Abstract
The efficacy of vaccination with Toxoplasma gondii recombinant GRA4 (rGRA4) and ROP2 (rRPO2) proteins and a mix of both combined with alum were evaluated in C57BL/6 and C3H mice. In C57BL/6 mice, rGRA4 and rGRA4-rROP2 immunizations generated similar levels of immunoglobulin G1 (IgG1) and IgG2a isotypes against GRA4, whereas immunizations with rROP2 and the mix induced a predominant IgG1 production against ROP2. All groups of C3H vaccinated mice exhibited higher levels of IgG1 than IgG2a. rGRA4-stimulated splenocytes from vaccinated mice produced primarily gamma interferon while those stimulated with rROP2 produced interleukin-4. Challenge of rGRA4- or rGRA4-rROP2-vaccinated mice from both strains with ME49 cysts resulted in fewer brain cysts than the controls, whereas vaccination with rROP2 alone only conferred protection to C3H mice. Immunization with a plasmid carrying the entire open reading frame of GRA4 showed a protective level similar to that of rGRA4 combined with alum. These results suggest that GRA4 can be a good candidate for a multiantigen anti-T. gondii vaccine based on the use of alum as an adjuvant.
Toxoplasma gondii is an obligate intracellular parasite, capable of infecting a variety of mammals and birds. Infection of immunocompetent humans is usually asymptomatic, with clinical disease largely confined to risk groups. Primary infection during pregnancy can result in severe neonatal malformations and ocular complications in the fetus. Since the emergence of AIDS, toxoplasmosis in the immunocompromised host is of great significance, as the recrudescence of a latent infection often results in a fatal encephalitis (19). Transmission to humans occurs through the ingestion of oocysts shed by felines or cysts from infected farm animal meat, mainly pigs (46). Toxoplasmosis is also of veterinary importance, since infection during pregnancy, especially in sheep, often results in abortion, representing considerable economic losses (14, 15).
It is known that chronically infected individuals and animals develop lifelong immune protection against reinfection. The effective immune response is mediated by CD4+ and CD8+ T cells and is associated with the production of gamma interferon (IFN-γ) (20, 21, 26, 44). Recently, the contribution of B cells against parasite infection was further examined (23, 24, 42). A live vaccine based on an attenuated strain of T. gondii is being used in farm animals (8). However, such a vaccine is not suitable for human use due to reactivation to the pathogenic form. For this reason, the use of recombinant technology arises as a powerful and interesting tool for the development of a vaccine for humans. In the quest for promissory molecules exhibiting protective value, T. gondii SAG1 (P30) has been the most exhaustively tested (2, 3, 7, 9-11, 16, 18, 25, 27, 34-36, 40). Most of these studies have shown that vaccination with SAG1 induced different levels of partial protection.
Apart from SAG1, other T. gondii proteins have also been tested (12, 16, 28, 29, 33, 34, 39, 49). Recombinant GRA4 (rGRA4) combined with cholera toxin induced partial protection in C57BL/6 immunized mice against a nonlethal challenge with the 76,000-molecular-weight T. gondii strain (33). On the other hand, immunization of C57BL/6 mice with a plasmid expressing the mature GRA4 protein protected mice against a lethal challenge with the same parasite strain (12). Gene vaccination with the dense granule proteins GRA1 and GRA7, and the ropthry protein ROP2 induced protection against infection with different avirulent T. gondii strains in C3H mice but not in BALB/c and C57BL/6 mice (49). In addition, ROP2 gene vaccination of BALB/c mice did not show any protection against a challenge with the RH strain (28). Mishima et al. (34) observed partial protection against a lethal challenge with the Beverly strain in BALB/c mice immunized with SRS1, SAG2, rROP2, and a mix of recombinant antigens combined with Freund's complete and incomplete adjuvant but not with SAG1.
It is evident that it would be interesting to include an adjuvant like alum, which can be used in humans, in the development of a vaccine against toxoplasmosis. However, alum was shown to promote the production of Th2 cytokines with a low level of CD8+-T-cell activation, converse to the requirements for inducing immunity against T. gondii in mice (1). In spite of this, Petersen et al. (40) showed protection against RH infection by using rSAG1 adsorbed to alum.
In an effort to find other T. gondii recombinant antigens with protective values similar to those produced by rSAG1, we have examined the efficacy of rGRA4 and rROP2 to confer immunity when combined with alum. In the future this would allow the generation of a multiantigen vaccine based on the use of alum as the adjuvant. In the present study, we used two different strains of inbred mice with different major histocompatibility haplotypes and different levels of susceptibility to T. gondii-induced morbidity and mortality: C57BL/6 (H-2b) and C3H (H-2k). C57BL/6 mice are highly susceptible, and oral infection with low numbers of encysted bradyzoites leads to a high mortality rate in the acute phase (31). C3H mice can survive oral infection (4), and the cyst load in the brains of these infected mice is high (6, 45). In addition, a DNA vaccine vector expressing the whole GRA4 protein was designed and used in an immunization assay to compare the protective value of the vaccine to rGRA4 plus alum.
MATERIALS AND METHODS
Parasites.
T. gondii tissue cysts of the ME49 strain were used to challenge mice. Brain tissue cysts of this strain were obtained by passage through C57BL/6 mice and maintained by passage of 20 tissue cysts administered by intraperitoneal injection.
Recombinant protein and plasmid constructions.
rROP2 (residues 196 to 561) and rGRA4 protein (residues 163 to the end, residue 345) were generated by the fusion of a six-histidine-linked tag at the N-terminal part of the truncated forms (30, 38). rROP2 has been constructed containing all of the three potential T-cell epitopes already described (41). rGRA4 comprises the C-terminal region and contains all of the B-cell epitopes as well as the T-cell epitope (positions 326 to 334) already described (32, 33). To obtain the plasmid encoding the GRA4 gene, the DNA sequence of the gra4 gene (32) was obtained from the GenBank database (accession number M76432). The entire gra4 open reading frame was amplified from T. gondii RH genomic DNA by PCR. An upstream sense primer (Gra4-F, 5′-CGCGGGTACCATGCAGGGCACTTGGTTTTC-3′), with an additional recognition sequence for the endonuclease KpnI, and a downstream antisense primer (Gra4-R, 5′-CGCGGAATTCTCACTCTTTGCGCATTCTTT-3′), with an additional recognition sequence for the endonuclease EcoRI, were synthesized. The PCR product was digested with KpnI and EcoRI restriction enzymes, purified from an agarose gel (Qiaex II; QIAGEN), cloned into the corresponding sites of pcDNA3 (pGRA4), and sequenced.
