick, S. A. Lira, and T. J. Standiford. 1998. Lung-specific transgenic expression of KC enhances resistance to Klebsiella pneumoniae in mice. J. Immunol. 161:2435-2440. [PubMed] [Google Scholar]
- 71.Uthaisangook, S., N. K. Day, S. L. Bahna, R. A. Good, and S. Haragucki. 2002. Innate immunity and its role against infections. Ann. Allergy Asthma Immunol. 88:253-265. [DOI] [PubMed] [Google Scholar]
- 72.Vincent, J. 2003. Nosocomial infections in adult intensive-care units. Lancet 361:2068-2077. [DOI] [PubMed] [Google Scholar]
- 73.Wagner, J. G., J. R. Harkema, and R. A. Roth. 2002. Pulmonary leukostasis and the inhibition of airway neutrophil recruitment are early events in the endotoxemic rat. Shock 17:151-158. [DOI] [PubMed] [Google Scholar]
- 74.Wakefield, C. H., P. D. Carey, S. Foulds, J. R. Monson, and P. J. Guillou. 1993. Changes in major histocompatibility complex class II expression in monocytes and T cells of patients developing infection after surgery. Br. J. Surg. 80:205-209. [DOI] [PubMed] [Google Scholar]
- 75.Williams, J. G., G. J. Jurkovich, and R. V. Maier. 1993. Interferon-γ: a key immunoregulatory lymphokine. J. Surg. Res. 54:79-93. [DOI] [PubMed] [Google Scholar]
- 76.Williams, J. H., S. K. Patel, D. Hatakeyama, R. Arian, K. Guo, T. J. Hickey, S. Y. Liao, and T. R. Ulich. 1993. Activated pulmonary vascular neutrophils as early mediators of endotoxin-induced lung inflammation. 1993. Am. J. Respir. Cell Mol. Biol. 8:134-144. [DOI] [PubMed] [Google Scholar]
- 77.Yamashiro, S., H. Kamohara, and T. Yoshimura. 1999. MCP-1 is selectively expressed in the late phase by cytokine-stimulated human neutrophils: TNF-α plays a role in MCP-1 mRNA expression. J. Leukoc. Biol. 65:671-679. [DOI] [PubMed] [Google Scholar]
- 78.Zhang, P., W. R. Summer, G. J. Bagby, and S. Nelson. 2000. Innate immunity and pulmonary host defense. Immunol. Rev. 173:39-51. [DOI] [PubMed] [Google Scholar]