Expression and purification of recombinant proteins.
The recombinant proteins were expressed in Escherichia coli strain M15 (QIAGEN) and purified by nondenaturing conditions on nitrilotriacetic acid-Ni2+ columns (QIAGEN) as already described (37). The purity of the recombinant proteins was analyzed by sodium dodecyl sulfate-polyacrylamide gel electrophoresis on 10% acrylamide gels in the Mini-Protean system (Bio-Rad) and stained with Coomassie blue. rROP2 migrated with an apparent molecular mass of 44 kDa (30), and rGRA4 migrated with an apparent molecular mass of 29 kDa (38). The purified recombinant proteins were quantified by the Bradford method.
Purification of pGRA4 plasmid and transfection of cells.
The plasmid was purified with a column chromatography kit (QIAGEN) according to the instructions of the manufacturer. Plasmid expression was analyzed by transfection of Cos-7 cells with the Profection mammalian transfection system (Promega). Calcium phosphate transfection of the gra4 gene in Cos-7 cells was performed according to the instructions of the manufacturer (technical manual no. O12; Promega). After 2 days, cell monolayers and supernatants were collected and stored at −20°C until use. Expression of transfected and nontransfected control cells was analyzed by Western blotting (16).
Immunization and challenge.
Eight-week-old C57BL/6 and C3H female inbred mice were immunized with 10 μg of either rROP2, rGRA4, or a mix of 10 μg of each recombinant antigen (R+G) adsorbed to 0.5 mg of Al(OH)3 (alhydrogel; Superfos Biosector a/s) by intramuscular injection into the hindquarters. Mice were boosted with the same dose three more times in a period of 2 or 8 weeks. Phosphate-buffered saline (PBS) plus alum was injected to the control group mice. For the DNA vaccination, C3H mice were immunized three times at 3-week intervals by intramuscular injection with 100 μg of pGra4 or the empty vector pcDNA3.
Fourteen days after the last booster, mice were challenged by oral infection with 20 (sublethal dose) or 100 (lethal dose) ME49 tissue cysts. Mice were observed daily for mortality. One month after the challenge, survivors were killed and their brains were removed and homogenized in 1 ml of PBS by 8 passages through a 21-gauge needle. The mean number of cysts per brain was determined by observation under an optical microscope, counting four samples of 25-μl aliquots for each brain.
After the last booster (day 14), serum samples were obtained at different time points: day 28 (same day of challenge) and day 56 (4 weeks after the challenge). Preimmune serum samples (day 0) were used as negative controls.
IgG and subclasses determination.
Antigen-specific antibodies were analyzed by enzyme-linked immunosorbent assay (ELISA) as previously described (30). Briefly, each well of 96-well microtiter plates (Immuno Plate Maxisorp; Nunc) was coated overnight at 4°C with 100 μl of 5-μg/ml recombinant proteins diluted in 0.05 M carbonate buffer (pH 9.6). Mouse sera were diluted in PBS and applied to the wells, followed by goat anti-mouse immunoglobulin G (IgG)-horseradish peroxidase conjugate as a secondary antibody (Jackson Immunoresearch Laboratories) diluted 1:4,000. For isotype analysis, mouse sera diluted 1:2,000 were applied to the plates and developed with goat anti-mouse IgG1- or IgG2a-horseradish peroxidase conjugate (Serotec) diluted 1:2,000 and 1:4,000, respectively. Immune complexes were revealed with orthophenylene diamine (Sigma) as the chromogen and 0.15% H2O2 as the substrate. Absorbance at 450 nm (A450) was measured with an automatic ELISA reader (Dynatech MR4000). Results were expressed as A450s, determined in duplicate for each serum sample from at least three independent ELISAs. The antigen-specific antibody titer was calculated as the reciprocal of the highest dilution whose absorbance was >3 standard deviations above the mean value obtained with the preimmune sera.
In vitro spleen cell proliferation.
Spleens were aseptically removed from 3 mice per group 2 weeks after the last booster injection. Single-cell preparations were obtained by crushing spleens through stainless steel meshes followed by suspension in erythrocyte lysis buffer (139.5 mM NH4Cl, 17 mM Tris-HCl [pH 7.4]). The viability of the cells used in the experiments was always higher than 80%, as measured by trypan blue exclusion (Sigma). The cells were then suspended in RPMI 1640 medium (Life Technologies) supplemented with 10% fetal calf serum, 2-mercaptoethanol (final concentration, 5 × 10−5 M), penicillin (100 U/ml), and streptomycin (100 mg/ml) and seeded at 5 × 105 cells per well in triplicate in flat-bottom 96-well microtiter plates (Costar, Cambridge, Mass.) in 200 μl of culture medium alone or with an optimal concentration of 10 μg of rGRA4 or rROP2 or 5 μg of concanavalin A/ml as a positive control for proliferation. The plates were incubated in 5% CO2 at 37°C for 4 days and 1 μCi of [3H]thymidine (specific activity, 5 Ci/mmol; Amersham Corp.)/well was added for the final 24 h of culture. The cells were harvested, and radioactivity incorporation was measured in a liquid scintillation counter (LKB, Gaithersburg, Md.). Results were expressed as the stimulation index (SI), which is the mean value of counts per minute for recombinant antigen-stimulated cells/mean value of counts per minute for nonstimulated cells. This analysis was performed in three independent experiments.
Cytokine analysis.
Two weeks after the last booster injection, culture supernatants from spleen cells stimulated in vitro with 10 μg of rROP2 or rGRA4 or with concanavalin A were collected at 72 h (5 × 106 cells/ml of complete medium by duplicate in flat-bottom 24-well microtiter plates), harvested, and stored at −70°C until IFN-γ and interleukin-4 (IL-4) release was measured by ELISA. Briefly, microtiter plates (Immuno Plate Maxisorp; Nunc) were coated overnight at 4°C with 3 μg of capturing rat anti-mouse IFN-γ or IL-4 monoclonal antibodies (Pharmingen)/ml diluted in 0.1 M Na2HPO4 (pH 9). The wells were washed thoroughly with 0.05% Tween 20 in PBS. Empty binding sites were blocked by 2 h of incubation at 37°C with 1% bovine serum albumin in PBS. The supernatants from the cell cultures were tested in triplicate at 100 μl per well, and serial dilutions of recombinant murine IFN-γ or IL-4 (Pharmingen) were used at 20 to 4,000 pg/ml for the standard curves. After incubation for 1 h at 37°C, the plates were washed four times and 1 μg of biotinylated rat anti-mouse IFN-γ or IL-4 monoclonal antibodies (Pharmingen)/ml were added for 1 h at 37°C. Streptavidin peroxidase conjugate (Sigma) diluted 1:1,000 was then added to the washed wells and allowed to react for 30 min at 37°C. Bound complexes were detected by a solution of 0.15% H2O2-0.15% orthophenylene diamine (Sigma) in citrate (0.1 M)-phosphate (0.1 M) buffer (pH 4.5). Absorbance was read at 450 nm in an automatic ELISA reader (Dynatech MR4000). At least two independent ELISAs were performed for each supernatant, and the analysis was performed with results from three independent experiments.
Statistical analysis.
For statistical evaluation of data from proliferation assays, IFN-γ and IL-4 production, and brain cyst loads, results from vaccinated mice were compared to those from controls by a Student t test with Prism 2.01 software (GraphPad).
RESULTS
Protection efficacy of rGRA4- and rROP2-alum vaccination in mice.
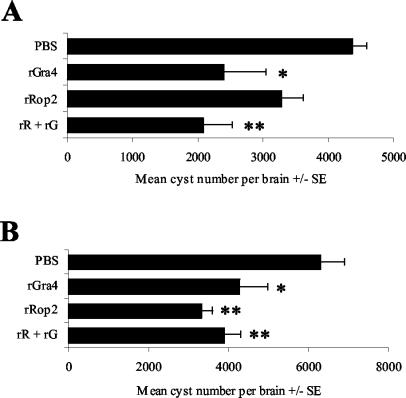
To evaluate the immunoprotective value of rGRA4 and rROP2 combined with alum against T. gondii infection, C57BL/6 and C3H mice were immunized intramuscularly with rGRA4- or rROP2-alum. Untreated mice or those that received PBS plus alum were used as negative controls. To determine whether the vaccination would provide any protection against the formation of T. gondii tissue cysts in the brain, vaccinated mice were challenged with a low dose of tissue cysts by peroral administration of 20 ME49 tissue cysts (Fig. 1). In these experiments, mice from both control and vaccinated groups survived for 4 weeks, at which time the animals were killed and their brains were removed for T. gondii tissue cyst enumeration. Control and vaccinated mice did not show any differences either in morbidity or in their mortality rates until the end of the experiment. C57BL/6 mice vaccinated with rGRA4 showed a significantly lower brain cyst burden (P < 0.01) than the control group (Fig. 1A), whereas vaccination with rROP2 failed to provide significant protection (P > 0.05). Mice receiving a mix of rGRA4 and rROP2 exhibited a significant resistance to cyst formation compared to the PBS-alum-vaccinated group (P < 0.01), but this resistance was not significantly different from that induced by rGRA4 alone (P > 0.05).
FIG. 1.
Assay for protection against oral challenge. C57BL/6 (A) and C3H (B) mice were given four intramuscular injections of PBS (control), rROP2, rGRA4, or rR+rG (mix of rROP2 and rGRA4) combined with alum, and 2 weeks after the last booster, they were orally infected with 20 cysts of the ME49 T. gondii strain. The brain parasite load was evaluated 1 month after infection. Values are means ± standard errors (SE) of the results from two similar experiments performed with groups of 8 mice. **, P < 0.01; *, P < 0.05 (Student's t test for comparisons between the PBS control group and the recombinant antigen treatment groups).
On the other hand, the brain cyst burden from C3H mice vaccinated with rGRA4 or rROP2 was significantly lower than that of the control group (P < 0.01) (Fig. 1B). Although rROP2-vaccinated mice showed fewer brain cysts than mice immunized with rGRA4, the difference between both groups was not significant. Coimmunization with rGRA4 and rROP2 also induced a significantly lower number of brain cysts than in the control group (P < 0.01), and this reduction had results similar to those of the rROP2-vaccinated mice. A parallel analysis of two infected naive mice showed a number of brain cysts similar to that of PBS-alum-immunized animals (data not shown).
In addition to determining whether it is possible to produce protection against chronic infection, we determined whether these vaccinations would provide any protection against mortality from acute toxoplasmosis. For these experiments, rGRA4-, rROP2-, or rGRA4-rROP2-immunized C57BL/6 and C3H mice were challenged perorally with a lethal dose of 100 ME49 tissue cysts. There were no significant differences in the survival rates from both strains of immunized mice compared to the control groups (data not shown).
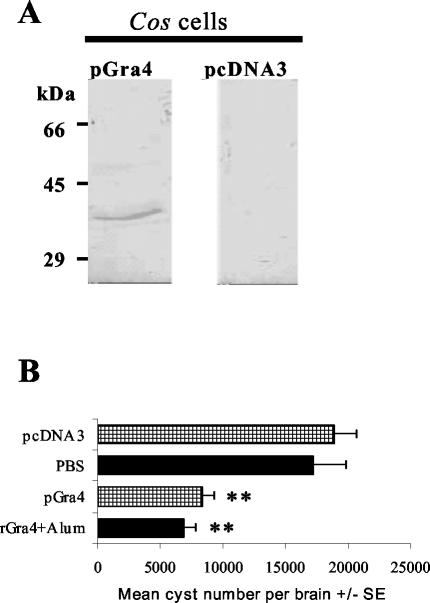
Since DNA vaccines showed induction of both humoral and cell-mediated immune responses with a Th1 pattern that could potentially provide protection against toxoplasmosis, we analyzed a gra4 gene (pGRA4) vaccination. Western blot analysis of a lysate from transfected Cos-7 cells indicated a band of the expected size for the complete protein (40 kDa) only in cells transfected with pGRA4 (Fig. 2A). Figure 2B shows that pGRA4-vaccinated C3H mice presented a significantly lower brain cyst burden than the control mice vaccinated with pcDNA3 alone (P < 0.01), and this protection result was similar to that induced by rGRA4-alum.
FIG. 2.
(A) Western blot analysis of a lysate of Cos-7 cells transfected with pGra4 or with empty pcDNA3 as a control. A mouse anti-rGRA4 serum sample was used as the first antibody. Molecular masses are given on the left. (B) Numbers of cysts per brain in C3H mice immunized with the gra4 gene (pcDNA3-GRA4), the empty plasmid (pcDNA3), the recombinant protein (rGRA4-alum), and its control (PBS-alum) after infection with 20 cysts of the ME49 T. gondii strain 2 weeks after the last booster. Brain parasite loads were evaluated 1 month after infection. **, P < 0.01 (Student's t test for comparisons between the control groups and the immunized groups).
Humoral responses.
To examine the vaccine potential of the recombinant proteins adsorbed to alum, blood samples were obtained 14 days after the immunization schedule was completed and IgG antibody titers were determined by ELISA, with the corresponding antigen as the bound target. All immunized C57BL/6 and C3H mice showed high IgG titers against rGRA4 and rROP2 (ranging from 48,600 to 97,200), whereas control groups did not react. Four weeks after the challenge with cysts (56 days after the last booster), all immunized mice showed increased levels of IgG titers (higher than 145,800) against the respective recombinant antigen used for the immunization. Control groups also showed reactivity to the recombinant antigens at this time (ranging from 24,300 to 145,800).
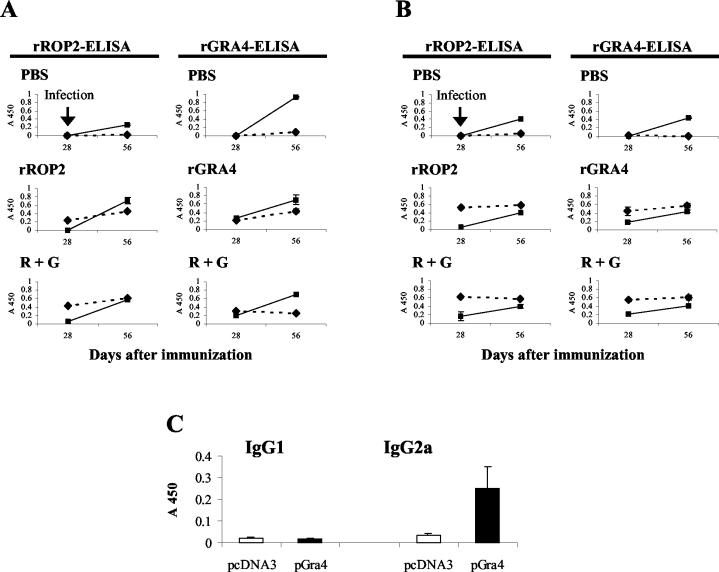
Figure 3A shows that, before the challenge (day 28 after the last booster), C57BL/6 mice immunized with rROP2 alone or with rROP2-rGRA4 exhibited a predominant anti-rROP2 IgG1 antibody response. In contrast, rGRA4- and rROP2-rGRA4-immunized mice showed similar anti-rGRA4 IgG1 and IgG2a isotype profiles (Fig. 3A). After the challenge (day 56), a remarkable anti-rROP2 IgG2a response was fired off in rROP2- and rROP2-rGRA4-vaccinated mice, changing the isotype pattern in mice immunized with rROP2 alone (Fig. 3A). Similarly, anti-rGRA4 IgG2a levels increased after infection compared to the IgG1 levels in both rGRA4- and rROP2-rGRA4-immunized mice (Fig. 3A). All groups of vaccinated C3H mice exhibited a predominant anti-rROP2 and -rGRA4 IgG1 antibody response after the last booster (Fig. 3B). The analysis of the response after the challenge showed an increase in the IgG2a levels for both proteins that was less pronounced against rGRA4 (Fig. 3B) and similar to the pattern observed in C57BL/6 mice (Fig. 3A). In contrast, the IgG1 levels from all immunized groups showed a slight increase or remained largely unaltered (Fig. 3B). Control groups from both strains of mice showed predominant anti-rROP2 and -rGRA4 IgG2a responses only after the oral challenge (Fig. 3A and B) that were similar to results with C3H mice, whereas C57BL/6 showed higher anti-rGRA4 antibody levels than rROP2.
FIG. 3.
Serum IgG subclass profiles from C57BL/6 (A) and C3H (B) mice vaccinated with rROP2, rGRA4, R + G (mix of rROP2 and rGRA4), or PBS combined with alum were determined 2 weeks after the last booster (day 28) and 4 weeks after oral infection with T. gondii cysts (day 56). Serum samples were analyzed by rROP2 or rGRA4 ELISA for the detection of IgG1 (▴ and dashed line) and IgG2a (▪). Sera were used in a 1:2,000 optimal dilution, as established in previous assays. Preimmune sera, obtained on day 0, were used in a 1:100 dilution, and no reactivity was observed. (C) Serum IgG subclass determination in sera from C3H mice immunized with the gra4 gene was analyzed by rGRA4 ELISA in a 1:100 dilution. All of the results were expressed as the A450 and are representative of one of two similar experiments.
All mice immunized with pGra4 showed low values of anti-rGRA4 IgG reactivity, with IgG2a, a marker of Th1 response, being the only isotype specifically detected (Fig. 3C).
Cellular proliferative responses and cytokine production.
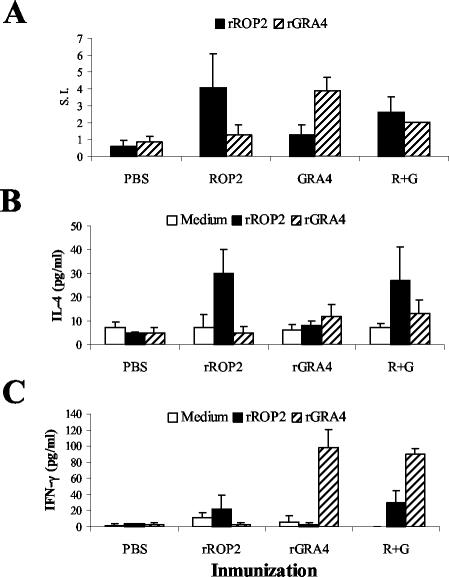
Two weeks after the last booster, single-cell preparations from spleens of rGRA4-, rROP2-, and rGRA4-rROP2-immunized C57BL/6 mice were prepared to assess the systemic proliferative responses to rGRA4 or rROP2 stimulation. Splenocytes from rROP2- or rGRA4-vaccinated mice showed specific proliferative responses to the respective antigen stimulation (Fig. 4A). Spleen cell suspensions from mice injected with rGRA4-rROP2 responded to both antigen stimulations (Fig. 4A). These proliferations were specific, since there was no expansion of spleen cells when they were cultured in the presence of the heterologous antigens (Fig. 4A), and control mice vaccinated with PBS-alum did not respond to rROP2 or rGRA4 stimulation. In vitro stimulation with concanavalin A induced the highest splenocyte proliferative responses, with the SI ranging from 14.3 to 20.5. Specific but less vigorous proliferative responses were also observed in spleen cell cultures from vaccinated C3H mice (data not shown).
FIG. 4.
Cellular response of splenocytes from C57BL/6 immunized mice. Splenocytes from vaccinated mice were harvested 2 weeks after the last booster. (A) Analysis of the proliferative response of cells from PBS-, rROP2-, rGRA4- and R+G (mix of rROP2 and rGRA4)-immunized mice combined with alum. After 4 days of in vitro stimulation with medium or 10 μg of rROP2 or rGRA4, [3H]thymidine was added for 24 h. The incorporated radioactivity was then measured. Results are expressed as the SI (the mean counts per minute of rROP2- or rGRA4-stimulated cells divided by the mean counts per minute of medium-stimulated cells). After 72 h of stimulation with medium, rROP2, or rGRA4, supernatants from cultured splenocytes were harvested and analyzed for the presence of IL-4 (B) and IFN-γ production (C) by ELISA. Results are representative of one of two similar experiments.
IL-4 and IFN-γ released from immune spleen cells stimulated in vitro with the recombinant antigens were measured in the culture supernatants. Following stimulation with rROP2, significant levels of IL-4 (Fig. 4B) and low levels of IFN-γ (Fig. 4C) were observed in supernatants of rROP2- and rROP2-rGRA4-primed splenocytes compared to unstimulated cells. In contrast, in response to rGRA4, stimulated rGRA4- and rROP2-rGRA4-primed splenocytes were found to produce lower IL-4 (Fig. 4B) and higher IFN-γ amounts (Fig. 4C) than cells from rROP2-inmmunized mice. In the same experiment, concanavalin A induced the highest cytokine release levels (120 pg/ml) for IL-4 and for IFN-γ (1,021 ng/ml). On the other hand, no IL-4 and IFN-γ release was evident in supernatants from spleen cells of immunized C3H mice after in vitro stimulation (data not shown).
DISCUSSION
This study has shown that vaccination with recombinant forms of GRA4 and ROP2 proteins of T. gondii and the mix of both combined with alum can elicit both humoral and cellular responses in mice. The responses elicited by rGRA4 are capable of reducing the levels of tissue cysts in the brains of infected mice with different genetic backgrounds, whereas those elicited by rROP2 result in protection only in the C3H strain. In addition, we show that in the C3H strain, gra4 gene vaccination reduces the parasite load to a similar level as rGRA4-alum vaccination.
Regarding ROP2 antigen, Vercammen et al. (49) showed that immunization with the rop2 gene, as well as with the gra1 and gra7 genes, protected C3H mice against a lethal challenge but not BALB/c or C57BL/6 mice. Similarly, in our study we observed that rROP2 vaccination resulted in protection in C3H but not in C57BL/6 mice after a nonlethal challenge with cysts. These results make evident the importance of using several mouse strains in the analysis of a vaccine against toxoplasmosis.
Vaccination of both mouse strains with rGRA4 leads to a substantial reduction in the cyst burden. These results are consistent with those of the study by Mévélec et al. (33) in which C57BL/6 mice were orally immunized with an rGRA4 combined with cholera toxin. In addition, Desolme et al. (12) observed that vaccination with the gra4 gene induced a protective immunity in C57BL/6 mice against a lethal challenge with tissue cysts, suggesting protection against acute infection. In our study, gra4 gene vaccination induces partial protection against a nonlethal challenge for C3H mice. These data suggest that GRA4 is a good candidate for the development of a vaccine against toxoplasmosis.
Fachado et al. (16) have recently shown that immunization with a mix of sag1 and rop2 genes induced higher protection against the highly virulent RH strain than single-gene vaccination of BALB/c mice. However, in our study, immunization with a mix of rGRA4 and rROP2 results in significant protection in both mouse strains compared to the control groups, but the level of protection is similar to that obtained after immunization with the single antigens. Moreover, both proteins elicit a complementary immune response: rGRA4 induces higher protection in C57BL/6 mice and rROP2 induces higher protection in C3H mice. One possible explanation for this could be that the immune response elicited against each of the parasite antigens differs with the mouse strain. Recently, it was observed that 22 women with documented exposure to T. gondii showed a heterogeneous cellular stimulatory response against GRA1, GRA6, GRA7, and SAG1 (17).
Adjuvants are an essential part in the design of a vaccine due to both the immunoadjuvant effect and the possible use for animals and humans. DNA vaccines have been shown to elicit a predominant Th1 response (47), suitable for the requirements to generate immunity against toxoplasmosis. In fact, several studies regarding systemic DNA vaccination against T. gondii were conducted with mice (see reference 5). However, DNA vaccines were not shown to be very immunogenic in humans (43). In contrast, aluminum compounds have been used as human vaccine adjuvants for more than 70 years. Although alum has been associated with the induction of high levels of antibodies and Th2-type responses (22), rSAG1 combined with alum (40), and here, rGRA4 and rROP2, was shown to induce partial immunity against T. gondii infection in mice. An expected strong humoral response against rGRA4 and rROP2 was generated for both mouse strains. Immunization of C57BL/6 mice with rGRA4-alum leads to the production of similar IgG1 and IgG2a levels, whereas rROP2-alum immunization results in a predominant IgG1 production characteristic of a Th2-type response. The same isotype pattern is observed with coimmunization with both antigens. In contrast, the response in vaccinated C3H mice was polarized towards a Th2-type response for each antigen and the mix. In addition, the results of cytokine production by in vitro-stimulated splenocytes from immunized C57BL/6 mice support the conclusion that vaccination with rGRA4 preferentially induces a Th1 response and rROP2 induces a Th2 response. In fact, little production of IFN-γ was observed in C57BL/6 mice immunized with rGRA4. We consider that immunity can be due, in part, to the presence of IFN-γ and/or by a specific B-cell-response induction. Recently, the B cell contribution to protection against parasite infection was further demonstrated (23, 24, 42). In our study, even though immunization of C3H mice with the recombinant antigens induced protection, we were unable to detect IFN-γ in cultured splenocytes; and although both C57BL/6 and C3H mice vaccinated with rROP2 elicit an IgG1-polarized humoral response, significant protection was observed only in C3H animals. It could be that T-cell presentation of rROP2 is under haplotype restriction. So other factors might be taken into account to explain these results.
Alum has been shown to increase the expression of major histocompatibility complex class II and costimulatory or adhesion molecules (ICAM-1, LFA-3, and CD40) associated with mature dendritic cells (DCs) (48). In addition, stimulation of peripheral blood monocytes with alum induced an increase in IL-1α, IL-1β, and tumor necrosis factor mRNA expression as well as IL-4 and IL-6 (48). Dimier-Poisson et al. (13) have recently demonstrated that mesenteric lymph node DCs pulsed ex vivo with T. gondii antigens led to a Th2 cytokine secretion pattern, eliciting strong protection either in C57BL/6 or CBA/J mice, whereas spleen DCs led to a Th1 cytokine pattern, also producing protection but with lower rates than mucosal DCs. On one hand, it is likely that a more efficient antigenic presentation due to the immunoadjuvant effect of alum could also be an important factor in conferring immunity, possibly allowing the host to induce a faster increase of systemic IFN-γ production after infection. Interestingly, in this study, immunized mice show an important postchallenge increased of anti-recombinant antigen IgG2a antibodies but not of the IgG1 isotype. On the other hand, a Th2-related response could have a certain protective value. Interestingly, these data suggest that the immune response generated by immunization with alum does not seem to interfere with the immune response generated by T. gondii, nor does it produce adverse pathological effects after infection.
Our results and those obtained by Petersen et al. (40) suggest that rGRA4, rROP2, and rSAG1 should be components of an alum-based multiantigen vaccine against toxoplasmosis. Finally, our results reinforce the value of alum as a possible adjuvant to be used in immunization against T. gondii, allowing the development of a vaccine for wide application for either humans or animals. We consider that combinations with other effective antigens that generate immunity by different strategies should also be taken into account in the future.
Acknowledgments
This work was supported by an ANPCyT grant (BID802/OC-AR-PICT 05-04831) and INEI (ANLIS Dr. Carlos G. Malbran). S.O.A. (Researcher), V.M. (Fellow), and P.E. (Fellow) are members of the National Council of Research (CONICET). S.O.A. is also member of the University of Buenos Aires (Departamento de Fisiología, Biología Molecular y Celular, FCEyN). V.M. (Fellow) is also a member of the Fundación Antorchas.
We thank Mara Rosenzvit for critical reading of the manuscript and helpful suggestions. We acknowledge the contribution of Angel Sinagra (animal manipulation assistance).
REFERENCES
- 1.Alexander, J., H. Jebbari, H. Bluethmann, A. Satoskar, and C. W. Roberts. 1996. Immunological control of Toxoplasma gondii vaccine design. Curr. Top. Microbiol. Immunol. 219:183-195. [DOI] [PubMed] [Google Scholar]
- 2.Angus, C. W., D. Klivington-Evans, J. P. Dubey, and J. A. Kovacs. 2000. Immunization with a DNA plasmid encoding the SAG1 (P30) protein of Toxoplasma gondii is immunogenic and protective in rodents. J. Infect. Dis. 181:317-324. [DOI] [PubMed] [Google Scholar]
- 3.Aosai, F., H.-S. Mun, K. Norose, M. Chen, H. Hata, M. Kobayashi, M. Kiuchi, H. J. Stauss, and A. Yano. 1999. Protective immunity induced by vaccination with SAG1 gene-transfected cells against Toxoplasma gondii infection in mice. Microbiol. Immunol. 43:87-91. [DOI] [PubMed] [Google Scholar]
- 4.Blackwell, J. M., C. W. Roberts, and J. Alexander. 1993. Influence of genes within the MHC on mortality and brain cyst development in mice infected with Toxoplasma gondii: kinetics of immune regulation in BALB H-2 congenic mice. Parasite Immunol. 15:317-324. [DOI] [PubMed] [Google Scholar]
- 5.Bout, D. T., M.-N. Mévélec, F. Velge-Roussel, I. Dimier-Poisson, and M. Lebrun. 2002. Prospects for a human Toxoplasma vaccine. Curr. Drug Targets Immune Endocr. Metabol. Disord. 2:227-234. [DOI] [PubMed] [Google Scholar]
- 6.Brown, C. R., C. A. Hunter, R. G. Estes, E. Beckmann, J. Forman, C. David, J. S. Remington, and R. McLeod. 1995. Definitive identification of a gene that confers resistance against toxoplasmosis. Immunology 85:419-428. [PMC free article] [PubMed] [Google Scholar]
- 7.Bulow, R., and J. C. Boothroyd. 1991. Protection of mice from fatal Toxoplasma gondii infection by immunization with p30 antigen in liposomes. J. Immunol. 147:3496-3500. [PubMed] [Google Scholar]
- 8.Buxton, D., K. Thomson, S. Maley, S. Wright, and H. J. Bos. 1991. Vaccination of sheep with a live incomplete strain (S48) of Toxoplasma gondii and their immunity to challenge when pregnant. Vet. Rec. 129:89-93. [DOI] [PubMed] [Google Scholar]
- 9.Couper, K. N., H. V. Nielsen, E. Petersen, F. Roberts, C. W. Roberts, and J. Alexander. 2003. DNA vaccination with the immunodominant tachyzoite surface antigen (SAG-1) protects against adult acquired Toxoplasma gondii infection but does not prevent maternofetal transmission. Vaccine 21:2813-2820. [DOI] [PubMed] [Google Scholar]
- 10.Darcy, F., P. Maes, H. Gras-Masse, C. Auriault, M. Bossus, D. Deslee, I. Godard, M. F. Cesbron, A. Tartar, and A. Capron. 1998. Protection of mice and nude rats against toxoplasmosis by a multiple antigenic peptide construction derived from Toxoplasma gondii P30 antigen. J. Immunol. 149:3636-3641. [PubMed] [Google Scholar]
- 11.Debard, N., D. Buzoni-Gatel, and D. Bout. 1996. Intranasal immunization with SAG1 protein of Toxoplasma gondii in association with cholera toxin dramatically reduces development of cerebral cysts after oral infection. Infect. Immun. 64:2158-2166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Desolme, B., M.-N. Mévélec, D. Buzoni-Gatel, and D. Bout. 2000. Induction of protective immunity against toxoplasmosis in mice by DNA immunization with a plasmid encoding Toxoplasma gondii Gra4 gene. Vaccine 18:2512-2521. [DOI] [PubMed] [Google Scholar]
- 13.Dimier-Poisson, I., F. Aline, M.-N. Mévélec, C. Beauvillain, D. Buzoni-Gatel, and D. Bout. 2003. Protective mucosal Th2 immune response against Toxoplasma gondii by murine mesenteric lymph node dendritic cells. Infect. Immun. 71:5254-5264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dubey, J. P. 1990. Status of toxoplasmosis in sheep and goats in the United States. J. Am. Vet. Med. Assoc. 196:259-262. [PubMed] [Google Scholar]
- 15.Duncanson, P., R. S. Terry, J. E. Smith, and G. Hide. 2001. High levels of congenital transmission of Toxoplasma gondii in a commercial sheep flock. Int. J. Parasitol. 31:1699-1703. [DOI] [PubMed] [Google Scholar]
- 16.Fachado, A., A. Rodriguez, S. O. Angel, D. Pinto, I. Vila, A. Acosta, R. M. Amendoeira, and J. Lannes-Vieira. 2003. Protective effect of a naked DNA vaccine cocktail against lethal toxoplasmosis in mice. Vaccine 21:1327-1335. [DOI] [PubMed] [Google Scholar]
- 17.Fatoohi, A. F., G. J. N. Cozon, T. Greenland, J. Ferrandiz, J. Bievenu, S. Picot, and F. Peyron. 2002. Cellular immune responses to recombinant antigens in pregnant women chronically infected with Toxoplasma gondii. Clin. Diagn. Lab. Immunol. 9:704-707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fermin, Z., D. Bout, P. Ricciardi-Castagnoli, and J. Hoebeke. 1999. Salbutamol as an adjuvant for nasal vaccination. Vaccine 17:1936-1941. [DOI] [PubMed] [Google Scholar]
- 19.Frenkel, J. K., and A. Escajadillo. 1987. Cyst rupture as a pathogenic mechanism of toxoplasmic encephalitis. Am. J. Trop. Med. Hyg. 36:517-522. [DOI] [PubMed] [Google Scholar]
- 20.Gazzinelli, R. T., F. T. Hakim, S. Hieny, G. M. Shearer, and A. Sher. 1991. Synergistic role of CD4+ and CD8+ T lymphocytes in IFN-gamma production and protective immunity induced by an attenuated Toxoplasma gondii vaccine. J. Immunol. 146:286-293. [PubMed] [Google Scholar]
- 21.Gazzinelli, R. T., M. Wysocka, S. Hayashi, E. Y. Denkers, S. Hieny, P. Caspar, G. Trinchieri, and A. Sher. 1994. Parasite induced IL-12 stimulates early IFN-γ synthesis and resistance during acute infection with Toxoplasma gondii. J. Immunol. 153:2533-2543. [PubMed] [Google Scholar]
- 22.Grun, J. L., and P. H. Maurer. 1989. Different T helper cell subsets elicited in mice utilising two different adjuvant vehicles: the role of endogenous IL-1 in proliferative responses. Cell Immunol. 121:134-145. [DOI] [PubMed] [Google Scholar]
- 23.Johnson, L. L., and P. C. Sayles. 2002. Deficient humoral responses underlie susceptibility to Toxoplasma gondii in CD4-deficient mice. Infect. Immun. 70:185-191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang, H., J. S. Remington, and Y. Suzuki. 2000. Decreased resistance of B cell-deficient mice to infection with Toxoplasma gondii despite unimpaired expression of IFN-gamma, TNF-alpha, and inducible nitric oxide synthase. J. Immunol. 164:2629-2634. [DOI] [PubMed] [Google Scholar]
- 25.Kasper, L. H., K. M. Currie, and M. S. Bradley. 1985. An unexpected response to vaccination with purified major membrane tachyzoite antigen (P30) of Toxoplasma gondii. J. Immunol. 134:3426-3431. [PubMed] [Google Scholar]
- 26.Khan, I. A., K. H. Ely, and L. H. Kasper. 1994. Antigen-specific CD8+ T cell clone protects against acute Toxoplasma gondii infection in mice. J. Immunol. 152:1856-1860. [PubMed] [Google Scholar]
- 27.Letscher-Bru, V., O. Villard, B. Risse, M. Zauke, J. P. Klein, and T. T. Kien. 1998. Protective effect of vaccination with a combination of recombinant surface antigen 1 and interleukin-12 against toxoplasmosis in mice. Infect. Immun. 66:4503-4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leyva, R., P. Herion, and R. Saavedra. 2001. Genetic immunization with plasmid DNA coding for the ROP2 protein of Toxoplasma gondii. Parasitol. Res. 87:70-79. [DOI] [PubMed] [Google Scholar]
- 29.Lunden, A., S. F. Parmley, K. L. Bengtsson, and F. G. Araujo. 1997. Use of a recombinant antigen, SAG2, expressed as a glutathione-S-transferase fusion protein to immunize mice against Toxoplasma gondii. Parasitol. Res. 83:6-9. [DOI] [PubMed] [Google Scholar]
- 30.Martin, V., M. Arcavi, M. R. R. Amendoeira, E. de Souza Neves, G. Santillán, E. Guarnera, J. C. Garberi, and S. O. Angel. 1998. Detection of human anti-Toxoplasma specific immunoglobulins A, M and G by a recombinant Toxoplasma gondii Rop2 protein. Clin. Diagn. Lab. Immunol. 5:627-631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McLeod, R., P. Eisenhauer, D. Mack, C. Brown, G. Filice, and G. Spitainy. 1989. Immune responses associated with early survival after peroral infection with Toxoplasma gondii. J. Immunol. 142:3247-3255. [PubMed] [Google Scholar]
- 32.Mévélec, M.-N., T. Chardès, O. Mercereau-Puijalon, I. Bourguin, A. Achbarou, J. F. Dubremetz, and D. Bout. 1992. Molecular cloning of GRA4, a Toxoplasma gondii dense granule protein, recognized by mucosal IgA antibodies. Mol. Biochem. Parasitol. 56:227-238. [DOI] [PubMed] [Google Scholar]
- 33.Mévélec, M. N., O. Merecerau-Puijalon, D. Buzoni-Gatel, I. Bourguin, T. Chardes, J. F. Dubremetz, and D. Bout. 1998. Mapping of B epitopes in Gra4, a dense granule antigen of Toxoplasma gondii and protection studies using recombinant proteins administered by the oral route. Parasite Immunol. 20:183-195. [PubMed] [Google Scholar]
- 34.Mishima, M., X. Xuan, A. Shioda, Y. Omata, K. Fujisaki, H. Nagasawa, and T. Mikami. 2001. Modified protection against Toxoplasma gondii lethal infection and brain cyst formation by vaccination with SAG2 and SRS1. J. Vet. Med. Sci. 63:433-438. [DOI] [PubMed] [Google Scholar]
- 35.Mohamed, R. M., F. Aosai, M. Chen, H.-S. Mun, K. Norose, U. S. Belal, L.-X. Piao, and A. Yano. 2003. Induction of protective immunity by DNA vaccination with Toxoplasma gondii HSP70, HSP30 and SAG1 genes. Vaccine 21:2852-2861. [DOI] [PubMed] [Google Scholar]
- 36.Nielsen, H. V., S. L. Lauemoller, L. Christiansen, S. Buus, A. Fomsgaard, and E. Petersen. 1999. Complete protection against lethal Toxoplasma gondii infection in mice immunized with a plasmid encoding the SAG1 gene. Infect. Immun. 67:6358-6363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Nigro, M., V. Martin, F. Kaufer, L. Carral, S. O. Angel, and V. Pszenny. 2001. High level of expression of the Toxoplasma gondii-recombinant Rop2 protein in Escherichia coli as a soluble form for optimal use in diagnosis. Mol. Biotechnol. 18:269-273. [DOI] [PubMed] [Google Scholar]
- 38.Nigro, M., A. Gutierrez, A. M. Hoffer, M. Clemente, F. Kaufer, L. Carral, V. Martin, E. A. Guarnera, and S. O. Angel. 2003. Evaluation of Toxoplasma gondii recombinant proteins for the diagnosis of recently acquired toxoplasmosis by an immunoglobulin G analysis. Diagn. Microbiol. Infect. Dis. 47:609-613. [DOI] [PubMed] [Google Scholar]
- 39.Parmley, S., T. Slifer, and F. Araujo. 2002. Protective effects of immunization with a recombinant cyst antigen in mouse models of infection with Toxoplasma gondii tissue cysts. J. Infect. Dis. 15:90-95. [DOI] [PubMed] [Google Scholar]
- 40.Petersen, E., H. V. Nielsen, L. Christiansen, and J. Spenter. 1998. Immunization with E. coli produced recombinant T. gondii SAG1 with alum as adjuvant protect mice against lethal infection with Toxoplasma gondii. Vaccine 16:1283-1289. [DOI] [PubMed] [Google Scholar]
- 41.Saavedra, R., F. de Meuter, J.-L. Decourt, and P. Hérion. 1991. Human T cell clone identifies a potentially partially protective 54-kDa protein antigen of Toxoplasma gondii cloned and expressed in Escherichia coli. J. Immunol. 147:1975-1982. [PubMed] [Google Scholar]
- 42.Sayles, P. C., G. W. Gibson, and L. L. Johnson. 2000. B cells are essential for vaccination-induced resistance to virulent Toxoplasma gondii. Infect. Immun. 68:1026-1033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Scheerlinck, J. P., G. Casey, P. McWaters, J. Kelly, D. Woollard, M. W. Lightowlers, J. M. Tennent, and P. J. Chaplin. 2001. The immune response to a DNA vaccine can be modulated by co-delivery of cytokine genes using a DNA prime-protein boost strategy. Vaccine 19:4053-4060. [DOI] [PubMed] [Google Scholar]
- 44.Suzuki, Y., M. A. Orellana, R. D. Schreiber, and J. S. Remington. 1988. Interferon-gamma: the major mediator of resistance against Toxoplasma gondii. Science 240:516-518. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki, Y., K. Joh, M. A. Orellana, F. K. Conley, and J. S. Remington. 1991. A gene(s) within the H-2D region determines the development of toxoplasmic encephalitis in mice. Immunology 74:732-739. [PMC free article] [PubMed] [Google Scholar]
- 46.Tenter, A. M., A. R. Heckeroth, and L. M. Weiss. 2000. Toxoplasma gondii: from animals to humans. Int. J. Parasitol. 30:1217-1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Tighe, H., M. Corr, M. Roman, and E. Raz. 1998. Gene vaccination: plasmid DNA is more than just a blueprint. Immunol. Today 19:89-97. [DOI] [PubMed] [Google Scholar]
- 48.Ulanova, M., A. Tarkowski, M. Hahn-Zoric, and L. A. Hanson. 2001. The common vaccine adjuvant aluminum hydroxide up-regulates accessory properties of human monocytes via an interleukin-4-dependent mechanism. Infect. Immun. 69:1151-1159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vercammen, M., T. Scorza, K. Huygen, J. De Braekeleer, R. Diet, D. Jacobs, E. Saman, and H. Verschueren. 2000. DNA vaccination with genes encoding Toxoplasma gondii antigens GRA1, GRA7, and ROP2 induces partially protective immunity against lethal challenge in mice. Infect. Immun. 68:38-45. [DOI] [PMC free article] [PubMed] [Google Scholar]